mTOR Signaling Pathway in Bone Diseases Associated with Hyperglycemia
Abstract
1. Introduction
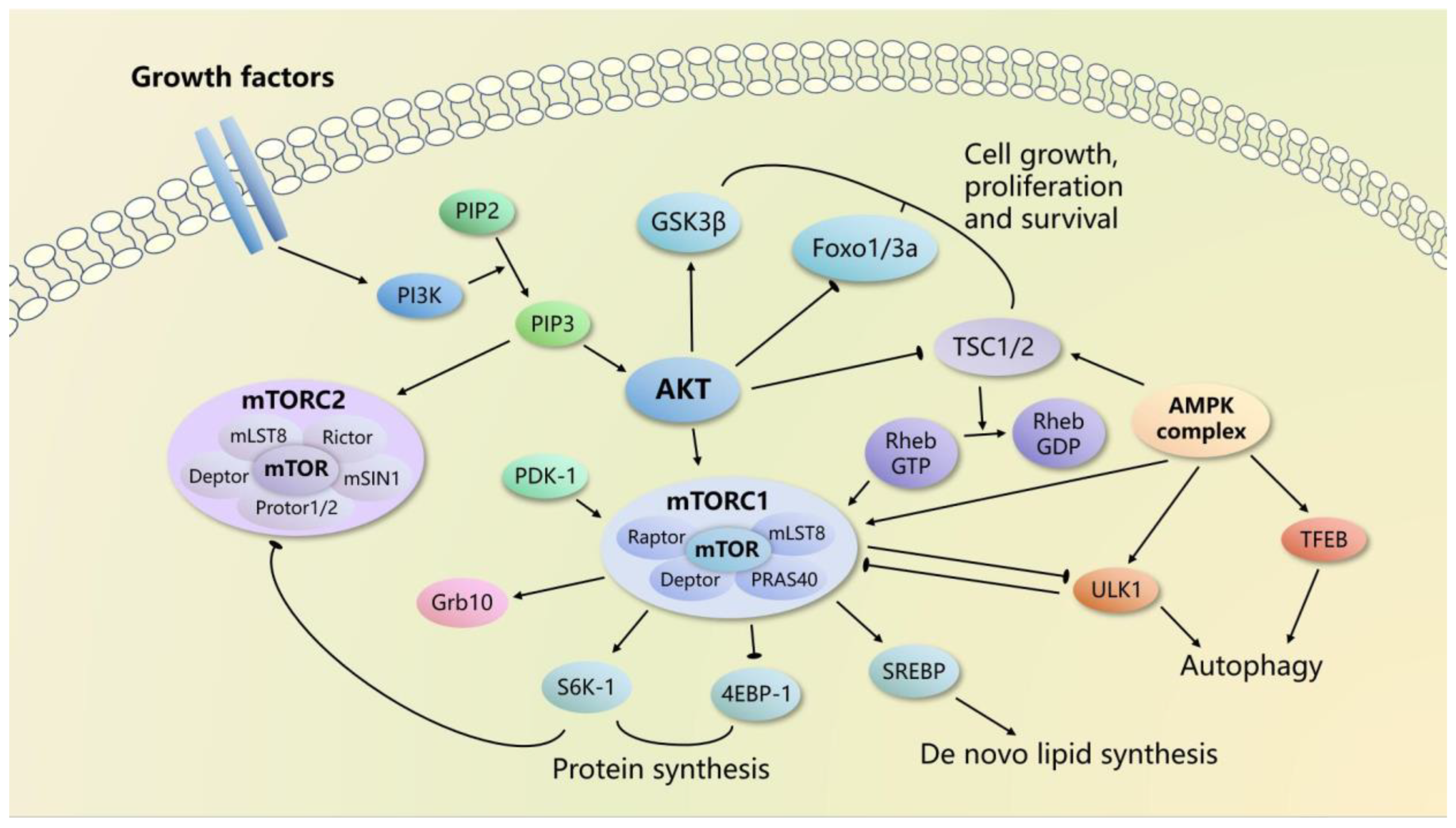
2. mTOR Signaling Pathways
2.1. mTOR Complexes
2.2. mTORC1 Signaling
2.3. mTORC2 Signaling
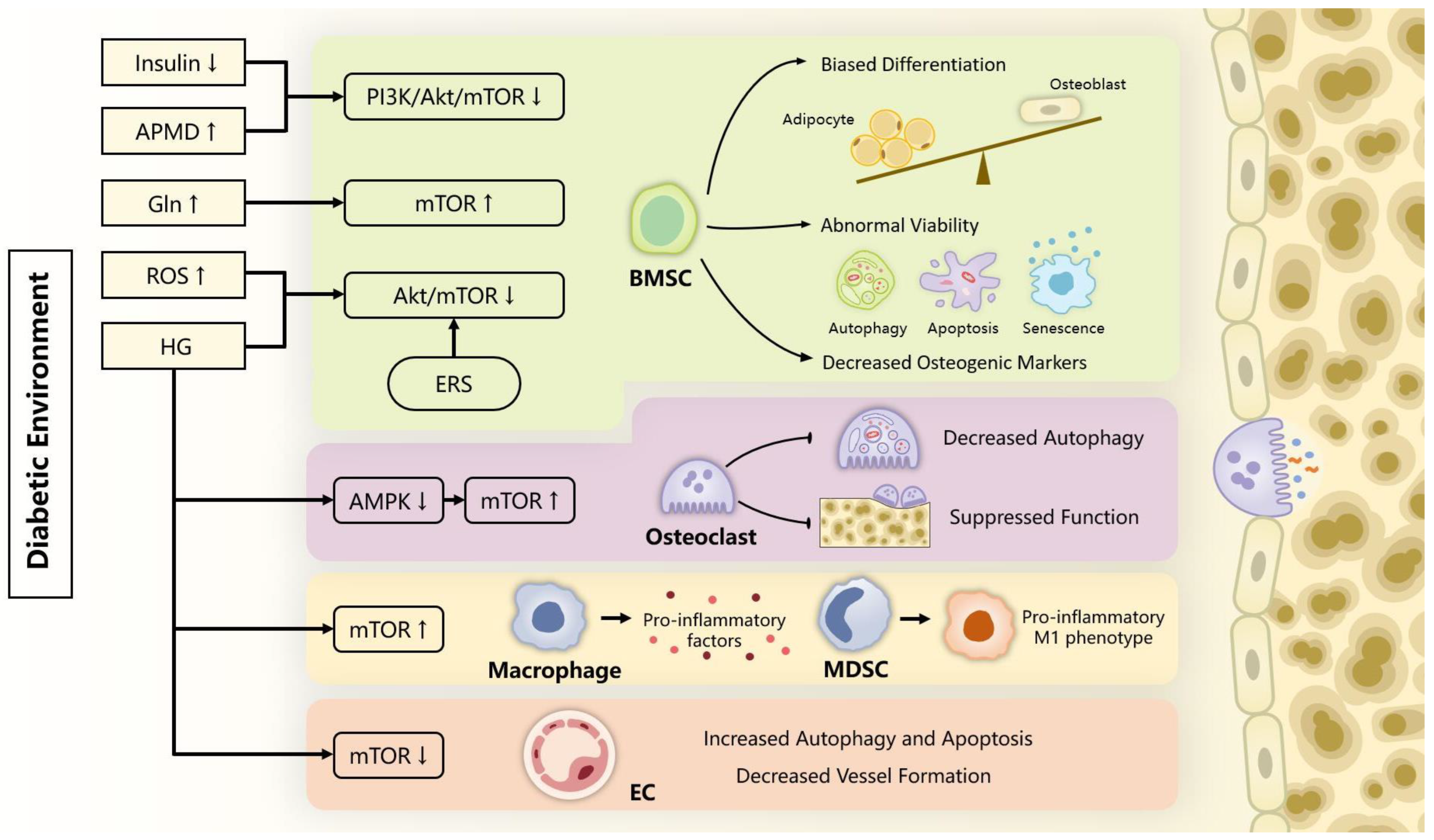
3. mTOR in Bone Metabolism
3.1. mTOR in BMSCs Osteogenesis and Bone Formation
3.2. mTOR in Osteoclast Formation and Bone Resorption
3.3. mTOR in Inflammatory Response
3.4. mTOR in Bone Vascularity
4. Therapeutic Prospects
4.1. Inhibition of mTOR Pathway
4.2. Activation of mTOR Pathway
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lovic, D.; Piperidou, A.; Zografou, I.; Grassos, H.; Pittaras, A.; Manolis, A. The Growing Epidemic of Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Ku, H.; Park, K.S. Prevalence and socioeconomic burden of diabetes mellitus in South Korean adults: A population-based study using administrative data. BMC Public Health 2021, 21, 548. [Google Scholar] [CrossRef] [PubMed]
- Hygum, K.; Starup-Linde, J.; Langdahl, B.L. Diabetes and bone. Osteoporos. Sarcopenia 2019, 5, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díaz, C.; Duarte-Montero, D.; Gutiérrez-Romero, S.A.; Mendivil, C.O. Diabetes and Bone Fragility. Diabetes Ther. 2021, 12, 71–86. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Lane, N.E. Bone and Joint Complications in Diabetes. In Diabetes in America, 3rd ed.; Cowie, C.C., Casagrande, S.S., Menke, A., Cissell, M.A., Eberhardt, M.S., Meigs, J.B., Gregg, E.W., Knowler, W.C., Barrett-Connor, E., Becker, D.J., et al., Eds.; National Institute of Diabetes and Digestive and Kidney Diseases (US): Bethesda, MD, USA, 2018. [Google Scholar]
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—A meta-analysis. Osteoporos. Int. 2007, 18, 427–444. [Google Scholar] [CrossRef]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. The effect of diabetes mellitus on osseous healing. Clin. Oral. Implant. Res. 2010, 21, 673–681. [Google Scholar] [CrossRef]
- Wang, H.; Ba, Y.; Xing, Q.; Du, J.L. Diabetes mellitus and the risk of fractures at specific sites: A meta-analysis. BMJ Open 2019, 9, e024067. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Howell, J.J.; Manning, B.D. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 2011, 22, 94–102. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Swierczynska, M.M.; Hall, M.N. mTOR in Metabolic and Endocrine Disorders. In Molecules to Medicine with mTOR; Maiese, K., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 347–364. [Google Scholar]
- Albert, V.; Hall, M.N. mTOR signaling in cellular and organismal energetics. Curr. Opin. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Abou Daher, A.; Alkhansa, S.; Azar, W.S.; Rafeh, R.; Ghadieh, H.E.; Eid, A.A. Translational Aspects of the Mammalian Target of Rapamycin Complexes in Diabetic Nephropathy. Antioxid. Redox Signal. 2022, 37, 802–819. [Google Scholar] [CrossRef] [PubMed]
- Suhara, T.; Baba, Y.; Shimada, B.K.; Higa, J.K.; Matsui, T. The mTOR Signaling Pathway in Myocardial Dysfunction in Type 2 Diabetes Mellitus. Curr. Diabetes Rep. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Burillo, J.; Marqués, P.; Jiménez, B.; González-Blanco, C.; Benito, M.; Guillén, C. Insulin Resistance and Diabetes Mellitus in Alzheimer's Disease. Cells 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Long, F. mTOR signaling in skeletal development and disease. Bone Res. 2018, 6, 1. [Google Scholar] [CrossRef]
- Singha, U.K.; Jiang, Y.; Yu, S.; Luo, M.; Lu, Y.; Zhang, J.; Xiao, G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J. Cell. Biochem. 2008, 103, 434–446. [Google Scholar] [CrossRef]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef]
- Lai, P.; Song, Q.; Yang, C.; Li, Z.; Liu, S.; Liu, B.; Li, M.; Deng, H.; Cai, D.; Jin, D.; et al. Loss of Rictor with aging in osteoblasts promotes age-related bone loss. Cell Death Dis. 2016, 7, e2408. [Google Scholar] [CrossRef]
- Gayatri, M.B.; Gajula, N.N.; Chava, S.; Reddy, A.B.M. High glutamine suppresses osteogenesis through mTORC1-mediated inhibition of the mTORC2/AKT-473/RUNX2 axis. Cell Death Discov. 2022, 8, 277. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Wainwright, J.V.; Mohan, A.L.; Tobias, M.E.; Murali, R.; Gandhi, C.D.; Schmidt, M.H. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 2019, 72, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.P.; Viji, R.; Dan, V.M.; Sajitha, I.S.; Prakash, R.; Rahul, P.V.; Santhoshkumar, T.R.; Lakshmi, S.; Pillai, M.R. DEPTOR promotes survival of cervical squamous cell carcinoma cells and its silencing induces apoptosis through downregulating PI3K/AKT and by up-regulating p38 MAP kinase. Oncotarget 2016, 7, 24154–24171. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Thoreen, C.C.; Peterson, T.R.; Lindquist, R.A.; Kang, S.A.; Spooner, E.; Carr, S.A.; Sabatini, D.M. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell 2007, 25, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Maruki, Y.; Long, X.; Yoshino, K.; Oshiro, N.; Hidayat, S.; Tokunaga, C.; Avruch, J.; Yonezawa, K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 2002, 110, 177–189. [Google Scholar] [CrossRef]
