Calcitriol, an Active Form of Vitamin D3, Mitigates Skin Barrier Dysfunction in Atopic Dermatitis NC/Nga Mice
(This article belongs to the Section Molecular Biology)
Abstract
1. Introduction
2. Results
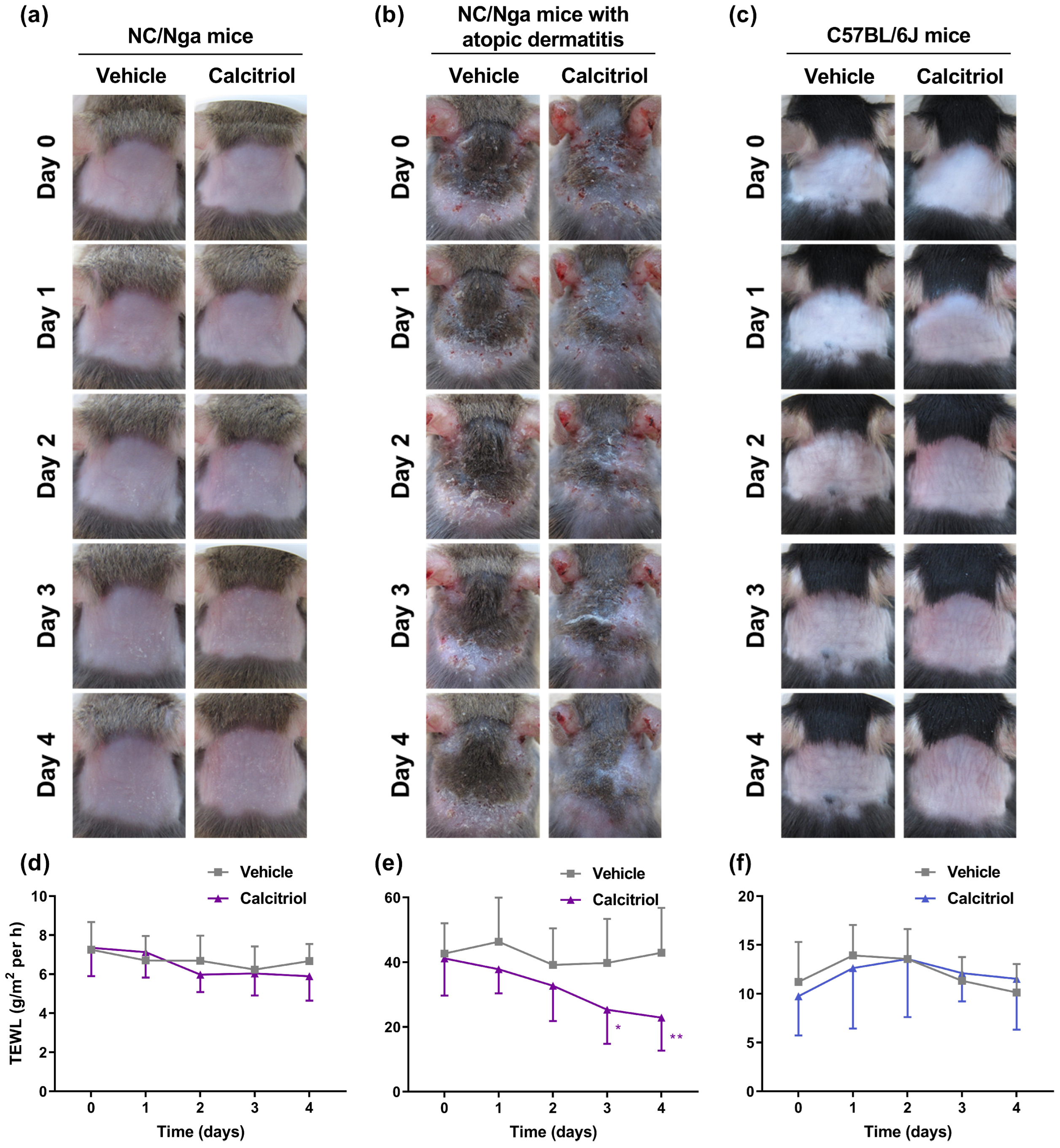
2.1. Topical Application of Calcitriol Improves the Skin Condition and TEWL in NC/Nga Mice with Atopic Dermatitis
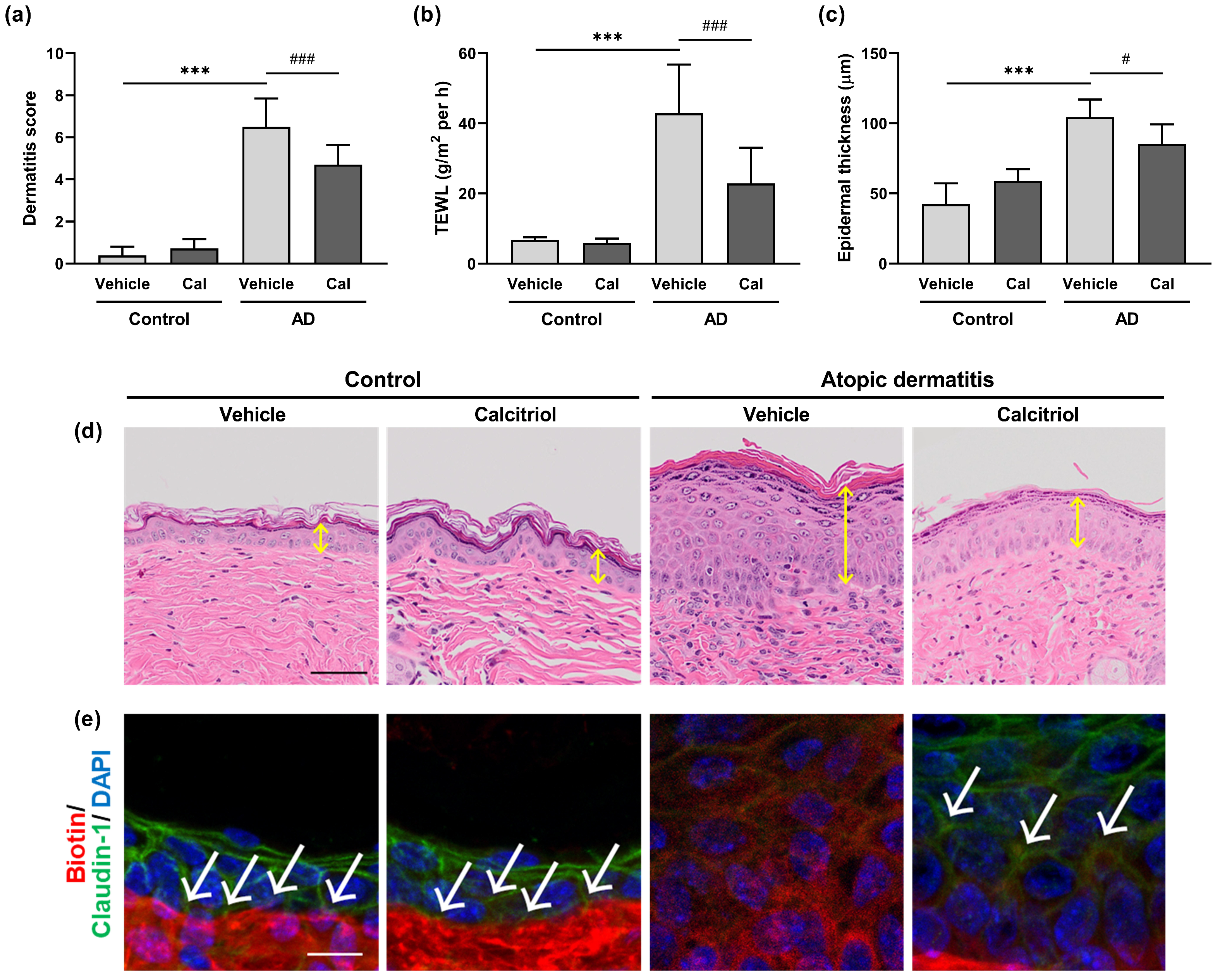
2.2. Calcitriol Improves the Symptoms of Atopic Dermatitis in Dfb-Treated NC/Nga Mice
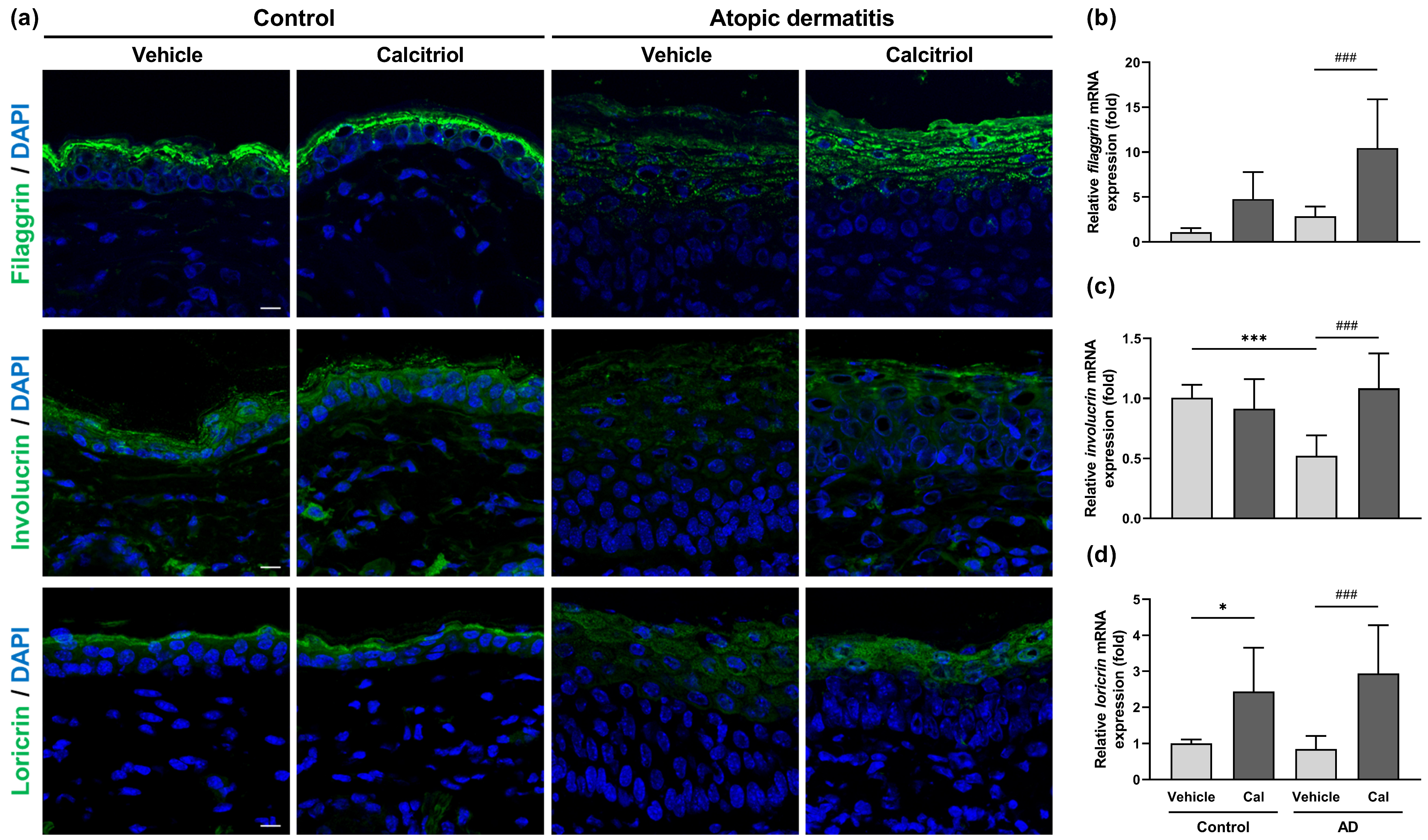
2.3. Effects of Calcitriol on the Expression of Skin Barrier-Related Proteins
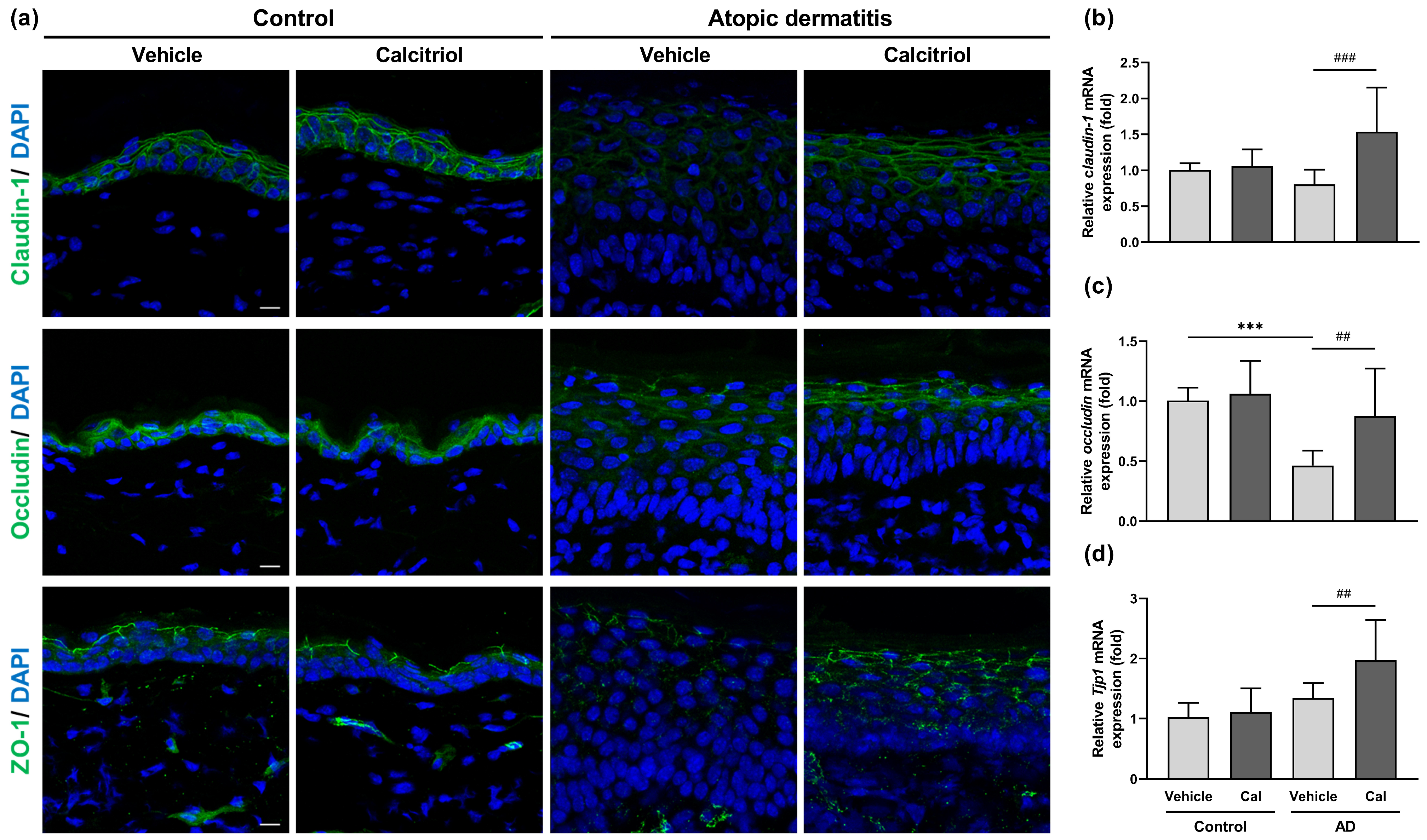
2.4. Effects of Calcitriol on the Expression of Tight Junction-Related Proteins
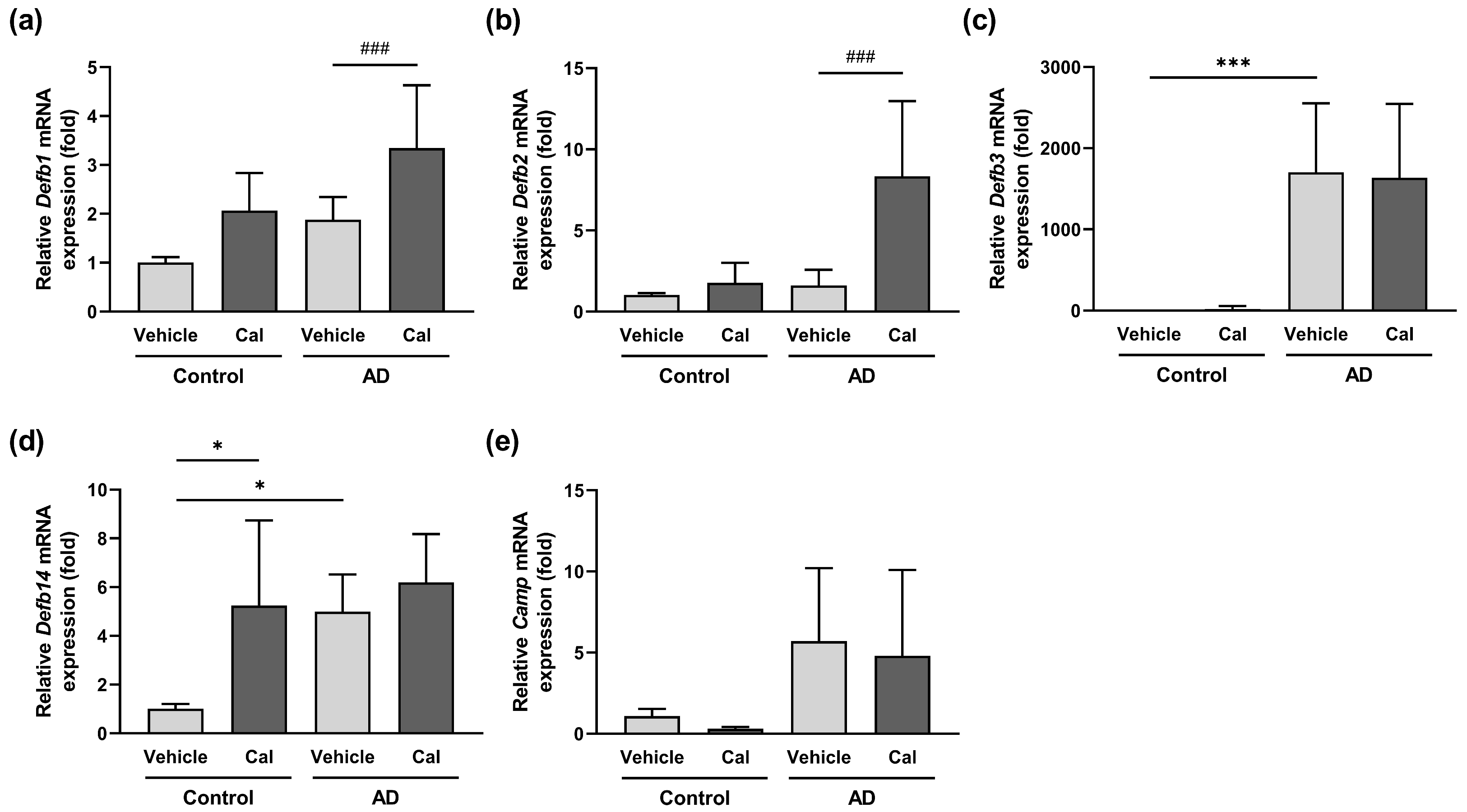
2.5. Effects of Calcitriol on the Expression of Skin-Derived Antimicrobial Peptides in NC/Nga Mice
2.6. Effects of Calcitriol on Cytokine Expression in the Skin of NC/Nga Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
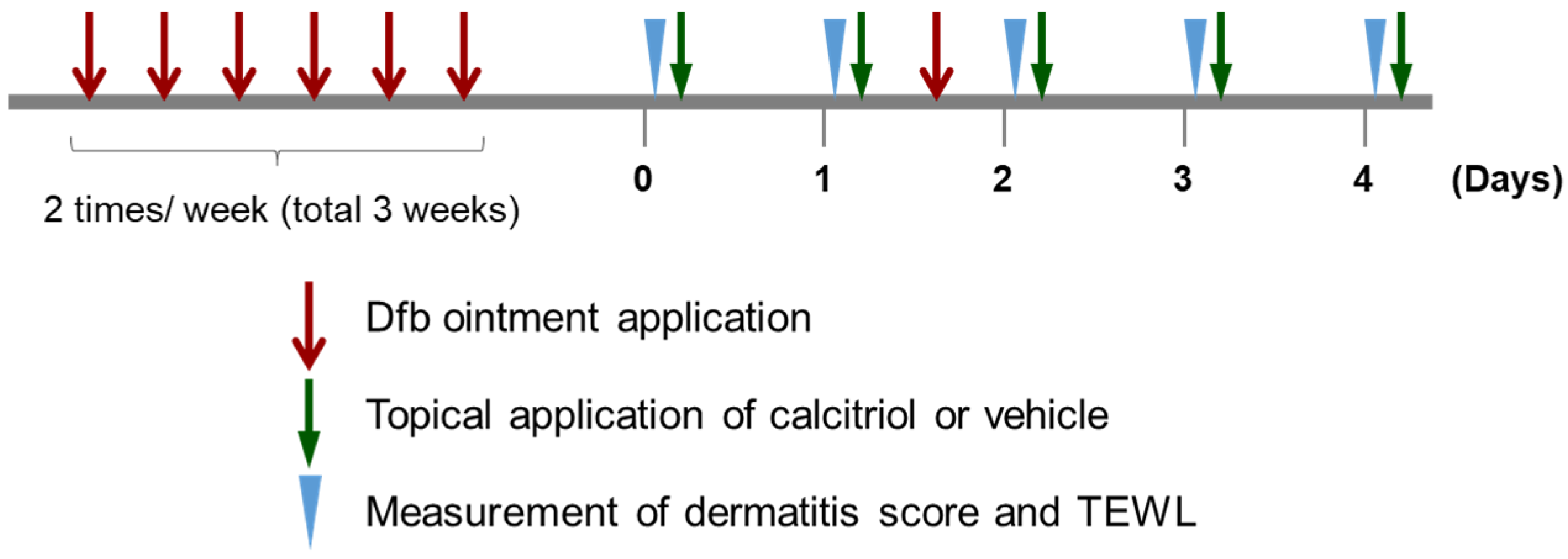
4.3. Induction of Dermatitis and Treatment of Mice
4.4. Evaluation of Skin Lesions and Condition
4.5. Histological and Immunohistochemical Analysis
4.6. Tight Junction Permeability Assay
4.7. Total RNA Preparation and Quantitative Real-Time PCR Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular mechanisms of atopic dermatitis pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Umehara, Y.; Kiatsurayanon, C.; Trujillo-Paez, J.V.; Chieosilapatham, P.; Peng, G.; Yue, H.; Nguyen, H.L.T.; Song, P.; Okumura, K.; Ogawa, H.; et al. Intractable itch in atopic dermatitis: Causes and treatments. Biomedicines 2021, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Cork, M.J.; Amagai, M.; De Benedetto, A.; Kabashima, K.; Hamilton, J.D.; Rossi, A.B. Type 2 inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innov. 2022, 2, 100131. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e1-7. [Google Scholar] [CrossRef]
