Repeated Rounds of Gonadotropin Stimulation Induce Imbalance in the Antioxidant Machinery and Activation of Pro-Survival Proteins in Mouse Oviducts
Abstract
1. Introduction
2. Results
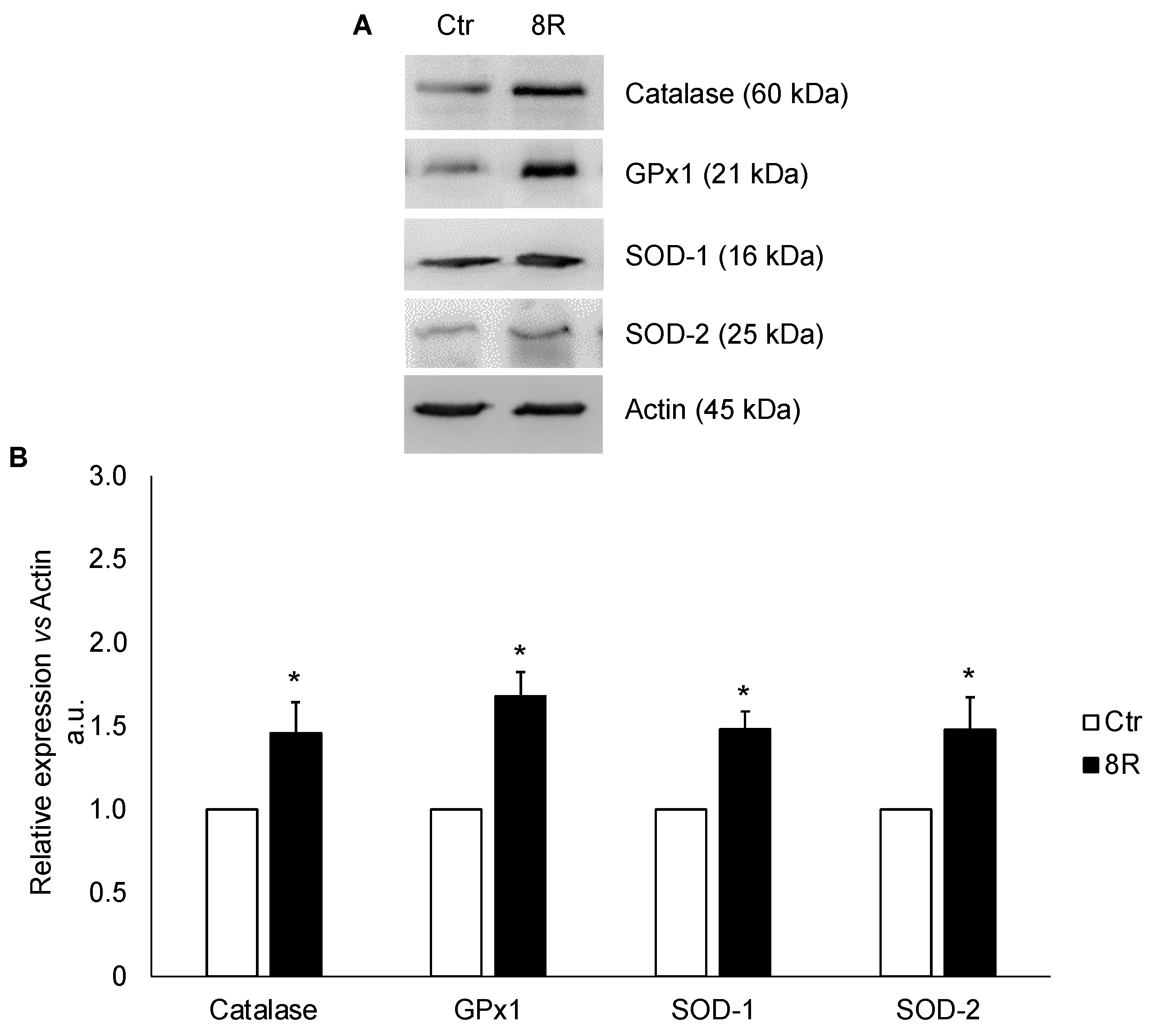
2.1. Protein Content of Oxidative Stress Enzymes in Unstimulated and Hyperstimulated Mouse Oviducts
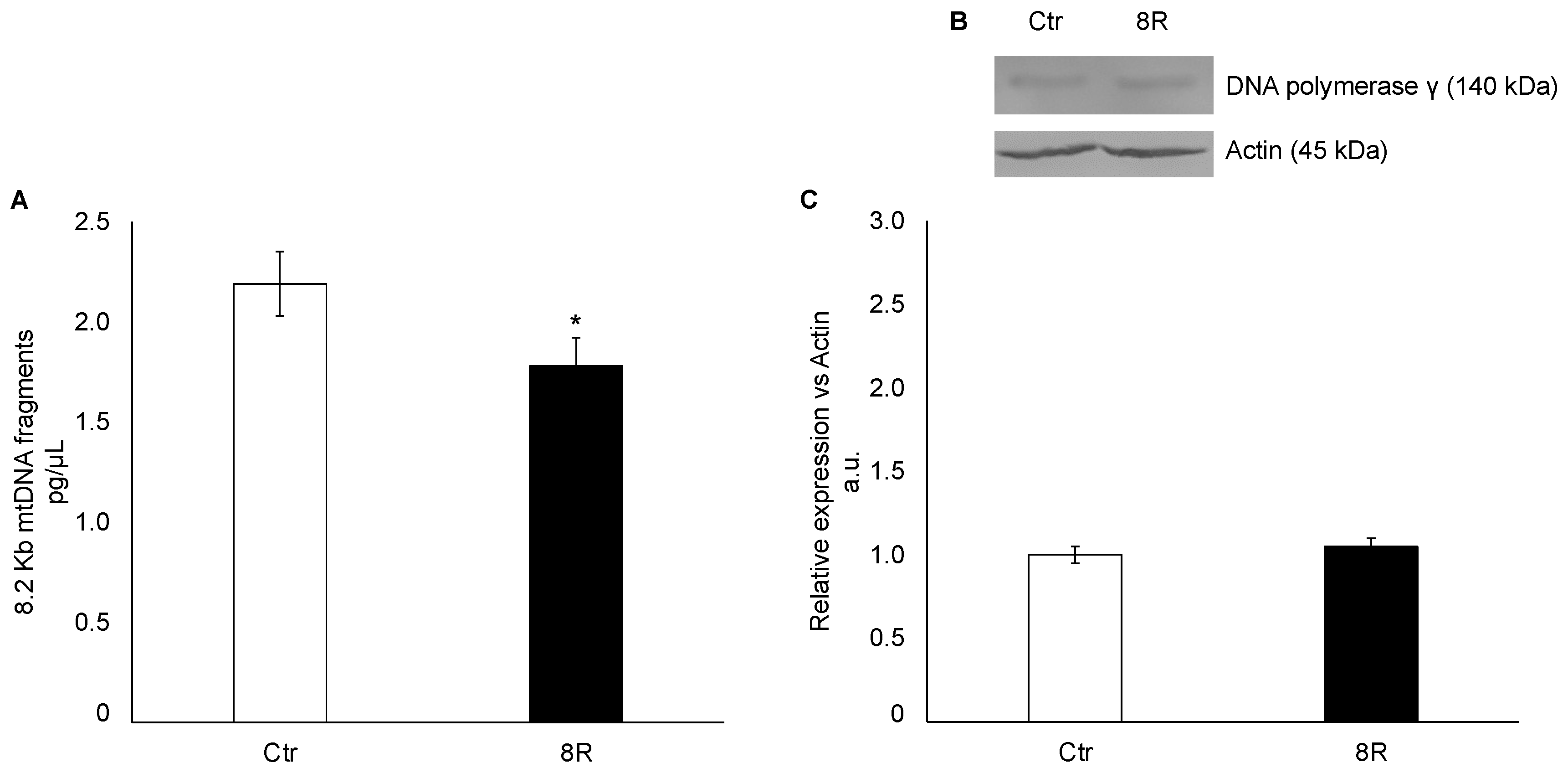
2.2. Mitochondrial Damage in Unstimulated and Hyperstimulated Mouse Oviducts
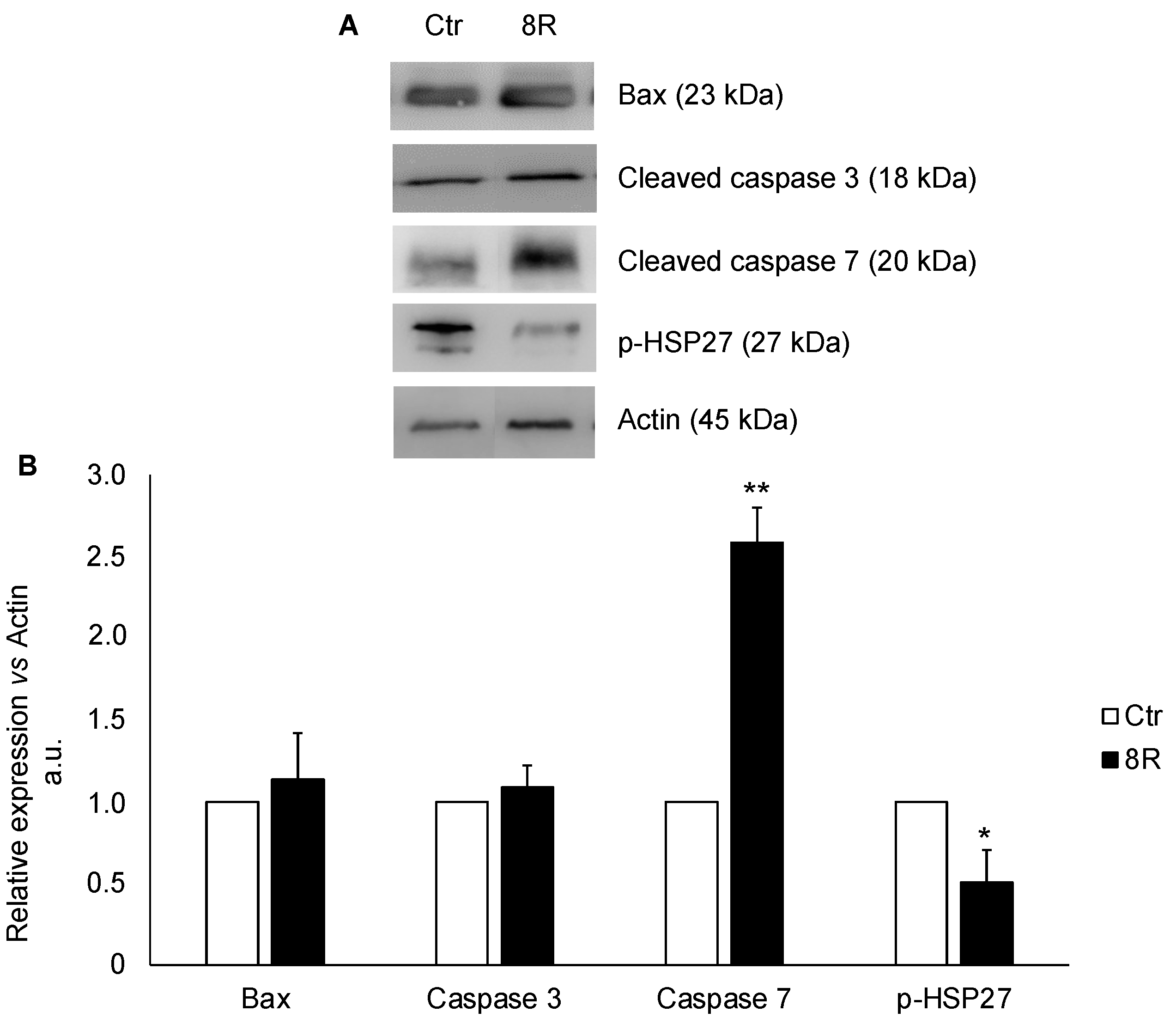
2.3. Content of Apoptotic Proteins in Unstimulated and Hyperstimulated Mouse Oviducts
2.4. Content of Cell-Cycle-Related Proteins in Unstimulated and Hyperstimulated Mouse Oviducts
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Collection of Animals and Oviducts
4.3. Mitochondrial DNA Damage
4.4. Western Blotting
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canipari, R.; De Santis, L.; Cecconi, S. Female Fertility and Environmental Pollution. Int. J. Environ. Res. Public Health 2020, 17, 8802. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Di Nisio, V.; Macchiarelli, G.; Nottola, S.A.; Halvaei, I.; De Santis, L.; Cecconi, S. Technologies for the Production of Fertilizable Mammalian Oocytes. Appl. Sci. 2019, 9, 1536. [Google Scholar] [CrossRef]
- Martinez, F.; Racca, A.; Rodríguez, I.; Polyzos, N.P. Ovarian stimulation for oocyte donation: A systematic review and meta-analysis. Hum. Reprod. Updat. 2021, 27, 673–696. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.; Atilan, O.; Tulay, P. The effect of repeated controlled ovarian stimulation cycles on the gamete and embryo development. Zygote 2019, 27, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Orvieto, R. Controlled Ovarian Hyperstimulation—An Inflammatory State. J. Soc. Gynecol. Investig. 2004, 11, 424–426. [Google Scholar] [CrossRef]
- Williams, C.L.; Jones, M.; Swerdlow, A.; Botting, B.J.; Davies, M.C.; Jacobs, I.; Bunch, K.J.; Murphy, M.F.G.; Sutcliffe, A.G. Risks of ovarian, breast, and corpus uteri cancer in women treated with assisted reproductive technology in Great Britain, 1991-2010: Data linkage study including 2.2 million person years of observation. BMJ 2018, 362, k2644. [Google Scholar] [CrossRef]