- Yang, H.; Rudge, D.G.; Koos, J.D.; Vaidialingam, B.; Yang, H.J.; Pavletich, N.P. mTOR kinase structure, mechanism and regulation. Nature 2013, 497, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.R.; Huang, X.; Boudeau, J.; Pawłowski, R.; Wullschleger, S.; Deak, M.; Ibrahim, A.F.; Gourlay, R.; Magnuson, M.A.; Alessi, D.R. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem. J. 2007, 405, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sahra, I.; Manning, B.D. mTORC1 signaling and the metabolic control of cell growth. Curr. Opin. Cell Biol. 2017, 45, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Shimobayashi, M.; Hall, M.N. Making new contacts: The mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 2014, 15, 155–162. [Google Scholar] [CrossRef]
- Tee, A.R.; Manning, B.D.; Roux, P.P.; Cantley, L.C.; Blenis, J. Tuberous Sclerosis Complex Gene Products, Tuberin and Hamartin, Control mTOR Signaling by Acting as a GTPase-Activating Protein Complex toward Rheb. Curr. Biol. 2022, 32, 733–734. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Xu, T.; Guan, K.L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003, 17, 1829–1834. [Google Scholar] [CrossRef]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Schweitzer, L.D.; Zoncu, R.; Sabatini, D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012, 150, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Chantranupong, L.; Cherniack, A.D.; Chen, W.W.; Ottina, K.A.; Grabiner, B.C.; Spear, E.D.; Carter, S.L.; Meyerson, M.; Sabatini, D.M. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 2013, 340, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Holz, M.K.; Ballif, B.A.; Gygi, S.P.; Blenis, J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 2005, 123, 569–580. [Google Scholar] [CrossRef]
- Dorrello, N.V.; Peschiaroli, A.; Guardavaccaro, D.; Colburn, N.H.; Sherman, N.E.; Pagano, M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 2006, 314, 467–471. [Google Scholar] [CrossRef]
- Ma, X.M.; Yoon, S.O.; Richardson, C.J.; Jülich, K.; Blenis, J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell 2008, 133, 303–313. [Google Scholar] [CrossRef]
- Hsieh, A.C.; Liu, Y.; Edlind, M.P.; Ingolia, N.T.; Janes, M.R.; Sher, A.; Shi, E.Y.; Stumpf, C.R.; Christensen, C.; Bonham, M.J.; et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012, 485, 55–61. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Chantranupong, L.; Keys, H.R.; Wang, T.; Gray, N.S.; Sabatini, D.M. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012, 485, 109–113. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Zoncu, R.; Medina, D.L.; Vetrini, F.; Erdin, S.; Erdin, S.; Huynh, T.; Ferron, M.; Karsenty, G.; Vellard, M.C.; et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012, 31, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Martina, J.A.; Chen, Y.; Gucek, M.; Puertollano, R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 2012, 8, 903–914. [Google Scholar] [CrossRef]
- Roczniak-Ferguson, A.; Petit, C.S.; Froehlich, F.; Qian, S.; Ky, J.; Angarola, B.; Walther, T.C.; Ferguson, S.M. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 2012, 5, ra42. [Google Scholar] [CrossRef]
- Yoon, M.S. The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef]
- Liu, P.; Gan, W.; Chin, Y.R.; Ogura, K.; Guo, J.; Zhang, J.; Wang, B.; Blenis, J.; Cantley, L.C.; Toker, A.; et al. PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex. Cancer Discov. 2015, 5, 1194–1209. [Google Scholar] [CrossRef]
- Zinzalla, V.; Stracka, D.; Oppliger, W.; Hall, M.N. Activation of mTORC2 by association with the ribosome. Cell 2011, 144, 757–768. [Google Scholar] [CrossRef]
- Hsu, P.P.; Kang, S.A.; Rameseder, J.; Zhang, Y.; Ottina, K.A.; Lim, D.; Peterson, T.R.; Choi, Y.; Gray, N.S.; Yaffe, M.B.; et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 2011, 332, 1317–1322. [Google Scholar] [CrossRef]
- Yu, Y.; Yoon, S.O.; Poulogiannis, G.; Yang, Q.; Ma, X.M.; Villén, J.; Kubica, N.; Hoffman, G.R.; Cantley, L.C.; Gygi, S.P.; et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 2011, 332, 1322–1326. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Guertin, D.A.; Stevens, D.M.; Thoreen, C.C.; Burds, A.A.; Kalaany, N.Y.; Moffat, J.; Brown, M.; Fitzgerald, K.J.; Sabatini, D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 2006, 11, 859–871. [Google Scholar] [CrossRef]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Rüegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Alessi, D.R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 2008, 416, 375–385. [Google Scholar] [CrossRef]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef]
- Bakker, A.D.; Gakes, T.; Hogervorst, J.M.; de Wit, G.M.; Klein-Nulend, J.; Jaspers, R.T. Mechanical Stimulation and IGF-1 Enhance mRNA Translation Rate in Osteoblasts Via Activation of the AKT-mTOR Pathway. J. Cell. Physiol. 2016, 231, 1283–1290. [Google Scholar] [CrossRef]
- Riddle, R.C.; Frey, J.L.; Tomlinson, R.E.; Ferron, M.; Li, Y.; DiGirolamo, D.J.; Faugere, M.C.; Hussain, M.A.; Karsenty, G.; Clemens, T.L. Tsc2 is a molecular checkpoint controlling osteoblast development and glucose homeostasis. Mol. Cell. Biol. 2014, 34, 1850–1862. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, N.; Cheng, G.; Zhang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Zhu, B.; Zhang, Q.; Qin, L. Rehmannia glutinosa Libosch Extracts Prevent Bone Loss and Architectural Deterioration and Enhance Osteoblastic Bone Formation by Regulating the IGF-1/PI3K/mTOR Pathway in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 3964. [Google Scholar] [CrossRef]
