Abstract
A phyloprofile of Frankia genomes was carried out to identify those genes present in symbiotic strains of clusters 1, 1c, 2 and 3 and absent in non-infective strains of cluster 4. At a threshold of 50% AA identity, 108 genes were retrieved. Among these were known symbiosis-associated genes such as nif (nitrogenase), and genes which are not know as symbiosis-associated genes such as can (carbonic anhydrase, CAN). The role of CAN, which supplies carbonate ions necessary for carboxylases and acidifies the cytoplasm, was thus analyzed by staining cells with pH-responsive dyes; assaying for CO2 levels in N-fixing propionate-fed cells (that require a propionate-CoA carboxylase to yield succinate-CoA), fumarate-fed cells and N-replete propionate-fed cells; conducting proteomics on N-fixing fumarate and propionate-fed cells and direct measurement of organic acids in nodules and in roots. The interiors of both in vitro and nodular vesicles were found to be at a lower pH than that of hyphae. CO2 levels in N2-fixing propionate-fed cultures were lower than in N-replete ones. Proteomics of propionate-fed cells showed carbamoyl-phosphate synthase (CPS) as the most overabundant enzyme relative to fumarate-fed cells. CPS combines carbonate and ammonium in the first step of the citrulline pathway, something which would help manage acidity and NH4+. Nodules were found to have sizeable amounts of pyruvate and acetate in addition to TCA intermediates. This points to CAN reducing the vesicles’ pH to prevent the escape of NH3 and to control ammonium assimilation by GS and GOGAT, two enzymes that work in different ways in vesicles and hyphae. Genes with related functions (carboxylases, biotin operon and citrulline-aspartate ligase) appear to have undergone decay in non-symbiotic lineages.
1. Introduction
Actinobacterial genus Frankia contains several symbiotic lineages that fix nitrogen within the root nodules of plants belonging to the Rosales, Fagales and Cucurbitales orders [1]. A phylogenetic analysis showed that these symbiotic Frankia formed three clusters [2]. Conversely there are numerous strains that have lost various symbiotic features; these form a coherent cluster [3]. These non-symbiotic strains fall into two categories: those that are non-infective such as CN3 or DC12 [4,5] and those strains that are infective but non-nitrogen-fixing such as EuI1c or AgB1.9 [6,7]. The relative position of the clusters has been found to fluctuate depending on the marker used or the strains studied, [8,9] but all are robust.
It has been estimated that the symbiotic Frankia strains diverged from other soil actinobacteria around 80–100 MY [2], a time length that corresponds roughly to that of the emergence of the plant lineages in symbiosis with Frankia, predating the emergence of the Fabaceae which is estimated at 60 MY [10]. This is thus a lengthy period of time that could have impacted the Frankia genomes with genes which are non-essential for symbiosis, presumably costly to express and dispensable in free-living lineages, and during which these genes could have been subject to negative evolutionary pressure leading them to be lost in a manner similar to that occurring in non-symbiotic host plant lineages in relation to symbiosis-associated genes [1].
More than 40 Frankia genomes from all clusters have become available since the first ones were published in 2007 [11,12]. These genomes have been compared and have been found to fluctuate in terms of parameters such as size [11], GC% [12], codon usage [13] and Ka/Ks [14] as they have been subjected to different evolutionary pressures in the soil, on the one hand, or in plant tissues, on the other. There has been massive genome erosion between non-sporulating (Sp−) and sporulating strains (Sp+) [15], for instance, in Alnus-infective strains that have lost around 2500 genes, or one third of the total number.
There have also been studies targeted at groups of genes which have shown the presence of canonical nod genes in some cluster 2 strains [16,17] and one cluster 3 strain [18], the absence of nif, sodF and rhb genes in non-symbiotic cluster 4 strains [12], the presence of celA-bcsA genes in symbiotic strains [19] and the loss of saprophytic genes such as gvp in cluster 1c strains and cluster 2 strains that have reduced genomes [11,16]. These genomes are thus a wealth of data that can be analyzed to try to understand the genetic determinants underpinning symbiosis in the absence of a proven genetic transformation protocol. We decided to compare symbiotic and non-symbiotic genomes to determine if the estimated 100 MY since their separation had resulted in the loss of symbiosis-associated determinants in those strains that had lost the ability to establish nodules. One such determinant that emerged was the can genes that code for carbonic anhydrase (CAN), an enzyme that converts CO2 into carbonic ions and protons mainly for pH homeostasis and the functioning of carboxylases. There are 23 of these genes in Frankia alni, and we investigated them from a physiological viewpoint.
2. Results
2.1. Genome Mining
When a search was made for those proteins conserved at a threshold of 50% identity between symbiotic cluster1 (cl1) strains (ACN14a, QA3, CcI3), cluster 2 (cl2) strains (BMG5.1 and Dg1) and cluster 3 (cl3) strains (EaN1pec and EUN1f) and absent from non-symbiotic non-infective cluster 4 (cl4) strains (CN3 and DC12), 108 hits were obtained (Table 1). Among those genes retrieved were nif (nitrogenase, FRAAL6800-6814); rhbBCEF (rhizobactin-related metabolite; FRAAL6422- FRAAL6426); sodF (Fe-superoxide dismutase, FRAAL4337); accA (acetyl carboxylase, FRAAL3196); argF (argininosuccinate lyase, FRAAL5202) and two can (carbonic anhydrase; FRAAL1222, FRAAL4889). These two CANs belong to the Beta class prevalent in prokaryotes which is more active than the Gamma CANs [20]. The can genes were found to be present in some genomes belonging to cluster 4, such as Frankia AgW1.1 and AgB1.8 strains, isolated from Alnus and able to reinfect Alnus but unable to fix nitrogen in pure culture or in vitro.

Table 1.
