Evaluation of Serum Albumin-Coated Bone Allograft for Bone Regeneration: A Seven-Year Follow-Up Study of 26 Cases
Abstract
1. Introduction
2. Results
2.1. Descriptive Statistics
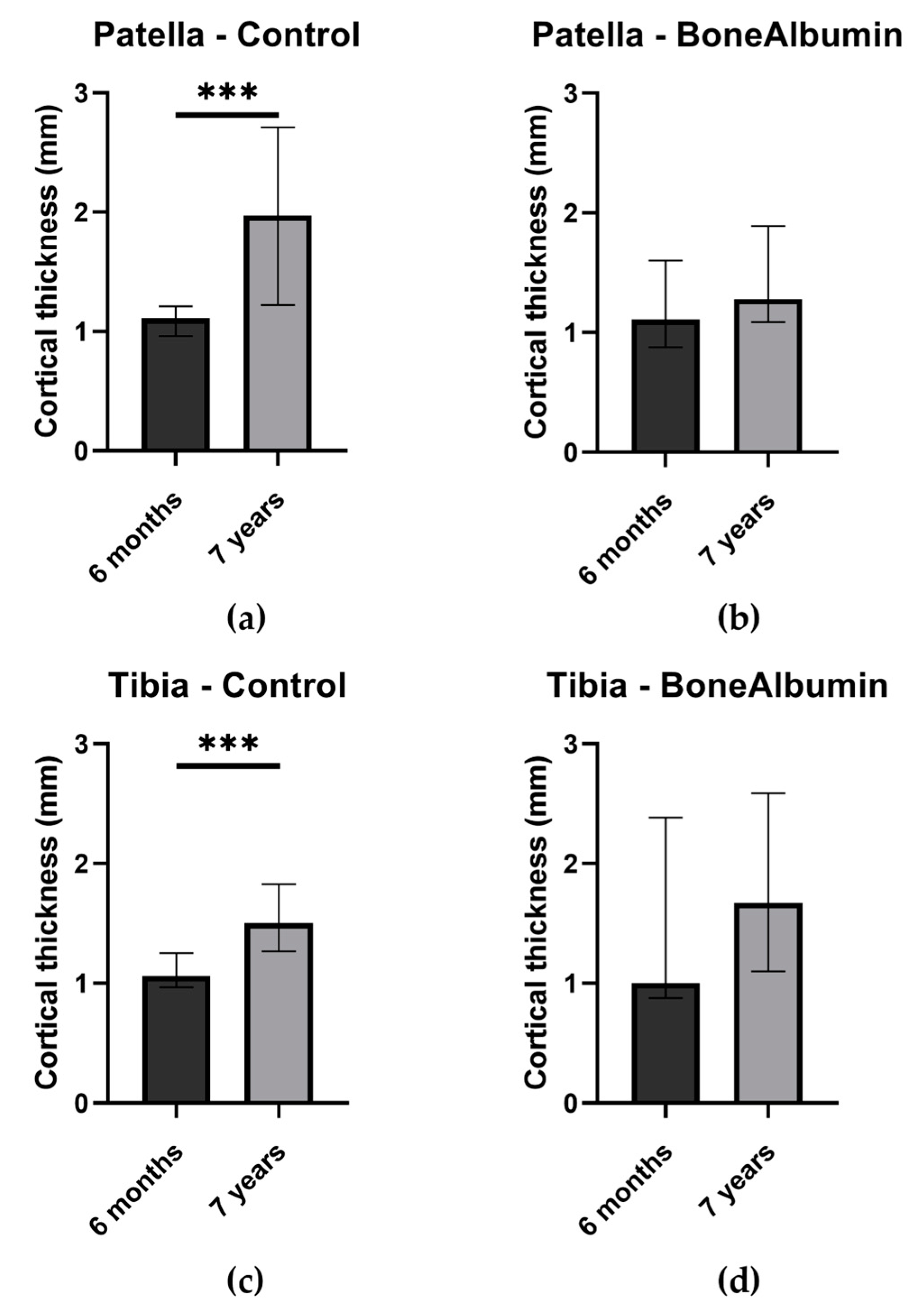
2.2. Bone Quality: Cortical Thickness
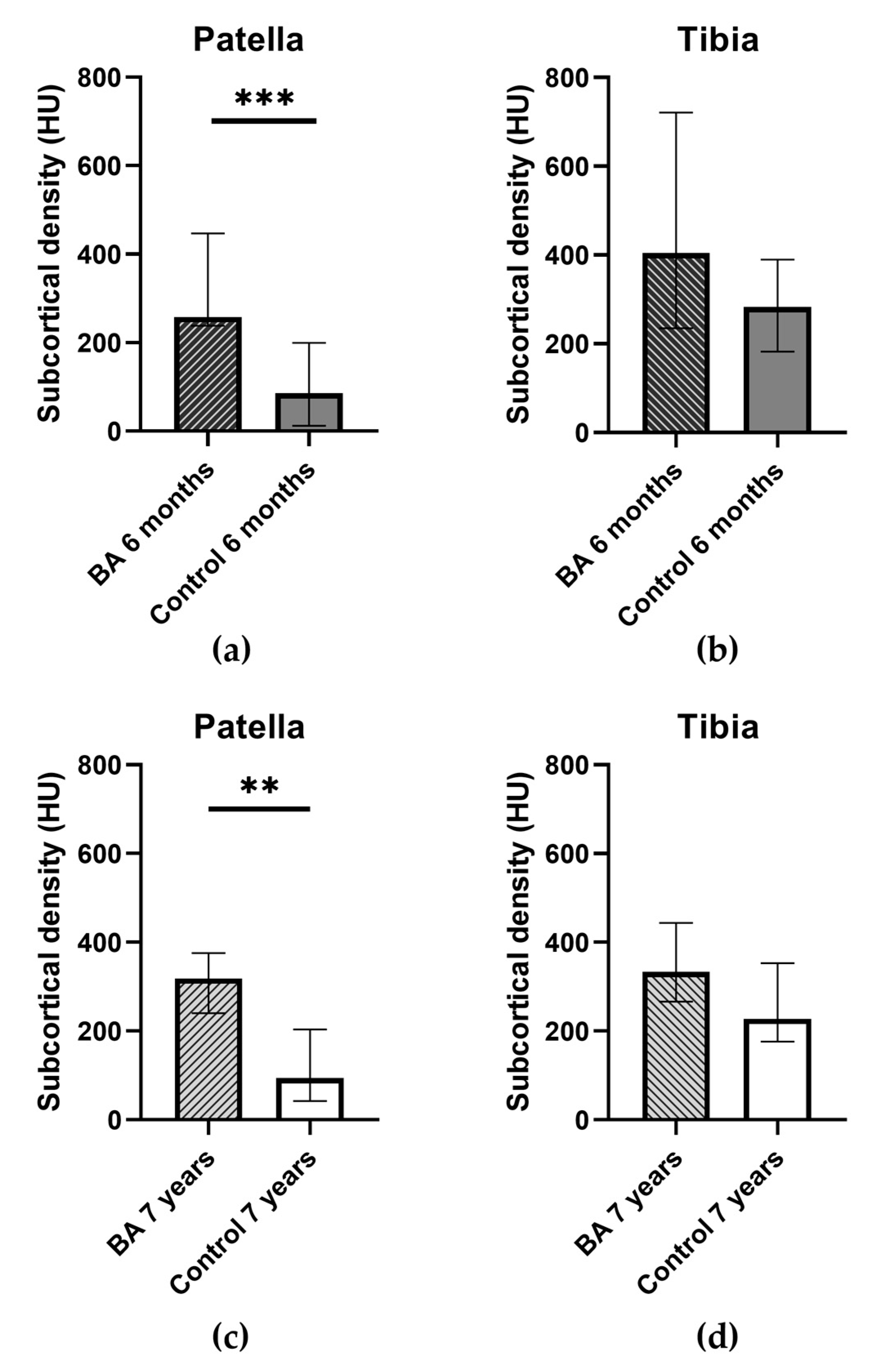
2.3. Bone Quality: Subcortical Density
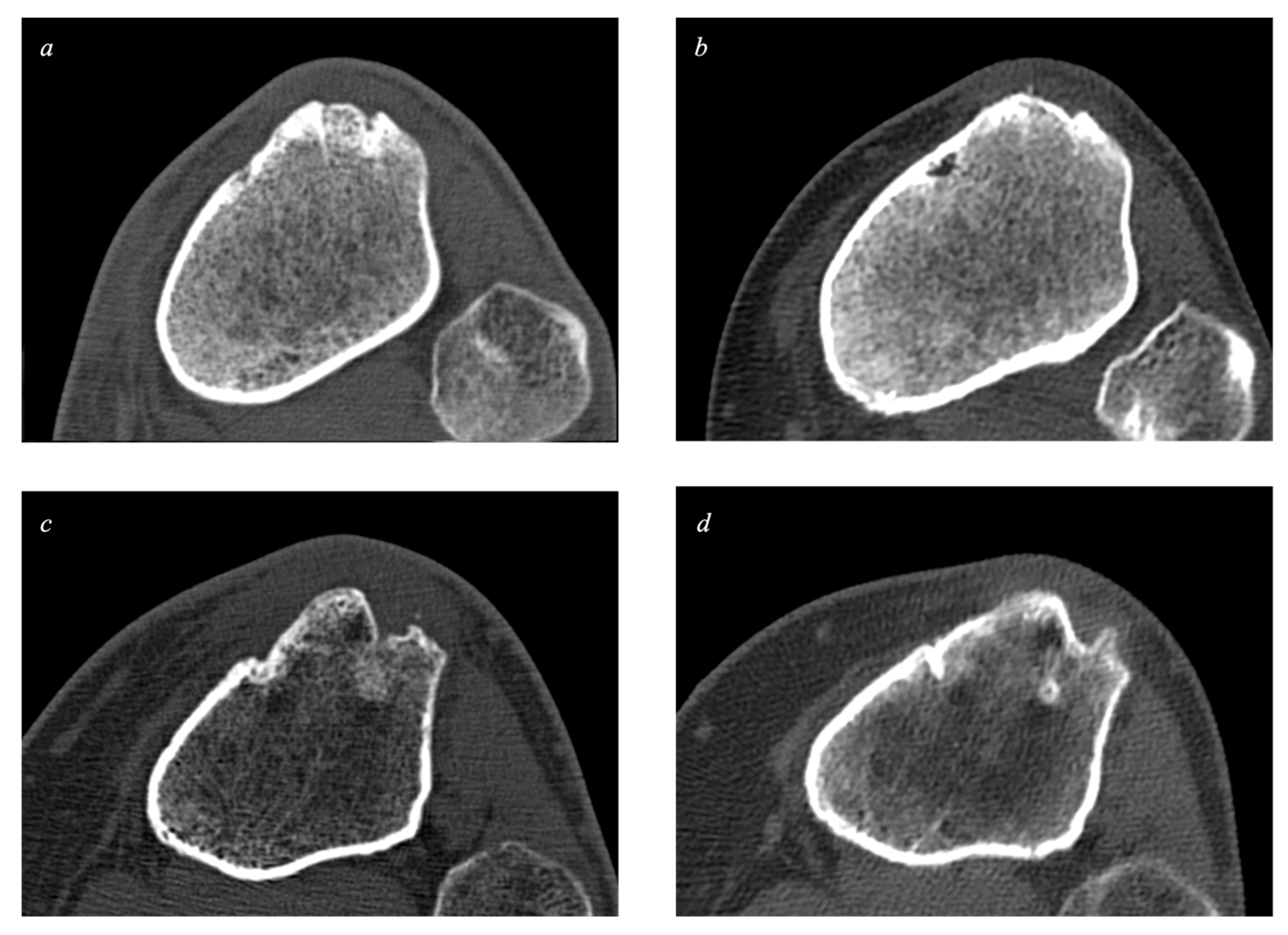
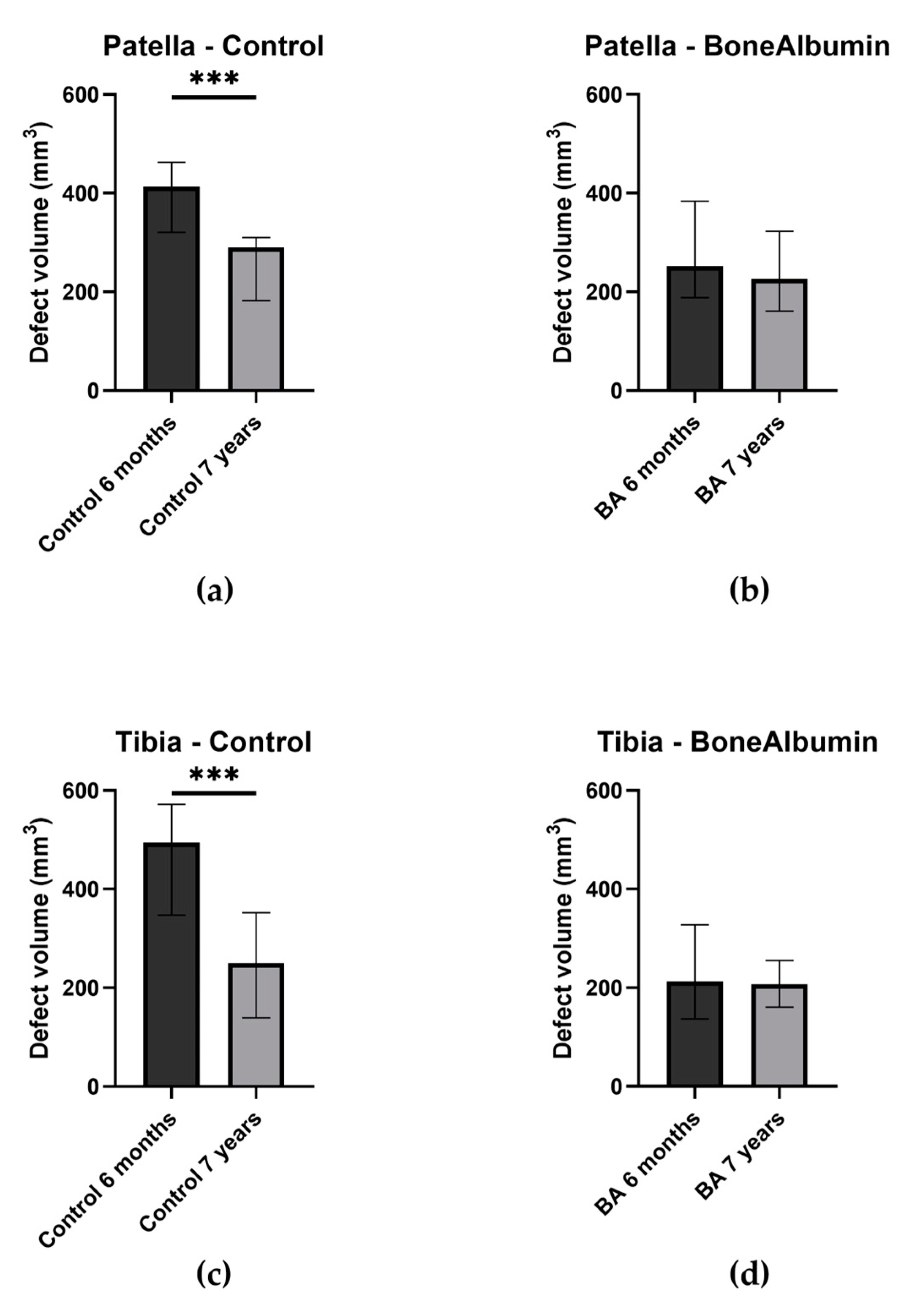
2.4. The Volume of Bone Defects
2.5. Complications
3. Discussion
4. Materials and Methods
4.1. Study Protocol and Patients
4.2. BoneAlbumin-Serum Albumin-Coated Bone Chips
4.3. Surgical Protocol
4.4. Computed Tomography
4.5. Statistical Analysis
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chechik, O.; Amar, E.; Khashan, M.; Lador, R.; Eyal, G.; Gold, A. An international survey on anterior cruciate ligament reconstruction practices. Int. Orthop. 2013, 37, 201–206. [Google Scholar] [CrossRef]
- Buerba, R.A.; Boden, S.A.; Lesniak, B. Graft Selection in Contemporary Anterior Cruciate Ligament Reconstruction. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e21.00230. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. Anterior Cruciate Ligament Reconstruction: Is Biological Augmentation Beneficial? Int. J. Mol. Sci. 2021, 22, 12566. