JAK Signaling Is Critically Important in Cytokine-Induced Viral Susceptibility of Keratinocytes
Abstract
1. Introduction
2. Results
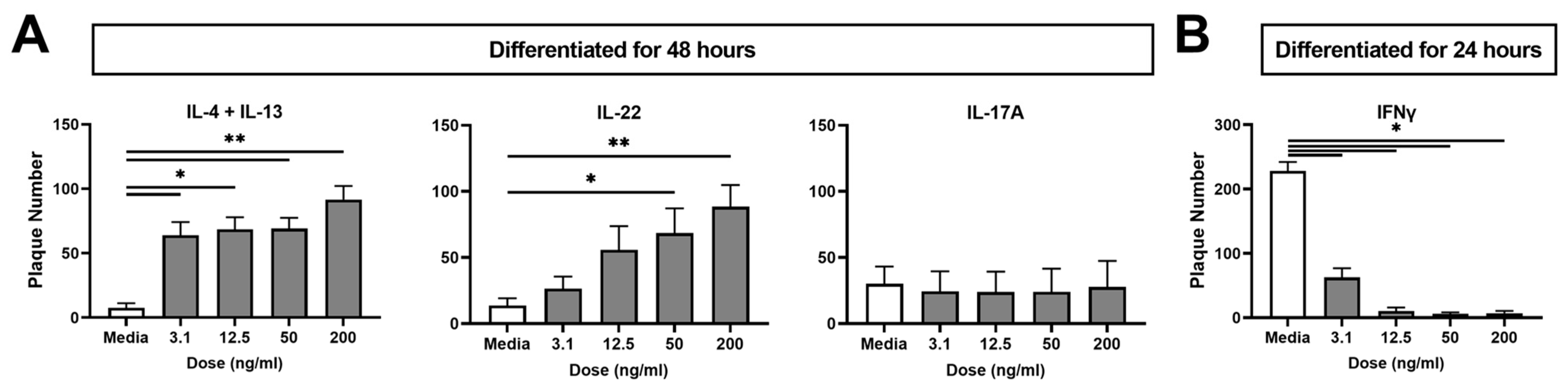
2.1. Type 2 (IL-4 + IL-13) and Type 3 (IL-22) Cytokines Increase KC Viral Susceptibility
2.2. The Type 1 Cytokine IFNγ Decreases KC Viral Susceptibility
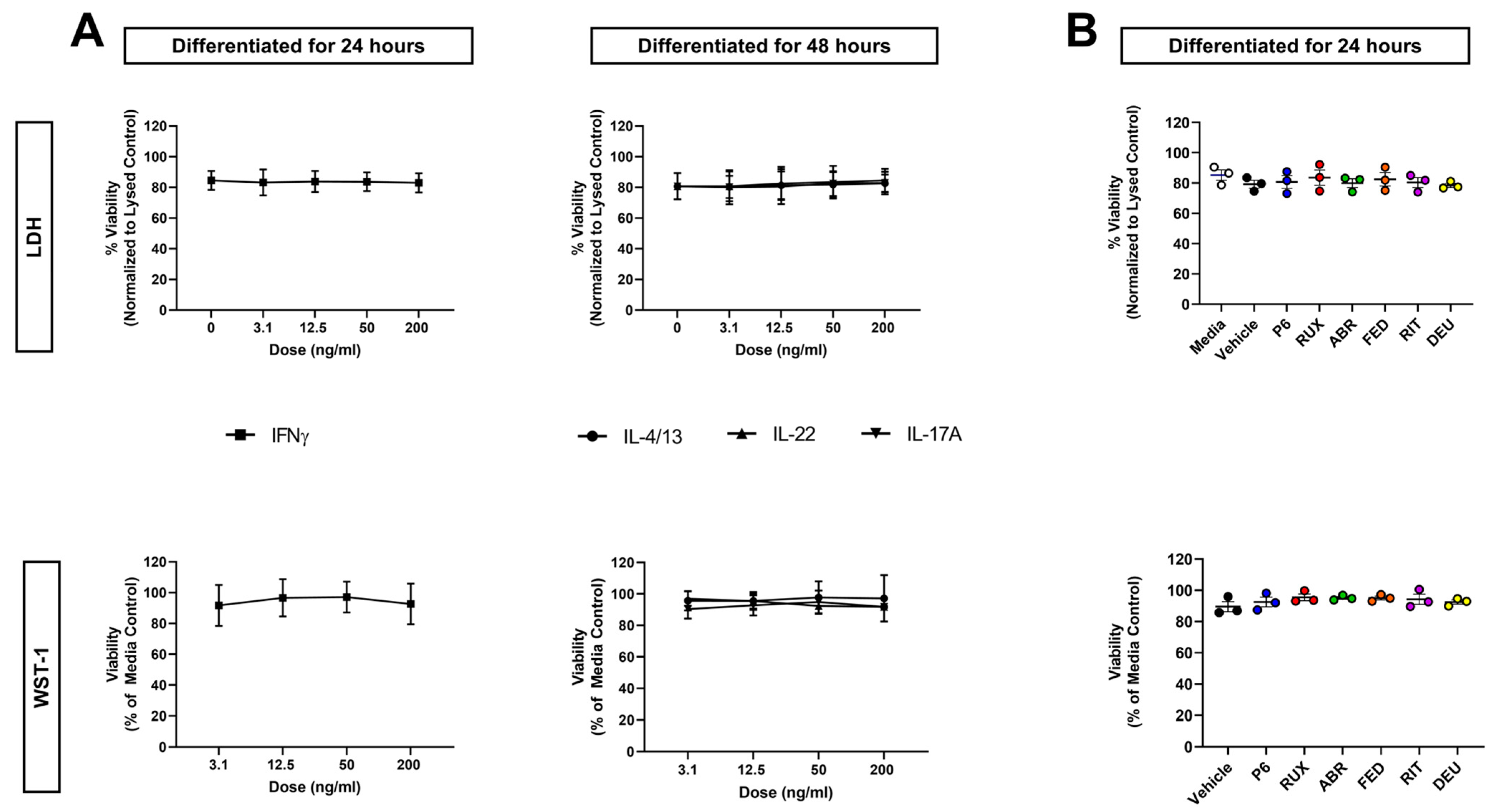
2.3. Neither Cytokine Nor JAKi Exposure Affects KC Viability
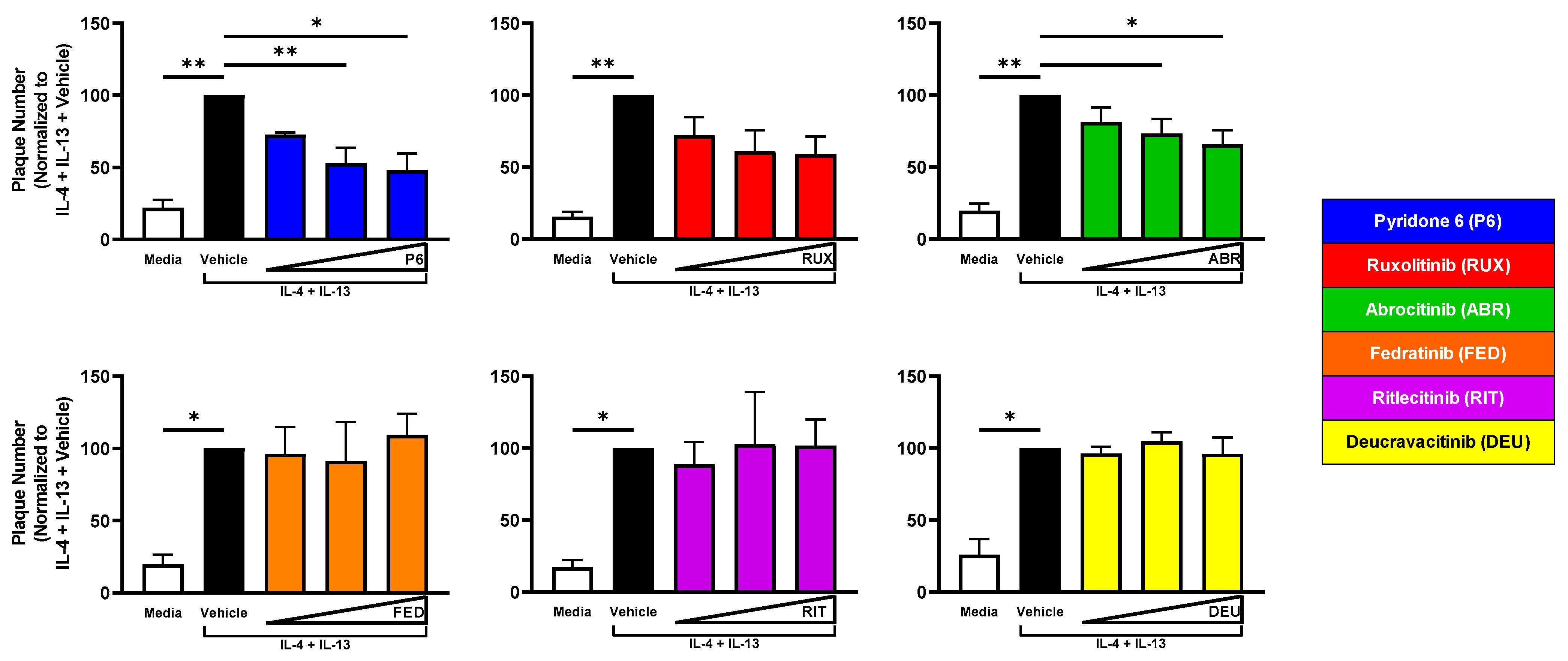
2.4. JAK1 Inhibition Reduces Viral Susceptibility of IL-4 + IL-13-Treated KC
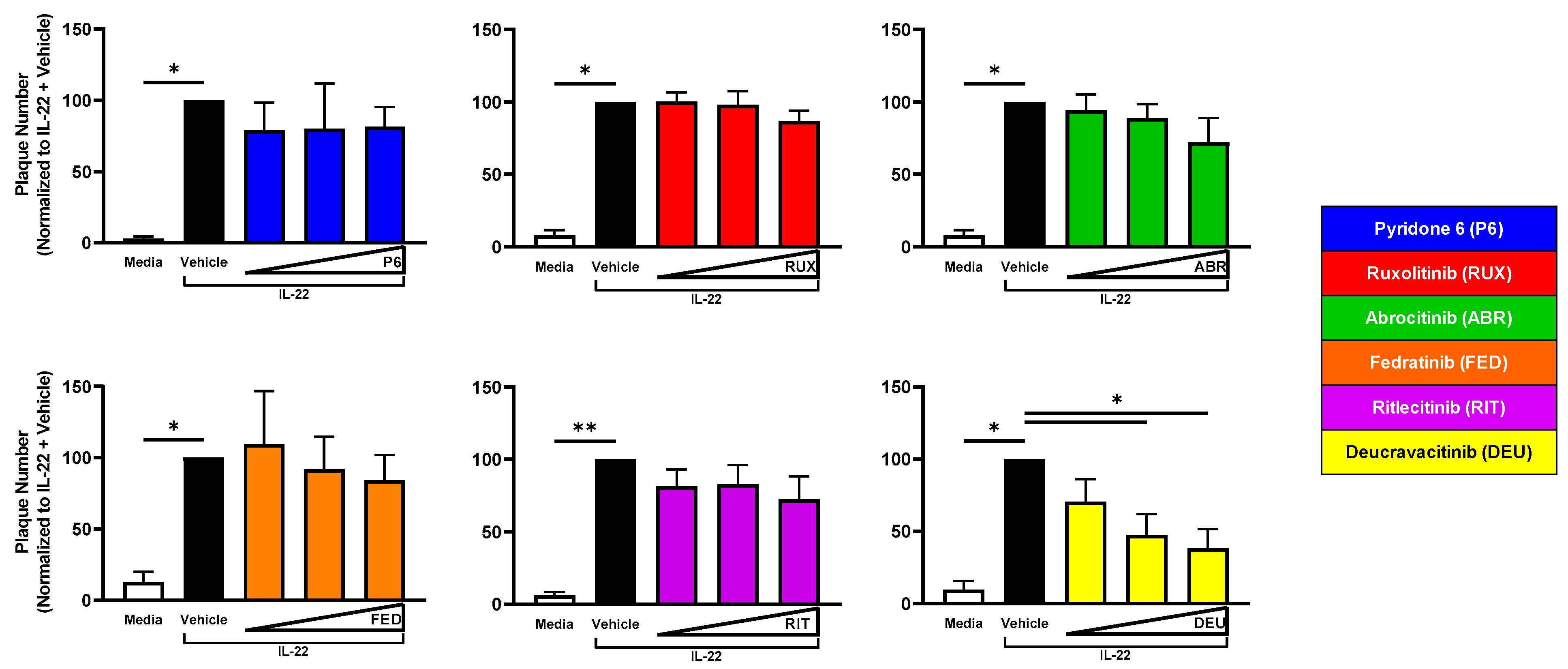
2.5. TYK2 Inhibition Reduces Viral Susceptibility of IL-22-Treated KC
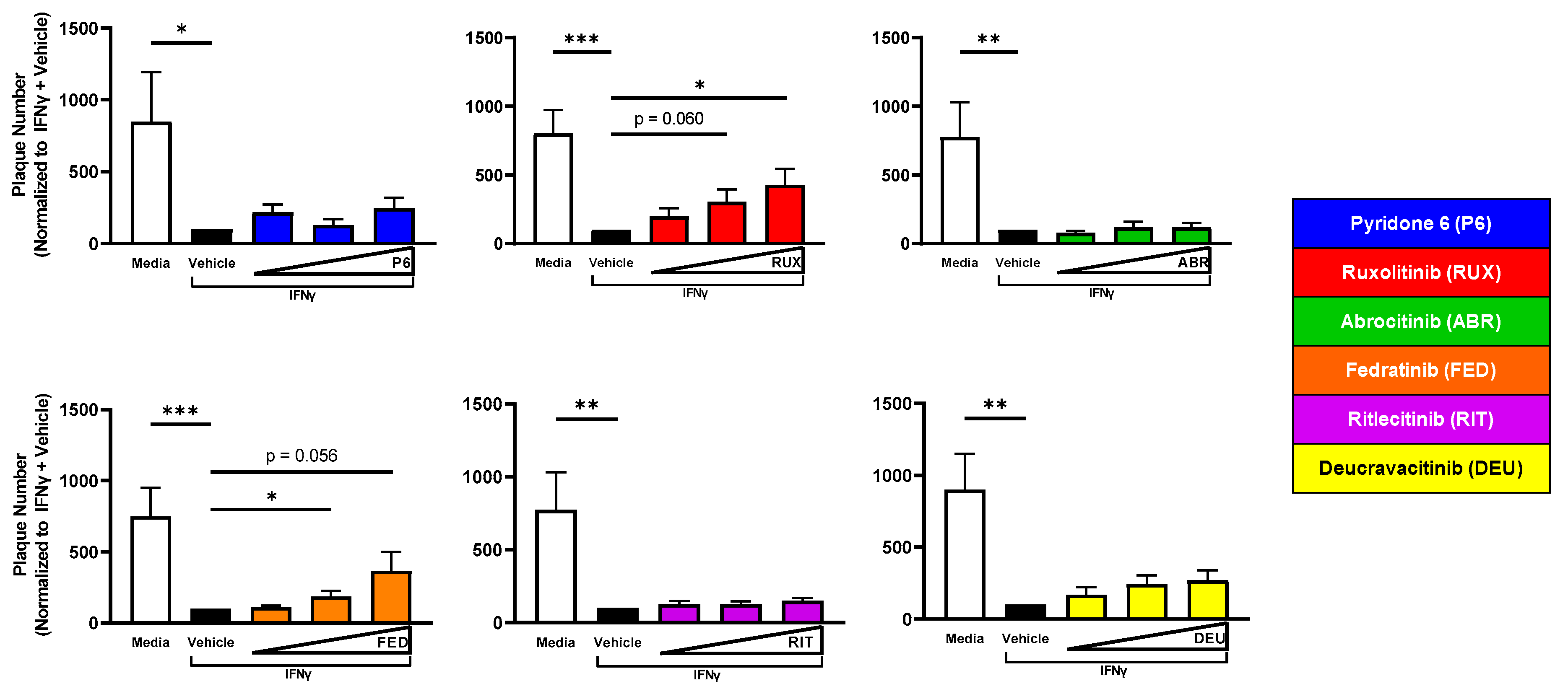
2.6. JAK2 Inhibition Reverses IFNγ-Induced Resistance to VV Infection
2.7. A JAK1/JAK2-Selective Inhibitor (Ruxolitinib) Increases Virion Production from IFNγ-Treated KC
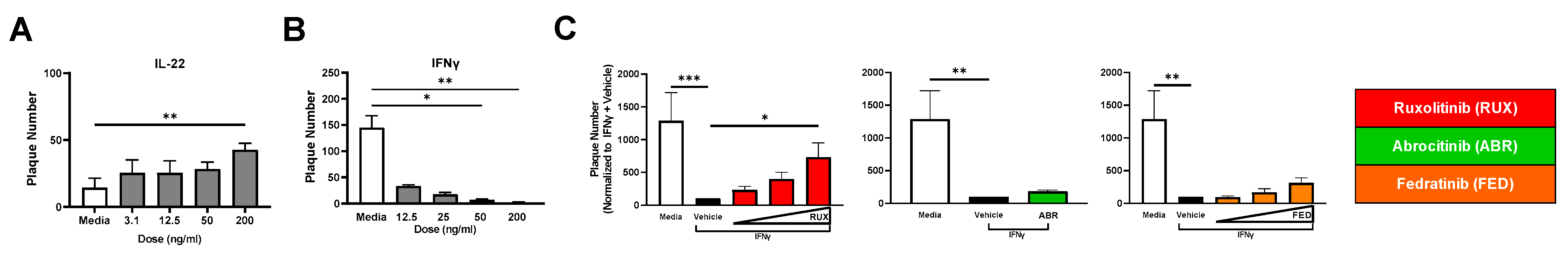
2.8. Primary KC Substantiates the Importance of Cytokine Profiles and JAKi in Cutaneous Viral Susceptibility
3. Discussion
4. Materials and Methods
4.1. Cells and Culture Techniques
4.2. Cytokines and JAKi
4.3. Cell Viability Assays
4.4. Viral Plaque Assay
4.5. Viral Titering
4.6. Statistical and Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| HSV-1 | Herpes simplex virus-1 |
| JAK | Janus kinase |
| JAKi | Janus kinase inhibitor |
| KC | Keratinocyte |
| MOI | Multiplicity of infection |
| PHFK | Primary human foreskin keratinocytes |
| STAT | Signal transducer and activator of transcription |
| VV | Vaccinia virus |
References
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [PubMed]
- Nograles, K.E.; Zaba, L.C.; Guttman-Yassky, E.; Fuentes-Duculan, J.; Suarez-Farinas, M.; Cardinale, I.; Khatcherian, A.; Gonzalez, J.; Pierson, K.C.; White, T.R.; et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br. J. Dermatol. 2008, 159, 1092–1102. [Google Scholar] [CrossRef]