- Meng, D.; Yang, Q.; Wang, H.; Melick, C.H.; Navlani, R.; Frank, A.R.; Jewell, J.L. Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J. Biol. Chem. 2020, 295, 2890–2899. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Luo, Y.F.; Guo, Z.; Qian, Q.; Meng, X.B.; Mo, Z.H. MicroRNA-139-5p mediates BMSCs impairment in diabetes by targeting HOXA9/c-Fos. FASEB J. 2023, 37, e22697. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Hsu, M.F.; Wu, K.K. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS ONE 2015, 10, e0126537. [Google Scholar] [CrossRef] [PubMed]
- Teissier, T.; Temkin, V.; Pollak, R.D.; Cox, L.S. Crosstalk Between Senescent Bone Cells and the Bone Tissue Microenvironment Influences Bone Fragility During Chronological Age and in Diabetes. Front. Physiol. 2022, 13, 812157. [Google Scholar] [CrossRef]
- Meng, Y.; Ji, J.; Tan, W.; Guo, G.; Xia, Y.; Cheng, C.; Gu, Z.; Wang, Z. Involvement of autophagy in the procedure of endoplasmic reticulum stress introduced apoptosis in bone marrow mesenchymal stem cells from nonobese diabetic mice. Cell Biochem. Funct. 2016, 34, 25–33. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Li, J.; Chen, L.; Tang, W. High glucose induces autophagy of MC3T3-E1 cells via ROS-AKT-mTOR axis. Mol. Cell. Endocrinol. 2016, 429, 62–72. [Google Scholar] [CrossRef]
- Kaludercic, N.; Di Lisa, F. Mitochondrial ROS Formation in the Pathogenesis of Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 12. [Google Scholar] [CrossRef]
- Chen, M.; Jing, D.; Ye, R.; Yi, J.; Zhao, Z. PPARβ/δ accelerates bone regeneration in diabetic mellitus by enhancing AMPK/mTOR pathway-mediated autophagy. Stem Cell Res. Ther. 2021, 12, 566. [Google Scholar] [CrossRef]
- Hu, X.; Li, B.; Wu, F.; Liu, X.; Liu, M.; Wang, C.; Shi, Y.; Ye, L. GPX7 Facilitates BMSCs Osteoblastogenesis via ER Stress and mTOR Pathway. J. Cell. Mol. Med. 2021, 25, 10454–10465. [Google Scholar] [CrossRef]
- Li, S.; Yang, D.; Gao, X.; Yao, S.; Wang, S.; Zhu, J.; Shu, J. Argpyrimidine bonded to RAGE regulates autophagy and cell cycle to cause periodontal destruction. J. Cell. Physiol. 2022, 237, 4460–4476. [Google Scholar] [CrossRef]
- Li, C.J.; Cheng, P.; Liang, M.K.; Chen, Y.S.; Lu, Q.; Wang, J.Y.; Xia, Z.Y.; Zhou, H.D.; Cao, X.; Xie, H.; et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Investig. 2015, 125, 1509–1522. [Google Scholar] [CrossRef]
- Ouyang, Z.; Kang, D.; Li, K.; Liang, G.; Liu, Z.; Mai, Q.; Chen, Q.; Yao, C.; Wei, R.; Tan, X.; et al. DEPTOR exacerbates bone-fat imbalance in osteoporosis by transcriptionally modulating BMSC differentiation. Biomed. Pharmacother. 2022, 151, 113164. [Google Scholar] [CrossRef]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef]
- Rastaldo, R.; Vitale, E.; Giachino, C. Dual Role of Autophagy in Regulation of Mesenchymal Stem Cell Senescence. Front. Cell Dev. Biol. 2020, 8, 276. [Google Scholar] [CrossRef]
- Zheng, Y.; Lei, Y.; Hu, C.; Hu, C. p53 regulates autophagic activity in senescent rat mesenchymal stromal cells. Exp. Gerontol. 2016, 75, 64–71. [Google Scholar] [CrossRef]
- Al-Azab, M.; Wang, B.; Elkhider, A.; Walana, W.; Li, W.; Yuan, B.; Ye, Y.; Tang, Y.; Almoiliqy, M.; Adlat, S.; et al. Indian Hedgehog regulates senescence in bone marrow-derived mesenchymal stem cell through modulation of ROS/mTOR/4EBP1, p70S6K1/2 pathway. Aging 2020, 12, 5693–5715. [Google Scholar] [CrossRef]
- Liu, F.; Yuan, Y.; Bai, L.; Yuan, L.; Li, L.; Liu, J.; Chen, Y.; Lu, Y.; Cheng, J.; Zhang, J. LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox. Biol. 2021, 43, 101963. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Karalazou, P.; Ntelios, D.; Chatzopoulou, F.; Fragou, A.; Taousani, M.; Mouzaki, K.; Galli-Tsinopoulou, A.; Kouidou, S.; Tzimagiorgis, G. OPG/RANK/RANKL signaling axis in patients with type I diabetes: Associations with parathormone and vitamin D. Ital. J. Pediatr. 2019, 45, 161. [Google Scholar] [CrossRef]
- An, Y.; Zhang, H.; Wang, C.; Jiao, F.; Xu, H.; Wang, X.; Luan, W.; Ma, F.; Ni, L.; Tang, X.; et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019, 33, 12515–12527. [Google Scholar] [CrossRef]
- Tian, Y.; Ming, J. Melatonin inhibits osteoclastogenesis via RANKL/OPG suppression mediated by Rev-Erbα in osteoblasts. J. Cell. Mol. Med. 2022, 26, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Gong, K.; Yang, H.; Li, Y.; Jiang, T.; Zeng, Z.; Cao, Z.; Pan, X. SIRT1 suppresses high glucose and palmitate-induced osteoclast differentiation via deacetylating p66Shc. Mol. Cell. Endocrinol. 2018, 474, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ma, C.; Liang, Y.; Zou, S.; Liu, X. Osteoclasts in bone regeneration under type 2 diabetes mellitus. Acta Biomater. 2019, 84, 402–413. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, R.; Desta, T.; Leone, C.; Gerstenfeld, L.C.; Graves, D.T. Diabetes causes decreased osteoclastogenesis, reduced bone formation, and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endocrinology 2004, 145, 447–452. [Google Scholar] [CrossRef]