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Merchán, E.C.; Durán, D.; Revilla, C.; Gómez-Cardero, P.; Martínez-Lloreda, A.; Bello, S. Arthroscopic BPTB graft reconstruction in ACL ruptures: 15-year results and survival. Knee 2014, 21, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C.; Ribbans, W.J. The role of vancomycin-soaking of the graft in anterior cruciate ligament reconstruction. J isakos 2022, 7, 94–98. [Google Scholar] [CrossRef] [PubMed]
- DeFazio, M.W.; Curry, E.J.; Gustin, M.J.; Sing, D.C.; Abdul-Rassoul, H.; Ma, R.; Fu, F.; Li, X. Return to Sport After ACL Reconstruction With a BTB Versus Hamstring Tendon Autograft: A Systematic Review and Meta-analysis. Orthop. J. Sport. Med. 2020, 8, 2325967120964919. [Google Scholar] [CrossRef]
- Patel, J.V.; Church, J.S.; Hall, A.J. Central third bone-patellar tendon-bone anterior cruciate ligament reconstruction: A 5-year follow-up. Arthroscopy 2000, 16, 67–70. [Google Scholar] [CrossRef]
- Migliorini, F.; Torsiello, E.; Trivellas, A.; Eschweiler, J.; Hildebrand, F.; Maffulli, N. Bone-patellar tendon-bone versus two- and four-strand hamstring tendon autografts for ACL reconstruction in young adults: A Bayesian network meta-analysis. Sci. Rep. 2023, 13, 6883. [Google Scholar] [CrossRef]
- Xie, X.; Liu, X.; Chen, Z.; Yu, Y.; Peng, S.; Li, Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee 2015, 22, 100–110. [Google Scholar] [CrossRef]
- Kartus, J.; Movin, T.; Karlsson, J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy 2001, 17, 971–980. [Google Scholar] [CrossRef]