- Toro, J.R.; Finlay, D.; Dou, X.; Zheng, S.C.; LeBoit, P.E.; Connolly, M.K. Detection of type 1 cytokines in discoid lupus erythematosus. Arch. Dermatol. 2000, 136, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.C.; Sanyal, R.D.; Pavel, A.B.; Glickman, J.; Zheng, X.; Xu, H.; Cho, Y.T.; Tsai, T.F.; Wen, H.C.; Peng, X.; et al. Atopic dermatitis in Chinese patients shows T. J. Allergy Clin. Immunol. 2018, 142, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Brunner, P.M.; Renert-Yuval, Y.; Czarnowicki, T.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; Johnson, D.B.; et al. Early-onset pediatric atopic dermatitis is T. J. Allergy Clin. Immunol. 2016, 138, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, R.D.; Pavel, A.B.; Glickman, J.; Chan, T.C.; Zheng, X.; Zhang, N.; Cueto, I.; Peng, X.; Estrada, Y.; Fuentes-Duculan, J.; et al. Atopic dermatitis in African American patients is T. Ann. Allergy Asthma Immunol. 2019, 122, 99–110.e116. [Google Scholar] [CrossRef]
- Gittler, J.K.; Shemer, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef]
- Ong, P.Y.; Leung, D.Y. Bacterial and Viral Infections in Atopic Dermatitis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 329–337. [Google Scholar] [CrossRef]
- Vora, S.; Damon, I.; Fulginiti, V.; Weber, S.G.; Kahana, M.; Stein, S.L.; Gerber, S.I.; Garcia-Houchins, S.; Lederman, E.; Hruby, D.; et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 2008, 46, 1555–1561. [Google Scholar] [CrossRef]
- Wollenberg, A.; Wetzel, S.; Burgdorf, W.H.; Haas, J. Viral infections in atopic dermatitis: Pathogenic aspects and clinical management. J. Allergy Clin. Immunol. 2003, 112, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Copeman, P.W.; Wallace, H.J. Eczema Vaccinatum. Br. Med. J. 1964, 2, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Xia, J. Eczema Monkeypoxicum: Report of Monkeypox Transmission in Atopic Dermatitis. JAAD Case Rep. 2022, 29, 95–99. [Google Scholar] [CrossRef]
- Simpson, E.L.; Papp, K.A.; Blauvelt, A.; Chu, C.Y.; Hong, H.C.; Katoh, N.; Calimlim, B.M.; Thyssen, J.P.; Chiou, A.S.; Bissonnette, R.; et al. Efficacy and Safety of Upadacitinib in Patients With Moderate to Severe Atopic Dermatitis: Analysis of Follow-up Data From the Measure Up 1 and Measure Up 2 Randomized Clinical Trials. JAMA Dermatol. 