- Eckert, R.L.; Sturniolo, M.T.; Broome, A.-M.; Ruse, M.; Rorke, E.A. Transglutaminase function in epidermis. J. Investig. Derm. 2005, 124, 481–492. [Google Scholar] [CrossRef]
- Brown, S.J.; Irwin McLean, W.H. One remarkable molecule: Filaggrin. J. Investig. Derm. 2012, 132, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, Y.; Roop, D.R. Loricrin: Past, present, and future. Int. J. Mol. Sci. 2020, 21, 2271. [Google Scholar] [CrossRef]
- Brandner, J.M.; Kief, S.; Grund, C.; Rendl, M.; Houdek, P.; Kuhn, C.; Tschachler, E.; Franke, W.W.; Moll, I. Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur. J. Cell Biol. 2002, 81, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Tokumasu, R.; Yamaga, K.; Yamazaki, Y.; Murota, H.; Suzuki, K.; Tamura, A.; Bando, K.; Furuta, Y.; Katayama, I.; Tsukita, S. Dose-dependent role of claudin-1 in vivo in orchestrating features of atopic dermatitis. Proc. Natl. Acad. Sci. USA 2016, 113, E4061–E4068. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell. Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Sugawara, T.; Iwamoto, N.; Akashi, M.; Kojima, T.; Hisatsune, J.; Sugai, M.; Furuse, M. Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice. J. Derm. Sci. 2013, 70, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chen, S.-C.; Hsu, S.-Y.; Lin, Y.-A.; Shih, C.-M.; Huang, C.-Y.; Wang, K.-H.; Lee, A.-W. Annoying psoriasis and atopic dermatitis: A narrative review. Int. J. Mol. Sci. 2022, 23, 4898. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Tsai, T.-F. Overlapping features of psoriasis and atopic dermatitis: From genetics to immunopathogenesis to phenotypes. Int. J. Mol. Sci. 2022, 23, 5518. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Wierzbicka, J.; Żmijewski, M.A. Vitamin D in the skin physiology and pathology. Acta Biochim. Pol. 2016, 63, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Kechichian, E.; Ezzedine, K. Vitamin D and the skin: An update for dermatologists. Am. J. Clin. Derm. 2017, 19, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Searing, D.A.; Leung, D.Y. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol. Allergy Clin. N. Am. 2010, 30, 397–409. [Google Scholar] [CrossRef]