List of genes conserved between Frankia strains of clusters 1, 2 and 3 but absent in cluster 4 genomes at a level of 50% Id. Bold letters indicate carbonic anhydrase.
When a search for gene annotations was carried out in the MAGE data base using “carbonic anhydrase” as keyword, supplementary hits were obtained in several lineages including Frankia alni, FRAAL2078, that would code for a distant Gamma CAN with homologs in all Frankia strains. Finally, the same keyword search yielded a Beta CAN present in cluster3 and cluster4 strains with a very low ID (<30% AA) with FRAAL1222 and FRAAL4889 CANs.
2.2. Cells Growth
Growth, N-fixation and CO2 levels were monitored over liquid N-fixing and N-replete cultures. Frankia grew rapidly on propionate and ammonium, reaching a plateau after only 3 days, whereas it took 6 days when fixing nitrogen on propionate, and on fumarate it took 13 days (Figure 1). The CO2 in the propionate+ammonium-fed culture reached a plateau at day 4, whereas in the propionate-fed nitrogen fixing culture, CO2 increased steadily over seven days as the cells multiplied. CO2 in the fumarate-fed culture was low at first and increased slowly thereafter.
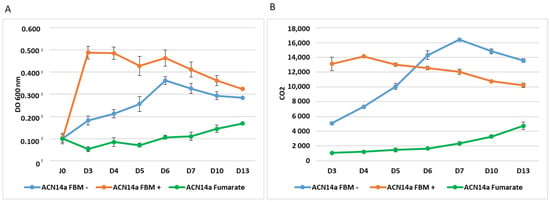
Figure 1.
(A) Growth of Frankia alni strain ACN14a (DO 600 nm) in FBM medium with 5 mM propionate and 5 mM NH4Cl (yellow dots), in FBM medium with 5 mM propionate without NH4Cl (blue dots) and in FBM medium without propionate but with 5 mM fumarate and without NH4Cl (green dots) followed over a time course of 13 days; (B) CO2 in the gas phase over strain ACN14a (in the same conditions as in (A)). Bars are +/− standard error.
2.3. Physiology Measurements of Cells
Frankia alni cells grown on an FBM-medium were stained with DND-189 and DND-99 fluorescent probes and observed under fluorescent light with filters as well as with SYTO-9, which showed the vesicles to be fluorescent (Figure 2) thus indicating their pH is acidic, more so than that of the hyphae that did not fluoresce with the pH-responsive stains. After staining 21 days post infection (dpi), nodule sections showed strong fluorescence with DND-99 and SYTO-9 stains, thus indicating acidity was found inside the cytoplasm of the vesicles (Figure 3).
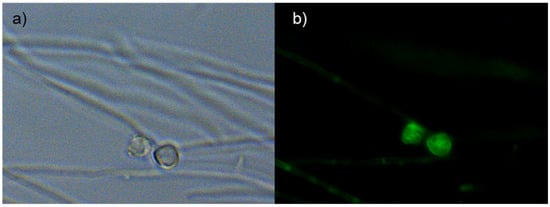
Figure 2.
Free cells cultures of Frankia alni ACN14a grown for 7 days in BAP− medium observed under visible light and UV microscope. Phase contrast (a) and green filter DND-189 probe-treated (b). Strong fluorescence indicative of acidity is found inside N2-fixing Frankia vesicles.
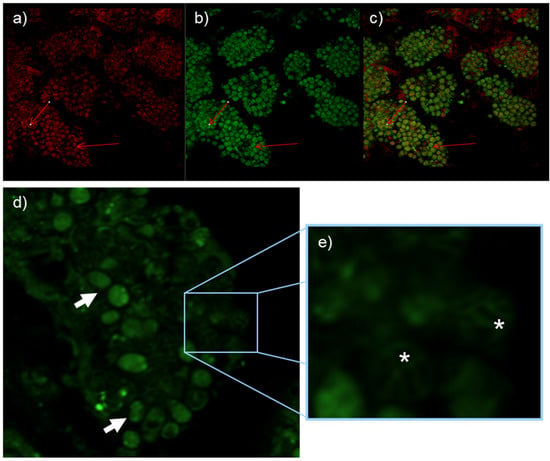
Figure 3.
Alnus glutinosa nodules resulting from inoculation of Frankia alni ACN14a. Nodules treated with DND-99 and SYTO-9 under red filter (a) green filter (b) and overlay (c) show acidity inside vesicles. Nodules treated with DND-189 probe show absence of acidity in intervesicular spaces (arrows), (d) and in intermembrane spacers (*), (e).
2.4. Gene Expression
Expression levels of can1 (FRAAL1222), can2 (FRAAL4889), ctp (FRAAL4724) and two carbamoyl phosphate synthases cpsA (FRAAL4684) and cspB (FRAAL4683) are shown in Figure 4. The can1 and ctp genes were highly upregulated under nitrogen starvation in FBM− media and in symbiosis. The second can gene (FRAAL4889) was not differentially expressed under all conditions tested. The two carbamoylphosphate synthase genes, cpsA and cpsB genes, were up-regulated in free-living cultures without nitrogen and down-regulated in symbiosis. A low level of expression was observed under growth as free culture in FBM+ with supplied ammonium.
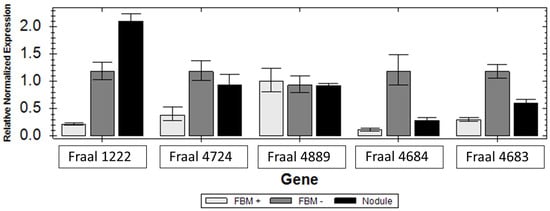
Figure 4.
