Abstract
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and is one of the leading causes of cancer-related deaths worldwide. Despite advances in medicine, it is still a cancer with a very poor prognosis. Both imaging and liver biopsy still have important limitations, especially in very small nodules and those which show atypical imaging features. In recent years, liquid biopsy and molecular analysis of tumor breakdown products have become an attractive source of new biomarkers. Patients with liver and biliary malignancies, including hepatocellular carcinoma (HCC), may greatly benefit from ctDNA testing. These patients are often diagnosed at an advanced stage of the disease, and relapses are common. Molecular analysis may indicate the best cancer treatment tailored to particular patients with specific tumor DNA mutations. Liquid biopsy is a minimally invasive technique that facilitates the early detection of cancer. This review summarizes the knowledge of ctDNA in liquid biopsy as an indicator for early diagnosis and monitoring of hepatocellular cancer.
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most commonly occurring cancer worldwide and, due to its constantly increasing incidence, has become the third leading cause of cancer-related death among general populations. Moreover, it represents the most common cause of death in patients with cirrhosis [1,2]. HCC is more common in men than in women [3,4]. There has been an increase in HCC incidence in recent years, a trend particularly seen in Sub-Saharan Africa, Eastern Asia, and the Southeast, primarily as a result of high HBV prevalence rates [5,6].
The definitive therapies for HCC remain surgical resection and liver transplantation that can be performed only in patients at very early (0) and early (A) stages. However, given the similar survival benefit paired with the less invasiveness and lower costs compared to surgical resection, percutaneous ablative therapies such as radiofrequency ablation (RFA) and microwave ablation (MWA) are now considered the first treatment approach in both very early and early stages [7].
Patients with hepatitis B or C (HBV or HCV), alcohol abuse or metabolic syndrome are predisposed to developing HCC [3,8,9,10,11]. Imaging, alpha-fetoprotein (AFP) levels, and tissue biopsy are the main methods used to detect and monitor relapses of HCC [9,12]. Despite the improvement in screening and surveillance programs, most patients with HCC (about 65–70%) are still diagnosed in the intermediate (B) or advanced (C) tumoral stages, thus resulting ineligible for radical therapies, treatment options for advanced disease are limited [8,13,14]; therefore, patients with intermediate and/or advanced HCCs are considered for transarterial therapies or systemic therapies which, albeit effective, are deemed non-curative or “palliative” and still yield a lower 5-year survival rate [9,15,16].
One of the keys to personalized treatment is gene profiling. Harvesting tissue samples via biopsy is an effective way to collect the right specimen for further analysis. However, liver biopsy is an invasive procedure. Biopsies often yield insufficient amounts of cancer cells and are not always available [17]. According to Renzulli et al., 2022 the use of liver biopsy is limited by a raised rate of false negative results (30%). Another limitation is an insufficient sampling rate of up to 15% [18]. The role of imaging studies in HCC diagnosis has a significant value, especially in cirrhotic patients. The diagnosis of HCC in this group of patients can be based on imaging findings alone, and treatment decisions can be made without tissue sampling. Despite the recent technological developments, including the use of hepato-specific contrast media in MRI, imaging diagnosis still has important limitations, especially in non-cirrhotic patients, in those with very small nodules (<1 cm), and in those who have nodules lacking the characteristic imaging features of HCC [19]. The serum concentration level of alpha-fetoprotein (AFP) is the most commonly used biomarker of HCC. However, the sensitivity and specificity of this compound are mediocre [20,21]. Furthermore, elevated AFP is associated more strongly with advanced stages of HCC than with early stages of the disease, thus questioning the efficacy of AFP measurement for early HCC screening [22,23].
Currently, no diagnostic method has 100% sensitivity and specificity and can be used in every patient. Commonly used markers used to detect HCC have poor diagnostic efficacy, while imaging and histopathology have limitations in diagnostic accuracy and sensitivity [9,24]. Therefore, it is very important to find an effective method for detecting HCC at an early stage of the disease and monitoring its recurrence.
2. Liquid Biopsy
In 1977, researchers first described circulating tumor DNA in human plasma, confirming that it contained mutations characteristic of cancerous cells [25,26,27,28].
Liquid biopsy is the collection for analysis of a sample of unstable biological tissue of the body [9,29], and it is a minimally invasive technique that facilitates early detection of cancer [30,31]. Over the past few years, the development of molecular techniques enabled the detection of circulating tumor cells (CTCs) and cell-free DNA (ctDNA). Cancer patients present a higher level of ctDNA compared to healthy people. In addition to this, in a liquid biopsy, cancer patients also present cell-free microRNA and extracellular RNA, such as exosomes or tumor-educated platelets (TEPS) (Figure 1) Ref. [9]. Circulating tumor DNA (ctDNA) is tumor-specific DNA in the circulation released after metabolic secretion, apoptosis, or necrosis [17], and the circulating half-life of ctDNA is up to two hours [32]. The ctDNA provides dynamic, detailed information about tumor biology without the need for frequent biopsies [33,34]. Performing a liquid biopsy is particularly advantageous when the amount of tissue is too small to perform repeated tissue biopsies [3]. Serial sampling can be accomplished with minimal invasive sample collection [27]. Currently, research is underway to optimize ctDNA technology for use in clinical practice [17]. Combining cell-free DNA with AFP marker and age might be a promising tool in HCC diagnosis [35]. DNA methylation signatures, copy number aberrations, and somatic mutations have all been characterized to date in ctDNA [9,26,36,37,38,39]. Knowledge of genetic mutations affecting the development and progression of HCC allows for a better understanding of this disease [3]. The ctDNA can accurately classify tumor stage, dynamically adjust treatment plans, and plan surgical resection and postoperative therapy [33]. Performing liquid ctDNA biopsies can help monitor response to therapy, early detection of non-response and knowledge of tumor recurrence even months before clinical signs of recurrence [26,40,41].
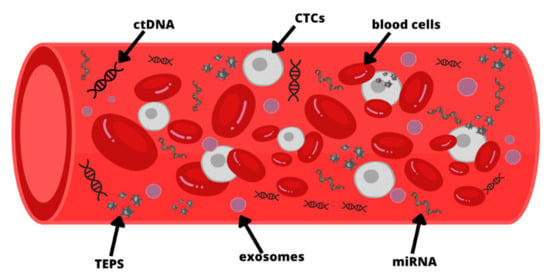
Figure 1.
Molecules detected in peripheral blood for liquid biopsy: circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), tumor-educated platelets (TEPS), microRNA (miRNA). The figure is only for illustrative purposes. There is no indication of the actual size of each element.
Liquid biopsies require two 10 mL blood samples that together contain 5 ng of DNA for ctDNA analysis [8,11,42]. The physical characteristics and molecular characteristics of CTCs can be used to distinguish them from nucleated cells or normal epithelial cells, including electric charge, density, measurement, transfer capacity, and deformation ability, as well as biological parameters, such as cell surface markers, or a combination of both characteristics [43]. The methods of detection can be divided into the following groups: methods targeted to assay a few known mutations using PCR (digital PCR, amplification-refractory mutation system (ARMS)-PCR, etc.) and untargeted methods to sequence millions of DNA fragments (Sanger sequencing, next-generation sequencing (NGS), etc.) [44,45,46]. All of them must be highly sensitive and specific due to the very small amount of DNA material, which can be extracted from a 1 mL blood sample [47]. In addition to single-locus/multiplexed assays, targeted sequencing and genome-wide sequencing are available, depending on the size of the assay panel. Sequencing using PCR can be used for single loci and multiplexed analyses, and NGS can be applied to any size panel [45]. NGS plasma ctDNA, the so-called liquid biopsy, is an analytically very sensitive and specific test, detecting single molecules of tumor DNA, >85% of single nucleotide polymorphisms (SNPs) that are present in patients with advanced tumors, with analytical specificity > 99.9999% [48].
2.1. Early Diagnosis
The utility of ctDNA assessment may be particularly important in patients with suspected HCC, as these patients are often not biopsied due to potential complications [8]. The use of ctDNA in oncological diagnostics may, in the future, replace standard cancer markers such as AFP. The ctDNA is more specific because it is detected based on the genetic changes present in the cancer cells. Another advantage of ctDNA over AFP is that AFP is secreted in only 70% of HCC patients [5,40]. Patients with non-elevated AFP in their blood benefited most from the genomic aspects. The study detected 44 of 76 HCCs (57.9%) by elevating AFP, a widely used diagnostic biomarker for HCC. As a comparison, DNA detection of 14 additional HCCs (18.4%) was achieved by combining the genomic classifier copynumber aberrations and fragment size in patients whose HCC had been missed by AFP testing alone [6]. Wang J. et al., 2020 conducted a study of 81 patients with HCC undergoing hepatectomy. Peripheral blood samples were taken before and after surgery. In total, 70.4% (57/81) of them had detectable ctDNA prior to surgery, while the positive AFP rate was only 56.8%—the ctDNA diagnostic capability was better than AFP. Positive preoperative ctDNA status was associated with larger tumor size, multiple tumor lesions and Microvascular Invasion, advanced stage BCLC (Barcelona Clinic Liver Cancer), shorter Disease-Free Survival (DFS) and Overall Survival (OS). The analysis showed that ctDNA was an independent risk factor for postoperative recovery [49].