2022, 158, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Eichenfield, L.F.; Leung, D.Y.M.; Forman, S.B.; Venturanza, M.E.; Sun, K.; Kuligowski, M.E.; et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J. Am. Acad. Dermatol. 2021, 85, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Simpson, E.L.; Thyssen, J.P.; Gooderham, M.; Chan, G.; Feeney, C.; Biswas, P.; Valdez, H.; DiBonaventura, M.; Nduaka, C.; et al. Efficacy and Safety of Abrocitinib in Patients With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Thaci, D.; Strober, B.; Gordon, K.B.; Foley, P.; Gooderham, M.; Morita, A.; Papp, K.A.; Puig, L.; Menter, M.A.; Colombo, M.J.; et al. Deucravacitinib in Moderate to Severe Psoriasis: Clinical and Quality-of-Life Outcomes in a Phase 2 Trial. Dermatol. Ther. 2022, 12, 495–510. [Google Scholar] [CrossRef]
- Hoisnard, L.; Lebrun-Vignes, B.; Maury, S.; Mahevas, M.; El Karoui, K.; Roy, L.; Zarour, A.; Michel, M.; Cohen, J.L.; Amiot, A.; et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci. Rep. 2022, 12, 7140. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Eichenfield, L.F.; Forman, S.B.; Kuligowski, M.E.; Kallender, H.; Sun, K.; Ren, H.; et al. Long-Term Safety and Disease Control With Ruxolitinib Cream in Atopic Dermatitis: Results From Two Phase 3 Studies. J. Am. Acad. Dermatol. 2023, 88, 1008–1016. [Google Scholar] [CrossRef]
- Simpson, E.L.; Silverberg, J.I.; Nosbaum, A.; Winthrop, K.L.; Guttman-Yassky, E.; Hoffmeister, K.M.; Egeberg, A.; Valdez, H.; Zhang, M.; Farooqui, S.A.; et al. Integrated Safety Analysis of Abrocitinib for the Treatment of Moderate-to-Severe Atopic Dermatitis From the Phase II and Phase III Clinical Trial Program. Am. J. Clin. Dermatol. 2021, 22, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T.; Simpson, E.L.; Silverberg, J.I.; Thaci, D.; Paul, C.; Pink, A.E.; Kataoka, Y.; Chu, C.Y.; DiBonaventura, M.; Rojo, R.; et al. Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1101–1112. [Google Scholar] [CrossRef]