- Diakosavvas, M.; Fasoulakis, Z.; Ntounis, T.; Koutras, A.; Angelou, K.; Tsatsaris, G.; Syllaios, A.; Garmpis, N.; Kontomanolis, E.N. A Potential Pathogenic Link Between Cancer of Female Reproductive System and Infertile Women Treated with Assisted Reproduction Techniques. In Vivo 2021, 35, 1393–1399. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Allen, N.E.; Key, T.J.; Dossus, L.; Lukanova, A.; Bakken, K.S.; Lund, E.; Fournier, A.; Overvad, K.; Hansen, L.B.; et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 2011, 105, 1436–1442. [Google Scholar] [CrossRef]
- Burger, H.G.; Dudley, E.C.; Robertson, D.M.; Dennerstein, L. Hormonal Changes in the Menopause Transition. Recent. Prog. Horm. Res. 2002, 57, 257–275. [Google Scholar] [CrossRef]
- Burdette, J.E.; Kurley, S.; Kilen, S.M.; Mayo, K.E.; Woodruff, T. Gonadotropin-Induced Superovulation Drives Ovarian Surface Epithelia Proliferation in CD1 Mice. Endocrinology 2006, 147, 2338–2345. [Google Scholar] [CrossRef]
- Song, K.; Dai, L.; Long, X.; Wang, W.; Di, W. Follicle-stimulating hormone promotes the proliferation of epithelial ovarian cancer cells by activating sphingosine kinase. Sci. Rep. 2020, 10, 13834. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Mhawech-Fauceglia, P.; Tsao-Wei, D.; Roman, L.; Gaur, R.K.; Epstein, A.L.; Pinski, J. Expression of the luteinizing hormone receptor (LHR) in ovarian cancer. BMC Cancer 2019, 19, 1114. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, I.; Behrens, R.F.; Smith, L.A. Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database Syst. Rev. 2019, 2019, CD008215. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-W.; Miao, Y.; Liang, C.-N.; Wang, N.; Jiang, B.; Wang, Q.-Y.; Kang, J.; Hou, G.; Yin, Y. Malignant Transformation of a Borderline Ovarian Tumor with Pulmonary and Pleural Metastases after Years of Latency: A Case Report and Literature Review. Front. Med. 2020, 7, 571348. [Google Scholar] [CrossRef] [PubMed]
