Long-Term Refrigerated Storage of Beef Using an Active Edible Film Reinforced with Mesoporous Silica Nanoparticles Containing Oregano Essential Oil (Lippia graveolens Kunth)
Abstract
1. Introduction
2. Results and Discussion
2.1. Amaranth Protein Isolate Production
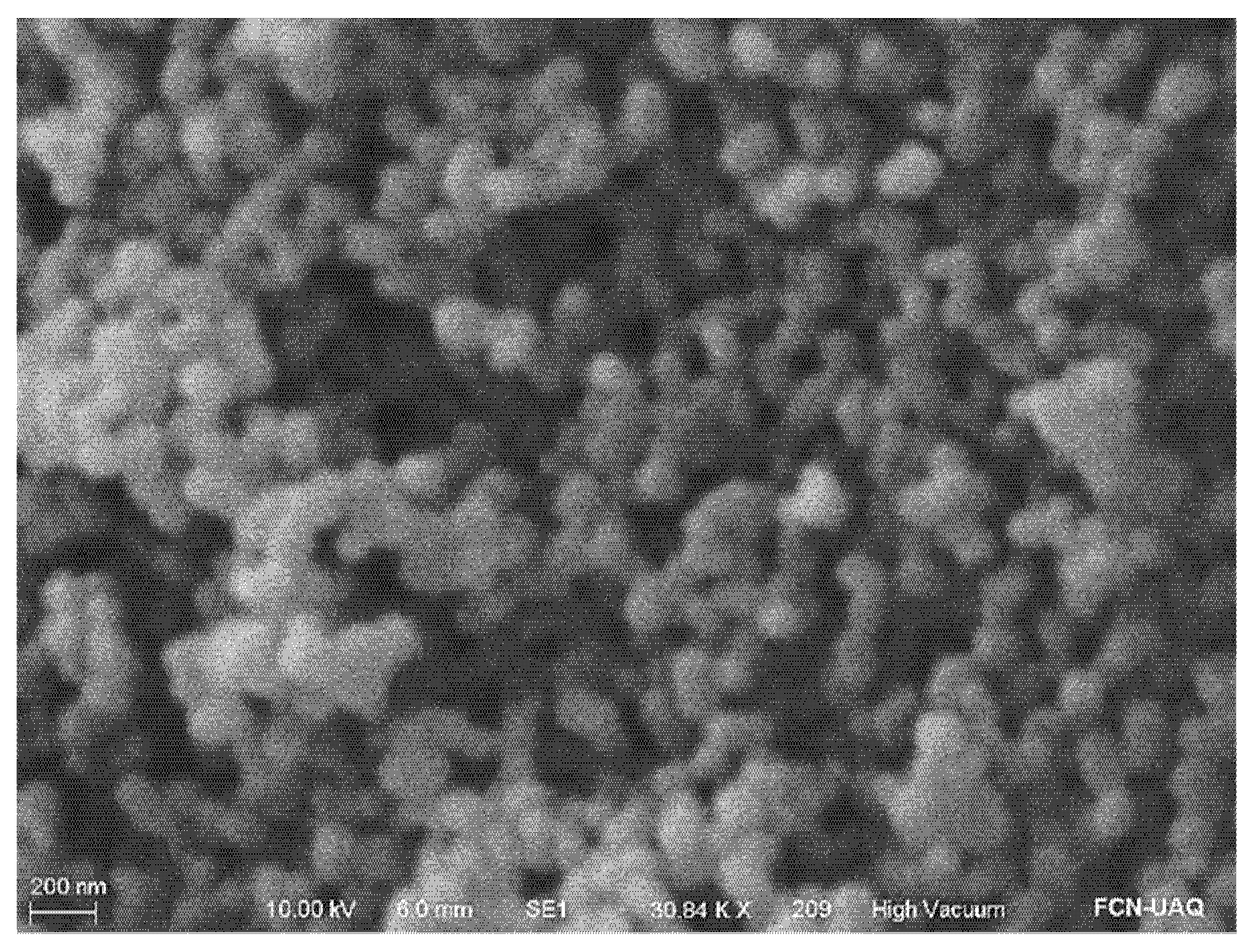
2.2. Mesoporous Silica Nanoparticles Morphology
2.3. Antimicrobial Effect of Oregano Essential Oil and Chitosan
2.3.1. Minimum Inhibitory Concentration of Oregano Essential Oil and Chitosan against E. coli O157:H7
2.3.2. Combined Minimum Inhibitory Concentration of Oregano Essential Oil and Chitosan against E. coli O157:H7
2.4. Loading Capacity of Mesoporous Silica Nanoparticles Loaded with Oregano Essential Oil
2.5. In Vitro Antimicrobial Activity of Active Edible Films
2.6. Stability of Film-Forming Solutions
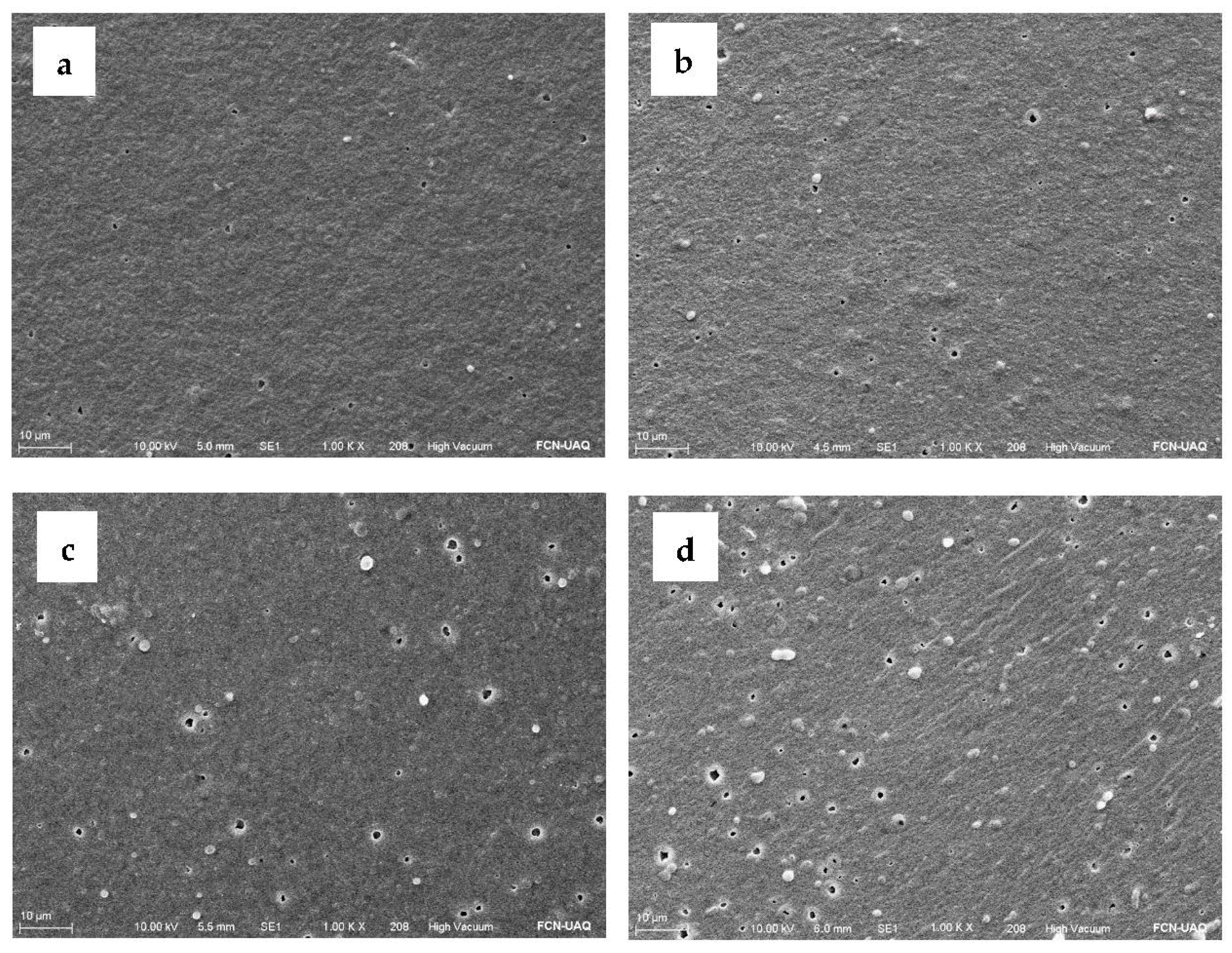