- Oren, E.; Banerji, A.; Camargo, C.A. Vitamin D and atopic disorders in an obese population screened for vitamin D deficiency. J. Allergy Clin. Immun. 2008, 121, 533–534. [Google Scholar] [CrossRef]
- Kanda, N.; Hau, C.S.; Tada, Y.; Sato, S.; Watanabe, S. Decreased serum LL-37 and vitamin D3 levels in atopic dermatitis: Relationship between IL-31 and oncostatin M. Allergy 2012, 67, 804–812. [Google Scholar] [CrossRef]
- Samochocki, Z.; Bogaczewicz, J.; Jeziorkowska, R.; Sysa-Jedrzejowska, A.; Glinska, O.; Karczmarewicz, E.; McCauliffe, D.P.; Wozniacka, A. Vitamin D effects in atopic dermatitis. J. Am. Acad. Derm. 2013, 69, 238–244. [Google Scholar] [CrossRef]
- Camargo, C.A.; Ganmaa, D.; Sidbury, R.; Erdenedelger, K.; Radnaakhand, N.; Khandsuren, B. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. J. Allergy Clin. Immun. 2014, 134, 831–835.e831. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.; Riedel, R.; Jörß, K.; Loddenkemper, C.; Steinmeyer, A.; Zügel, U.; Babina, M.; Radbruch, A.; Worm, M. Vitamin D receptor activation improves allergen-triggered eczema in mice. J. Investig. Derm. 2012, 132, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Tukaj, S.; Zillikens, D.; Kasperkiewicz, M. Topically applied low-dose calcitriol ameliorates atopic eyelid dermatitis. JAAD Case Rep. 2019, 5, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Watanabe, N.; Geba, G.P.; Sperl, J.; Tsudzuki, M.; Hiroi, J.; Matsumoto, M.; Ushio, H.; Saito, S.; Askenase, P.W.; et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Reich, K.; Keren, A.; Kabashima, K.; Steinhoff, M.; Paus, R. Mouse models of atopic dermatitis: A critical reappraisal. Exp. Derm. 2021, 30, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Maillet, I.; Mackowiak, C.; Viala, C.; Di Padova, F.; Li, M.; Togbe, D.; Quesniaux, V.; Lai, Y.; Ryffel, B. Experimental atopic dermatitis depends on IL-33R signaling via MyD88 in dendritic cells. Cell Death Dis. 2017, 8, e2735. [Google Scholar] [CrossRef]