- Hacken, B.A.; Keyt, L.K.; Leland, D.P.; LaPrade, M.D.; Camp, C.L.; Levy, B.A.; Stuart, M.J.; Krych, A.J. A Novel Scoring Instrument to Assess Donor Site Morbidity After Anterior Cruciate Ligament Reconstruction With a Patellar Tendon Autograft at 2-Year Follow-up Using Contemporary Graft-Harvesting Techniques. Orthop. J. Sport. Med. 2020, 8, 2325967120925482. [Google Scholar] [CrossRef] [PubMed]
- Beynnon, B.D.; Johnson, R.J.; Fleming, B.C.; Kannus, P.; Kaplan, M.; Samani, J.; Renström, P. Anterior cruciate ligament replacement: Comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts. A prospective, randomized study. J. Bone Joint Surg. Am. 2002, 84, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Boszotta, H.; Prünner, K. Refilling of Removal Defects: Impact on Extensor Mechanism Complaints After Use of a Bone-Tendon-Bone Graft for Anterior Cruciate Ligament Reconstruction. Arthrosc. J. Arthrosc. Relat. Surg. 2000, 16, 160–164. [Google Scholar] [CrossRef]
- Herzog, M.M.; Marshall, S.W.; Lund, J.L.; Pate, V.; Mack, C.D.; Spang, J.T. Trends in Incidence of ACL Reconstruction and Concomitant Procedures Among Commercially Insured Individuals in the United States, 2002–2014. Sport. Health 2018, 10, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.L.; Maradit Kremers, H.; Bryan, A.J.; Larson, D.R.; Dahm, D.L.; Levy, B.A.; Stuart, M.J.; Krych, A.J. Incidence of Anterior Cruciate Ligament Tears and Reconstruction: A 21-Year Population-Based Study. Am. J. Sport. Med. 2016, 44, 1502–1507. [Google Scholar] [CrossRef]