2.2. Guiding Personalized Therapy
The use of ctDNA liquid biopsy is very useful in the search for drug-sensitive gene markers and the identification of possible resistance mechanisms in cancer patients [50]. Mody K. et al., 2017 conducted a study in which 35 patients with HCC were subjected to ctDNA testing. It was found that ctDNA can be a very good non-tissue alternative to genomic profiling in patients with HCC [17]. The main advantage of ctDNA is believed to be its ability to store comprehensive somatic information about primary HCC as well as metastasis [51]. The profile of genetic mutations may change over time; the advantage of ctDNA is the possibility of repeating it during treatment. Sadakatsu Ikeda et al., 2018 point out that changes detected by ctDNA analysis were present in approximately 90% of HCC patients who had at least one potentially drug-sensitive lesion [8]. Matsumae T et al. performed ctDNA profiling using a panel for detecting mutations in 25 HCC-related cancer genes in 85 patients with non-surgical HCC (u-HCC) who received atezolizumab plus bevacizumab. CfDNA/ctDNA profiling has been shown to be a good biomarker for predicting the prognosis of patients with u-HCC treated with combined anti-PD-L1 and anti-VEGF immunotherapy [52].
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a primary liver cancer (PLC) and shows both hepatic and biliary differentiation [43,53]. Diagnosis of cHCC-CCA on the basis of tumor biopsy alone remains difficult [43,54], as it has characteristics of both HCC and CCA, so a tissue sample from an area similar to HCC or CCA may lead to misdiagnosis [43,55]. A histopathological evaluation of the biopsy or surgical material playsa key role in the diagnosis of cHCC-CCA. A lack of distinguishing features of cHCC-CCA imaging distinguishes the disease from HCC and CCA [43,56]. The cfDNA study of the bile of CCA patients showed high sensitivity and specificity in detecting single nucleotide changes, insertions and deletions (94.7 and 99.9%, respectively), and copy number variation (75.0 and 98.9%, respectively) [43,57]. Bile cfDNA has been demonstrated to be a reliable source of tumor genetic information, so bile fluid biopsy might be a promising approach for identifying CCAs. It has been proven that cHCC-CCA tumor tissue has a lower frequency of CTNNB1 mutations (only 6%) compared to HCC. Compared to CCAs, cHCC-CCAs had a significantly lower KRAS mutation rate (0%). As a result of the lack of mutations in both CTNNB1 and KRAS in cHCC-CCA is a unique feature [43,58]. Liquid biopsy might even be able to replace traditional tissue biopsy in the future by analyzing molecular changes in circulating tumor-derived molecules [43].
2.3. Prognostic Value
The detection of early HCC recurrence may be enhanced by ctDNA. Which are mainly caught by AFP levels and liver ultrasound, which has a sensitivity of only 63% [59,60]. In a Chinese study, it was confirmed that the majority of samples taken from patients at the time of suspected recurrence (97.4%, 75/77) taken at a time when the tumor could be confirmed by CT/MRI imaging methods were positive. In contrast, samples (100%, 42/42) from non-relapsed patients were, respectively, negative. In addition, in a few cases, positive ctDNA was detected in plasma samples before disease recurrence was seen on MRI, so cancer recurrence could be detected a median of 4.6 months in advance [51].
Zhu GQ et al. investigated the value of ctDNA in predicting early postoperative tumor recurrence and monitoring tumor burden in patients with HCC. They examined 41 patients who had undergone liver resection leading to a cure, with a confirmed radiological diagnosis. The genetic changes identified in the ctDNA were shown to be consistent with mutations present in the matched HCC tissue. Researchers have detected mutations of genes that were tested in ctDNA tissues and which are relevant in carcinogenesis: CTNNB1, TP53, NRAS, BRAF, and NFE2L2 [33]. A total number of 96 operatively treated patients diagnosed with primary HCC were enrolled in a study aiming to verify the prognostic value of ctDNA. It was found that ctDNA positivity in the immediate postoperative period was significantly associated with worse disease-free survival (DFS) and overall survival (OS). That indicates the clinical significance of ctDNA-based minimal residual disease (MRD) detection. Patients with positive postoperative ctDNA-AFP (H) subgroups and patients with positive ctDNA and Barcelona Clinic Liver Cancer (BCLC) staging C subgroups had the worst prognosis. Liquid biopsy markers such as postoperative ctDNA have a significant value in detecting high-risk recurrence patients [61].
Before surgery, ctDNA was detected in 63.4% of patients, while after radical liver surgery, only in 46% of patients. Serial ctDNA marking has been shown to reflect changes in real-time tumor mass well. There is a relationship between preoperative ctDNA and tumor size, differentiation, Microvascular Invasion, and early recurrence. In addition, preoperative detection of ctDNA has been shown to correlate with a high risk of tumor recurrence, confirming the role of ctDNA in monitoring disease progression. The most indicative time-point is probably postoperative, because, in most cases, the ctDNA is negative postoperatively, but in those who have prolonged positive results, the recurrence risk is high [33].
The results of a study involving 38 patients with HCC treated according to the trans-arterial chemoembolization (TACE) procedure suggest that cfDNA and ctDNA dynamics may become promising markers in assessing the effectiveness of treatment after the first TACE procedure. The changes in values between blood samples taken the day before TACE and values one month after TACE were +31.4% for cfDNA and 0% for ctDNA. As a result, they significantly predicted the worsening of the disease and were associated with worse PFS. In this study, the combined cfDNA and ctDNA scores were able to stratify patients into high- or low-risk groups for progressive disease (PD) one month after TACE, with a PD rate of 80.0% vs. 4.3% (p = 0.001) and a median progression-free survival of 1.3 vs. 10.3 months (p = 0.002) [40].
3. Comparison of ctDNA and Tissue NGS Results
NGS (next-generation sequencing) is a novel gene detection technology. It can detect many types of gene mutations in different types of chests and body fluid samples [11]. The ctDNA NGS differs from tissue NGS in that ctDNA shows genomic changes from excreted DNA from the primary tumor site and multiple metastatic sites, while tissue NGS shows changes present in the tissue fragment being assessed. Not every tumor releases DNA into the bloodstream at the same time, and the high vascularity of tumors increases the availability and speed of detecting changes in blood [62]. On the other hand, in patients with brain tumors, the blood–brain barrier may be an obstacle. One alternative procedure in the mentioned case may be a biopsy of the cerebrospinal fluid [63].
In the study by Sadakatsu Ikeda et al. in 26 patients with HCC in 6 cases, gene changes were found in both tissue NGS and ctDNA NGS, 14 changes were present only in tissue NGS, and another 18 changes were detected only in ctDNA NGS. The agreement in both studies for the most common mutations was 50% for TP53, 100% for CTNNB1, and 90% for ARID1A, respectively [8]. However, in a study of 213 patients with a diagnosis of gastrointestinal cancer, overall concordance was analyzed for 68 genes contained in tissue and liquid biopsy panels and found a concordance ratio of 96%, a McNemar P. value of 0.68, meaning ctDNA and tissue NGS is not significantly different [34]. Another study presented by An et al., 2019 showed a comparison between the ctDNA sequencing and tDNA (tumor DNA) results in a group of 26 patients. Out of 139 mutations detected in ctDNA, 69 (49.6%) could be validated in paired tumor DNA (tDNA). Another 70 mutations (50.4%) were only in plasma samples. At least one overlapping mutation could be detected in 23 patients (88.5%). Moreover, seven patients presented the same ctDNA mutations set in both ctDNA and matched tDNA. Nine presumptive driver genes for HCC, including TP53, AXIN1, CTNNB1, CDKN2A, ARID1A, ARID2, SMARCA4, KEAP1, and NFE2L2, were selected to explore their concordance between ctDNA and tDNA. A total of 37 driver events were identified in 23 patients (88.5%). Three patients did not present the presence of conventional driver events in both ctDNA and tDNA. Among driver events, 25 (67.6%) were shared in paired ctDNA and tDNA, 3 (8.1%) were found in plasma only, and 9 (24.3%) were exclusively in tissue material. Moreover, the concordance rate was higher among drivers than non-driver mutations (25/37, 67.6% versus 44/130, 33.8%, Chi-square p-value = 0.0002) [64].
Studies show that there is a strong correlation between ctDNA and tissue NGS. However, the advantage of ctDNA may be a higher rate of detection of MET changes, and they can detect primary tumor DNA shedding and MET changes, while tank assays detect biopsy localized changes. A blood test is less invasive compared to a biopsy, which may encourage repeat testing. The disadvantage of ctDNA testing, however, may be its lower sensitivity in detecting lesions that are easily identifiable by tissue testing, and not all disease sites may release DNA into the circulation, especially in early stages [62,65]. Both methods can independently detect neoplastic changes, emphasizing the clinical value in detecting and monitoring treatment and recurrence of the disease; it should be emphasized that they are complementary.
4. Circulating Marker Characteristics
The exact mechanisms of ctDNA release remain unknown. It is assumed that DNA fragments are disposed into the bloodstream by apoptosis and necrosis of cancer cells. There is no evidence regarding the variety of release among cancer clones. An experimental model in the study of Labgaa I. et al., 2021 aimed to assess the role of xenografts in monitoring ctDNA and CTC dynamics. The authors tried to check the possibility of DNA detection coming from different cell lines in xenografts, which would recapitulate intratumoral heterogeneity (ITH) as a result of clonal evolution. DNA specific and exclusive to each cell line mutations (APOB to Huh7 and FGA to HepG2) were targeted. It was found that the release of DNA fragments between the two clones varied. Further results showed that the concentration of ctDNA and the presence of tumor-specific mutations reflected tumor progression. The presented ITH model suggested a clone-dependent release of ctDNA. Differences in the release of DNA fragments were also caused by the treatment (sorafenib) [66].