- Keith, Y.H.; Honda, T.; Ono, S.; Lee, B.; Shibuya, R.; Hanakawa, S.; Ishida, Y.; Nakamizo, S.; Kabashima, K. Infiltration and local differentiation of bone marrow-derived integrinβ7-positive mast cell progenitors in atopic dermatitis-like skin. J. Allergy Clin. Immunol. 2023, 151, 159–171.e158. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yokozeki, H.; Karasuyama, H.; Satoh, T. IL-31-generating network in atopic dermatitis comprising macrophages, basophils, thymic stromal lymphopoietin, and periostin. J. Allergy Clin. Immunol. 2022, 151, 737–746.e6. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhu, Z.; Liu, X.; Chen, B.; Yin, H.; Gu, C.; Fang, X.; Zhu, R.; Yu, T.; Mi, W.; et al. A dysregulated sebum–microbial metabolite–IL-33 axis initiates skin inflammation in atopic dermatitis. J. Exp. Med. 2022, 219, e20212397. [Google Scholar] [CrossRef]
- Naidoo, K.; Jagot, F.; van den Elsen, L.; Pellefigues, C.; Jones, A.; Luo, H.; Johnston, K.; Painter, G.; Roediger, B.; Lee, J.; et al. Eosinophils determine dermal thickening and water loss in an MC903 model of atopic dermatitis. J. Investig. Derm. 2018, 138, 2606–2616. [Google Scholar] [CrossRef]
- Kim, S.H.; Seong, G.S.; Choung, S.Y. Fermented Morinda citrifolia (Noni) alleviates DNCB-induced atopic dermatitis in NC/Nga mice through modulating immune balance and skin barrier junction. Nutrients 2020, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, M.J.; Bak, D.H.; Lee, B.C.; Ko, E.J.; Ahn, G.R.; Ahn, S.W.; Kim, M.J.; Na, J.; Kim, B.J. Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice. Sci. Rep. 2018, 8, 11895. [Google Scholar] [CrossRef]
- Tominaga, M.; Ozawa, S.; Ogawa, H.; Takamori, K. A hypothetical mechanism of intraepidermal neurite formation in NC/Nga mice with atopic dermatitis. J. Derm. Sci. 2007, 46, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Nestel, F.P.; Bourdeau, V.r.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.I.; Morrison, G.M.; Dorin, J.R. Rapid sequence divergence in mammalian β-defensins by adaptive evolution. Mol. Immunol. 2003, 40, 413–421. [Google Scholar] [CrossRef]