Relative expression of can1 (FRAAL1222), can2 (FRAAL4889), ctp (FRAAL4724), cpsA and cpsB (FRAAL4684 and FRAAL4683) genes in Frankia alni ACN14a grown under nitrogen-free conditions in FBM− (grey) media, with 5 mM ammonium in FBM+ media (light grey) and in symbiosis within Alnus glutinosa nodules (dark).
2.5. Proteomics
The proteomic analysis results of fumarate- and propionate-fed cells under nitrogen fixing conditions are shown in Table 2 with the 40 most up-regulated proteins of each condition. Fumarate-fed cells had a high overabundance of acyl-CoA synthase, three dicarboxylate transporters and proteases. Propionate-fed cells had carbamoyl-phosphate synthase, nitrogenase, SHC and lipid synthase as the most overabundant proteins.

Table 2.
List of the 40 most overabundant proteins in fumarate-fed cells (section a) or in propionate-fed cells (section b).
2.6. Metabolomics of Organic Acids
The small organic acids present in roots and 21 dpi nodules were formic, acetic, pyruvic, propionic, malonic, malic, fumaric, α-ketoglutaric, succinic, oxalic and citric acids (Table 3). Among these, citric, malic and oxalic acids were the most abundant, while α-ketoglutaric acid fumaric acid and the monocarboxylates pyruvic acid and acetic acid were those with the highest FCs of 2.1, 2.1, 1.9 and 1.6, respectively, in nodules relative to roots.

Table 3.
Abundance of organic acids in A. glutinosa nodules (N1 and N2) and roots (R1 and R2).
3. Discussion
Frankia symbiotic determinants are still poorly understood mostly due to the absence of an effective genetic transformation protocol. Omics, transcriptomics and proteomics are promising new approaches for the study of plant—bacteria interactions, and have been used to circumvent this drawback in Frankia. A transcriptomic study of 21 dpi symbiotic cells showed upregulation of the genes nif, hup, suf and shc [21]. More recently, it was shown that cellulase and cellulose synthase were upregulated upon early (2 dpi) contact even although Frankia alni feeds neither on glucose nor on cellulose [19], suggesting a role in penetration into the root hair. However, few hints have emerged through omics on the nature of the trophic relations between symbiont and host.
Carbonic anhydrase (CAN) is a zinc-dependent metallo enzyme that catalyzes the reversible reaction of CO2 with water into carbonic ions and protons, thereby modifying pH and permitting the subsequent action of carboxylases. In animal lungs, carbonic anhydrase converts blood bicarbonate to carbon dioxide that is then exhaled. In the stomach, the CAN of parietal cells produces acid, in the kidneys it helps maintain the blood pH level by forming protons that are then secreted into urine. In plants, CAN helps increase chloroplast CO2 concentration to facilitate the carboxylation of RuBisCO. Bacteria need to adapt to niches with low pH, for example Helicobacter pylori in the stomach [22] or Mycobacterium tuberculosis in macrophages [23], and CAN has been shown to permit adaptation to these biotopes. An Escherichia coli can- mutant can only survive if the atmospheric partial pressure of CO2 is high or during anaerobic growth in a closed vessel at low pH where copious CO2 is generated endogenously [24]. Pathogen CANs are also targets of specific enzyme inhibitors and fight antibiotic resistant cells. This mechanism is known to be used against Mycobacterium tuberculosis [23,25], M. leprae [26], Legionella pneumophila [22] and several other bacteria [27]. Thus, CAN activity is a critical feature for these bacteria. Many CAN enzymes have been described with less sequence identity which suggests the convergent evolution of the enzymatic function [28]. Carboxylases play important roles in the cell; for instance phosphoenolpyruvate carboxylase feeds pyruvate into the TCA, propionyl-CoA carboxylase yields methylmalonyl-CoA for feeding succinyl-CoA into the TCA, acetyl-CoA carboxylase yields malonyl-CoA for the lipid synthesis or carbamoyl phosphate synthetase that then permit L-ornithine to transform into L-citrulline.
Propionate and acetate are known antibacterial compounds which are active against a wide range of microbes. Their mechanism of action has been postulated to be a drop in cytoplasmic pH [29] or synergy and potentiation of the anti-bacterial activity of transition metals [30]. They can freely cross membranes when they are not charged (they are neutral at their pKa, 4.8 for acetate, 4.9 for propionate, and much lower at 2.4 for pyruvate) and thus require no dedicated transporter [31] except at low concentrations or at a pH above neutrality [32]. They are used as food preservatives, yet propionate and acetate are the most efficiently used carbon sources in Frankia [33], presumably because Frankia has been selected over millions of years to use them and can counter their toxic properties. One study claimed that one Frankia strain needed a transporter for propionate, but it is probable that the inhibitors used inhibited incorporation into the TCA and the labeled propionate could then simply move freely in and out of the cells [34]. The present study found three expressed dicarboxylate transporters when Frankia was fed the dicarboxylate fumarate but no likely propionate transporter when fed propionate. Furthermore, the onset of nitrogen fixation was much more rapid when Frankia was fed monocarboxylates than with dicarboxylates, suggesting there was no need for the synthesis of a dedicated monocarboxylates transporter [33].
Symbiotic Frankia lives in a sheltered niche, the root nodular cortex, but it nevertheless faces strong physiological constraints. One of those constraints is pH homeostasis as it is modified by several enzymes such as upregulated nitrogenase that requires protons and ATP to reduce dinitrogen to ammonium or to uptake hydrogenase that recycles hydrogen into protons and ATP. Rhizobium, that has similar constraints, has its peribacteroid space made acidic by the host to provide a proton-motive force, presumably to permit uptake of malate and activate proteases [35] and also to trap fixed nitrogen as ammonium and not as gaseous ammonia that would otherwise escape at a pH above 6 [36].