The ctDNA contains information about tumor genome profiles, particularly single nucleotide variants (SNVs) and copy number variants (CNVs). High fractions of somatic SNVs and CNVs in plasma were detected in patients before surgery. On the other hand, after tumor resection, SNV and CNV were significantly reduced [51]. In a study by David Sefrioui and co-authors, it was observed that in patients with HCC after cytotoxic treatment, the release of ctDNA increased, which may indicate the process of apoptosis in cancer cells several hours after the administration of the drug [40].
Detection of ctDNA is different for individual cancers and depends on their stage of advancement. In a massive study involving 11,525 patients diagnosed with various cancers (HCC, n = 571), DNA was isolated from plasma. The sensitivity of ctDNA detection for HCC in stage I–III disease was 68.0%, 95% confidence interval was 62.6–73.4%, respectively. Stage IV ctDNA detection was similar for most tumors and was >70% [50].
5. Mutation in Hepatocellular Carcinoma
Cancer-specific mutations have been reported in ctDNA from the peripheral blood of patients with HCC. These mutations include CTNNB1, TERT, TP53, TMBIM6 and MLH1 [3,67,68,69,70,71]. They are described in detail below. Other frequently detected genes in HCC were: EGRF, MYC, CDK6, TMEM141, UBB, and ADGRV1, etc. [3,72]. Moreover, there were no patients with the same ctDNA-derived somatic mutation pattern [72]. The less common genetic mutations and the characteristics associated with them are included in Table 1.

Table 1.
The less common genetic mutations detected in the ctDNA, and the characteristics associated with them.
5.1. CTNNB1
One of the most prevalent genetic alterations in HCC is the mutation of the CTNNB1 gene, which can appear at any moment during the evolution of the HCC [88,89]. In the Jian Gao study, mutations in the CTNNB1 gene were diagnosed in 15% of patients with HBV-related HCC [3].
One of the most frequent genetic events in HCC is a gain of function (GOF) mutation of CTNNB1, and it could be found in 15 to 30% of human HCCs [90,91]. It was found that proteins that are involved in various metabolic activities, such as drug and amino acid metabolisms, glycolysis, and gluconeogenesis, are enriched in CTNNB1 mutant tumors [92,93]. CTNNB1 mutation usually occurs at a later stage of HCC progression, and there is still no adequate study of its mechanism [92].
A metabolic morphotype of CTNNB1-mutated HCC is specific, andit is often cholestatic and sometimes steatotic [89,92,94]. It was shown that HCCs with GOF (gain of function) CTNNB1 mutations display a particular, well-differentiated phenotype, infrequent microvascular invasion, low α-fetoprotein (AFP) levels, and better outcomes [90].
The oncogenic Wnt/β-catenin pathway, activated by the mutated CTNNB1, plays an important role in the metabolic regulation in the liver [75,92]. Studies have shown the presence of the Wnt/β-catenin pathway for ex. CTNNB1 gene mutation indicates a weaker response to immunotherapy [95]. However, ORR (overall response rate), DCR (disease control rate), PFS (progression-free survival), and OS (overall survival) have been shown to be similar for both CTNNB1 mutation and CTNNB1 mutation absence [52].
One of the aims of a study by Oversoe, S.K. et al., 2021 was to evaluate the concordance rates between plasma and tissue samples. Results of the study showed that analysis of ctDNA can reveal tumor mutations that are not apparent in single tumor biopsies. The detection rate of CTNNB1 mutation in HCC patients may be increased by combining those two methods. Serial analysis of ctDNA enables monitoring of tumor mutation profiles and may help with creating personalized therapeutic strategies [96].
5.2. TERT
The presence of TERT mutations has been shown to be associated with poor prognosis in patients with different types of cancer [97]. The TERT gene is involved in the telomeric cycle and is altered in approximately 60% of HCC cases. These TERT changes consist of two hotspot mutations within the gene promoter [40].
Mutations in the TERT gene may be a potential screening target for both early stage and advanced HCC. In a study by Zhang Y. and others, TERT promoter SNVs were most commonly found among HCC patients with early stage cancer in 22.3% of cases [50]. The aim of the study by Hirai et al., 2021 was to detect TERT mutations in the ctDNA of patients with advanced HCC. The study group consisted of 130 patients: 86 were treated with systemic chemotherapy (sorafenib and/or lenvatinib) and 44 with transcatheter arterial chemoembolization. The TERT promoter mutations in ctDNA were detected in 71 patients (54.6%) [98]. The presence of TERT mutations, as well as its high fractional abundance (≥1%), was associated with shorter overall survival than in patients without them [52,98].
The study by Ge, Z. et al. aimed to detect the oncogenic mutations in paired circulating tumor DNA and circulating tumor cells in patients with HCC. It was found that the correlations between liquid biopsy results and clinicopathologic parameters vary between ctDNA status and CTC count. TERT C228T (or ctDNA positivity) correlated with macrovascular invasion (MVI) as found in CT/MRI imaging. Such correlation was not observed for CTCs. Results of the linear regression analysis showed a positive correlation between ctDNA VAF and the size of the largest tumor as well as AFP level. Similarly, this correlation with AFP level and tumor diameter was observed for TERT C228T VAF. However, CTC count did not correlate with the largest tumor diameter or AFP [13].
5.3. TP53
Wild-type TP53 is involved in cell cycle regulation and apoptosis following DNA damage [99]. If the TP53 gene is mutated, DNA-damaged cells can escape apoptosis and become cancerous. Additionally, mutant TP53 proteins lose their wild-type functions and accumulate in the nucleus. There is a strong correlation between this accumulation and malignant tumors [100].
In a study that isolated ctDNA from 26 patients with advanced HCC, the most common (61.5%) mutations concerned the TP53 gene. They observed the highest frequency of mutant ctDNA alleles in the TP53 mutation at 12%. This is an important fact because TP53 overexpression is a factor in poor prognosis in patients with HCC [8]. In a Chinese study on 26 HCC patients, the NGS method was used for ctDNA profiling. Accordingly, 96.2% of patients with HCC had ctDNA mutations that could be validated through matched tumor tissue testing. A potential screening marker and the most common mutation was TP53 R249S. Since the detection sensitivity of this mutation in ctDNA was 83.3%. Several cfDNA parameters were linearly related to tumor diameter. In the future, the use of ctDNA for postoperative HCC observation may be attempted [64].
TP53 mutations may be involved in the regulation of HBV-associated HCC. In Jian Gao’s study, mutations in the TP53 gene were present in 38% of HCC patients due to HBV infection, while in HCC and HCV patients, it was present in 20% of patients [3]. In the group of 96 patients in the study by Ye, K. et al., 2022 it was observed that mutations of TP53 were associated with relapses (p = 0.0106). It may be closely associated with cancer due to HBV infection and exposure to aflatoxin [61,101,102].
5.4. TMBIM6
BAX is a member of the Bcl-2 family of pro-apoptotic proteins [103]. It is involved in both intrinsic and extrinsic apoptotic signaling and concentrates mainly in the cytosol. The intrinsic way can be triggered by oncogenic stress, chemotherapeutic agents or metabolic stress, while the extrinsic pathway (death receptor pathway) activation depends on the interactions between death receptors and their ligands of the tumor necrosis factor (TNF) family [104]. Apoptosis is dependent on the pro-apoptotic BCL-2 proteins BAX and BAK can be induced by cytotoxic cell stress. BAX protein activity is restricted by the constant shift from the mitochondria to the cytosol. Changes in the BCL-2 regulation and the appearance of antiapoptotic effects are one of the most remarkable changes in HCC development. Abnormalities regarding BAX, such as inactivated mutations or down-regulations, can affect the ratio of BAX/BCL-2, resulting in resistance to cell death [104].
Funk et al., 2022 presented a study on the effects of TMBIM6 localization equilibrium changes in hepatocarcinogenesis. There are two groups of HCC regarding TMBIM6 protection status. The first group is BAX protected tumors, which experience elevated levels of oxidative stress and cytosolic concentration of TMBIM6. Those cells are prone to cellular stress resulting in DNA damage and further activation of DNA repair mechanisms. The second group is TMBIM6 non-protected tumors. Those tumor cells present mitochondrial dysfunction and higher proliferative capacity. Differences between those groups are followed by various responses to treatment. TMBIM6 protected tumors present increased sensitivity to PARP inhibitors, while TMBIM6 non-protected HCC may respond better to classical chemotherapy [105]. In a study by Alunni-Fabbroni, M. et al., 2019 in 13 male HCC patients treated with sorafenib, one of the most frequently detected mutant genes was TMBIM6 (69.2% of patients). TMBIM6 may cause low efficacy of sorafenib therapy by inhibiting the apoptosis pathway induced by this drug. However, this study did not show a significant correlation between TMBIM6 and survival time. Researchers found a significant correlation between the presence of TMBIM6 variants and portal vein invasion [106].