- Dong, W.; Qi, M.; Wang, Y.; Feng, X.; Liu, H. Zoledronate and high glucose levels influence osteoclast differentiation and bone absorption via the AMPK pathway. Biochem. Biophys. Res. Commun. 2018, 505, 1195–1202. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Aung, Z.M.; Han, W.; Zhang, Y.; Chai, G. LY3023414 inhibits both osteogenesis and osteoclastogenesis through the PI3K/Akt/GSK3 signalling pathway. Bone Jt. Res. 2021, 10, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wu, W.; Sun, X.; Zhang, P. Glucocorticoids Enhanced Osteoclast Autophagy Through the PI3K/Akt/mTOR Signaling Pathway. Calcif. Tissue Int. 2020, 107, 60–71. [Google Scholar] [CrossRef]
- Hiraiwa, M.; Ozaki, K.; Yamada, T.; Iezaki, T.; Park, G.; Fukasawa, K.; Horie, T.; Kamada, H.; Tokumura, K.; Motono, M.; et al. mTORC1 Activation in Osteoclasts Prevents Bone Loss in a Mouse Model of Osteoporosis. Front. Pharmacol. 2019, 10, 684. [Google Scholar] [CrossRef]
- Huynh, H.; Wan, Y. mTORC1 impedes osteoclast differentiation via calcineurin and NFATc1. Commun. Biol. 2018, 1, 29. [Google Scholar] [CrossRef]
- Bae, S.; Oh, B.; Tsai, J.; Park, P.S.U.; Greenblatt, M.B.; Giannopoulou, E.G.; Park-Min, K.H. The crosstalk between MYC and mTORC1 during osteoclastogenesis. Front. Cell Dev. Biol. 2022, 10, 920683. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, Z.; Ma, Y.; Song, R.; Yuan, Y.; Bian, J.; Gu, J.; Liu, Z. Antiosteoclastic bone resorption activity of osteoprotegerin via enhanced AKT/mTOR/ULK1-mediated autophagic pathway. J. Cell. Physiol. 2020, 235, 3002–3012. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Gu, J.; Song, R.; Wang, D.; Sun, Z.; Sui, C.; Zhang, C.; Liu, X.; Bian, J.; Liu, Z. Osteoprotegerin inhibit osteoclast differentiation and bone resorption by enhancing autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J. Cell. Biochem. 2019, 120, 1630–1642. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zhang, C.; Wang, D.; Song, R.; Ma, Y.; Cao, Y.; Zhao, H.; Bian, J.; Gu, J.; Liu, Z. Suppression of AMP-activated protein kinase reverses osteoprotegerin-induced inhibition of osteoclast differentiation by reducing autophagy. Cell Prolif. 2020, 53, e12714. [Google Scholar] [CrossRef] [PubMed]
- Montaseri, A.; Giampietri, C.; Rossi, M.; Riccioli, A.; Del Fattore, A.; Filippini, A. The Role of Autophagy in Osteoclast Differentiation and Bone Resorption Function. Biomolecules 2020, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.Y.; Yang, B.; Shi, Y.X.; Zhang, W.L.; Liu, F.; Zhao, W.; Yang, M.W. High glucose downregulates the effects of autophagy on osteoclastogenesis via the AMPK/mTOR/ULK1 pathway. Biochem. Biophys. Res. Commun. 2018, 503, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, J.; Li, S.; Cui, H.; Zhang, G.; Ni, X.; Wang, J. S-Equol enhances osteoblastic bone formation and prevents bone loss through OPG/RANKL via the PI3K/Akt pathway in streptozotocin-induced diabetic rats. Front. Nutr. 2022, 9, 986192. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Shahen, V.A.; Gerbaix, M.; Koeppenkastrop, S.; Lim, S.F.; McFarlane, K.E.; Nguyen, A.N.L.; Peng, X.Y.; Weiss, N.B.; Brennan-Speranza, T.C. Multifactorial effects of hyperglycaemia, hyperinsulinemia and inflammation on bone remodelling in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 2020, 55, 109–118. [Google Scholar] [CrossRef]
- Olson, N.C.; Doyle, M.F.; de Boer, I.H.; Huber, S.A.; Jenny, N.S.; Kronmal, R.A.; Psaty, B.M.; Tracy, R.P. Associations of Circulating Lymphocyte Subpopulations with Type 2 Diabetes: Cross-Sectional Results from the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS ONE 2015, 10, e0139962. [Google Scholar] [CrossRef]
- Cifuentes-Mendiola, S.E.; Solis-Suarez, D.L.; Martínez-Dávalos, A.; Godínez-Victoria, M.; García-Hernández, A.L. CD4+ T-cell activation of bone marrow causes bone fragility and insulin resistance in type 2 diabetes. Bone 2022, 155, 116292. [Google Scholar] [CrossRef]
- Newman, H.; Shih, Y.V.; Varghese, S. Resolution of inflammation in bone regeneration: From understandings to therapeutic applications. Biomaterials 2021, 277, 121114. [Google Scholar] [CrossRef]
- Qing, L.; Fu, J.; Wu, P.; Zhou, Z.; Yu, F.; Tang, J. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am. J. Transl. Res. 2019, 11, 655–668. [Google Scholar]
- Jamalpoor, Z.; Asgari, A.; Lashkari, M.H.; Mirshafiey, A.; Mohsenzadegan, M. Modulation of Macrophage Polarization for Bone Tissue Engineering Applications. Iran. J. Allergy Asthma Immunol. 2018, 17, 398–408. [Google Scholar] [CrossRef]
- Horwood, N.J. Macrophage Polarization and Bone Formation: A review. Clin. Rev. Allergy Immunol. 2016, 51, 79–86. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, W.; Hao, D.; He, B.; Kong, L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int. Immunopharmacol. 2019, 70, 459–466. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Tan, L.; Xu, Y.; Xie, X.; Zhao, Y. The Regulatory Effects of mTOR Complexes in the Differentiation and Function of CD4+ T Cell Subsets. J. Immunol. Res. 2020, 2020, 3406032. [Google Scholar] [CrossRef]