Among Frankia cells, the hyphae and vesicles differ from each other in terms of physiology, with hyphae having a complete ammonia assimilation pathway (GS and GOGAT) and vesicles only having an active GS that would lead to large amounts of glutamine under low O2 [37]. It has been hypothesized that glutamine could then diffuse toward the plant as a consequence of several peptides which are upregulated in nodules and which increase porosity towards AA [38]. One of the vesicles’ main characteristics is to have a multi-lamellate wall with hopanoid lipids providing a passive diffusion barrier to oxygen, yielding an anaerobic environment in which nitrogenase can function [39]. GSII, the primary enzyme responsible for NH4+ assimilation, was found to have an optimum pH of 6.4 [40], whereas GOGAT and GDH had optimums of 7.6 and 8.6, respectively [37], which is 1 or 2 pH units above that of GSII. This difference in the pH response of the two enzymes could be explained by the more acidic conditions in the vesicles and is consistent with the vesicles only having GSII activity while the hyphae have a complete cycle. In this scenario, the GS would be complemented by carbamoyl-phosphate synthase (CPS) and ornithine carbamoyl transferase (OCT) which use HCO3− to incorporate NH4+ into ornithine and yield citrulline [41]. OCT is also found in the phyloprofile, with only a distant homolog (32% identities) present in non-symbiotic lineages, with its closest relatives being found in the Planctomyces.
Propionate is synthesized through the catabolism of chlorophyll, valine and fatty acids [42]. The synthesis of acetate as acetyl-CoA in plant cells occurs through pyruvate dehydrogenase entering the TCA cycle and yields lipids and flavonoids [43]. Propionate is known to be upregulated as a response to different situations such as drought [44] and high levels of CO2. The catabolism of lipids is also known to yield acetate [43]. However, in nodules we see much higher levels of dicarboxylates than of monocarboxylates, suggesting Frankia could be fed a mixture of both types of organic acids, with monocarboxylates also serving to modulate cytoplasmic acidity.
Nodules are known to fix CO2 at five times the level of adjacent roots [45], with the labeled carbon ending up as amino acids (glutamate, aspartate and citrulline) and organic acids (malate, citrate and succinate) through the action of PEP carboxylase or pyruvate carboxylase, an enzyme that would be active when the carbon source is pyruvate. Frankia strain ArI3 in pure culture was also shown to fix more nitrogen when supplied supplementary CO2, but only when fed monocarboxylates [46] and not TCA intermediates such as succinate, malate, etc. Pyruvate is assimilated through a dedicated carboxylase and transformed into oxalacetate; propionate is assimilated through the propionyl CoA carboxylase and transformed into succinate. Both oxalacetate and succinate use biotin, and acetate is assimilated through acetyl-CoA carboxylase into malonyl-CoA and lipids. Another possible pathway for the assimilation of propionate is the methyl citrate cycle that is also present in symbiotic strains and absent in non-symbiotic strains. This pathway does not require carboxylase and could function to complement the methyl-malonyl pathway. It has long been assumed that the host feeds Frankia with dicarboxylates since the expression of AgDCAT, a transporter specific for dicarboxylates, was nodule-specific [47]. However, monocarboxylates could also contribute to the nutrition of Frankia since pyruvate, for instance, is present in the roots and nodules of Casuarina and Alnus [48]. The transport of monocarboxylates appears to be facilitated in Coryebacterium by a specific sodium solute symporter at a high pH, but import can nevertheless occur through simple diffusion at a neutral pH [32]. When Frankia is fed a dicarboxylate, it upregulates three transporters, all of which were not up-regulated in 21 dpi nodules [21].
The most overabundant proteins in propionate-fed in-vitro-grown cells were CPS subunit, proteins that use ammonium, phosphate and HCO3− to yield carbamoyl that could then be used to transform L-ornithine into L-citrulline, the compound shown to carry the most labels after 15N has been fed to A. glutinosa [49]. This could be a pathway for the assimilation of ammonium which is complementary to the GS-GOGAT pathway. Frankia has been shown to be able to use L-ornithine as source of nitrogen without inhibition of nitrogenase. This could also serve to select efficient nitrogen fixers that can provide NH4+ to quench carbonate and maintain a slightly acidic pH.
The genes encoding CANs are differentially expressed in nodules (FRAAL1222: 1.20; FRAAL2078: 0.86; FRAAL4724: 1.15; FRAAL4889: 0.71, relative to BAP+, [21]), a process which is compatible with the requirement for CO2 when grown on propionate in vitro and the very high efficiency of the CAN that have a low Km [50]. It is also compatible with the host feeding the symbiont a mixture of dicarboxylates and monocarboxylates. The amount of organic acids measured in this study and by previous authors [33,48] do lead to a certain conclusion in relation to the photosynthates fed to the microsymbiont because of likely compartmentation in the mitochondria, chloroplasts and vacuoles. Nevertheless yet these organic acids are good candidates for the role.
Carbonic anhydrase would thus be added to the list of symbiosis-related genes such as those coding for nitrogenase, hopanoid biosynthesis, iron-sulfur cluster synthesis, hydrogen-uptake [21] and cellulase and cellulose synthase [19]. Similar to hydrogen-uptake and cellulase, there are copies that have diverged early in the evolutionary history of the genus. Whenever genetic editing becomes practical, it will be of interest to assay this symbiosis determinant.
4. Materials and Methods
4.1. Genome Mining
Genome comparisons were made on the Mage platform [51]. Conserved genes present in representative genomes from clusters 1, 2 and 3 were extracted, and those present in non-symbiotic lineage 4 were subtracted using as the threshold 50% of identities over 80% of the length of the shorter AA sequence. For cluster 4, only CN3 [52] and DC12 [5] were used because these are unable to induce nodules in any of the hosts tested other than EuI1c and AgB1.9 that can induce inefficient nodules [53]. A list of the strains and a flow chart of research directions are given in the Supplementary Materials (see Supplementary Materials Table S1 and Figure S1).