5.5. MLH1
The MLH1 gene plays a key role in the repair of DNA strand breaks, and it has been shown that MLH1 is immediately recruited to sites of DNA strand breaks caused by DNA-damaging agents [107]. It also has a significant role in the repair of mismatched bases in the DNA strand [108]. The MLH1 polymorphism has been detected in colorectal cancer, endometrial cancer, prostate cancer, pancreatic cancer, head and neck squamous cell carcinoma, and oral squamous cell carcinoma [109,110,111,112,113]. However, there are only a few studies that support an association between MLH1 polymorphisms and HCC.
In one Korean study, 107 HCC patients were assessed for ctDNA amounts. The patients were divided into two groups according to the median concentration of recovered ctDNA: low (<5.77 ng/mL) and high (≥5.77 ng/mL). There was a significant increase in the proportion of patients with high ctDNA levels with the progression of the stage of HCC and those with vascular invasion. Patients with MLH1 or NPM1 mutations were found to have poorer Overall Survival compared to non-mutated patients. The SNVMLH1 was proven to be associated with BCLC (Barcelona Clinic Liver Cancer) (p = 0.025). After analyzing subgroups of patients with highly advanced HCC (BCLC stage C/D or modified Union for International Cancer Control stage IV), it turned out that patients with MLH1 SNV detected in ctDNA had lower survival rates than those without this mutation. The absence of MLH1 mutations correlated with low AFP levels and was associated with the best overall survival (p = 0.005) [113]. In a study by Xiao Nian Zhu et al., 2017 including 436 patients with HCC, the presence of single nucleotide polymorphism (SNP) MLH1, rs1800734, was shown to correlate with tumor size, stage, and AFP level of HCC patients (p < 0.05). However, between the other three MLH1 SNPs: rs10849, rs3774343, and rs1540354, no such associations were observed. Each of these MLH1 polymorphisms interacted with HBV infection, alcohol consumption, and smoking. The MLH1 rs1800734 SNP had an interaction with the other SNPs rs10849, rs3774343, and rs1540354, these SNP-SNP interactions resulted in a higher incidence of HCC, but no association was detected between the presence of MLH1 SNPs and a positive family history of HCC [114]. These reports suggest that MLH1 mutations may have a significant role in the development and prognosis of HCC patients.
5.6. DNA Methylation Changes
The abnormal methylation of DNA has been considered to be one of the key factors involved in the development and progression of cancer [115]. It has been demonstrated that global hypomethylation is associated with genome instability and loss of imprinting, resulting in increased cancer risk [116]. During the transformation from normal to cancerous tissue, DNA hypermethylation occurs at the promoter regions of tumor suppressor genes [117,118]. Almost half of the studies on biomarkers in HCC have focused on gene methylation in the last five years. In ctDNA, methylation biomarkers can be classified into three categories: the number of methylations, the expression of methylations, and the detection of 5-hydroxymethylcytosin [119].
In a study of 227 tissue samples from HCC patients that assessed DNA methylation profiles, HCC tissues showed a much greater variation in DNA methylation compared to non-malignant tissue. Additionally, the methylome DNA of adjacent cirrhotic non-cancerous tissue from HCC captures prognostic features in HCC patients. Researchers detected promoter hypermethylation in four genes (TSPYL5, KCNA3, LDHB, and SPINT2) that are involved in the regulation of TP53, cAMP, serine protease, and NADH, and a corresponding decrease in gene expression in early HCC [120]. HCC ctDNA samples contained a wide range of hypermethylated gene sites, including THY1, DBX1, GPBAR1, CDKN2A, VIM, FBLN1, RGS10, RUNX, MT1M, MT1G, and RGS10 [121]. In an interesting finding, researchers found that hypomethylation in HBV integration regions is associated with a higher sensitivity for diagnosing HCC [122]. Na Hu et al., 2017 in their study of 80 patients with HBV-related HCC, showed hypomethylation of the UBE2Q1 gene in the sera of HBV-associated HCC patients and also noted a negative correlation between UBE2Q1 gene methylation and the TNM stage of the tumor [123].
6. Limitations of ctDNA
Although liquid biopsy seems to be a promising tool in HCC management with its non-invasiveness and possible repetition of tumor biology examinations, it comes with limitations. There is still a fraction of tumor-derived mutations that do not appear in ctDNA profiling, mostly subclonal mutations that have inferior allele frequencies in tissue samples [124]. The molecular profiles of tumors differ due to their heterogeneity. Thus, in a person without confirmed cancer, it can be difficult to determine whether a nucleic acid signature is a ctDNA molecule, especially in the early stages, where a tumor may show a small subset of mutations. Even in a plasma sample with the same tumor genotype, intratumoral heterogeneity may lead to differences in ctDNA mutation concentrations [125]. It is common for studies to have a limited sample size as one of their limitations. The challenging aspect of a noninvasive blood test is determining the sample size needed to adequately capture heterogeneous genomic aberrations in tumors with extensive heterogeneity [6,124,126]. The widespread clinical application of liquid biopsy is not yet on the horizon due to both the related cost and technology. Moreover, the majority of data supporting its utility derives from proof-of-concept studies, mainly retrospective, and not validated by different researchers, and there needs to be a standardized assay protocol with high sensitivity and specificity [127,128].
7. Conclusions
Liquid biopsy might decrease the need for invasive diagnostic procedures in patients with HCC. Currently, known markers need further research on their specificity in detecting HCC and application in disease monitoring. Molecular diagnostics enables the creation of new therapeutic approaches. ctDNA provides detailed and dynamic tumor data, making it a hopeful marker for both prognostic and diagnostic use in patients with hepatocellular carcinoma; it is a promising material for planning a personalized treatment. Further extended studies on the use of ctDNA in patients with HCC are needed; currently, this technique is quite time-consuming. Although costs are currently high, they decrease over time, and the usefulness of ctDNA in liquid biopsy may significantly change the clinical management of HCC. The replacement of currently used tools in the management of HCC patients by liquid biopsy biomarkers is unrealistic, but they will likely be integrated into the process, providing a stronger predictive power.
Author Contributions
Conceptualization, A.K. and M.L.; methodology, R.P. and I.K.; software, I.K. and J.B.; formal analysis, A.K., J.B. and I.K.; investigation, A.K.; resources, R.P. and M.L.; writing—original draft preparation, M.L. and J.B.; writing—review and editing, all authors; visualization, A.K., M.L. and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liver-Fact-Sheet. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (accessed on 17 May 2023).
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Clark, P.J.; Aitken, J.F.; Baade, P.D. Global Trends in Incidence Rates of Primary Adult Liver Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xi, L.; Yu, R.; Xu, H.; Wu, M.; Huang, H. Differential Mutation Detection Capability Through Capture-Based Targeted Sequencing in Plasma Samples in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 596789. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Hanna, D.L.; Usher, J.; LoCoco, J.; Chaudhari, P.; Lenz, H.J.; Setiawan, V.W.; El-Khoueiry, A. Impact of Sex on the Survival of Patients with Hepatocellular Carcinoma: A Surveillance, Epidemiology, and End Results Analysis. Cancer 2014, 120, 3707–3716. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Allah Bakeshei, K.; Mohammadian-Hafshejani, A. International Epidemiology of Liver Cancer: Geographical Distribution, Secular Trends and Predicting the Future. J. Prev. Med. Hyg. 2020, 61, E259–E289. [Google Scholar] [CrossRef]