- Chen, S.; van Tok, M.N.; Knaup, V.L.; Kraal, L.; Pots, D.; Bartels, L.; Gravallese, E.M.; Taurog, J.D.; van de Sande, M.; van Duivenvoorde, L.M.; et al. mTOR Blockade by Rapamycin in Spondyloarthritis: Impact on Inflammation and New Bone Formation in vitro and in vivo. Front. Immunol. 2019, 10, 2344. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, M.; Liu, Y.; Xu, S.; Ouyang, Y.; Shi, W.; Jian, D.; Wang, B.; Liu, F.; Li, J.; et al. A positive feedback loop between mTORC1 and cathelicidin promotes skin inflammation in rosacea. EMBO Mol. Med. 2021, 13, e13560. [Google Scholar] [CrossRef]
- Zhang, X.J.; Shang, K.; Pu, Y.K.; Wang, Q.; Wang, T.T.; Zou, Y.; Wang, Y.M.; Xu, Y.J.; Li, X.L.; Zhang, R.H.; et al. Leojaponin inhibits NLRP3 inflammasome activation through restoration of autophagy via upregulating RAPTOR phosphorylation. J. Ethnopharmacol. 2021, 278, 114322. [Google Scholar] [CrossRef]
- Wang, Q.; Nie, L.; Zhao, P.; Zhou, X.; Ding, Y.; Chen, Q.; Wang, Q. Diabetes fuels periodontal lesions via GLUT1-driven macrophage inflammaging. Int. J. Oral Sci. 2021, 13, 11. [Google Scholar] [CrossRef]
- Zhao, Z.; Ming, Y.; Li, X.; Tan, H.; He, X.; Yang, L.; Song, J.; Zheng, L. Hyperglycemia Aggravates Periodontitis via Autophagy Impairment and ROS-Inflammasome-Mediated Macrophage Pyroptosis. Int. J. Mol. Sci. 2023, 24, 6309. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sun, L.; Ma, H.; Peng, J.; Zhen, Y.; Duan, K.; Liu, G.; Ding, W.; Zhao, Y. The phenotype and functional alterations of macrophages in mice with hyperglycemia for long term. J. Cell. Physiol. 2012, 227, 1670–1679. [Google Scholar] [CrossRef]
- Kawai, H.; Oo, M.W.; Tsujigiwa, H.; Nakano, K.; Takabatake, K.; Sukegawa, S.; Nagatsuka, H. Potential role of myeloid-derived suppressor cells in transition from reaction to repair phase of bone healing process. Int. J. Med. Sci. 2021, 18, 1824–1830. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, X.; Yan, X.; Lin, Y.; Tan, Q.; Hou, Y. mTOR inhibitor INK128 promotes wound healing by regulating MDSCs. Stem Cell Res. Ther. 2021, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Huang, C.C.; Lu, Y.; Shirazi, S.; Gajendrareddy, P.; Ravindran, S.; Cooper, L.F. Bone regeneration is mediated by macrophage extracellular vesicles. Bone 2020, 141, 115627. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhu, T.; Zhang, T.; Wang, X.; Li, W.; Chen, D.; Meng, H.; An, S. M1 macrophage-derived exosomes transfer miR-222 to induce bone marrow mesenchymal stem cell apoptosis. Lab. Investig. 2021, 101, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Müller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone fragility in diabetes: Novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220. [Google Scholar] [CrossRef]
- Peng, J.; Hui, K.; Hao, C.; Peng, Z.; Gao, Q.X.; Jin, Q.; Lei, G.; Min, J.; Qi, Z.; Bo, C.; et al. Low bone turnover and reduced angiogenesis in streptozotocin-induced osteoporotic mice. Connect. Tissue Res. 2016, 57, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Mangialardi, G.; Ferland-McCollough, D.; Maselli, D.; Santopaolo, M.; Cordaro, A.; Spinetti, G.; Sambataro, M.; Sullivan, N.; Blom, A.; Madeddu, P. Bone marrow pericyte dysfunction in individuals with type 2 diabetes. Diabetologia 2019, 62, 1275–1290. [Google Scholar] [CrossRef]
- Ribot, J.; Denoeud, C.; Frescaline, G.; Landon, R.; Petite, H.; Pavon-Djavid, G.; Bensidhoum, M.; Anagnostou, F. Experimental Type 2 Diabetes Differently Impacts on the Select Functions of Bone Marrow-Derived Multipotent Stromal Cells. Cells 2021, 10, 268. [Google Scholar] [CrossRef]
- Hu, X.F.; Xiang, G.; Wang, T.J.; Ma, Y.B.; Zhang, Y.; Yan, Y.B.; Zhao, X.; Wu, Z.X.; Feng, Y.F.; Lei, W. Impairment of type H vessels by NOX2-mediated endothelial oxidative stress: Critical mechanisms and therapeutic targets for bone fragility in streptozotocin-induced type 1 diabetic mice. Theranostics 2021, 11, 3796–3812. [Google Scholar] [CrossRef] [PubMed]
- Samakkarnthai, P.; Sfeir, J.G.; Atkinson, E.J.; Achenbach, S.J.; Wennberg, P.W.; Dyck, P.J.; Tweed, A.J.; Volkman, T.L.; Amin, S.; Farr, J.N.; et al. Determinants of Bone Material Strength and Cortical Porosity in Patients with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2020, 105, e3718–e3729. [Google Scholar] [CrossRef] [PubMed]
- Caliaperoumal, G.; Souyet, M.; Bensidhoum, M.; Petite, H.; Anagnostou, F. Type 2 diabetes impairs angiogenesis and osteogenesis in calvarial defects: MicroCT study in ZDF rats. Bone 2018, 112, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Chen, Y.; Fu, J.; Lei, Y.; Sun, J.; Tang, B. Platelet-derived growth factor D promotes the angiogenic capacity of endothelial progenitor cells. Mol. Med. Rep. 2019, 19, 125–132. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Yang, J.; Huang, J.; Luo, C.; Zhang, J.; Yan, W.; Ao, Y. Bone morphogenetic protein 9 enhances osteogenic and angiogenic responses of human amniotic mesenchymal stem cells cocultured with umbilical vein endothelial cells through the PI3K/AKT/m-TOR signaling pathway. Aging 2021, 13, 24829–24849. [Google Scholar] [CrossRef]
- Tian, D.; Xiang, Y.; Tang, Y.; Ge, Z.; Li, Q.; Zhang, Y. Circ-ADAM9 targeting PTEN and ATG7 promotes autophagy and apoptosis of diabetic endothelial progenitor cells by sponging mir-20a-5p. Cell Death Dis. 2020, 11, 526. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, F.; Liu, Q.; Deng, X.; Hai, R.; He, X.; Zhou, X. Downregulation of miRNA-328 promotes the angiogenesis of HUVECs by regulating the PIM1 and AKT/mTOR signaling pathway under high glucose and low serum condition. Mol. Med. Rep. 2020, 22, 895–905. [Google Scholar] [CrossRef]