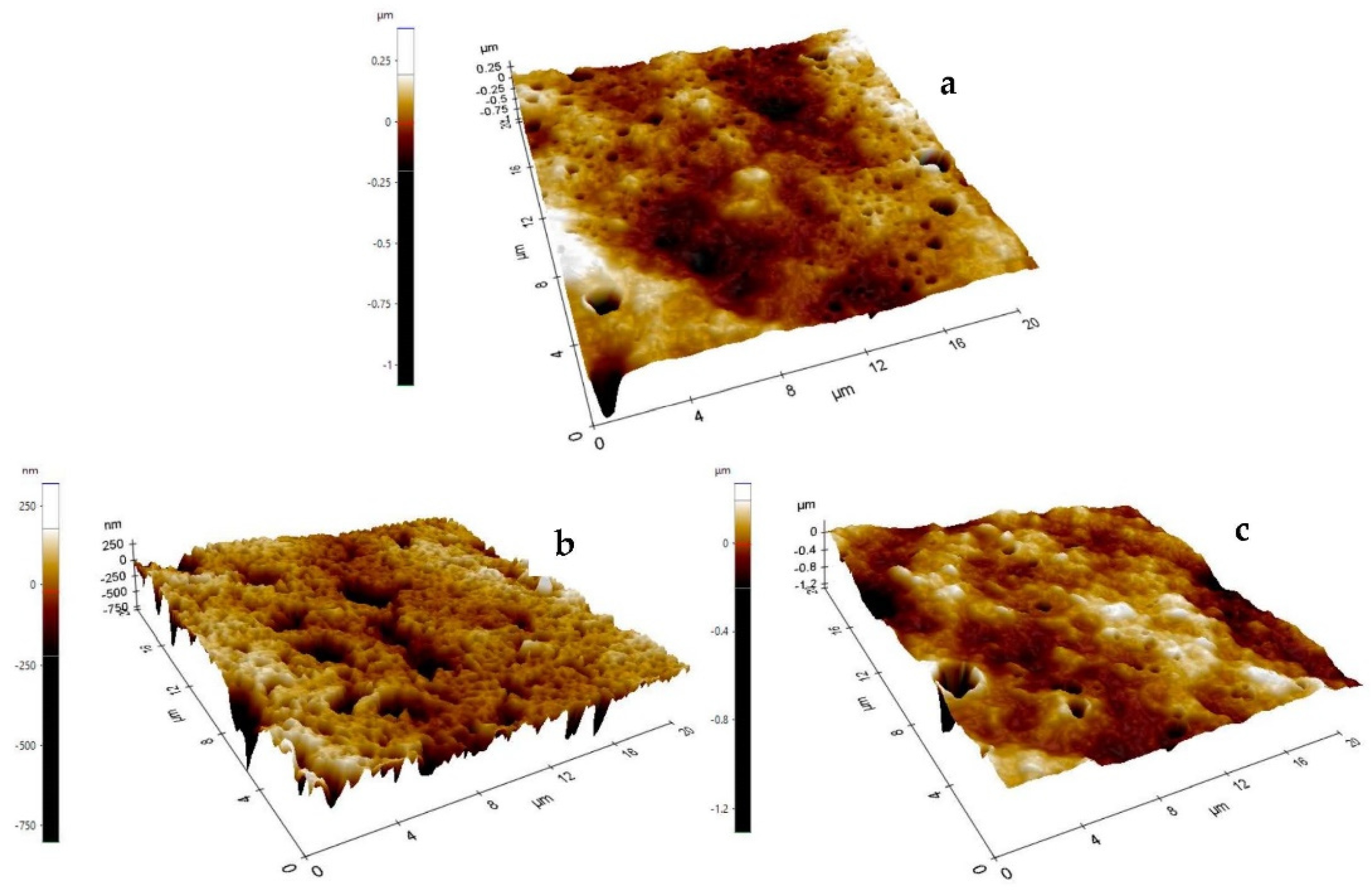
2.7. Color, Morphology, and Topography of Active Edible Films
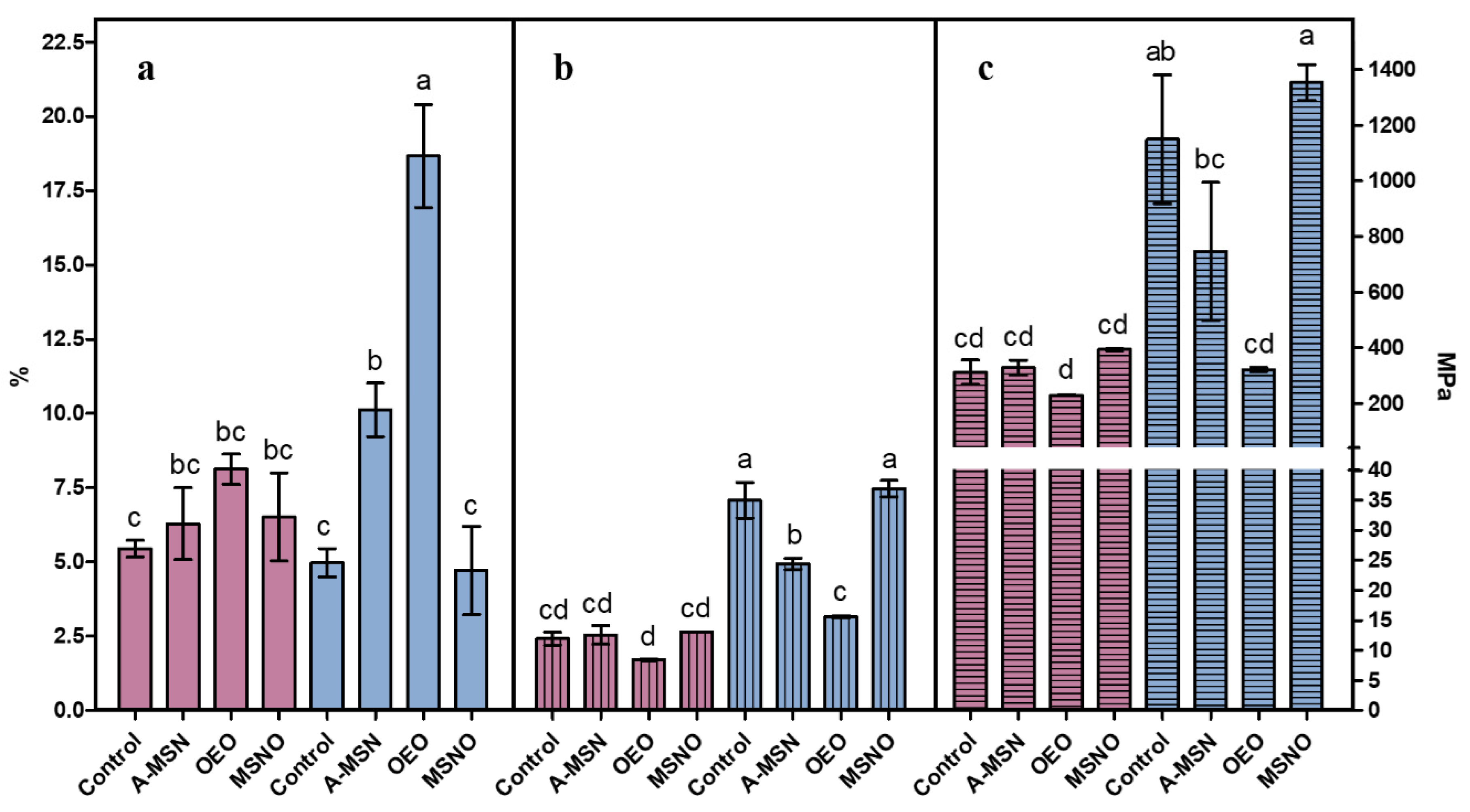
2.8. Thickness, Mechanical Properties, and Water Vapor Permeability (WVP) of Active Edible Films
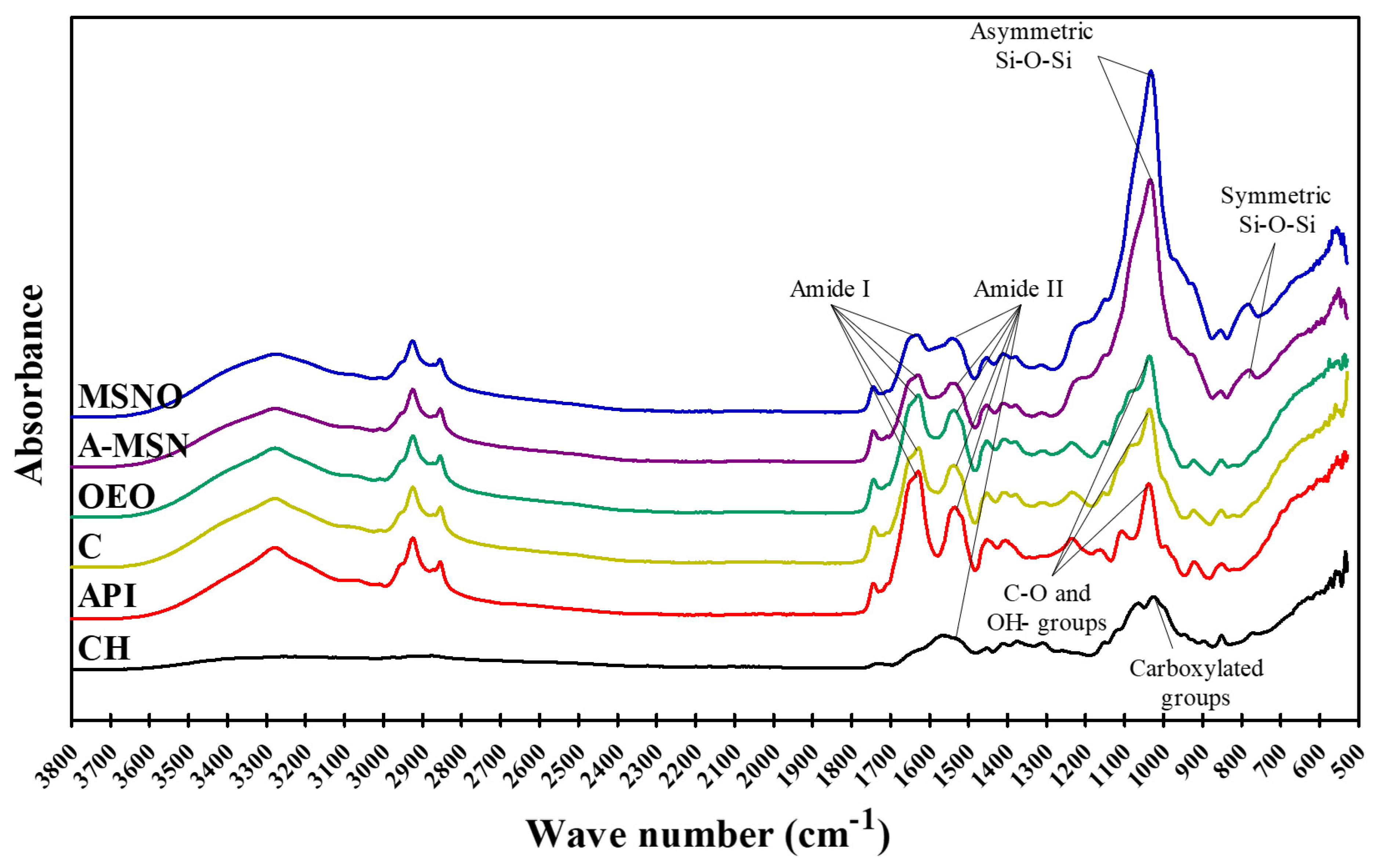
2.9. Fourier Transform Infrared Spectroscopy (FT-IR)
2.10. Effect of Edible Coatings on Fresh Beef Shelf Life
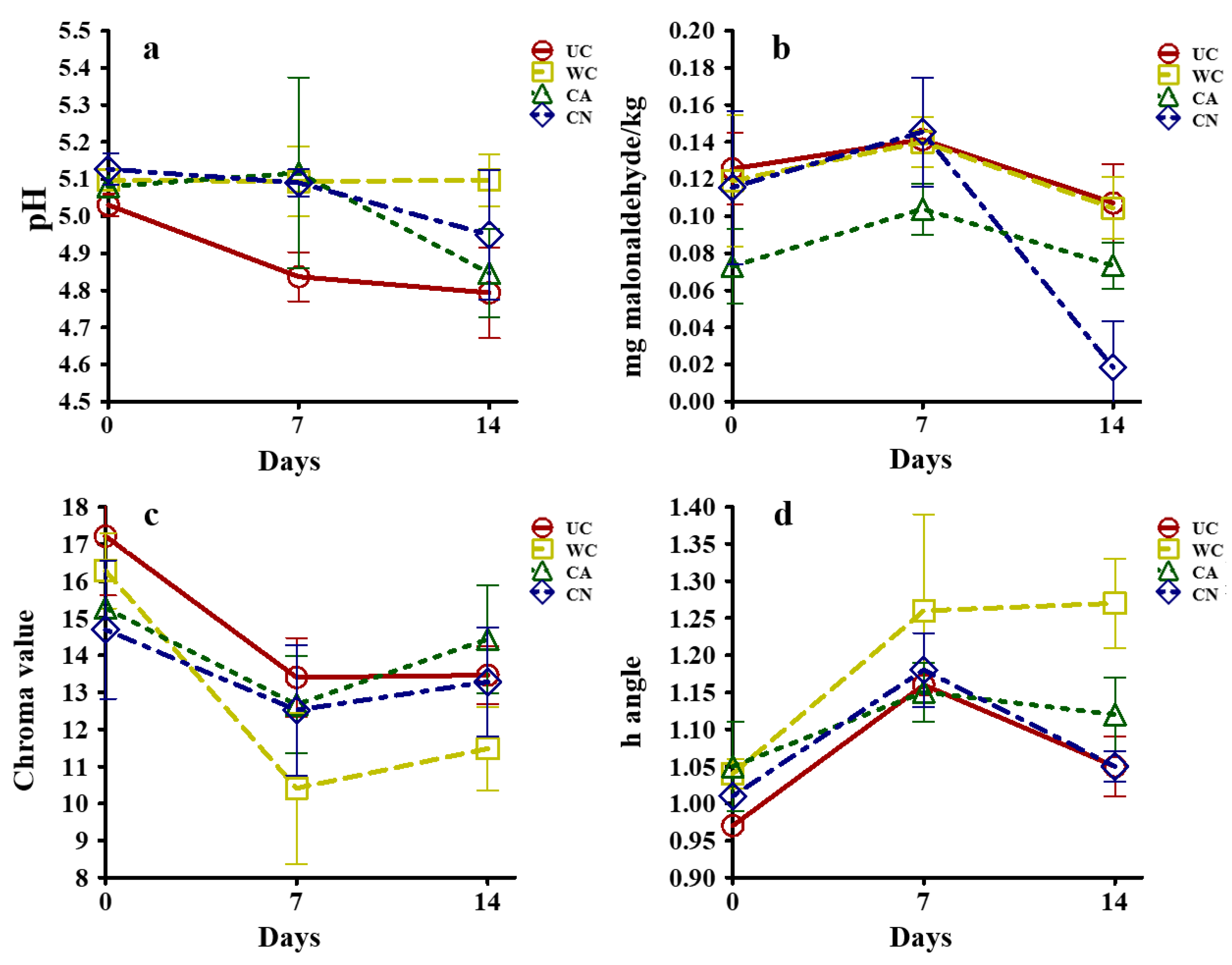
2.10.1. Physical and Chemical Properties
2.10.2. Antimicrobial Properties
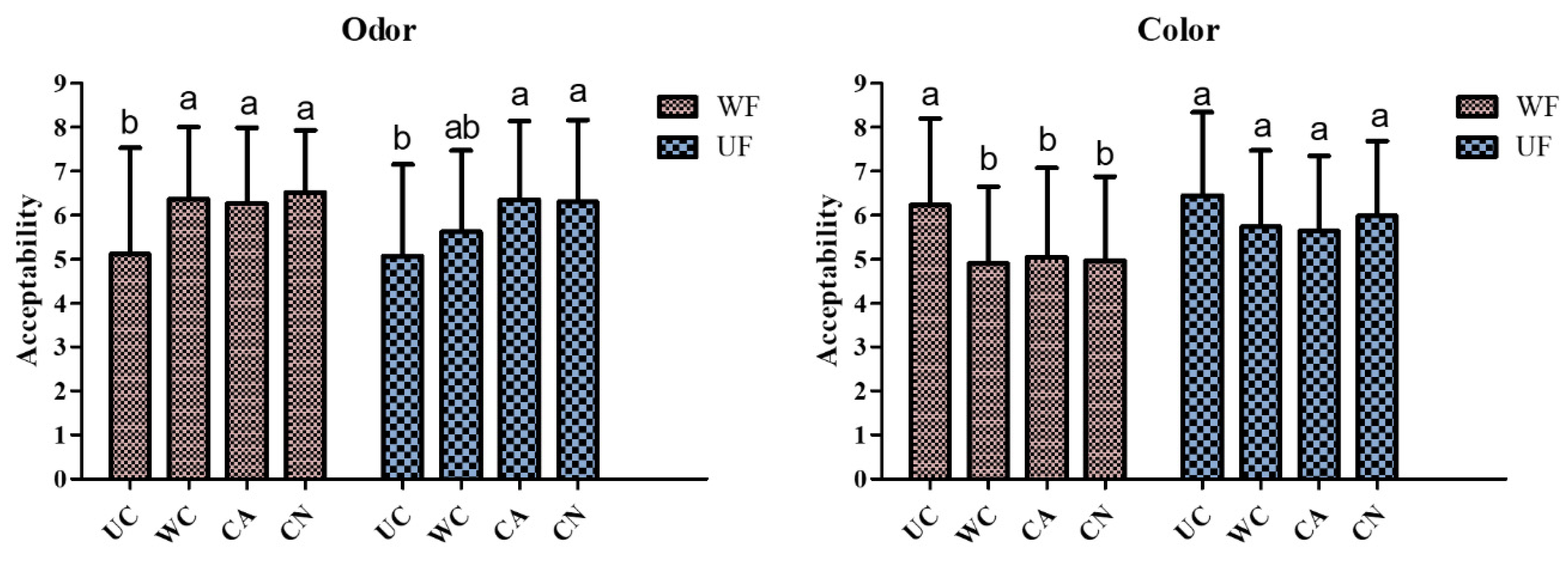
2.11. Sensory Acceptability
3. Materials and Methods
3.1. Materials and Culture Media
3.2. Biological Materials and Microbial Strains
3.3. Oregano (Lippia graveolens Kunth) Essential Oil (OEO) Extraction
3.4. Amaranth Protein Isolate (API) Production
3.5. Mesoporous Silica Nanoparticles (MSN), Amino-Functionalized Mesoporous Silica Nanoparticles (A-MSN) and Mesoporous Silica Nanoparticles Loaded with Oregano Essential Oil (MSNO) Synthesis