- Meng, Z.; Ren, Q.; Zhong, G.; Li, S.; Chen, Y.; Wu, W.; Feng, Y.; Mao, M.; Zhang, F.; Long, G. Noninvasive Detection of Hepatocellular Carcinoma with Circulating Tumor DNA Features and α-Fetoprotein. J. Mol. Diagn. 2021, 23, 1174–1184. [Google Scholar] [CrossRef]
- Cucchetti, A.; Piscaglia, F.; Cescon, M.; Colecchia, A.; Ercolani, G.; Bolondi, L.; Pinna, A.D. Cost-Effectiveness of Hepatic Resection versus Percutaneous Radiofrequency Ablation for Early Hepatocellular Carcinoma. J. Hepatol. 2013, 59, 300–307. [Google Scholar] [CrossRef]
- Ikeda, S.; Lim, J.S.; Kurzrock, R. Analysis of Tissue and Circulating Tumor DNA by Next-Generation Sequencing of Hepatocellular Carcinoma: Implications for Targeted Therapeutics. Mol. Cancer Ther. 2018, 17, 1114–1122. [Google Scholar] [CrossRef]
- Ye, Q.; Ling, S.; Zheng, S.; Xu, X. Liquid Biopsy in Hepatocellular Carcinoma: Circulating Tumor Cells and Circulating Tumor DNA. Mol. Cancer 2019, 18, 114. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer Statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Ding, Y.; Yao, J.; Wen, M.; Liu, X.; Huang, J.; Zhang, M.; Zhang, Y.; Lv, Y.; Xie, Z.; Zuo, J.H. The Potential, Analysis and Prospect of CtDNA Sequencing in Hepatocellular Carcinoma. PeerJ 2022, 10, e13473. [Google Scholar] [CrossRef]
- Colombo, F.; Baldan, F.; Mazzucchelli, S.; Martin-Padura, I.; Marighetti, P.; Cattaneo, A.; Foglieni, B.; Spreafico, M.; Guerneri, S.; Baccarin, M.; et al. Evidence of Distinct Tumour-Propagating Cell Populations with Different Properties in Primary Human Hepatocellular Carcinoma. PLoS ONE 2011, 6, e21369. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Helmijr, J.C.A.; Jansen, M.P.H.M.; Boor, P.P.C.; Noordam, L.; Peppelenbosch, M.; Kwekkeboom, J.; Kraan, J.; Sprengers, D. Detection of Oncogenic Mutations in Paired Circulating Tumor DNA and Circulating Tumor Cells in Patients with Hepatocellular Carcinoma. Transl. Oncol. 2021, 14, 101073. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Garzillo, G.; Ascanio, S.; Renzulli, M. Focal Lesions in the Cirrhotic Liver: Their Pivotal Role in Gadoxetic Acid-Enhanced MRI and Recognition by the Western Guidelines. Dig. Dis. 2014, 32, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Facciorusso, A.; Sacco, R.; Bartalena, L.; Mosconi, C.; Cea, U.V.; Cappelli, A.; Antonino, M.; Modestino, F.; Brandi, N.; et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J. Pers. Med. 2021, 11, 1041. [Google Scholar] [CrossRef]
- Guarino, M.; Viganò, L.; Ponziani, F.R.; Giannini, E.G.; Lai, Q.; Morisco, F.; Vitale, A.; Russo, F.P.; Cillo, U.; Burra, P.; et al. Recurrence of Hepatocellular Carcinoma after Direct Acting Antiviral Treatment for Hepatitis C Virus Infection: Literature Review and Risk Analysis. Dig. Liver Dis. 2018, 50, 1105–1114. [Google Scholar] [CrossRef]
- Mody, K.; Kasi, P.M.; Yang, J.D.; Surapaneni, P.K.; Ritter, A.; Roberts, A.; Nagy, R.; Borad, M.J. Feasibility of Circulating Tumor DNA Testing in Hepatocellular Carcinoma. J. Gastrointest. Oncol. 2019, 10, 745–750. [Google Scholar] [CrossRef]
- Renzulli, M.; Pecorelli, A.; Brandi, N.; Brocchi, S.; Tovoli, F.; Granito, A.; Carrafiello, G.; Ierardi, A.M.; Golfieri, R. The Feasibility of Liver Biopsy for Undefined Nodules in Patients under Surveillance for Hepatocellular Carcinoma: Is Biopsy Really a Useful Tool? J. Clin. Med. 2022, 11, 4399. [Google Scholar] [CrossRef]
- Renzulli, M.; Golfieri, R. Proposal of a New Diagnostic Algorithm for Hepatocellular Carcinoma Based on the Japanese Guidelines but Adapted to the Western World for Patients under Surveillance for Chronic Liver Disease. J. Gastroenterol. Hepatol. 2016, 31, 69–80. [Google Scholar] [CrossRef]
- Tang, J.C.; Feng, Y.L.; Guo, T.; Xie, A.Y.; Cai, X.J. Circulating Tumor DNA in Hepatocellular Carcinoma: Trends and Challenges. Cell Biosci. 2016, 6, 32. [Google Scholar] [CrossRef]
- Attwa, M.H.; El-Etreby, S.A. Guide for Diagnosis and Treatment of Hepatocellular Carcinoma. World J. Hepatol. 2015, 7, 1632–1651. [Google Scholar] [CrossRef]
- Nakazawa, T.; Hidaka, H.; Takada, J.; Okuwaki, Y.; Tanaka, Y.; Watanabe, M.; Shibuya, A.; Minamino, T.; Kokubu, S.; Koizumi, W. Early Increase in α-Fetoprotein for Predicting Unfavorable Clinical Outcomes in Patients with Advanced Hepatocellular Carcinoma Treated with Sorafenib. Eur. J. Gastroenterol. Hepatol. 2013, 25, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Matsumura, S.; Ban, D.; Irie, T.; Ochiai, T.; Tanaka, S.; Arii, S.; Tanabe, M. Does the Preoperative Alpha-Fetoprotein Predict the Recurrence and Mortality after Hepatectomy for Hepatocellular Carcinoma without Macrovascular Invasion in Patients with Normal Liver Function? Hepatol. Res. 2014, 44, E437–E446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, J.; Luo, F. Serum Tumor Markers for Detection of Hepatocellular Carcinoma. World J. Gastroenterol. 2006, 12, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Leon, S.A.; Shapiro, B.; Sklaroff, D.M.; Yaros, M.J. Free DNA in the Serum of Cancer Patients and the Effect of Therapy. Cancer Res. 1977, 37, 646–650. [Google Scholar] [PubMed]
- Krishnamurthy, N.; Spencer, E.; Torkamani, A.; Nicholson, L. Liquid Biopsies for Cancer: Coming to a Patient near You. J. Clin. Med. 2017, 6, 3. [Google Scholar] [CrossRef]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid Biopsy: Monitoring Cancer-Genetics in the Blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ducreux, M.; Lencioni, R.; Di Bisceglie, A.M.; Galle, P.R.; Dufour, J.F.; Greten, T.F.; Raymond, E.; Roskams, T.; De Baere, T.; et al. EASL-EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-Free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef]
- Liang, W.; Xu, Z.; Kong, F.; Huang, X.; Xiao, Y.; Zhou, W.; Ye, S.; Ye, Q. Circulating Tumour Cell Combined with DNA Methylation for Early Detection of Hepatocellular Carcinoma. Front. Genet. 2022, 13, 1065693. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and Future Perspectives of Liquid Biopsies in Genomics-Driven Oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and Death of Circulating Cell-Free DNA. Cancer Biol. Ther. 2019, 20, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.Q.; Liu, W.R.; Tang, Z.; Qu, W.F.; Fang, Y.; Jiang, X.F.; Song, S.S.; Wang, H.; Tao, C.Y.; Zhou, P.Y.; et al. Serial Circulating Tumor DNA to Predict Early Recurrence in Patients with Hepatocellular Carcinoma: A Prospective Study. Mol. Oncol. 2022, 16, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Riviere, P.; Fanta, P.T.; Ikeda, S.; Baumgartner, J.; Heestand, G.M.; Kurzrock, R. The Mutational Landscape of Gastrointestinal Malignancies as Reflected by Circulating Tumor DNA. Mol. Cancer Ther. 2018, 17, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Chen, Y.; Zhou, J.; Zhao, H.; Zhang, H.; Wang, G. Diagnostic Value of Circulating Cell-Free DNA Levels for Hepatocellular Carcinoma. Int. J. Infect. Dis. 2018, 67, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.H.; Wei, W.; Krawczyk, M.; Wang, W.; Luo, H.; Flagg, K.; Yi, S.; Shi, W.; Quan, Q.; Li, K.; et al. Circulating Tumour DNA Methylation Markers for Diagnosis and Prognosis of Hepatocellular Carcinoma. Nat. Mater. 2017, 16, 1155–1162. [Google Scholar] [CrossRef]
- Lehmann-Werman, R.; Neiman, D.; Zemmour, H.; Moss, J.; Magenheim, J.; Vaknin-Dembinsky, A.; Rubertsson, S.; Nellgård, B.; Blennow, K.; Zetterberg, H.; et al. Identification of Tissue-Specific Cell Death Using Methylation Patterns of Circulating DNA. Proc. Natl. Acad. Sci. USA 2016, 113, E1826–E1834. [Google Scholar] [CrossRef]