- Wei, P.; Zhong, C.; Yang, X.; Shu, F.; Xiao, S.; Gong, T.; Luo, P.; Li, L.; Chen, Z.; Zheng, Y.; et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burn. Trauma 2020, 8, tkaa020. [Google Scholar] [CrossRef]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int. J. Mol. Sci. 2022, 23, 15743. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, S.J.; Kim, D.J.; Jang, H.C.; Lim, S.; Choi, S.H.; Kim, Y.H.; Shin, D.H.; Kim, S.H.; Kim, T.H.; et al. Effects of valsartan and amlodipine on oxidative stress in type 2 diabetic patients with hypertension: A randomized, multicenter study. Korean J. Intern. Med. 2017, 32, 497–504. [Google Scholar] [CrossRef]
- Langlais, P.; Yi, Z.; Finlayson, J.; Luo, M.; Mapes, R.; De Filippis, E.; Meyer, C.; Plummer, E.; Tongchinsub, P.; Mattern, M.; et al. Global IRS-1 phosphorylation analysis in insulin resistance. Diabetologia 2011, 54, 2878–2889. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Ren, L.; Zhi, L.; Yu, Z.; Lv, F.; Xu, F.; Peng, W.; Bai, X.; Cheng, K.; Quan, L.; et al. Negative regulation of AMPK signaling by high glucose via E3 ubiquitin ligase MG53. Mol. Cell 2021, 81, 629–637.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, B.; Peng, S.; Xiao, J. Metformin Rescues the Impaired Osteogenesis Differentiation Ability of Rat Adipose-Derived Stem Cells in High Glucose by Activating Autophagy. Stem Cells Dev. 2021, 30, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Brooks, R.; Houskeeper, J.; Bremner, S.K.; Dunlop, J.; Viollet, B.; Logan, P.J.; Salt, I.P.; Ahmed, S.F.; Yarwood, S.J. Metformin suppresses adipogenesis through both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. Mol. Cell. Endocrinol. 2017, 440, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Yook, J.Y.; Son, M.Y.; Kim, M.J.; Koo, D.B.; Han, Y.M.; Cho, Y.S. Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells Dev. 2010, 19, 557–568. [Google Scholar] [CrossRef]
- Phornphutkul, C.; Lee, M.; Voigt, C.; Wu, K.Y.; Ehrlich, M.G.; Gruppuso, P.A.; Chen, Q. The effect of rapamycin on bone growth in rabbits. J. Orthop. Res. 2009, 27, 1157–1161. [Google Scholar] [CrossRef]
- Hu, X.K.; Yin, X.H.; Zhang, H.Q.; Guo, C.F.; Tang, M.X. Liraglutide attenuates the osteoblastic differentiation of MC3T3-E1 cells by modulating AMPK/mTOR signaling. Mol. Med. Rep. 2016, 14, 3662–3668. [Google Scholar] [CrossRef]
- Lan, D.; Yao, C.; Li, X.; Liu, H.; Wang, D.; Wang, Y.; Qi, S. Tocopherol attenuates the oxidative stress of BMSCs by inhibiting ferroptosis through the PI3k/AKT/mTOR pathway. Front. Bioeng. Biotechnol. 2022, 10, 938520. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Dolkart, O.; Salai, M.; Barak, S.; Piattelli, A.; Amir-Barak, H.; Zavan, B. Pulsed electromagnetic fields increase osteogenetic commitment of MSCs via the mTOR pathway in TNF-α mediated inflammatory conditions: An in-vitro study. Sci. Rep. 2018, 8, 5108. [Google Scholar] [CrossRef]
- Karner, C.M.; Lee, S.Y.; Long, F. Bmp Induces Osteoblast Differentiation through both Smad4 and mTORC1 Signaling. Mol. Cell. Biol. 2017, 37, e00253-16. [Google Scholar] [CrossRef]
- Ge, X.; Zhou, G. Protective effects of naringin on glucocorticoid-induced osteoporosis through regulating the PI3K/Akt/mTOR signaling pathway. Am. J. Transl. Res. 2021, 13, 6330–6341. [Google Scholar] [PubMed]
- Zhou, H.; Jiao, G.; Dong, M.; Chi, H.; Wang, H.; Wu, W.; Liu, H.; Ren, S.; Kong, M.; Li, C.; et al. Orthosilicic Acid Accelerates Bone Formation in Human Osteoblast-Like Cells Through the PI3K-Akt-mTOR Pathway. Biol. Trace Elem. Res. 2019, 190, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Zhao, D.; Liu, B.; Wang, B.; Yu, W.; Li, J.; Yu, X.; Cao, F.; Zheng, G.; et al. TGF-β1 promotes the osteoinduction of human osteoblasts via the PI3K/AKT/mTOR/S6K1 signalling pathway. Mol. Med. Rep. 2019, 19, 3505–3518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xiong, Y.; Zhang, Y.; Jia, L.; Zhang, W.; Xu, X. Rutin promotes osteogenic differentiation of periodontal ligament stem cells through the GPR30-mediated PI3K/AKT/mTOR signaling pathway. Exp. Biol. Med. 2020, 245, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Luo, W.; Wang, B.; Yi, Z.; Gong, P.; Xiong, Y. 1α,25-Dihydroxyvitamin D3 ameliorates diabetes-induced bone loss by attenuating FoxO1-mediated autophagy. J. Biol. Chem. 2021, 296, 100287. [Google Scholar] [CrossRef]
- Mizerska-Kowalska, M.; Sławińska-Brych, A.; Kaławaj, K.; Żurek, A.; Pawińska, B.; Rzeski, W.; Zdzisińska, B. Betulin Promotes Differentiation of Human Osteoblasts In Vitro and Exerts an Osteoinductive Effect on the hFOB 1.19 Cell Line Through Activation of JNK, ERK1/2, and mTOR Kinases. Molecules 2019, 24, 2637. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Agarwal, S.; Bell, C.M.; Rothbart, S.B.; Moran, R.G. AMP-activated Protein Kinase (AMPK) Control of mTORC1 Is p53- and TSC2-independent in Pemetrexed-treated Carcinoma Cells. J. Biol. Chem. 2015, 290, 27473–27486. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zheng, Y.D.; Yuan, Y.; Chen, S.C.; Xie, B.C. Effects of Anti-Diabetic Drugs on Fracture Risk: A Systematic Review and Network Meta-Analysis. Front. Endocrinol. 2021, 12, 735824. [Google Scholar] [CrossRef]