- Ewald, D.A.; Noda, S.; Oliva, M.; Litman, T.; Nakajima, S.; Li, X.; Xu, H.; Workman, C.T.; Scheipers, P.; Svitacheva, N.; et al. Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling. J. Allergy Clin. Immun. 2017, 139, 562–571. [Google Scholar] [CrossRef]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immun. 2019, 143, 1–11. [Google Scholar] [CrossRef]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immun. 2015, 136, 1254–1264. [Google Scholar] [CrossRef]
- Hata, T.R.; Kotol, P.; Jackson, M.; Nguyen, M.; Paik, A.; Udall, D.; Kanada, K.; Yomasaki, K.; Alexandrescu, D.; Gallo, R.L. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J. Allergy Clin. Immun. 2008, 122, 829–831. [Google Scholar] [CrossRef]
- Gonzalez-Curiel, I.; Trujillo, V.; Montoya-Rosales, A.; Rincon, K.; Rivas-Calderon, B.; deHaro-Acosta, J.; Marin-Luevano, P.; Lozano-Lopez, D.; Enciso-Moreno, J.A.; Rivas-Santiago, B. 1,25-dihydroxyvitamin D3 induces LL-37 and HBD-2 production in keratinocytes from diabetic foot ulcers promoting wound healing: An in vitro model. PLoS ONE 2014, 9, e111355. [Google Scholar] [CrossRef]
- Dimitrov, V.; White, J.H. Species-specific regulation of innate immunity by vitamin D signaling. J. Steroid Biochem. Mol. Biol. 2016, 164, 246–253. [Google Scholar] [CrossRef]
- Lowry, M.B.; Guo, C.; Zhang, Y.; Fantacone, M.L.; Logan, I.E.; Campbell, Y.; Zhang, W.; Le, M.; Indra, A.K.; Ganguli-Indra, G.; et al. A mouse model for vitamin D-induced human cathelicidin antimicrobial peptide gene expression. J. Steroid Biochem. Mol. Biol. 2020, 198, 105552. [Google Scholar] [CrossRef]
- Gambichler, T.; Skrygan, M.; Tomi, N.S.; Othlinghaus, N.; Brockmeyer, N.H.; Altmeyer, P.; Kreuter, A. Differential mRNA expression of antimicrobial peptides and proteins in atopic dermatitis as compared to psoriasis vulgaris and healthy skin. Int. Arch. Allergy Immunol. 2008, 147, 17–24. [Google Scholar] [CrossRef]