- Chan, K.C.A.; Woo, J.K.S.; King, A.; Zee, B.C.Y.; Lam, W.K.J.; Chan, S.L.; Chu, S.W.I.; Mak, C.; Tse, I.O.L.; Leung, S.Y.M.; et al. Analysis of Plasma Epstein-Barr Virus DNA to Screen for Nasopharyngeal Cancer. N. Engl. J. Med. 2017, 377, 513–522. [Google Scholar] [CrossRef]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct Detection of Early-Stage Cancers Using Circulating Tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef]
- Sefrioui, D.; Verdier, V.; Savoye-Collet, C.; Beaussire, L.; Ghomadi, S.; Gangloff, A.; Goria, O.; Riachi, G.; Montialoux, H.; Schwarz, L.; et al. Circulating DNA Changes Are Predictive of Disease Progression after Transarterial Chemoembolization. Int. J. Cancer 2022, 150, 532–541. [Google Scholar] [CrossRef]
- Reinert, T.; Schøler, L.V.; Thomsen, R.; Tobiasen, H.; Vang, S.; Nordentoft, I.; Lamy, P.; Kannerup, A.S.; Mortensen, F.V.; Stribolt, K.; et al. Analysis of Circulating Tumour DNA to Monitor Disease Burden Following Colorectal Cancer Surgery. Gut 2016, 65, 625–634. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, H.; An, K.; Liu, Z.; Hai, L.; Li, R.; Zhou, Y.; Zhao, W.; Jia, Y.; Wu, N.; et al. Whole-Genome Bisulfite Sequencing Analysis of Circulating Tumour DNA for the Detection and Molecular Classification of Cancer. Clin. Transl. Med. 2022, 12, e1014. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.C.; Teng, P.C.; Chen, P.J.; Posadas, E.; Tseng, H.R.; Lu, S.C.; Yang, J.D. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology 2021, 73, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, X.; Yu, X.; Xu, Z.; Yang, G.; Liu, B.; Xiu, P. Clinical Applications of Liquid Biopsy as Prognostic and Predictive Biomarkers in Hepatocellular Carcinoma: Circulating Tumor Cells and Circulating Tumor DNA. J. Exp. Clin. Cancer Res. 2018, 37, 213. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, H. Next-Generation Sequencing in Liquid Biopsy: Cancer Screening and Early Detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid Biopsy: Current Technology and Clinical Applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Yong, E. Cancer Biomarkers: Written in Blood. Nature 2014, 511, 524–526. [Google Scholar] [CrossRef]
- Lanman, R.B.; Mortimer, S.A.; Zill, O.A.; Sebisanovic, D.; Lopez, R.; Blau, S.; Collisson, E.A.; Divers, S.G.; Hoon, D.S.B.; Scott Kopetz, E.; et al. Analytical and Clinical Validation of a Digital Sequencing Panel for Quantitative, Highly Accurate Evaluation of Cell-Free Circulating Tumor DNA. PLoS ONE 2015, 10, e0140712. [Google Scholar] [CrossRef]
- Wang, J.; Huang, A.; Wang, Y.-P.; Yin, Y.; Fu, P.-Y.; Zhang, X.; Zhou, J. Circulating Tumor DNA Correlates with Microvascular Invasion and Predicts Tumor Recurrence of Hepatocellular Carcinoma. Ann. Transl. Med. 2020, 8, 237. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Y.; Xu, Y.; Li, L.; Gong, Y.; Zhang, K.; Zhang, M.; Guan, Y.; Chang, L.; Xia, X.; et al. Pan-Cancer Circulating Tumor DNA Detection in over 10,000 Chinese Patients. Nat. Commun. 2021, 12, 11. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, G.; Zeng, Y.; Dong, X.; Li, Z.; Huang, Y.; Xin, F.; Qiu, L.; Xu, H.; Zhang, W.; et al. Comprehensive Liquid Profiling of Circulating Tumor DNA and Protein Biomarkers in Long-Term Follow-Up Patients with Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 5284–5294. [Google Scholar] [CrossRef]
- Matsumae, T.; Kodama, T.; Myojin, Y.; Maesaka, K.; Sakamori, R.; Takuwa, A.; Oku, K.; Motooka, D.; Sawai, Y.; Oshita, M.; et al. Circulating Cell-Free DNA Profiling Predicts the Therapeutic Outcome in Advanced Hepatocellular Carcinoma Patients Treated with Combination Immunotherapy. Cancers 2022, 14, 3367. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.; Aishima, S.; Clavien, P.A.; Fowler, K.; Goodman, Z.; Gores, G.; Gouw, A.; Kagen, A.; Klimstra, D.; Komuta, M.; et al. CHCC-CCA: Consensus Terminology for Primary Liver Carcinomas with Both Hepatocytic and Cholangiocytic Differentation. Hepatology 2018, 68, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Gigante, E.; Ronot, M.; Bertin, C.; Ciolina, M.; Bouattour, M.; Dondero, F.; Cauchy, F.; Soubrane, O.; Vilgrain, V.; Paradis, V. Combining Imaging and Tumour Biopsy Improves the Diagnosis of Combined Hepatocellular-Cholangiocarcinoma. Liver Int. 2019, 39, 2386–2396. [Google Scholar] [CrossRef] [PubMed]
- Gera, S.; Ettel, M.; Acosta-Gonzalez, G.; Xu, R. Clinical Features, Histology, and Histogenesis of Combined Hepatocellular-Cholangiocarcinoma. World J. Hepatol. 2017, 9, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.J.; Sheybani, A.; Parke, R.A.; Doherty, S.; Brunt, E.M.; Chapman, W.C.; Menias, C.O. Combined Hepatocellular and Cholangiocarcinoma (Biphenotypic) Tumors: Imaging Features and Diagnostic Accuracy of Contrast-Enhanced CT and MRI. AJR Am. J. Roentgenol. 2013, 201, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Zhang, D.; Yin, L.; Qiu, Y.; Liu, J.; Yu, W.; Fu, X.; Zhu, B.; Xu, X.; Duan, A.; et al. Bile Cell-Free DNA as a Novel and Powerful Liquid Biopsy for Detecting Somatic Variants in Biliary Tract Cancer. Oncol. Rep. 2019, 42, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Chen, L.; Zhang, C.; Fujita, M.; Li, R.; Yan, S.M.; Ong, C.K.; Liao, X.; Gao, Q.; Sasagawa, S.; et al. Genomic and Transcriptomic Profiling of Combined Hepatocellular and Intrahepatic Cholangiocarcinoma Reveals Distinct Molecular Subtypes. Cancer Cell 2019, 35, 932.e8–947.e8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Wu, L.; Li, J.; Ji, J.; Yu, Q.; Dai, W.; Feng, J.; Wu, J.; Guo, C. Current Status of CtDNA in Precision Oncology for Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 140. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-Analysis. Gastroenterology 2018, 154, 1706.e1–1718.e1. [Google Scholar] [CrossRef]
- Ye, K.; Fan, Q.; Yuan, M.; Wang, D.; Xiao, L.; Long, G.; Chen, R.; Fang, T.; Li, Z.; Zhou, L. Prognostic Value of Postoperative Circulating Tumor DNA in Patients with Early- and Intermediate-Stage Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 834992. [Google Scholar] [CrossRef]
- Schwaederle, M.; Husain, H.; Fanta, P.T.; Piccioni, D.E.; Kesari, S.; Schwab, R.B.; Patel, S.P.; Harismendy, O.; Ikeda, M.; Parker, B.A.; et al. Use of Liquid Biopsies in Clinical Oncology: Pilot Experience in 168 Patients. Clin. Cancer Res. 2016, 22, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Gu, W.; Nagpal, S.; Gephart, M.H.; Quake, S.R. Brain Tumor Mutations Detected in Cerebral Spinal Fluid. Clin. Chem. 2015, 61, 514–522. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Guan, Y.; Xu, Y.; Han, Y.; Wu, C.; Bao, C.; Zhou, B.; Wang, H.; Zhang, M.; Liu, W.; et al. The Diagnostic and Prognostic Usage of Circulating Tumor DNA in Operable Hepatocellular Carcinoma. Am. J. Transl. Res. 2019, 11, 6462–6474. [Google Scholar] [PubMed]
- Schwaederle, M.; Husain, H.; Fanta, P.T.; Piccioni, D.E.; Kesari, S.; Schwab, R.B.; Banks, K.C.; Lanman, R.B.; Talasaz, A.A.; Parker, B.A.; et al. Detection Rate of Actionable Mutations in Diverse Cancers Using a Biopsy-Free (Blood) Circulating Tumor Cell DNA Assay. Oncotarget 2016, 7, 9707–9716. [Google Scholar] [CrossRef]
- Labgaa, I.; von Felden, J.; Craig, A.J.; Martins-Filho, S.N.; Villacorta-Martin, C.; Demartines, N.; Dormond, O.; D’Avola, D.; Villanueva, A. Experimental Models of Liquid Biopsy in Hepatocellular Carcinoma Reveal Clone-Dependent Release of Circulating Tumor DNA. Hepatol. Commun. 2021, 5, 1095–1105. [Google Scholar] [CrossRef]
- Franses, J.W.; Lim, M.; Burgoyne, A.M.; Mody, K.; Lennerz, J.; Chang, J.; Imperial, R.; Dybel, S.N.; Dinh, T.M.; Masannat, J.; et al. Profile and Predictors of Blood Tumor Mutational Burden in Advanced Hepatocellular Carcinoma. Oncologist 2022, 27, E908–E911. [Google Scholar] [CrossRef]