- Blauvelt, A.; Silverberg, J.I.; Lynde, C.W.; Bieber, T.; Eisman, S.; Zdybski, J.; Gubelin, W.; Simpson, E.L.; Valenzuela, F.; Criado, P.R.; et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: Results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J. Am. Acad. Dermatol. 2022, 86, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Gooderham, M.; Warren, R.B.; Papp, K.A.; Strober, B.; Thaci, D.; Morita, A.; Szepietowski, J.C.; Imafuku, S.; Colston, E.; et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J. Am. Acad. Dermatol. 2023, 88, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.G.; Monticelli, S.R.; Moran, M.C.; Miller, B.L.; Beck, L.A.; Ward, B.M. Conditions That Simulate the Environment of Atopic Dermatitis Enhance Susceptibility of Human Keratinocytes to Vaccinia Virus. Cells 2022, 11, 1337. [Google Scholar] [CrossRef]
- Moran, M.C.; Chinchilli, E.; Kenney, H.M.; Pope, E.M.; Scott, G.; Brewer, M.G.; Beck, L.A. Stage of Keratinocyte Differentiation is a Key Determinant of Viral Susceptibility in Human Skin. J. Investig. Dermatol. 2023, S0022-202X(23)01937-1, ahead of print. [Google Scholar] [CrossRef]
- Solimani, F.; Meier, K.; Ghoreschi, K. Emerging Topical and Systemic JAK Inhibitors in Dermatology. Front. Immunol. 2019, 10, 2847. [Google Scholar] [CrossRef]
- Fetter, T.; Smith, P.; Guel, T.; Braegelmann, C.; Bieber, T.; Wenzel, J. Selective Janus Kinase 1 Inhibition Is a Promising Therapeutic Approach for Lupus Erythematosus Skin Lesions. Front. Immunol. 2020, 11, 344. [Google Scholar] [CrossRef]
- Nogueira, M.; Puig, L.; Torres, T. JAK Inhibitors for Treatment of Psoriasis: Focus on Selective TYK2 Inhibitors. Drugs 2020, 80, 341–352. [Google Scholar] [CrossRef]
- Rhein, B.A.; Powers, L.S.; Rogers, K.; Anantpadma, M.; Singh, B.K.; Sakurai, Y.; Bair, T.; Miller-Hunt, C.; Sinn, P.; Davey, R.A.; et al. Interferon-gamma Inhibits Ebola Virus Infection. PLoS Pathog. 2015, 11, e1005263. [Google Scholar] [CrossRef]
- Shrestha, B.; Wang, T.; Samuel, M.A.; Whitby, K.; Craft, J.; Fikrig, E.; Diamond, M.S. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J. Virol. 2006, 80, 5338–5348. [Google Scholar] [CrossRef]