- Clausen, M.-L.; Slotved, H.C.; Krogfelt, K.A.; Agner, T. Measurements of AMPs in stratum corneum of atopic dermatitis and healthy skin–tape stripping technique. Sci. Rep. 2018, 8, 1666. [Google Scholar] [CrossRef] [PubMed]
- Ballardini, N.; Johansson, C.; Lilja, G.; Lindh, M.; Linde, Y.; Scheynius, A.; Agerberth, B. Enhanced expression of the antimicrobial peptide LL-37 in lesional skin of adults with atopic eczema. Br. J. Derm. 2009, 161, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Hongo, M.; Ohshima, H.; Kurasawa, M.; Hirakawa, S.; Kitajima, Y. Human beta defensin-1 regulates the development of tight junctions in cultured human epidermal keratinocytes. J. Derm. Sci. 2013, 71, 145–148. [Google Scholar] [CrossRef]
- Kiatsurayanon, C.; Niyonsaba, F.; Smithrithee, R.; Akiyama, T.; Ushio, H.; Hara, M.; Okumura, K.; Ikeda, S.; Ogawa, H. Host defense (antimicrobial) peptide, human β-defensin-3, improves the function of the epithelial tight-junction barrier in human keratinocytes. J. Investig. Derm. 2014, 134, 2163–2173. [Google Scholar] [CrossRef]
- Akiyama, T.; Niyonsaba, F.; Kiatsurayanon, C.; Nguyen, T.T.; Ushio, H.; Fujimura, T.; Ueno, T.; Okumura, K.; Ogawa, H.; Ikeda, S. The human cathelicidin LL-37 host defense peptide upregulates tight junction-related proteins and increases human epidermal keratinocyte barrier function. J. Innate Immun. 2014, 6, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.T.; Peng, G.; Trujillo-Paez, J.V.; Yue, H.; Ikutama, R.; Takahashi, M.; Umehara, Y.; Okumura, K.; Ogawa, H.; Ikeda, S.; et al. The antimicrobial peptide AMP-IBP5 suppresses dermatitis-like lesions in a mouse model of atopic dermatitis through the low-density lipoprotein receptor-related protein-1 receptor. Int. J. Mol. Sci. 2023, 24, 5200. [Google Scholar] [CrossRef]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Matsumoto, K.; Saito, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; et al. Antimicrobial peptides human β-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur. J. Immunol. 2007, 37, 434–444. [Google Scholar] [CrossRef]