- Yu, L.; Liu, X.; Han, C.; Lu, S.; Zhu, G.; Su, H.; Qi, W.; Liao, X.; Peng, T. XRCC1 Rs25487 Genetic Variant and TP53 Mutation at Codon 249 Predict Clinical Outcomes of Hepatitis B Virus-Related Hepatocellular Carcinoma after Hepatectomy: A Cohort Study for 10 Years’ Follow up. Hepatol. Res. 2016, 46, 765–774. [Google Scholar] [CrossRef]
- Huang, A.; Zhao, X.; Yang, X.R.; Li, F.Q.; Zhou, X.L.; Wu, K.; Zhang, X.; Sun, Q.M.; Cao, Y.; Zhu, H.M.; et al. Circumventing Intratumoral Heterogeneity to Identify Potential Therapeutic Targets in Hepatocellular Carcinoma. J. Hepatol. 2017, 67, 293–301. [Google Scholar] [CrossRef]
- Cai, Z.X.; Chen, G.; Zeng, Y.Y.; Dong, X.Q.; Lin, M.J.; Huang, X.H.; Zhang, D.; Liu, X.L.; Liu, J.F. Circulating Tumor DNA Profiling Reveals Clonal Evolution and Real-Time Disease Progression in Advanced Hepatocellular Carcinoma. Int. J. Cancer 2017, 141, 977–985. [Google Scholar] [CrossRef]
- Huang, A.; Zhang, X.; Zhou, S.L.; Cao, Y.; Huang, X.W.; Fan, J.; Yang, X.R.; Zhou, J. Detecting Circulating Tumor DNA in Hepatocellular Carcinoma Patients Using Droplet Digital PCR Is Feasible and Reflects Intratumoral Heterogeneity. J. Cancer 2016, 7, 1907–1914. [Google Scholar] [CrossRef]
- Ikeda, S.; Tsigelny, I.F.; Skjevik, Å.A.; Kono, Y.; Mendler, M.; Kuo, A.; Sicklick, J.K.; Heestand, G.; Banks, K.C.; Talasaz, A.; et al. Next-Generation Sequencing of Circulating Tumor DNA Reveals Frequent Alterations in Advanced Hepatocellular Carcinoma. Oncologist 2018, 23, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.P.; Wang, J.; Huang, J.H. CDKN2A Is a Prognostic Biomarker and Correlated with Immune Infiltrates in Hepatocellular Carcinoma. Biosci. Rep. 2021, 41, BSR20211103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.-B.; Qiu, X.-P.; Zhang, S.; Wang, C.; Zheng, F. CDKN2A Promoter Methylation and Hepatocellular Carcinoma Risk: A Meta-Analysis. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226.e4–1239.e4. [Google Scholar] [CrossRef]
- Abdel-Moety, A.; Baddour, N.; Salem, P.; Rady, A.; El-Shendidi, A. ARID1A Expression in Hepatocellular Carcinoma and Relation to Tumor Recurrence after Microwave Ablation. Clin. Exp. Hepatol. 2022, 8, 49–59. [Google Scholar] [CrossRef]
- Yim, S.Y.; Kang, S.H.; Shin, J.H.; Jeong, Y.S.; Sohn, B.H.; Um, S.H.; Lee, J.S. Low ARID1A Expression Is Associated with Poor Prognosis in Hepatocellular Carcinoma. Cells 2020, 9, 2002. [Google Scholar] [CrossRef]
- Loesch, R.; Chenane, L.; Colnot, S. ARID2 Chromatin Remodeler in Hepatocellular Carcinoma. Cells 2020, 9, 2152. [Google Scholar] [CrossRef]
- Jiang, H.; Cao, H.J.; Ma, N.; Bao, W.D.; Wang, J.J.; Chen, T.W.; Zhang, E.-B.; Yuan, Y.-M.; Ni, Q.-Z.; Zhang, F.-K.; et al. Chromatin Remodeling Factor ARID2 Suppresses Hepatocellular Carcinoma Metastasis via DNMT1-Snail Axis. Proc. Natl. Acad. Sci. USA 2020, 117, 4770–4780. [Google Scholar] [CrossRef]
- Xu, G.; Zhou, X.; Xing, J.; Xiao, Y.; Jin, B.; Sun, L.; Yang, H.; Du, S.; Xu, H.; Mao, Y. Identification of RASSF1A Promoter Hypermethylation as a Biomarker for Hepatocellular Carcinoma. Cancer Cell Int. 2020, 20, 547. [Google Scholar] [CrossRef]
- Pasha, H.F.; Mohamed, R.H.; Radwan, M.I. RASSF1A and SOCS1 Genes Methylation Status as a Noninvasive Marker for Hepatocellular Carcinoma. Cancer Biomark. 2019, 24, 241–247. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Huang, T.; Duan, S.; Dai, D.; Jiang, D.; Sui, X.; Li, D.; Chen, Y.; Ding, F.; et al. Meta-Analysis of DNA Methylation Biomarkers in Hepatocellular Carcinoma. Oncotarget 2016, 7, 81255–81267. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Run, Z.C.; Wang, Z.L.; Jiang, T.; An, Y.; Li, Z. RASSF1A Methylation as a Biomarker for Detection of Colorectal Cancer and Hepatocellular Carcinoma. World J. Gastrointest. Oncol. 2022, 14, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; He, H.; Zhang, W.; Yu, D.; Wang, X.; Chen, Y. Combination of Serum RASSF1A Methylation and AFP Is a Promising Non-Invasive Biomarker for HCC Patient with Chronic HBV Infection. Diagn. Pathol. 2015, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yue, F.; Dai, Y.; Shi, B.; Xu, G.; Jiang, X.; Zhou, X.; Pfeifer, G.P.; Liu, L. Suppressor of Hepatocellular Carcinoma RASSF1A Activates Autophagy Initiation and Maturation. Cell Death Differ. 2019, 26, 1379–1395. [Google Scholar] [CrossRef]
- Bannaga, A.S.; Alvarez, R.; Zhou, L.; Petchey, M.; Noufaily, A.; Hitchins, M.P.; Arasaradnam, R.P. Role of Methylated Septin 9 as an Adjunct Diagnostic and Prognostic Biomarker in Hepatocellular Carcinoma. HPB 2021, 23, 1595–1606. [Google Scholar] [CrossRef]
- Li, B.; Huang, H.; Huang, R.; Zhang, W.; Zhou, G.; Wu, Z.; Lv, C.; Han, X.; Jiang, L.; Li, Y.; et al. SEPT9 Gene Methylation as a Noninvasive Marker for Hepatocellular Carcinoma. Dis. Markers 2020, 2020, 6289063. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S. The Mutational Landscape of Hepatocellular Carcinoma. Clin. Mol. Hepatol. 2015, 21, 220–229. [Google Scholar] [CrossRef]
- Rebouissou, S.; Franconi, A.; Calderaro, J.; Letouzé, E.; Imbeaud, S.; Pilati, C.; Nault, J.C.; Couchy, G.; Laurent, A.; Balabaud, C.; et al. Genotype-Phenotype Correlation of CTNNB1 Mutations Reveals Different ß-Catenin Activity Associated with Liver Tumor Progression. Hepatology 2016, 64, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhou, Y.; Qian, M.; Xu, M.; Wang, J.; Zhang, Y.; Song, X.; Wang, H.; Lin, S.; Ren, C.; et al. TBX3 Functions as a Tumor Suppressor Downstream of Activated CTNNB1 Mutants during Hepatocarcinogenesis. J. Hepatol. 2021, 75, 120–131. [Google Scholar] [CrossRef]
- Perugorria, M.J.; Olaizola, P.; Labiano, I.; Esparza-Baquer, A.; Marzioni, M.; Marin, J.J.G.; Bujanda, L.; Banales, J.M. Wnt-β-Catenin Signalling in Liver Development, Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 121–136. [Google Scholar] [CrossRef]
- Huo, J.; Wu, L.; Zang, Y. Development and Validation of a CTNNB1-Associated Metabolic Prognostic Model for Hepatocellular Carcinoma. J. Cell. Mol. Med. 2021, 25, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhu, H.; Dong, L.; Shi, W.; Chen, R.; Song, Z.; Huang, C.; Li, J.; Dong, X.; Zhou, Y.; et al. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell 2019, 179, 561.e22–577.e22. [Google Scholar] [CrossRef]
- Senni, N.; Savall, M.; Cabrerizo Granados, D.; Alves-Guerra, M.C.; Sartor, C.; Lagoutte, I.; Gougelet, A.; Terris, B.; Gilgenkrantz, H.; Perret, C.; et al. β-Catenin-Activated Hepatocellular Carcinomas Are Addicted to Fatty Acids. Gut 2019, 68, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Nandakumar, S.; Armenia, J.; Khalil, D.N.; Albano, M.; Ly, M.; Shia, J.; Hechtman, J.F.; Kundra, R.; El Dika, I.; et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019, 25, 2116–2126. [Google Scholar] [CrossRef] [PubMed]
- Oversoe, S.K.; Clement, M.S.; Weber, B.; Grønbæk, H.; Hamilton-Dutoit, S.J.; Sorensen, B.S.; Kelsen, J. Combining Tissue and Circulating Tumor DNA Increases the Detection Rate of a CTNNB1 Mutation in Hepatocellular Carcinoma. BMC Cancer 2021, 21, 376. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Zhang, C.; Zhang, C.; Wang, H. TERT Mutations Correlate with Higher TMB Value and Unique Tumor Microenvironment and May Be a Potential Biomarker for Anti-CTLA4 Treatment. Cancer Med. 2020, 9, 7151–7160. [Google Scholar] [CrossRef]
- Hirai, M.; Kinugasa, H.; Nouso, K.; Yamamoto, S.; Terasawa, H.; Onishi, Y.; Oyama, A.; Adachi, T.; Wada, N.; Sakata, M.; et al. Prediction of the Prognosis of Advanced Hepatocellular Carcinoma by TERT Promoter Mutations in Circulating Tumor DNA. J. Gastroenterol. Hepatol. 2021, 36, 1118–1125. [Google Scholar] [CrossRef]