4.2. Plant Growth
Alnus seeds were surface-sterilized and germinated as described previously [19]. Seedlings were transferred to opaque pots, grown with N-free medium and inoculated with Frankia alni as before [1].
4.3. Culture of Cells and Physiology Measurements of Cells
Growth, N-fixation and CO2 levels were followed in FBM medium [54], both with and without 5 mM ammonium, and in the same medium with 5 mM fumarate instead of propionate. Growth was monitored as previously [33] and CO2 in the headspace was measured in the flask every day using a gas chromatograph (Micro GC R3000, SRA Instrument, Marcy L’Etoile, France) as described previously [55].
4.4. Microscopy
The staining of nodule slices and FBM− grown Frankia was carried out with DND-99 probes (LysoTracker Red) or DND-189 (LysoSensor Green, Molecular Probes, Eugene, OR, USA) in a 50 mm Tris pH = 7 buffer. Excess dye was removed by a short wash with buffer and samples were observed using a confocal microscope (LSM510 META, Carl Zeiss, Le Pecq, France) using a plan-Apo 63×/1.2 NA water immersion objective.
4.5. Phylogenomics
The amino acid sequences of the carbonic anhydrase genes from the representative Frankia species and related actinobacteria were retrieved using the BlastP method [26]; and were aligned using the Seaview platform [56] implementing the ClustalW2 v2.1 software [57]. The alignment was then used for a Maximum Likelihood tree reconstruction approach [58] with a bootstrap of 1000 replicates [59] to assess the robustness of the topology obtained.
4.6. Gene Expression
Expression analysis of the carbonic anhydrases FRAAL1222, can1 and FRAAL4889, can2; carbonate transporter FRAAL4724, ctp and two carbamoylphosphate synthase genes, FRAAL4883, (cspA) and FRAAL4884, (cspB), was carried out with cells grown on FBM+ medium [54] with 5 mM propionate and with 5 mM ammonium chloride and cells grown on FBM− medium without ammonium chloride and in 21 dpi nodules [21]. This was monitored by qPCR using the primers that are listed in Table 4 and following the procedure described previously [21].

Table 4.
List of the primers used for monitoring the expression level of genes in symbiosis and in fumarate-fed and propionate-fed cells.
4.7. Proteomics
Proteomic analysis of 5 mM propionate-fed and 5 mM fumarate-fed Frankia cells grown in BAP- was carried out as described before [60]. The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifiers PXD018045 and 10.6019/PXD018045.
4.8. Metabolomic of Organic Acids
The organic acids in the nodules and roots were quantified using 22-day-old nodules grown as described in Alloisio et al. [31]. These were extracted from liquid nitrogen ground tissues. Sterile ultrapure water was added to achieve a concentration of 1 g of nodule per mL of water. Samples were centrifuged at 13,000 rpm in Eppendorf centrifuge for 10 min.
After extraction, the supernatants of the nodules or roots were diluted (1:20) in ultrapure water and filtrated on a 0.45 µm PVDF filter prior to being analysed by ionic chromatography and tandem mass spectrometry (IC-MS/MS). IC-MS/MS was used to verify the presence of organic acids already quantified by IC-conductivity and to identify other unknown organic acids.
The IC-MS/MS instrument involved was the ICS-5000+ Ion Chromatography System combined with a TSQ Fortis Triple Quadrupole Mass Spectrometer (all Thermo Scientific, Waltham, MA, USA). The monitoring software was Chromeleon 7.2.10. The IC system was equipped with an AS-AP auto-sampler, a Dual Pump analytical gradient system, a suppressor ADRS 600 (2 mm, Thermo Scientific) and a conductivity detector (P/N 061830). The IC separation was realized using an anion-exchange column IonPac AS11-HC-4 µm (2 × 250 mm2, Thermo Scientific, Illkirch, France) preceded by a guard column IonPac AG11-HC-4 µm (2 × 50 mm2, Thermo Scientific) and with the same parameters as the IC-conductivity analysis. Concerning the mass spectrometer, the heated electrospray ionization source was used in negative mode with the following parameters: negative ion spray voltage 3.0 kV, sheath gas 52 Arb, auxiliary gas 13 Arb, sweep gas 16 Arb, ion transfer tube temperature 275 °C and vaporizer temperature 375 °C. Acquisitions were realized in full scan mode or in Single Ion Monitoring mode with analytes detected after deprotonation in ionization source.
The flow chart of Materials and Methods is presented in Supplementary Figure S1.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24119162/s1.