- Shen, M.; Yu, H.; Jin, Y.; Mo, J.; Sui, J.; Qian, X.; Chen, T. Metformin Facilitates Osteoblastic Differentiation and M2 Macrophage Polarization by PI3K/AKT/mTOR Pathway in Human Umbilical Cord Mesenchymal Stem Cells. Stem Cells Int. 2022, 2022, 9498876. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Kabiri, F.; Saliani, N.; Nourazarian, A.; Avci, Ç.B.; Rahbarghazi, R.; Nozad Charoudeh, H. Prolonged incubation with Metformin decreased angiogenic potential in human bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2018, 108, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.D.; Cortizo, A.M.; Sedlinsky, C. Metformin revisited: Does this regulator of AMP-activated protein kinase secondarily affect bone metabolism and prevent diabetic osteopathy. World J. Diabetes 2016, 7, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhao, Z.; Xu, H.H.K.; Dai, Z.; Yu, K.; Xiao, L.; Schneider, A.; Weir, M.D.; Oates, T.W.; Bai, Y.; et al. Effects of Metformin Delivery via Biomaterials on Bone and Dental Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 15905. [Google Scholar] [CrossRef]
- Al Jofi, F.E.; Ma, T.; Guo, D.; Schneider, M.P.; Shu, Y.; Xu, H.H.K.; Schneider, A. Functional organic cation transporters mediate osteogenic response to metformin in human umbilical cord mesenchymal stromal cells. Cytotherapy 2018, 20, 650–659. [Google Scholar] [CrossRef]
- Krebs, M.; Brunmair, B.; Brehm, A.; Artwohl, M.; Szendroedi, J.; Nowotny, P.; Roth, E.; Fürnsinn, C.; Promintzer, M.; Anderwald, C.; et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes 2007, 56, 1600–1607. [Google Scholar] [CrossRef]
- Houde, V.P.; Brûlé, S.; Festuccia, W.T.; Blanchard, P.G.; Bellmann, K.; Deshaies, Y.; Marette, A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes 2010, 59, 1338–1348. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, C.; Zhou, L.; Li, Y.; Wu, D. Osteogenic effects of rapamycin on bone marrow mesenchymal stem cells via inducing autophagy. J. Orthop. Surg. Res. 2023, 18, 129. [Google Scholar] [CrossRef]
- Isomoto, S.; Hattori, K.; Ohgushi, H.; Nakajima, H.; Tanaka, Y.; Takakura, Y. Rapamycin as an inhibitor of osteogenic differentiation in bone marrow-derived mesenchymal stem cells. J. Orthop. Sci. 2007, 12, 83–88. [Google Scholar] [CrossRef]
- Mabilleau, G.; Pereira, M.; Chenu, C. Novel skeletal effects of glucagon-like peptide-1 (GLP-1) receptor agonists. J. Endocrinol. 2018, 236, R29–R42. [Google Scholar] [CrossRef]
- Gao, L.; Li, S.L.; Li, Y.K. Liraglutide Promotes the Osteogenic Differentiation in MC3T3-E1 Cells via Regulating the Expression of Smad2/3 Through PI3K/Akt and Wnt/β-Catenin Pathways. DNA Cell Biol. 2018, 37, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, S.; Xue, P.; Li, Y. Liraglutide, a glucagon-like peptide-1 receptor agonist, facilitates osteogenic proliferation and differentiation in MC3T3-E1 cells through phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), extracellular signal-related kinase (ERK)1/2, and cAMP/protein kinase A (PKA) signaling pathways involving β-catenin. Exp. Cell Res. 2017, 360, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, S.; Xue, P.; Li, Y. Liraglutide Inhibits the Apoptosis of MC3T3-E1 Cells Induced by Serum Deprivation through cAMP/PKA/β-Catenin and PI3K/AKT/GSK3β Signaling Pathways. Mol. Cells 2018, 41, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Dibble, C.C.; Cantley, L.C. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015, 25, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, S.; Yang, P.; Kou, Y.; Li, C.; Liu, H.; Li, M. Exendin-4 and eldecalcitol synergistically promote osteogenic differentiation of bone marrow mesenchymal stem cells through M2 macrophages polarization via PI3K/AKT pathway. Stem Cell Res. Ther. 2022, 13, 113. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Zhang, M.; Xu, J. Cholesterol inhibits autophagy in RANKL-induced osteoclast differentiation through activating the PI3K/AKT/mTOR signaling pathway. Mol. Biol. Rep. 2022, 49, 9217–9229. [Google Scholar] [CrossRef]
- Stage, T.B.; Christensen, M.H.; Jørgensen, N.R.; Beck-Nielsen, H.; Brøsen, K.; Gram, J.; Frost, M. Effects of metformin, rosiglitazone and insulin on bone metabolism in patients with type 2 diabetes. Bone 2018, 112, 35–41. [Google Scholar] [CrossRef]
- Nordklint, A.K.; Almdal, T.P.; Vestergaard, P.; Lundby-Christensen, L.; Jørgensen, N.R.; Boesgaard, T.W.; Breum, L.; Gade-Rasmussen, B.; Sneppen, S.B.; Gluud, C.; et al. Effect of Metformin vs. Placebo in Combination with Insulin Analogues on Bone Markers P1NP and CTX in Patients with Type 2 Diabetes Mellitus. Calcif. Tissue Int. 2020, 107, 160–169. [Google Scholar] [CrossRef]
- Nordklint, A.K.; Almdal, T.P.; Vestergaard, P.; Lundby-Christensen, L.; Boesgaard, T.W.; Breum, L.; Gade-Rasmussen, B.; Sneppen, S.B.; Gluud, C.; Hemmingsen, B.; et al. The effect of metformin versus placebo in combination with insulin analogues on bone mineral density and trabecular bone score in patients with type 2 diabetes mellitus: A randomized placebo-controlled trial. Osteoporos. Int. 2018, 29, 2517–2526. [Google Scholar] [CrossRef]
| Agents | Target Cell/Tissue (Environment) | Target | Main Finding | Ref. |