- Meinhold-Heerlein, I.; Fotopoulou, C.; Harter, P.; Kurzeder, C.; Mustea, A.; Wimberger, P.; Hauptmann, S.; Sehouli, J. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch. Gynecol. Obstet. 2016, 293, 695–700. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017, 8, 1093. [Google Scholar] [CrossRef]
- Kyo, S.; Ishikawa, N.; Nakamura, K.; Nakayama, K. The fallopian tube as origin of ovarian cancer: Change of diagnostic and preventive strategies. Cancer Med. 2019, 9, 421–431. [Google Scholar] [CrossRef]
- Madsen, C.; Baandrup, L.; Dehlendorff, C.; Kjaer, S.K. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: A nationwide case-control study. Acta Obstet. Gynecol. Scand. 2014, 94, 86–94. [Google Scholar] [CrossRef]
- Hakim, A.A.; Barry, C.P.; Barnes, H.J.; Anderson, K.E.; Petitte, J.; Whitaker, R.; Lancaster, J.M.; Wenham, R.M.; Carver, D.K.; Turbov, J.; et al. Ovarian Adenocarcinomas in the Laying Hen and Women Share Similar Alterations in p53, ras, and HER-2/neu. Cancer Prev. Res. 2009, 2, 114–121. [Google Scholar] [CrossRef]
- Treviño, L.S.; Giles, J.R.; Wang, W.; Urick, M.E.; Johnson, P.A. Gene Expression Profiling Reveals Differentially Expressed Genes in Ovarian Cancer of the Hen: Support for Oviductal Origin? Horm. Cancer 2010, 1, 177–186. [Google Scholar] [CrossRef]
- Zhang, S.; Dolgalev, I.; Zhang, T.; Ran, H.; Levine, D.A.; Neel, B.G. Both fallopian tube and ovarian surface epithelium are cells-of-origin for high-grade serous ovarian carcinoma. Nat. Commun. 2019, 10, 5367. [Google Scholar] [CrossRef]
- Di Luigi, G.; Rossi, G.; Castellucci, A.; Leocata, P.; Carta, G.; Canipari, R.; Nottola, S.A.; Cecconi, S. Repeated ovarian stimulation does not affect the expression level of proteins involved in cell cycle control in mouse ovaries and fallopian tubes. J. Assist. Reprod. Genet. 