- Kanda, N.; Watanabe, S. Increased serum human β-defensin-2 levels in atopic dermatitis: Relationship to IL-22 and oncostatin M. Immunobiology 2012, 217, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Tominaga, M.; Takamori, K.; Kajiwara, N.; Saito, H.; Nagaoka, I.; Ogawa, H.; et al. Antimicrobial peptides human β-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J. Immunol. 2010, 184, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Y.; Pan, J.; Liu, N.; Qin, Y.; Qiu, L.; Liu, M.; Wang, T. The role of type 2 innate lymphoid cells in allergic diseases. Front. Immunol. 2021, 12, 586078. [Google Scholar] [CrossRef]
- Soleymani, T.; Hung, T.; Soung, J. The role of vitamin D in psoriasis: A review. Int. J. Derm. 2015, 54, 383–392. [Google Scholar] [CrossRef]
- Yamamoto, M.; Haruna, T.; Yasui, K.; Takahashi, H.; Iduhara, M.; Takaki, S.; Deguchi, M.; Arimura, A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. 2007, 56, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Haruna, T.; Ueda, C.; Asano, Y.; Takahashi, H.; Iduhara, M.; Takaki, S.; Yasui, K.; Matsuo, Y.; Arimura, A. Contribution of itch-associated scratch behavior to the development of skin lesions in Dermatophagoides farinae-induced dermatitis model in NC/Nga mice. Arch. Derm. Res. 2009, 301, 739–746. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Sequence (5′–3′) |
|---|---|---|
| Filaggrin | Forward | CATCCGTAAAGACCTCTATGCCAAC |
| Reverse | ATGGAGCCACCGATCCACA | |
| Involucrin | Forward | AACCACACCAGTGTCCCAGAG |
| Reverse | TGGGTGAGTAGGCCAGCTGA | |
| Loricrin | Forward | CTCACTCATCTTCCCTGGTGCTT |
| Reverse | CCTCCTCCACCAGAGGTCTTTC | |
| Claudin-1 | Forward | ACCGGGCAGATACAGTGCAA |
| Reverse | TGCCAATGGTGGACACAAAGA | |
| Occludin | Forward | GGCAAGCGATCATACCCAGAG |
| Reverse | AGGCTGCCTGAAGTCATCCAC | |
| Tjp1 | Forward | GTTGGTACGGTGCCCTGAAAGA |
| Reverse | GCTGACAGGTAGGACAGACGAT | |
| Defb1 | Forward | AGCCTCATCTGTCAGCCCAACTA |
| Reverse | TCCAAGACTTGTGAGAATGCCAAC | |
| Defb2 | Forward | TTTCTACCAGCCATGAGGACTCT |