- Kiss, Z.S.; Kellaway, D.P.; Cook, J.L.; Khan, K.M. Postoperative patellar tendon healing: An ultrasound study. VIS Tendon Study Group. Australas. Radiol. 1998, 42, 28–32. [Google Scholar] [CrossRef]
- Kartus, J.; Movin, T.; Karlsson, J. Donor-Site Morbidity after Anterior Cruciate Ligament Reconstruction Using Autografts. In Anterior Knee Pain and Patellar Instability; Sanchis-Alfonso, V., Ed.; Springer London: London, UK, 2006; pp. 305–319. [Google Scholar]
- Kartus, J.; Ejerhed, L.; Movin, T. Anterior Knee Pain with Special Emphasis on the Clinical, Radiographic, Histological, Ultrastructural, and Biochemical Aspects After Anterior Cruciate Ligament Reconstruction Using Autografts. In Anterior Knee Pain and Patellar Instability; Sanchis-Alfonso, V., Ed.; Springer London: London, UK, 2011; pp. 251–266. [Google Scholar]
- Marder, R.A.; Raskind, J.R.; Carroll, M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am. J. Sport. Med. 1991, 19, 478–484. [Google Scholar] [CrossRef]
- Treece, G.; Gee, A. Cortical Bone Mapping: Measurement and Statistical Analysis of Localised Skeletal Changes. Curr. Osteoporos Rep. 2018, 16, 617–625. [Google Scholar] [CrossRef]
- Poole, K.E.; Treece, G.M.; Ridgway, G.R.; Mayhew, P.M.; Borggrefe, J.; Gee, A.H. Targeted regeneration of bone in the osteoporotic human femur. PLoS ONE 2011, 6, e16190. [Google Scholar] [CrossRef]