2014, 31, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, V.; Rossi, G.; Palmerini, M.G.; Macchiarelli, G.; Tiboni, G.M.; Cecconi, S. Increased rounds of gonadotropin stimulation have side effects on mouse fallopian tubes and oocytes. Reproduction 2018, 155, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Antonouli, S.; Palmerini, M.G.; Bianchi, S.; Rossi, G.; Cecconi, S.; Belli, M.; Bernardi, S.; Khalili, M.A.; Familiari, G.; Nottola, S.A.; et al. Repeated hyperstimulation affects the ultrastructure of mouse fallopian tube epithelium. J. Reprod. Dev. 2020, 66, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-K.; Wang, Q.; Zhang, T.-T.; Yin, S.; Zhang, C.-L.; Ge, Z.-J. Repeated superovulation may affect mitochondrial functions of cumulus cells in mice. Sci. Rep. 2016, 6, 31368. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Ferreira, E.M.; Andrade, A.Z.; Jordão, A.A.; Ferriani, R.A.; Navarro, P.A. The Impact of Controlled Ovarian Stimulation on Serum Oxidative Stress Markers in Infertile Women with Endometriosis Undergoing ICSI. Antioxidants 2022, 11, 1161. [Google Scholar] [CrossRef]
- Concannon, C.G.; Gorman, A.M.; Samali, A. On the role of Hsp27 in regulating apoptosis. Apoptosis 2003, 8, 61–70. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2021, 11, 617843. [Google Scholar] [CrossRef]
- Sugino, N. Reactive oxygen species in ovarian physiology. Reprod. Med. Biol. 2005, 4, 31–44. [Google Scholar]
- Racchi, M.L. Antioxidant Defenses in Plants with Attention to Prunus and Citrus spp. Antioxidants 2013, 2, 340. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Miyamoto, K.; Sato, E.F.; Kasahara, E.; Jikumaru, M.; Hiramoto, K.; Tabata, H.; Katsuragi, M.; Odo, S.; Utsumi, K.; Inoue, M. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic. Biol. Med. 2010, 49, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, S.; Liu, H.; Gong, Y.; Bai, H.; Huang, W.; Liu, Q.; Guan, L.; Fan, P. Association of GPx1 P198L and CAT C-262T Genetic Variations with Polycystic Ovary Syndrome in Chinese Women. Front. Endocrinol. 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Katz-Jaffe, M.G.; Lane, S.L.; Parks, J.C.; McCallie, B.R.; Makloski, R.; Schoolcraft, W.B. Antioxidant Intervention Attenuates Aging-Related Changes in the Murine Ovary and Oocyte. Life 2020, 10, 250. [Google Scholar] [CrossRef]