Author Contributions
P.P. and P.N. designed research; P.P., L.C., P.F., J.A., G.M., N.A., N.D., C.B., X.S. and P.J. performed research; P.P., J.A., G.V.S. and P.N. analyzed data; and P.P. and P.N. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the French ANR (BugsInACell ANR-13-BSV7-0013-03) to P.N. and by an MEC postdoctoral fellowship from the Spanish government awarded to L.C. (Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional de I-D+i 2008–2011).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions generated for this study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
Thanks are expressed to Audrey Dubost (iBio, Université de Lyon) for help with data management, to Danis Abrouk for help with drawing the figures and to Elise Lacroix for Greenhouse management (Université de Lyon).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Griesmann, M.; Chang, Y.; Liu, X.; Spannagl, M.; Crook, M.B.; Billault-Penneteau, B.; Lauressergues, D.; Imanishi, L.; Kohlen, W.; Haberer, G.; et al. Phylogenomics reveals multiple independent losses of the nitrogen-fixing root nodule symbiosis. Science 2018, 361, 6398. [Google Scholar] [CrossRef] [PubMed]
- Normand, P.; Orso, S.; Cournoyer, B.; Jeannin, P.; Chapelon, C.; Dawson, J.; Evtushenko, L.; Misra, A.K. Molecular phylogeny of the genus Frankia and related genera and emendation of the family Frankiaceae. Int. J. Syst. Bacteriol. 1996, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Flandrois, J.; Brochier-Armanet, C.; Briolay, J.; Abrouk, D.; Schwob, G.; Normand, P.; Fernandez, M.P. Taxonomic assignment of uncultured prokaryotes with long range PCR targeting the spectinomycin operon. Res. Microbiol. 2019, 170, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Nouioui, I.; Ghodhbane-Gtari, F.; Del Carmen Montero-Calasanz, M.; Rohde, M.; Tisa, L.S.; Gtari, M.; Klenk, H.P. Frankia inefficax sp. nov., an actinobacterial endophyte inducing ineffective, non nitrogen-fixing, root nodules on its actinorhizal host plants. Antonie Leeuwenhoek 2017, 110, 313–320. [Google Scholar] [CrossRef]
- Tisa, L.S.; Beauchemin, N.; Cantor, M.N.; Furnholm, T.; Ghodhbane-Gtari, F.; Goodwin, L.; Copeland, A.; Gtari, M.; Huntemann, M.; Ivanova, N.; et al. Draft genome sequence of Frankia sp. strain DC12, an atypical, noninfective, ineffective isolate from Datisca cannabina. Genome Announc. 2015, 3, e00889-15. [Google Scholar] [CrossRef]
- Baker, D.; Newcomb, W.; Torrey, J. Characterization of an ineffective actinorhizal microsymbiont, Frankia sp. EuI1 (Actinomycetales). Can. J. Microbiol. 1980, 26, 1072–1089. [Google Scholar] [CrossRef]
- Hahn, D.; Starrenburg, M.; Akkermans, A. Variable compatibility of cloned Alnus glutinosa ecotypes against ineffective Frankia strains. Plant Soil 1988, 107, 233–243. [Google Scholar] [CrossRef]
- Pozzi, A.C.; Bautista-Guerrero, H.H.; Abby, S.S.; Herrera-Belaroussi, A.; Abrouk, D.; Normand, P.; Valdes, M.; Fernandez, M.P. Multi-Locus Sequence Analysis and extensive sampling bring new insights on Frankia phylogeny and phylogeography. Syst. Appl. Microbiol. 2018, 41, 311–323. [Google Scholar] [CrossRef]
- Sen, A.; Daubin, V.; Abrouk, D.; Gifford, I.; Berry, A.M.; Normand, P. Phylogeny of the class Actinobacteria revisited in the light of complete genomes. The orders ‘Frankiales’ and Micrococcales should be split into coherent entities: Proposal of Frankiales ord. nov., Geodermatophilales ord. nov., Acidothermales ord. nov. and Nakamurellales ord. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 3821–3832. [Google Scholar]
- Bell, C.D.; Soltis, D.E.; Soltis, P.S. The age and diversification of the Angiosperms re-revisited. Am. J. Bot. 2010, 97, 1296–1303. [Google Scholar] [CrossRef]
- Normand, P.; Lapierre, P.; Tisa, L.S.; Gogarten, J.P.; Alloisio, N.; Bagnarol, E.; Bassi, C.A.; Berry, A.M.; Bickhart, D.M.; Choisne, N.; et al. Genome characteristics of facultatively symbiotic Frankia sp. strains reflect host range and host plant biogeography. Genome Res. 2007, 17, 7–15. [Google Scholar] [CrossRef]
- Tisa, L.S.; Oshone, R.; Sarkar, I.; Ktari, A.; Sen, A.; Gtari, M. Genomic approaches toward understanding the actinorhizal symbiosis: An update on the status of Frankia genomes. Symbiosis 2016, 70, 5–16. [Google Scholar] [CrossRef]
- Sen, A.; Sur, S.; Bothra, A.K.; Benson, D.R.; Normand, P.; Tisa, L.S. The implication of life style on codon usage patterns and predicted highly expressed genes for three Frankia genomes. Antonie Leeuwenhoek 2008, 93, 335–346. [Google Scholar] [CrossRef]
- Thakur, S.; Normand, P.; Daubin, V.; Tisa, L.S.; Sen, A. Contrasted evolutionary constraints on secreted and non-secreted proteomes of selected Actinobacteria. BMC Genom. 2013, 14, 474. [Google Scholar] [CrossRef]
- Bethencourt, L.; Vautrin, F.; Taib, N.; Dubost, A.; Castro-Garcia, L.; Imbaud, O.; Abrouk, D.; Fournier, P.; Briolay, J.; Nguyen, A.; et al. Draft genome sequences for three unisolated Alnus-infective Frankia Sp+ strains, AgTrS, AiOr and AvVan, the first sequenced Frankia strains able to sporulate in-planta. J. Genom. 2019, 7, 50–55. [Google Scholar] [CrossRef]