3.6. Mesoporous Silica Nanoparticles Loading Capacity
3.7. Minimum Inhibitory Concentration (MIC) and Combined Minimum Inhibitory Concentration of Oregano Essential Oil and Chitosan against E. coli O157:H7
3.8. Preparation and Characterization of Film-Forming Solutions Added with Amino-Functionalized Mesoporous Silica Nanoparticles, Emulsified Oregano Essential Oil or Mesoporous Silica Nanoparticles Loades with Oregano Essential Oil, and Preparation of Active Edible Films (AEF)
3.8.1. Thickness, Mechanical Properties, and Water Vapor Permeability (WVP) of Active Edible Films
3.8.2. Color, Morphology and Topography of Active Edible Films
3.8.3. Fourier Transform Infrared Spectroscopy (FT-IR)
3.8.4. In Vitro Antimicrobial Activity of Active Edible Filsm
3.8.5. Active Edible Films Effect on Fresh Beef
3.8.6. Sensory Evaluation
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hernández-Hernández, E.; Castillo-Hernández, G.; González-Gutiérrez, C.J.; Silva-Dávila, A.J.; Gracida-Rodríguez, J.N.; García-Almendárez, B.E.; di Pierro, P.; Vázquez-Landaverde, P.; Regalado-González, C. Microbiological and physicochemical properties of meat coated with microencapsulated mexican oregano (Lippia graveolens Kunth) and basil (Ocimum basilicum L.) essential oils mixture. Coatings 2019, 9, 414. [Google Scholar] [CrossRef]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.D.L.S.; Gomide, L.A.D.M.; Ramos, E.M. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zakrys-Waliwander, P.; O’Sullivan, M.; O’Neill, E.; Kerry, J. The effects of high oxygen modified atmosphere packaging on protein oxidation of bovine M. longissimus dorsi muscle during chilled storage. Food Chem. 2012, 131, 527–532. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Singh, N.; Singh, P.; Shevkani, K.; Singh Virdi, A. Amaranth: Potential source for flour enrichment. In Flour and breads and Their Fortification in Health and Disease Prevention; Preedy, V., Watson, R., Patel, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–135. [Google Scholar]
- Tapia-Blácido, D.; do Amaral Sobral, P.; Menegalli, F. Optimization of amaranth flour films plasticized with glycerol and sorbitol by multi-response analysis. LWT 2011, 44, 1731–1738. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of chitosan for biomedical applications. In Chitosan Based Biomaterials; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Volume 1, pp. 3–20. [Google Scholar]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; DiVenere, D.; Salerno, M. Effects of pre- and postharvest chitosan treatments to control storage grey mold of table grapes. J. Food Sci. 2002, 67, 1862–1867. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Morsi, R.E.; Fathy, M. Chitosan-oregano essential oil blends use as antimicrobial packaging material. In Antimicrobial Food Packaging; Barros-Velázquez, Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 539–551. [Google Scholar]
- Ortega-Ramirez, L.A.; Rodríguez-García, I.; Silva-Espinoza, B.A.; Ayala-Zavala, J.F. Oregano (Origanum spp.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 625–631. [Google Scholar]
- Hernández-Hernández, E.; Regalado-González, C.; Vázquez-Landaverde, P.; Guerrero-Legarreta, I.; García-Almendárez, B.E. Microencapsulation, chemical characterization, and antimicrobial activity of Mexican (Lippia graveolens H.B.K.) and european (Origanum vulgare L.) oregano essential oils. Sci. World J. 2014, 2014, 641814. [Google Scholar] [CrossRef] [PubMed]