- Liang, J.; Gao, Y.; Feng, Z.; Zhang, B.; Na, Z.; Li, D. Reactive oxygen species and ovarian diseases: Antioxidant strategies. Redox Biol. 2023, 62, 102659. [Google Scholar] [CrossRef]
- Hance, N.; Ekstrand, M.; Trifunovic, A. Mitochondrial DNA polymerase gamma is essential for mammalian embryogenesis. Hum. Mol. Genet. 2005, 14, 1775–1783. [Google Scholar] [CrossRef]
- Maiuri, A.R.; Li, H.; Stein, B.D.; Tennessen, J.M.; O’hagan, H.M. Inflammation-induced DNA methylation of DNA polymerase gamma alters the metabolic profile of colon tumors. Cancer Metab. 2018, 6, 9. [Google Scholar] [CrossRef]
- Vidyasagar, A.; Wilson, N.A.; Djamali, A. Heat shock protein 27 (HSP27): Biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair 2012, 5, 7. [Google Scholar] [CrossRef]
- Al-Madhoun, A.S.; Chen, Y.-X.; Haidari, L.; Rayner, K.; Gerthoffer, W.; McBride, H.; O’brien, E.R. The interaction and cellular localization of HSP27 and ERβ are modulated by 17β-estradiol and HSP27 phosphorylation. Mol. Cell. Endocrinol. 2007, 270, 33–42. [Google Scholar] [CrossRef]
- Takaki, E.; Fujimoto, M.; Nakahari, T.; Yonemura, S.; Miyata, Y.; Hayashida, N.; Yamamoto, K.; Vallee, R.B.; Mikuriya, T.; Sugahara, K.; et al. Heat Shock Transcription Factor 1 Is Required for Maintenance of Ciliary Beating in Mice. J. Biol. Chem. 2007, 282, 37285–37292. [Google Scholar] [CrossRef]
- Jones, T.J.; Adapala, R.K.; Geldenhuys, W.J.; Bursley, C.; AbouAlaiwi, W.A.; Nauli, S.M.; Thodeti, C.K. Primary cilia regulates the directional migration and barrier integrity of endothelial cells through the modulation of Hsp27 dependent actin cytoskeletal organization. J. Cell. Physiol. 2011, 227, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wann, A.; Chapple, J.; Knight, M. The primary cilium influences interleukin-1β-induced NFκB signalling by regulating IKK activity. Cell. Signal. 2014, 26, 1735–1742. [Google Scholar] [CrossRef]
- Hayashi, N.; Peacock, J.W.; Beraldi, E.; Zoubeidi, A.; Gleave, M.E.; Ong, C.J. Hsp27 silencing coordinately inhibits proliferation and promotes Fas-induced apoptosis by regulating the PEA-15 molecular switch. Cell Death Differ. 2011, 19, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Farber, R.; Nakazawa, A.; Kumar, S.; Bharti, A.; Nalin, C.; Weichselbaum, R.; Kufe, D.; Kharbanda, S. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene 2000, 19, 1975–1981. [Google Scholar] [CrossRef]