- Persson, T.; Battenberg, K.; Demina, I.V.; Vigil-Stenman, T.; Vanden Heuvel, B.; Pujic, P.; Facciotti, M.T.; Wilbanks, E.G.; O’Brien, A.; Fournier, P.; et al. Candidatus Frankia datiscae Dg1, the actinobacterial microsymbiont of Datisca glomerata, expresses the canonical nod genes nodABC in symbiosis with its host plant. PLoS ONE 2015, 10, e0127630. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Wibberg, D.; Battenberg, K.; Blom, J.; Vanden Heuvel, B.; Berry, A.M.; Kalinowski, J.; Pawlowski, K. An assemblage of Frankia Cluster II strains from California contains the canonical nod genes and also the sulfotransferase gene nodH. BMC Genom. 2016, 17, 796. [Google Scholar] [CrossRef]
- Gtari, M.; Ghodhbane-Gtari, F.; Nouioui, I. Frankia soli sp. nov., an actinobacterium isolated from soil beneath Ceanothus jepsonii. Int. J. Syst. Evol. Microbiol. 2019, 70, 1203–1209. [Google Scholar] [CrossRef]
- Pujic, P.; Alloisio, N.; Fournier, P.; Roche, D.; Sghaier, H.; Armengaud, J.; Normand, P. Omics of the early molecular dialogue between Frankia alni and Alnus glutinosa and the cellulase synton. Env. Microbiol. 2019, 21, 3328–3345. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. An overview of the alpha-, beta- and gammacarbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzym. Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef]
- Alloisio, N.; Queiroux, C.; Fournier, P.; Pujic, P.; Normand, P.; Vallenet, D.; Medigue, C.; Yamaura, M.; Kakoi, K.; Kucho, K. The Frankia alni symbiotic transcriptome. Mol. Plant Microbe Interact. 2010, 23, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Legionella pneumophila carbonic anhydrases: Underexplored antibacterial drug targets. Pathogens 2016, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.K.; Colvin, C.J.; Needle, D.B.; Mba Medie, F.; Champion, P.A.; Abramovitch, R.B. The carbonic anhydrase inhibitor ethoxzolamide inhibits the Mycobacterium tuberculosis PhoPR Regulon and Esx-1 Secretion and Attenuates Virulence. Antimicrob. Agents Chemother. 2015, 59, 4436–4445. [Google Scholar] [CrossRef] [PubMed]
- Merlin, C.; Masters, M.; McAteer, S.; Coulson, A. Why Is carbonic anhydrase essential to Escherichia coli? J. Bacteriol. 2003, 185, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Buchieri, M.V.; Riafrecha, L.E.; Rodríguez, O.M.; Vullo, D.; Morbidoni, H.R.; Supuran, C.T.; Colinas, P.A. Inhibition of the β-carbonic anhydrases from Mycobacterium tuberculosis with C-cinnamoyl glycosides: Identification of the first inhibitor with anti-mycobacterial activity. Bioorganic Med. Chem. Lett. 2013, 23, 740–743. [Google Scholar] [CrossRef]
- Aspatwar, A.; Winum, J.Y.; Carta, F.; Supuran, C.T.; Hammaren, M.; Parikka, M.; Parkkila, S. Carbonic anhydrase inhibitors as novel drugs against mycobacterial β-Carbonic anhydrases: An update on in vitro and in vivo studies. Molecules 2018, 23, 2911. [Google Scholar] [CrossRef]
- Alyar, S.; Adem, S. Synthesis, characterization, antimicrobial activity and carbonic anhydrase enzyme inhibitor effects of salicilaldehyde-N-methyl p-toluenesulfonylhydrazone and its Palladium(II), Cobalt(II) complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 131, 294–302. [Google Scholar] [CrossRef]
- Smith, K.S.; Ferry, J.G. Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 2000, 24, 335–366. [Google Scholar] [CrossRef]
- Heseltine, W.W.; Galloway, L.D. Some antibacterial properties of sodium propionate. J. Pharm. Pharmacol. 1951, 3, 581–585. [Google Scholar] [CrossRef]
- Zhitnitsky, D.; Rose, J.; Lewinson, O. The highly synergistic, broad spectrum, antibacterial activity of organic acids and transition metals. Sci. Rep. 2017, 7, 44554. [Google Scholar] [CrossRef]
- Gibson, J. Movement of acetate across the cytoplasmic membrane of the unicellular Cyanobacteria Synechococcus and Aphanocapsa. Arch. Microbiol. 1981, 130, 175–179. [Google Scholar] [CrossRef]
- Jolkver, E.; Emer, D.; Ballan, S.; Kramer, R.; Eikmanns, B.J.; Marin, K. Identification and characterization of a bacterial transport system for the uptake of pyruvate, propionate, and acetate in Corynebacterium glutamicum. J. Bacteriol. 2009, 191, 940–948. [Google Scholar] [CrossRef]
- Carro, L.; Persson, T.; Pujic, P.; Alloisio, N.; Fournier, P.; Boubakri, H.; Pawlowski, K.; Normand, P. Organic acids metabolism in Frankia alni. Symbiosis 2016, 70, 37–48. [Google Scholar] [CrossRef]
- Stowers, M.D.; Kulkarni, R.K.; Steele, D.B. Intermediary carbon metabolism in Frankia. Arch. Microbiol. 1986, 143, 319–324. [Google Scholar] [CrossRef]
- Pierre, O.; Engler, G.; Hopkins, J.; Brau, F.; Boncompagni, E.; Herouart, D. Peribacteroid space acidification: A marker of mature bacteroid functioning in Medicago truncatula nodules. Plant Cell Environ. 2013, 36, 2059–2070. [Google Scholar]
- Day, D.A.; Poole, P.S.; Tyermanc, S.D.; Rosendahld, L. Ammonia and amino acid transport across symbiotic membranes in nitrogen-fixing legume nodules. Cell. Mol. Life Sci. 2001, 58, 61–71. [Google Scholar] [CrossRef]