- Genant, H.K.; Libanati, C.; Engelke, K.; Zanchetta, J.R.; Høiseth, A.; Yuen, C.K.; Stonkus, S.; Bolognese, M.A.; Franek, E.; Fuerst, T.; et al. Improvements in hip trabecular, subcortical, and cortical density and mass in postmenopausal women with osteoporosis treated with denosumab. Bone 2013, 56, 482–488. [Google Scholar] [CrossRef]
- Yazdanshenas, H.; Madadi, F.; Madadi, F.; Washington, E.R.; Jones, K.; Shamie, A.N. Patellar tendon donor-site healing during six and twelve months after Anterior Cruciate Ligament Reconstruction. J. Orthop. 2015, 12, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.T.; Scott, D.D. A review of bone substitutes. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Dhanakodi, N.; Thilak, J.; Varghese, J.; Menon, K.V.; Varma, H.; Tripathy, S.K. Ceramic Bone Graft Substitutes do not reduce donor-site morbidity in ACL reconstruction surgeries: A pilot study. Sicot J. 2019, 5, 14. [Google Scholar] [CrossRef]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Bernards, M.T.; Qin, C.; Jiang, S. MC3T3-E1 cell adhesion to hydroxyapatite with adsorbed bone sialoprotein, bone osteopontin, and bovine serum albumin. Colloids Surf B Biointerfaces 2008, 64, 236–247. [Google Scholar] [CrossRef]