- Bruey, J.-M.; Ducasse, C.; Bonniaud, P.; Ravagnan, L.; Susin, S.A.; Diaz-Latoud, C.; Gurbuxani, S.; Arrigo, A.-P.; Kroemer, G.; Solary, E.; et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2000, 2, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Manero, F.; Gonin, S.; Kretz-Remy, C.; Virot, S.; Arrigo, A.-P. Hsp27 as a Negative Regulator of Cytochrome c Release. Mol. Cell. Biol. 2002, 22, 816–834. [Google Scholar] [CrossRef] [PubMed]
- Parcellier, A.; Schmitt, E.; Gurbuxani, S.; Seigneurin-Berny, D.; Pance, A.; Chantôme, A.; Plenchette, S.; Khochbin, S.; Solary, E.; Garrido, C. HSP27 is a Ubiquitin-Binding Protein Involved in I-κBα Proteasomal Degradation. Mol. Cell. Biol. 2003, 23, 5790–5802. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamauchi, L.; Hunter, T.; Kikkawa, U.; Kamada, S. Possible involvement of caspase-7 in cell cycle progression at mitosis. Genes Cells 2008, 13, 609–621. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.-D. Caspase-7: A protease involved in apoptosis and inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Obexer, P.; Ausserlechner, M.J. X-Linked Inhibitor of Apoptosis (XIAP)—A Critical Death-Resistance Regulator and Therapeutic Target for Personalized Cancer Therapy. Front. Oncol. 2014, 4, 197. [Google Scholar] [CrossRef] [PubMed]
- Deveraux, Q.L.; Leo, E.; Stennicke, H.R.; Welsh, K.; Salvesen, G.S.; Reed, J.C. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999, 18, 5242–5251. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.L.; Denault, J.-B.; Riedl, S.J.; Shin, H.; Renatus, M.; Salvesen, G.S. XIAP inhibits caspase-3 and -7 using two binding sites: Evolutionarily conserved mechanism of IAPs. EMBO J. 2005, 24, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Wagner, E.F.; Nebreda, Á.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef]