- Hooks, J.J.; Wang, Y.; Detrick, B. The critical role of IFN-gamma in experimental coronavirus retinopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3402–3408. [Google Scholar] [CrossRef]
- Muller, U.; Steinhoff, U.; Reis, L.F.; Hemmi, S.; Pavlovic, J.; Zinkernagel, R.M.; Aguet, M. Functional role of type I and type II interferons in antiviral defense. Science 1994, 264, 1918–1921. [Google Scholar] [CrossRef]
- Kajita, A.I.; Morizane, S.; Takiguchi, T.; Yamamoto, T.; Yamada, M.; Iwatsuki, K. Interferon-Gamma Enhances TLR3 Expression and Anti-Viral Activity in Keratinocytes. J. Investig. Dermatol. 2015, 135, 2005–2011. [Google Scholar] [CrossRef]
- Strittmatter, G.E.; Sand, J.; Sauter, M.; Seyffert, M.; Steigerwald, R.; Fraefel, C.; Smola, S.; French, L.E.; Beer, H.D. IFN-gamma Primes Keratinocytes for HSV-1-Induced Inflammasome Activation. J. Investig. Dermatol. 2016, 136, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Tsoi, L.C.; Sarkar, M.K.; Xing, X.; Xue, K.; Uppala, R.; Berthier, C.C.; Zeng, C.; Patrick, M.; Billi, A.C.; et al. IFN-gamma enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci. Transl. Med. 2019, 11, eaav7561. [Google Scholar] [CrossRef]
- Qing, Y.; Stark, G.R. Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J. Biol. Chem. 2004, 279, 41679–41685. [Google Scholar] [CrossRef] [PubMed]
- Stabenow, J.; Buller, R.M.; Schriewer, J.; West, C.; Sagartz, J.E.; Parker, S. A mouse model of lethal infection for evaluating prophylactics and therapeutics against Monkeypox virus. J. Virol. 2010, 84, 3909–3920. [Google Scholar] [CrossRef]
- Pardanani, A.; Harrison, C.; Cortes, J.E.; Cervantes, F.; Mesa, R.A.; Milligan, D.; Masszi, T.; Mishchenko, E.; Jourdan, E.; Vannucchi, A.M.; et al. Safety and Efficacy of Fedratinib in Patients With Primary or Secondary Myelofibrosis: A Randomized Clinical Trial. JAMA Oncol. 2015, 1, 643–651. [Google Scholar] [CrossRef]
- Harrison, C.N.; Schaap, N.; Vannucchi, A.M.; Kiladjian, J.J.; Tiu, R.V.; Zachee, P.; Jourdan, E.; Winton, E.; Silver, R.T.; Schouten, H.C.; et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): A single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017, 4, e317–e324. [Google Scholar] [CrossRef]