- Haag, S.L.; Martinez-Alvarez, J.; Schiele, N.R.; Bernards, M.T. Delivery of bioactive albumin from multi-functional polyampholyte hydrogels. J. Appl. Polym. Sci. 2022, 139, e52846. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Simon, M.; Schwarz, C.M.; Masteling, M.; Vácz, G.; Hornyák, I.; Lacza, Z. Serum albumin as a local therapeutic agent in cell therapy and tissue engineering. BioFactors 2017, 43, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; Alkhutari, A.S.; Abotaleb, B.; Altairi, N.H.; Del Fabbro, M. Do osteoconductive bone substitutes result in similar bone regeneration for maxillary sinus augmentation when compared to osteogenic and osteoinductive bone grafts? A systematic review and frequentist network meta-analysis. Int. J. Oral. Maxillofac. Surg. 2020, 49, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, P.; Niculescu-Morzsa, E.; Zwickl, H.; Halbwirth, F.; Pichler, M.; Matzner, M.; Gottsauner-Wolf, F.; Nehrer, S. Investigation of bone allografts representing different steps of the bone bank procedure using the CAM-model. Altex 2010, 27, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, J.; Köttstorfer, J.; Schuster, R.; Kovar, F.M.; Platzer, P.; Aldrian, S. Primary anterior cruciate ligament reconstruction in athletes: A 5-year follow up comparing patellar tendon versus hamstring tendon autograft. Wien. Klin. Wochenschr. 2014, 126, 397–402. [Google Scholar] [CrossRef]
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42 (Suppl. S2), S16–S21. [Google Scholar] [CrossRef]
- European Union Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Off. J. Eur. Union 2004, 102, 48–58.