- Svensson, C.; Part, K.; Künnis-Beres, K.; Kaldmäe, M.; Fernaeus, S.Z.; Land, T. Pro-survival effects of JNK and p38 MAPK pathways in LPS-induced activation of BV-2 cells. Biochem. Biophys. Res. Commun. 2011, 406, 488–492. [Google Scholar] [CrossRef]
- Jia, D.; Nagaoka, Y.; Katsumata, M.; Orsulic, S. Inflammation is a key contributor to ovarian cancer cell seeding. Sci. Rep. 2018, 8, 12394. [Google Scholar] [CrossRef]
- Caligioni, C.S. Assessing Reproductive Status/Stages in Mice. Curr. Protoc. Neurosci. 2009, 48, A-4I. [Google Scholar] [CrossRef]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse Estrous Cycle Identification Tool and Images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nisio, V.; Antonouli, S.; Colafarina, S.; Zarivi, O.; Rossi, G.; Cecconi, S.; Poma, A.M.G. Repeated Rounds of Gonadotropin Stimulation Induce Imbalance in the Antioxidant Machinery and Activation of Pro-Survival Proteins in Mouse Oviducts. Int. J. Mol. Sci. 2023, 24, 9294. https://doi.org/10.3390/ijms24119294
Di Nisio V, Antonouli S, Colafarina S, Zarivi O, Rossi G, Cecconi S, Poma AMG. Repeated Rounds of Gonadotropin Stimulation Induce Imbalance in the Antioxidant Machinery and Activation of Pro-Survival Proteins in Mouse Oviducts. International Journal of Molecular Sciences. 2023; 24(11):9294. https://doi.org/10.3390/ijms24119294
Chicago/Turabian StyleDi Nisio, Valentina, Sevastiani Antonouli, Sabrina Colafarina, Osvaldo Zarivi, Gianna Rossi, Sandra Cecconi, and Anna Maria Giuseppina Poma. 2023. "Repeated Rounds of Gonadotropin Stimulation Induce Imbalance in the Antioxidant Machinery and Activation of Pro-Survival Proteins in Mouse Oviducts" International Journal of Molecular Sciences 24, no. 11: 9294. https://doi.org/10.3390/ijms24119294
APA StyleDi Nisio, V., Antonouli, S., Colafarina, S., Zarivi, O., Rossi, G., Cecconi, S., & Poma, A. M. G. (2023). Repeated Rounds of Gonadotropin Stimulation Induce Imbalance in the Antioxidant Machinery and Activation of Pro-Survival Proteins in Mouse Oviducts. International Journal of Molecular Sciences, 24(11), 9294. https://doi.org/10.3390/ijms24119294