- Howell, M.D.; Gallo, R.L.; Boguniewicz, M.; Jones, J.F.; Wong, C.; Streib, J.E.; Leung, D.Y. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 2006, 24, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E.; ElKhal, A.; Freyschmidt, E.J.; MacArthur, D.H.; McDonald, D.; Howell, M.D.; Leung, D.Y.; Laouar, A.; Manjunath, N.; Bianchi, T.; et al. Impaired immune response to vaccinia virus inoculated at the site of cutaneous allergic inflammation. J. Allergy Clin. Immunol. 2007, 120, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Marie, R.E.M.; Abuzeid, A.; Attia, F.M.; Anani, M.M.; Gomaa, A.H.A.; Atef, L.M. Serum level of interleukin-22 in patients with cutaneous warts: A case-control study. J. Cosmet. Dermatol. 2021, 20, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Boniface, K.; Bernard, F.X.; Garcia, M.; Gurney, A.L.; Lecron, J.C.; Morel, F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 2005, 174, 3695–3702. [Google Scholar] [CrossRef]
- Ekman, A.K.; Bivik Eding, C.; Rundquist, I.; Enerback, C. IL-17 and IL-22 Promote Keratinocyte Stemness in the Germinative Compartment in Psoriasis. J. Investig. Dermatol. 2019, 139, 1564–1573.e1568. [Google Scholar] [CrossRef]
- Moran, M.C.; Pandya, R.P.; Leffler, K.A.; Yoshida, T.; Beck, L.A.; Brewer, M.G. Characterization of Human Keratinocyte Cell Lines for Barrier Studies. JID Innov. 2021, 1, 100018. [Google Scholar] [CrossRef]
- Strober, B.; Thaci, D.; Sofen, H.; Kircik, L.; Gordon, K.B.; Foley, P.; Rich, P.; Paul, C.; Bagel, J.; Colston, E.; et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J. Am. Acad. Dermatol. 2023, 88, 40–51. [Google Scholar] [CrossRef]
- Marzano, A.V.; Genovese, G.; Moltrasio, C.; Gaspari, V.; Vezzoli, P.; Maione, V.; Misciali, C.; Sena, P.; Patrizi, A.; Offidani, A.; et al. The clinical spectrum of COVID-19-associated cutaneous manifestations: An Italian multicenter study of 200 adult patients. J. Am. Acad. Dermatol. 2021, 84, 1356–1363. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Antinori, A.; Orkin, C.M. Monkeypox Virus Infection across 16 Countries—April–June 2022. Reply. N. Engl. J. Med. 2022, 387, e69. [Google Scholar] [CrossRef]