- Lai, P.B.S.; Chi, T.Y.; Chen, G.G. Different Levels of P53 Induced Either Apoptosis or Cell Cycle Arrest in a Doxycycline-Regulated Hepatocellular Carcinoma Cell Line in Vitro. Apoptosis 2007, 12, 387–393. [Google Scholar] [CrossRef]
- Long, J.; Wang, A.; Bai, Y.; Lin, J.; Yang, X.; Wang, D.; Yang, X.; Jiang, Y.; Zhao, H. Development and Validation of a TP53-Associated Immune Prognostic Model for Hepatocellular Carcinoma. EBioMedicine 2019, 42, 363–374. [Google Scholar] [CrossRef]
- Ortiz-Cuaran, S.; Villar, S.; Gouas, D.; Ferro, G.; Plymoth, A.; Khuhaprema, T.; Kalalak, A.; Sangrajrang, S.; Friesen, M.D.; Groopman, J.D.; et al. Association between HBX Status, Aflatoxin-Induced R249S TP53 Mutation and Risk of Hepatocellular Carcinoma in a Case-Control Study from Thailand. Cancer Lett. 2013, 331, 46–51. [Google Scholar] [CrossRef]
- Villar, S.; Le Roux-Goglin, E.; Gouas, D.A.; Plymoth, A.; Ferro, G.; Boniol, M.; Lereau, M.; Bah, E.; Hall, A.J.; Wild, C.P.; et al. Seasonal Variation in TP53 R249S-Mutated Serum DNA with Aflatoxin Exposure and Hepatitis B Virus Infection. Environ. Health Perspect. 2011, 119, 1635–1640. [Google Scholar] [CrossRef]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 Regulated Apoptosis in Cancer. Open Biol. 2018, 8, 180002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ding, Y.; Ye, N.; Wild, C.; Chen, H.; Zhou, J. Direct Activation of Bax Protein for Cancer Therapy. Med. Res. Rev. 2016, 36, 313–341. [Google Scholar] [CrossRef] [PubMed]
- Funk, K.; Czauderna, C.; Klesse, R.; Becker, D.; Hajduk, J.; Oelgeklaus, A.; Reichenbach, F.; Fimm-Todt, F.; Lauterwasser, J.; Galle, P.R.; et al. BAX Redistribution Induces Apoptosis Resistance and Selective Stress Sensitivity in Human HCC. Cancers 2020, 12, 1437. [Google Scholar] [CrossRef] [PubMed]
- Alunni-Fabbroni, M.; Rönsch, K.; Huber, T.; Cyran, C.C.; Seidensticker, M.; Mayerle, J.; Pech, M.; Basu, B.; Verslype, C.; Benckert, J.; et al. Circulating DNA as Prognostic Biomarker in Patients with Advanced Hepatocellular Carcinoma: A Translational Exploratory Study from the SORAMIC Trial. J. Transl. Med. 2019, 17, 328. [Google Scholar] [CrossRef]
- Guan, J.; Lu, C.; Jin, Q.; Lu, H.; Chen, X.; Tian, L.; Zhang, Y.; Ortega, J.; Zhang, J.; Siteni, S.; et al. MLH1 Deficiency-Triggered DNA Hyperexcision by Exonuclease 1 Activates the CGAS-STING Pathway. Cancer Cell 2021, 39, 109.e5–121.e5. [Google Scholar] [CrossRef]
- Riazy, M.; Kalloger, S.E.; Sheffield, B.S.; Peixoto, R.D.; Li-Chang, H.H.; Scudamore, C.H.; Renouf, D.J.; Schaeffer, D.F. Mismatch Repair Status May Predict Response to Adjuvant Chemotherapy in Resectable Pancreatic Ductal Adenocarcinoma. Mod. Pathol. 2015, 28, 1383–1389. [Google Scholar] [CrossRef]
- Han, Y.; Peng, Y.; Fu, Y.; Cai, C.; Guo, C.; Liu, S.; Li, Y.; Chen, Y.; Shen, E.; Long, K.; et al. MLH1 Deficiency Induces Cetuximab Resistance in Colon Cancer via Her-2/PI3K/AKT Signaling. Adv. Sci. 2020, 7, 2000112. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, L.; Zang, Y.; Liu, W.; Liu, S.; Teng, F.; Xue, F.; Wang, Y. Endometrial Cancer in Lynch Syndrome. Int. J. Cancer 2022, 150, 7–17. [Google Scholar] [CrossRef]
- Vietri, M.T.; D’elia, G.; Caliendo, G.; Resse, M.; Casamassimi, A.; Passariello, L.; Albanese, L.; Cioffi, M.; Molinari, A.M. Hereditary Prostate Cancer: Genes Related, Target Therapy and Prevention. Int. J. Mol. Sci. 2021, 22, 3753. [Google Scholar] [CrossRef]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Albanese, L.; Signoriello, G.; Napoli, C.; Molinari, A.M. Pancreatic Cancer with Mutation in BRCA1/2, MLH1, and APC Genes: Phenotype Correlation and Detection of a Novel Germline BRCA2 Mutation. Genes 2022, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Eun, J.W.; Choi, J.H.; Woo, H.G.; Cho, H.J.; Ahn, H.R.; Suh, C.W.; Baek, G.O.; Cho, S.W.; Cheong, J.Y. MLH1 Single-Nucleotide Variant in Circulating Tumor DNA Predicts Overall Survival of Patients with Hepatocellular Carcinoma. Sci. Rep. 2020, 10, 17862. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, W.; Qiu, X.; Wang, Z.; Tan, C.; Bei, C.; Qin, L.; Ren, Y.; Tan, S. Single Nucleotide Polymorphisms in MLH1 Predict Poor Prognosis of Hepatocellular Carcinoma in a Chinese Population. Oncotarget 2017, 8, 80039–80049. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Suzuki, I.; Leodolter, A.; Alonso, S.; Horiuchi, S.; Yamashita, K.; Perucho, M. Global DNA Demethylation in Gastrointestinal Cancer Is Age Dependent and Precedes Genomic Damage. Cancer Cell 2006, 9, 199–207. [Google Scholar] [CrossRef]
- Cui, H.; Onyango, P.; Brandenburg, S.; Wu, Y.; Hsieh, C.L.; Feinberg, A.P. Loss of Imprinting in Colorectal Cancer Linked to Hypomethylation of H19 and IGF2. Cancer Res. 2002, 62, 6442–6446. [Google Scholar]
- Tsai, H.C.; Baylin, S.B. Cancer Epigenetics: Linking Basic Biology to Clinical Medicine. Cell Res. 2011, 21, 502–517. [Google Scholar] [CrossRef]
- Luo, H.; Wei, W.; Ye, Z.; Zheng, J.; Xu, R. hua Liquid Biopsy of Methylation Biomarkers in Cell-Free DNA. Trends Mol. Med. 2021, 27, 482–500. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Gassa, A.; Buchner, D.; Alakus, H.; Dong, Q.; Ren, N.; Liu, M.; Odenthal, M.; Stippel, D.; et al. Circulating Tumor DNA as an Emerging Liquid Biopsy Biomarker for Early Diagnosis and Therapeutic Monitoring in Hepatocellular Carcinoma. Int. J. Biol. Sci. 2020, 16, 1551–1562. [Google Scholar] [CrossRef]
- Hernandez-Meza, G.; von Felden, J.; Gonzalez-Kozlova, E.E.; Garcia-Lezana, T.; Peix, J.; Portela, A.; Craig, A.J.; Sayols, S.; Schwartz, M.; Losic, B.; et al. DNA Methylation Profiling of Human Hepatocarcinogenesis. Hepatology 2021, 74, 183–199. [Google Scholar] [CrossRef]
- Zhang, P.; Wen, X.; Gu, F.; Deng, X.; Li, J.; Dong, J.; Jiao, J.; Tian, Y. Methylation Profiling of Serum DNA from Hepatocellular Carcinoma Patients Using an Infinium Human Methylation 450 BeadChip. Hepatol. Int. 2013, 7, 893–900. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, P.; Guo, S.; Tao, C.; Chen, W.; Zhao, W.; Wang, J.; Cheung, R.; Villanueva, A.; Fan, J.; et al. Hypomethylation in HBV Integration Regions Aids Non-Invasive Surveillance to Hepatocellular Carcinoma by Low-Pass Genome-Wide Bisulfite Sequencing. BMC Med. 2020, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Fan, X.P.; Fan, Y.C.; Chen, L.Y.; Qiao, C.Y.; Han, L.Y.; Wang, K. Hypomethylated Ubiquitin-Conjugating Enzyme2 Q1 (UBE2Q1) Gene Promoter in the Serum Is a Promising Biomarker for Hepatitis B Virus-Associated Hepatocellular Carcinoma. Tohoku J. Exp. Med. 2017, 242, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, Y.; Goldstein, J.B.; Ye, K.; Hu, X.; Xiao, L.; Li, L.; Chang, L.; Guan, Y.; Long, G.; et al. Preoperative Evaluation of Microvascular Invasion with Circulating Tumour DNA in Operable Hepatocellular Carcinoma. Liver Int. 2020, 40, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Sun, K.; Tong, Y.K.; Cheng, S.H.; Cheng, T.H.T.; Heung, M.M.S.; Wong, J.; Wong, V.W.S.; Chan, H.L.Y.; Chan, K.C.A.; et al. Preferred End Coordinates and Somatic Variants as Signatures of Circulating Tumor DNA Associated with Hepatocellular Carcinoma. Proc. Natl. Acad. Sci. USA 2018, 115, E10925–E10933. [Google Scholar] [CrossRef]
- Tao, K.; Bian, Z.; Zhang, Q.; Guo, X.; Yin, C.; Wang, Y.; Zhou, K.; Wan, S.; Shi, M.; Bao, D.; et al. Machine Learning-Based Genome-Wide Interrogation of Somatic Copy Number Aberrations in Circulating Tumor DNA for Early Detection of Hepatocellular Carcinoma. EBioMedicine 2020, 56, 102811. [Google Scholar] [CrossRef]
- Pelizzaro, F.; Cardin, R.; Penzo, B.; Pinto, E.; Vitale, A.; Cillo, U.; Russo, F.P.; Farinati, F. Liquid Biopsy in Hepatocellular Carcinoma: Where Are We Now? Cancers 2021, 13, 2274. [Google Scholar] [CrossRef]
- Labgaa, I.; Villanueva, A.; Dormond, O.; Demartines, N.; Melloul, E. The Role of Liquid Biopsy in Hepatocellular Carcinoma Prognostication. Cancers 2021, 13, 659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).