|---|---|---|---|---|
| Inhibition of mTOR | ||||
| Metformin | adipose-derived stem cells (ASCs) (40 mM high glucose culture environment) | mTOR | 0.1 mM metformin reversed the osteogenesis inhibition of ASCs caused by high glucose via inhibiting mTOR and upregulating autophagy | [135] |
| C3H10T1/2 MSCs (10% FCS plus either IID or PIO) (10% FCS plus AGD) | mTOR/p70 | Metformin increased RUNX2 expression and inhibited PPARγ activity in MSCs through the suppression of the mTOR/p70S6K signaling pathway, thereby decreasing adipogenesis | [136] | |
| C3H10T1/2 MSCs (high-glucose conditions with glutamine) | AMPK/mTOR | Activation of AMPK by metformin inhibited high glutamine-induced mTORC1 hyperactivation and rescues RUNX2 through the mTORC2/AKT-473 axis | [22] | |
| Rapamycin | human embryonic stem cells (hESCs) (mouse embryonic fibroblast-conditioned medium/serum-free medium) | mTOR | Rapamycin functioned as a potent stimulator of osteoblastic differentiation of hESCs by modulating mTOR and BMP/Smad signaling | [137] |
| Five-week-old New Zealand White rabbits | mTOR | Direct infusion of rapamycin into proximal tibial growth plates decreased the size of the growth plate and inhibited overall long bone growth | [138] | |
| PPARβ/δ Agonist | rat BMSCs (high glucose environment) SD rats (1% streptozotocin injected) | AMPK/mTOR | PPARβ/δ agonist promoted osteogenic differentiation of rat BMSCs through activating AMPK/mTOR-regulated autophagy and improved bone regeneration in type 1 diabetic rats. | [70] |
| Liraglutide | MC3T3-E1 (DMEM medium) | AMPK/mTOR | Liraglutide reduced the differentiation of MC3T3-E1 osteoblasts by regulating AMPK/mTOR pathway | [139] |
| Activation of mTOR signaling | ||||
| Rehmannia glutinosa Libosch Extracts | MC3T3-E1 (high glucose α-MEM medium) Wistar rats (high-fat diet and streptozotocin injection) | IGF-1/ PI3K/mTOR | The extracts increased the proliferation and differentiation of osteoblastic MC3T3-E1 cells injured by high glucose by activating the IGF-1/PI3K/mTOR pathway. Rehmanniae Radix Praeparata could prevent bone loss in type 2 diabetic rats. | [61] |
| Tocopherol | rat BMSCs (treated with H2O2) | PI3K/AKT/ mTOR | Tocopherol protected rat BMSCs from oxidative stress damage by activating PI3K/AKT/mTOR pathway | [140] |
| Pulsed Electromagnetic Fields (PEMFs) | MSCs (treated with 0.1 mg/mL of TNFα) | mTOR | PEMF increased the expression of osteogenic markers and promoted osteogenic differentiation of MSCs under TNF-α-mediated inflammatory conditions via mTOR activation | [141] |
| BMP-2 | BMSCs (α-MEM medium) | mTOR | BMP-2 activated mTOR signaling pathway and downstream genes regulating protein anabolism to induce osteoblast differentiation | [142] |
| Naringin | Osteoblasts cultured from the differentiated BMSCs (DMEM medium) | PI3K/AKT/ mTOR | Naringin promoted proliferation and differentiation of osteoblasts by activating PI3K/AKT/mTOR pathway | [143] |
| Orthosilicic Acid | MG-63 and U2-OS (DMEM medium) | PI3K/AKT/ mTOR | Orthosilicic acid promoted osteogenesis in vitro by activating PI3K/AKT/mTOR signaling pathway | [144] |
| Transforming growth factor beta 1 (TGF-β1) | hFOB 1.19 (DMEM medium) | PI3K/AKT/ mTOR/S6K1 | TGF-β1 induced the survival, osteogenic differentiation and migration of human hFOB 1.19 osteoblasts by activating the PI3K/AKT/mTOR/S6K1 pathway | [145] |
| Rutin | Periodontal ligament stem cells (PDLSCs) (α-MEM medium) | PI3K/AKT/ mTOR | Rutin increased proliferation and osteogenic differentiation of PDLSCs through G protein-coupled receptor 30 (GPR30)-mediated PI3K/AKT/mTOR signal transduction | [146] |
| 1α,25-Dihydroxyvitamin D3 (1,25D) | Wild type mice (high-fat diet and streptozotocin injection) Osteoblasts (high glucose environment) | PI3K/AKT/ FoxO1, Sesn3/AMPK/ mTORC1 | 1,25D could reverse dysfunctional bone metabolism in type 2 diabetic mice through attenuating autophagy, by activating PI3K/AKT signaling, inhibiting FoxO1 and Sesn3/AMPK, and upregulating mTORC1. | [147] |
| Betulin (BET) | hFOB 1.19 (osteogenic medium and basal medium) | mTOR | BET increased the expression level of osteogenic differentiation markers and promoted mineralization by activating mitogen-activated protein kinases (MAPKs) and mTOR | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, J.; Wang, S.; Tao, R.; Yi, J.; Chen, M.; Zhao, Z. mTOR Signaling Pathway in Bone Diseases Associated with Hyperglycemia. Int. J. Mol. Sci. 2023, 24, 9198. https://doi.org/10.3390/ijms24119198
Wang S, Wang J, Wang S, Tao R, Yi J, Chen M, Zhao Z. mTOR Signaling Pathway in Bone Diseases Associated with Hyperglycemia. International Journal of Molecular Sciences. 2023; 24(11):9198. https://doi.org/10.3390/ijms24119198
Chicago/Turabian StyleWang, Shuangcheng, Jiale Wang, Shuangwen Wang, Ran Tao, Jianru Yi, Miao Chen, and Zhihe Zhao. 2023. "mTOR Signaling Pathway in Bone Diseases Associated with Hyperglycemia" International Journal of Molecular Sciences 24, no. 11: 9198. https://doi.org/10.3390/ijms24119198
APA StyleWang, S., Wang, J., Wang, S., Tao, R., Yi, J., Chen, M., & Zhao, Z. (2023). mTOR Signaling Pathway in Bone Diseases Associated with Hyperglycemia. International Journal of Molecular Sciences, 24(11), 9198. https://doi.org/10.3390/ijms24119198