- Beck, L.A.; Boguniewicz, M.; Hata, T.; Schneider, L.C.; Hanifin, J.; Gallo, R.; Paller, A.S.; Lieff, S.; Reese, J.; Zaccaro, D.; et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J. Allergy Clin. Immunol. 2009, 124, 260–269, 269.e261–269.e267. [Google Scholar] [CrossRef]
- Dickson, M.A.; Hahn, W.C.; Ino, Y.; Ronfard, V.; Wu, J.Y.; Weinberg, R.A.; Louis, D.N.; Li, F.P.; Rheinwald, J.G. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 2000, 20, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.G.; Yoshida, T.; Kuo, F.I.; Fridy, S.; Beck, L.A.; De Benedetto, A. Antagonistic Effects of IL-4 on IL-17A-Mediated Enhancement of Epidermal Tight Junction Function. Int. J. Mol. Sci. 2019, 20, 4070. [Google Scholar] [CrossRef] [PubMed]
- Etienne, L.; Joshi, P.; Dingle, L.; Huang, E.; Grzesik, P.; Desai, P.J. Visualization of herpes simplex virus type 1 virions using fluorescent colors. J. Virol. Methods 2017, 241, 46–51. [Google Scholar] [CrossRef] [PubMed]
| Janus Kinase Inhibitor (Shorthand Nomenclature) | For Treatment of: | IC50 (nM) | Cmax | ||||||
|---|---|---|---|---|---|---|---|---|---|
| JAK1 | JAK2 | JAK3 | TYK2 | REFERENCE | nM | REFERENCE | Human Dose | ||
| Pyridone 6 (P6) | Not in clinical development | 15 * | 1 | 5 | 1 | PMID: 11934592 Access Date: 15 July 2021 | N/A | N/A | N/A |
| Ruxolitinib (RUX) | Atopic dermatitis #, Myelofibrosis, Polycythemia Vera, Graft-Versus-Host Disease | 3.3 | 2.8 | 428 | 19 | PMID: 20130243 Access Date: 15 July 2021 | 650 | PMID: 35368221 Access Date: 7 December 2022 | 15 mg BID |
| Abrocitinib (ABR) | Atopic Dermatitis | 29.2 | 803 | >104 | 1250 | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/213871Orig1s000MultidisciplineR.pdf Access Date: 15 July 2021 | 2164 | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/213871Orig1s000MultidisciplineR.pdf Access Date: 7 December 2022 | 100 mg QD |
| Fedratinib (FED) | Myelofibrosis | 105 | 3 | 1002 | 405 | PMID: 18394554 Access Date: 15 July 2021 | 3438 | https://packageinserts.bms.com/pi/pi_inrebic.pdf Access Date: 7 December 2022 | 400 mg QD |