| Reverse | CAGTGGTCAAGTTCTGCTTCGT | |
| Defb3 | Forward | TCAGTCATGAGGATCCATTACCTTC |
| Reverse | GCCAATGCACCGATTCCAG | |
| Defb14 | Forward | ATCTTGTTCTTGGTGCCTGC |
| Reverse | CTTCTTTCGGCAGCATTTTC | |
| Camp | Forward | TGCTCCGAGCTGTGGATGAC |
| Reverse | CCTTCACTCGGAACCTCACAGAC | |
| Il4 | Forward | ACGGAGATGGATGTGCCAAAC |
| Reverse | AGCACCTTGGAAGCCCTACAGA | |
| Il13 | Forward | CGGCAGCATGGTATGGAGTG |
| Reverse | ATTGCAATTGGAGATGTTGGTCAG | |
| Il33 | Forward | GAGACTCCGTTCTGGCCTCA |
| Reverse | AATGTGTCAACAGACGCAGCAA | |
| Tslp | Forward | CGAGCAAATCGAGGACTGTGAG |
| Reverse | GCAGTGGTCATTGAGGGCTTC | |
| Actb | Forward | CATCCGTAAAGACCTCTATGCCAAC |
| Reverse | ATGGAGCCACCGATCCACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umehara, Y.; Trujillo-Paez, J.V.; Yue, H.; Peng, G.; Nguyen, H.L.T.; Okumura, K.; Ogawa, H.; Niyonsaba, F. Calcitriol, an Active Form of Vitamin D3, Mitigates Skin Barrier Dysfunction in Atopic Dermatitis NC/Nga Mice. Int. J. Mol. Sci. 2023, 24, 9347. https://doi.org/10.3390/ijms24119347
Umehara Y, Trujillo-Paez JV, Yue H, Peng G, Nguyen HLT, Okumura K, Ogawa H, Niyonsaba F. Calcitriol, an Active Form of Vitamin D3, Mitigates Skin Barrier Dysfunction in Atopic Dermatitis NC/Nga Mice. International Journal of Molecular Sciences. 2023; 24(11):9347. https://doi.org/10.3390/ijms24119347
Chicago/Turabian StyleUmehara, Yoshie, Juan Valentin Trujillo-Paez, Hainan Yue, Ge Peng, Hai Le Thanh Nguyen, Ko Okumura, Hideoki Ogawa, and François Niyonsaba. 2023. "Calcitriol, an Active Form of Vitamin D3, Mitigates Skin Barrier Dysfunction in Atopic Dermatitis NC/Nga Mice" International Journal of Molecular Sciences 24, no. 11: 9347. https://doi.org/10.3390/ijms24119347
APA StyleUmehara, Y., Trujillo-Paez, J. V., Yue, H., Peng, G., Nguyen, H. L. T., Okumura, K., Ogawa, H., & Niyonsaba, F. (2023). Calcitriol, an Active Form of Vitamin D3, Mitigates Skin Barrier Dysfunction in Atopic Dermatitis NC/Nga Mice. International Journal of Molecular Sciences, 24(11), 9347. https://doi.org/10.3390/ijms24119347