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts. J. Bone Jt. Surg. Br. Vol. 2007, 89-B, 574–580. [Google Scholar] [CrossRef]
- Weszl, M.; Skaliczki, G.; Cselenyák, A.; Kiss, L.; Major, T.; Schandl, K.; Bognár, E.; Stadler, G.; Peterbauer, A.; Csönge, L.; et al. Freeze-dried human serum albumin improves the adherence and proliferation of mesenchymal stem cells on mineralized human bone allografts. J. Orthop. Res. 2012, 30, 489–496. [Google Scholar] [CrossRef]
- Skaliczki, G.; Schandl, K.; Weszl, M.; Major, T.; Kovács, M.; Skaliczki, J.; Szendrői, M.; Dobó-Nagy, C.; Lacza, Z. Serum albumin enhances bone healing in a nonunion femoral defect model in rats: A computer tomography micromorphometry study. Int. Orthop. 2013, 37, 741–745. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Vácz, G.; Szabó, T.; Szigyártó, I.C.; Toró, I.; Vámos, B.; Hornyák, I.; Renner, K.; Klára, T.; Szabó, B.T.; et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 126–132. [Google Scholar] [CrossRef]
- Peña, G.; Gallego, L.; Redondo, L.M.; Junquera, L.; Doval, J.F.; Meana, Á. Comparative analysis of plasma-derived albumin scaffold, alveolar osteoblasts and synthetic membrane in critical mandibular bone defects: An experimental study on rats. J. Biomater. Appl. 2021, 36, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhu, S.; Hou, Y. Insight on Serum Albumin: From Structure and Biological Properties to Functional Biomaterials for Bone Repair. ACS Biomater. Sci. Eng. 2023, 9, 2235–2250. [Google Scholar] [CrossRef] [PubMed]
- Mehwish, N.; Chen, Y.; Zaeem, M.; Wang, Y.; Lee, B.H.; Deng, H. Novel biohybrid spongy scaffolds for fabrication of suturable intraoral graft substitutes. Int. J. Biol. Macromol. 2022, 214, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Ryser, M.F.; Thieme, S.; Bornhäuser, M.; Lehmann, R.; Brenner, S. Serum albumin strongly influences SDF-1 dependent migration. Int. J. Hematol. 2009, 89, 269–275. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Igarashi, A.; Misawa, H.; Tsurusaki, Y. Enhancement of albumin expression in bone tissues with healing rat fractures. J. Cell. Biochem. 2003, 89, 356–363. [Google Scholar] [CrossRef]
- Haag, S.L.; Schiele, N.R.; Bernards, M.T. Enhancement and mechanisms of MC3T3-E1 osteoblast-like cell adhesion to albumin through calcium exposure. Biotechnol. Appl. Biochem. 2022, 69, 492–502. [Google Scholar] [CrossRef]
- Ishida, K.; Yamaguchi, M. Role of albumin in osteoblastic cells: Enhancement of cell proliferation and suppression of alkaline phosphatase activity. Int. J. Mol. Med. 2004, 14, 1077–1081. [Google Scholar] [CrossRef]
- Schandl, K.; Horváthy, D.B.; Doros, A.; Majzik, E.; Schwarz, C.M.; Csönge, L.; Abkarovits, G.; Bucsi, L.; Lacza, Z. Bone-Albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autografts. Int. Orthop. 2016, 40, 2097–2104. [Google Scholar] [CrossRef]
- Brandsson, S.; Faxén, E.; Eriksson, B.I.; Kälebo, P.; Swärd, L.; Lundin, O.; Karlsson, J. Closing patellar tendon defects after anterior cruciate ligament reconstruction: Absence of any benefit. Knee Surg. Sport. Traumatol. Arthrosc. 1998, 6, 82–87. [Google Scholar] [CrossRef]
- Tsuda, E.; Okamura, Y.; Ishibashi, Y.; Otsuka, H.; Toh, S. Techniques for reducing anterior knee symptoms after anterior cruciate ligament reconstruction using a bone-patellar tendon-bone autograft. Am. J. Sport. Med. 2001, 29, 450–456. [Google Scholar] [CrossRef]