- Schultz, N.A.; Benson, D.R. Enzymes of ammonia assimilation in hyphae and vesicles of Frankia sp. strain CpI1. J. Bacteriol. 1990, 172, 1380–1384. [Google Scholar] [CrossRef]
- Carro, L.; Pujic, P.; Alloisio, N.; Fournier, P.; Boubakri, H.; Poly, F.; Rey, M.; Heddi, A.; Normand, P. Physiological effects of major upregulated Alnus glutinosa peptides on Frankia sp. ACN14a. Microbiology 2016, 162, 1173–1184. [Google Scholar] [CrossRef]
- Berry, A.M.; Harriott, O.T.; Moreau, R.A.; Osman, S.F.; Benson, D.R.; Jones, A.D. Hopanoid lipids compose the Frankia vesicle envelope. presumptive barrier of oxygen diffusion to nitrogenase. Proc. Natl. Acad. Sci. USA 1993, 90, 6091–6094. [Google Scholar] [CrossRef]
- Edmands, J.; Noridge, N.A.; Benson, D.R. The actinorhizal root-nodule symbiont Frankia sp. strain CpI1 has two glutamine synthetases. Proc. Natl. Acad. Sci. USA 1987, 84, 6126–6130. [Google Scholar] [CrossRef]
- Lundberg, P.; Lundquist, P.O. Primary metabolism in N2-fixing Alnus incana-Frankia symbiotic root nodules studied with 15N and 31P nuclear magnetic resonance spectroscopy. Planta 2004, 219, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.A.; Filley, J.R.; Erb, J.M.; Graybill, E.R.; Hawes, J.W. Peroxisomal metabolism of propionic acid and isobutyric acid in plants. J. Biol. Chem. 2007, 282, 24980–24989. [Google Scholar] [CrossRef] [PubMed]
- Perez de Souza, L.; Garbowicz, K.; Brotman, Y.; Tohge, T.; Fernie, A.R. The acetate pathway supports flavonoid and lipid biosynthesis in Arabidopsis. Plant Physiol. 2020, 182, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; To, T.K.; Matsui, A.; Tanoi, K.; Kobayashi, N.I.; Matsuda, F.; Habu, Y.; Ogawa, D.; Sakamoto, T.; Matsunaga, S.; et al. Acetate-mediated novel survival strategy against drought in plants. Nat. Plants 2017, 3, 17097. [Google Scholar] [CrossRef] [PubMed]
- McClure, P.R.; Coker, G.T.I.; Schubert, K.R. Carbon dioxide fixation in roots and nodules of Alnus glutinosa. 1. Role of phosphoenolpyruvate carboxylase and carbamyl phosphate synthetase in dark CO2 fixation, citrulline synthesis and N2 fixation. Plant Physiol. 1983, 71, 652–657. [Google Scholar] [CrossRef]
- Murry, M.A.; Fontaine, M.S.; Torrey, J.G. Growth kinetics and nitrogenase induction in Frankia sp. HFPArI3 grown in batch culture. Plant Soil 1984, 78, 61–78. [Google Scholar] [CrossRef]
- Jeong, J.; Suh, S.; Guan, C.; Tsay, Y.F.; Moran, N.; Oh, C.J.; An, C.S.; Demchenko, K.N.; Pawlowski, K.; Lee, Y. A nodule-specific dicarboxylate transporter from alder is a member of the peptide transporter family. Plant Physiol. 2004, 134, 969–978. [Google Scholar] [CrossRef]
- Brooks, J.M.; Benson, D.R. Comparative metabolomics of root nodules infected with Frankia sp. strains and uninfected roots from Alnus glutinosa and Casuarina cunninghamiana reflects physiological integration. Symbiosis 2016, 70, 87–96. [Google Scholar] [CrossRef]
- Leaf, G.; Gardner, I.; Bond, G. Observation on the composition and metabolism of the nitrogen-fixing nodules of Alnus. J. Exp. Bot. 1958, 9, 320–334. [Google Scholar] [CrossRef]
- Boone, C.D.; Gill, S.; Habibzadegan, A.; McKenna, R. Carbonic anhydrase: An efficient enzyme with possible global implications. Int. J. Chem. Eng. 2013, 2013, 813931. [Google Scholar] [CrossRef]
- Vallenet, D.; Calteau, A.; Cruveiller, S.; Gachet, M.; Lajus, A.; Josso, A.; Mercier, J.; Renaux, A.; Rollin, J.; Rouy, Z.; et al. MicroScope in 2017: An expanding and evolving integrated resource for community expertise of microbial genomes. Nucleic Acids Res. 2017, 45, D517–D528. [Google Scholar] [CrossRef]
- Ghodhbane-Gtari, F.; Beauchemin, N.; Bruce, D.; Chain, P.; Chen, A.; Walston Davenport, K.; Deshpande, S.; Detter, C.; Furnholm, T.; Goodwin, L.; et al. Draft genome sequence of Frankia sp. strain CN3, an atypical, noninfective (Nod-) ineffective (Fix-) isolate from Coriaria nepalensis. Genome Announc. 2013, 1, e0008513. [Google Scholar] [CrossRef]
- Tisa, L.; McBride, M.; Ensign, J.C. Studies on growth and morphology of Frankia strains EAN1pec, EuI1c, CpI1 and ACN1AG. Can. J. Bot. 1983, 61, 2768–2773. [Google Scholar] [CrossRef]
- Schwenke, J. Rapid, exponential growth and increased biomass yield of some Frankia strains in buffered and stirred mineral medium (BAP) with phosphatidyl choline. Plant Soil 1991, 137, 37–41. [Google Scholar] [CrossRef]
- Simonin, M.; Cantarel, A.A.M.; Crouzet, A.; Gervaix, J.; Martins, J.F.; Richaume, A. Negative effects of copper oxide nanoparticles on carbon and nitrogen cycle microbial activities in contrasting agricultural soils and in presence of plants. Front. Microbiol. 2018, 9, 3102. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Ghedira, K.; Harigua-Souiai, E.; Benhamda, C.; Fournier, P.; Pujic, P.; Guesmi, S.; Guizani, I.; Miotello, G.; Armengaud, J.; Normand, P.; et al. The PEG-responding desiccome of the alder microsymbiont Frankia alni. Sci. Rep. 2018, 8, 759. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).