| Ritlecitinib (RIT) | Not FDA approved (In multiple clinical trials) | >104 | >104 | 33.1 | >104 | PMID: 27791347 Access Date: 15 July 2021 | N/A | N/A | N/A |
| Deucravacitinib (DEU) | Plaque Psoriasis | >104 | >104 | >104 | 0.2 | PMID: 31318208 Access Date: 15 July 2021 | 105 | https://packageinserts.bms.com/pi/pi_sotyktu.pdf Access Date: 7 December 2022 | 6 mg QD |
| Effect on Viral Susceptibility | |||||||
| Ruxolitinib | Abrocitinib | Fedratinib | Ritlecitinib | Deucravacitinib | |||
| Clinical Observations | |||||||
| No JAKi | Observed (Significant) | Observed | Not Observed | No Reported Results | Observed | ||
| in vitro Observations (Vaccinia Virus) | |||||||
| Cytokine | IL-4 + IL-13 | Increase & | Decrease | Decrease | No effect | No effect | No effect |
| IL-22 | Increase #,& | No effect | No effect | No effect | No effect | Decrease | |
| IL-17A | No effect & | Not Determined | |||||
| IFNγ | Decrease #,& | Increase #,& | No effect | Increase #,& | No effect | No effect | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnold, K.A.; Peterson, L.F.; Beck, L.A.; Brewer, M.G. JAK Signaling Is Critically Important in Cytokine-Induced Viral Susceptibility of Keratinocytes. Int. J. Mol. Sci. 2023, 24, 9243. https://doi.org/10.3390/ijms24119243
Arnold KA, Peterson LF, Beck LA, Brewer MG. JAK Signaling Is Critically Important in Cytokine-Induced Viral Susceptibility of Keratinocytes. International Journal of Molecular Sciences. 2023; 24(11):9243. https://doi.org/10.3390/ijms24119243
Chicago/Turabian StyleArnold, Kimberly A., Liam F. Peterson, Lisa A. Beck, and Matthew G. Brewer. 2023. "JAK Signaling Is Critically Important in Cytokine-Induced Viral Susceptibility of Keratinocytes" International Journal of Molecular Sciences 24, no. 11: 9243. https://doi.org/10.3390/ijms24119243
APA StyleArnold, K. A., Peterson, L. F., Beck, L. A., & Brewer, M. G. (2023). JAK Signaling Is Critically Important in Cytokine-Induced Viral Susceptibility of Keratinocytes. International Journal of Molecular Sciences, 24(11), 9243. https://doi.org/10.3390/ijms24119243