- Riaz, O.; Nisar, S.; Phillips, H.; Siddiqui, A. Quantifying the problem of kneeling after a two incision bone tendon bone arthroscopic anterior cruciate ligament reconstruction. Muscles Ligaments Tendons J. 2015, 5, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Feller, J.A.; Webster, K.E. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am. J. Sport. Med. 2003, 31, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Kraeutler, M.J.; Bravman, J.T.; McCarty, E.C. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: A meta-analysis of 5182 patients. Am. J. Sport. Med. 2013, 41, 2439–2448. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Hang, D.W.; Gentili, A.; Finerman, G.A. MRI and morphology of the insertion of the patellar tendon after graft harvesting. J. Bone Jt. Surg. Br. 1996, 78, 823–826. [Google Scholar] [CrossRef]
- Even, J.; Eskander, M.; Kang, J. Bone morphogenetic protein in spine surgery: Current and future uses. J. Am. Acad. Orthop. Surg. 2012, 20, 547–552. [Google Scholar] [CrossRef]
- Seijas, R.; Rius, M.; Ares, O.; García-Balletbó, M.; Serra, I.; Cugat, R. Healing of donor site in bone-tendon-bone ACL reconstruction accelerated with plasma rich in growth factors: A randomized clinical trial. Knee Surg. Sport. Traumatol. Arthrosc. 2015, 23, 991–997. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]


| BA Ref. 6 Months | BA 6 Months | BA Ref. 7 Years | BA 7 Years | Control Ref. 6 Months | Control 6 Months | Control Ref. 7 Years | Control 7 Years | |
|---|---|---|---|---|---|---|---|---|
| Valid number | 10 | 10 | 10 | 10 | 16 | 16 | 16 | 16 |
| 25th percentile | 142.3 | 238.3 | 206.3 | 240.5 | 222.3 | 12 | 180.3 | 42.5 |
| Median | 275 | 258.5 | 264.5 | 318.5 | 247 | 85.5 | 244 | 94.5 |
| 75th percentile | 295.3 | 446.8 | 282.5 | 375.8 | 275.2 | 199.5 | 316 | 203.8 |
| BA Ref. 6 Months | BA 6 Months | BA Ref. 7 Years | BA 7 Years | Control Ref. 6 Months | Control 6 Months | Control Ref. 7 Years | Control 7 Years | |
|---|---|---|---|---|---|---|---|---|
| Valid number | 10 | 10 | 10 | 10 | 16 | 16 | 16 | 16 |
| 25th percentile | 227 | 234.8 | 181.5 | 266 | 214.8 | 182.3 | 222.5 | 176.5 |
| Median | 336.5 | 404 | 330.5 | 333.5 | 306 | 283.3 | 312 | 227.5 |
| 75th percentile | 405.5 | 721 | 377.5 | 443.8 | 382.8 | 389.8 | 374.3 | 352.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyulay, K.K.; Karászi, P.; Rédei, M.; Sólymos, P.; Schandl, K.; Lacza, Z.; Horváthy, D.B. Evaluation of Serum Albumin-Coated Bone Allograft for Bone Regeneration: A Seven-Year Follow-Up Study of 26 Cases. Int. J. Mol. Sci. 2023, 24, 9232. https://doi.org/10.3390/ijms24119232
Gyulay KK, Karászi P, Rédei M, Sólymos P, Schandl K, Lacza Z, Horváthy DB. Evaluation of Serum Albumin-Coated Bone Allograft for Bone Regeneration: A Seven-Year Follow-Up Study of 26 Cases. International Journal of Molecular Sciences. 2023; 24(11):9232. https://doi.org/10.3390/ijms24119232
Chicago/Turabian StyleGyulay, Kata K., Péter Karászi, Mátyás Rédei, Petra Sólymos, Károly Schandl, Zsombor Lacza, and Dénes B. Horváthy. 2023. "Evaluation of Serum Albumin-Coated Bone Allograft for Bone Regeneration: A Seven-Year Follow-Up Study of 26 Cases" International Journal of Molecular Sciences 24, no. 11: 9232. https://doi.org/10.3390/ijms24119232
APA StyleGyulay, K. K., Karászi, P., Rédei, M., Sólymos, P., Schandl, K., Lacza, Z., & Horváthy, D. B. (2023). Evaluation of Serum Albumin-Coated Bone Allograft for Bone Regeneration: A Seven-Year Follow-Up Study of 26 Cases. International Journal of Molecular Sciences, 24(11), 9232. https://doi.org/10.3390/ijms24119232