- Silva Damasceno, E.T.; Almeida, R.R.; de Carvalho SY, B.; de Carvalho GS, G.; Mano, V.; Pereira, A.C.; de Lima Guimarães, L.G. Lippia origanoides Kunth. essential oil loaded in nanogel based on the chitosan and ρ-coumaric acid: Encapsulation efficiency and antioxidant activity. Ind. Crops Prod. 2018, 125, 85–94. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Chkirida, S.; Zari, N.; Bouhfid, R.; Qaiss, A.E.K. Insight into the bionanocomposite applications on wastewater decontamination: Review. J. Water Process. Eng. 2021, 43, 102198. [Google Scholar] [CrossRef]
- Pal, N.; Lee, J.H.; Cho, E.B. Recent trends in morphology-controlled synthesis and application of mesoporous silica nanoparticles. Nanomaterials 2020, 10, 2122. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Mir, N.A.; Chandla, N.K.; Singh, S. Combined effect of pH treatment and the extraction pH on the physicochemical, functional and rheological characteristics of amaranth (Amaranthus hypochondriacus) seed protein isolates. Food Chem. 2021, 353, 129466. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Trejo, M.; Mendoza, S.; Loarca-Piña, G.; Figueroa-Cárdenas, J. Physicochemical characterization of protein isolates of amaranth and common bean and a study of their compatibility with xanthan gum. Int. J. Biol. Macromol. 2021, 166, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Gou, K.; Wang, Y.; Xie, L.; Guo, X.; Guo, Y.; Ke, J.; Wu, L.; Li, S.; Li, H. Synthesis, structural properties, biosafety and applications of chiral mesoporous silica nanostructures. Chem. Eng. J. 2021, 421, 127862. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.H.; Hung, Y.; Mou, C.Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef]
- Lin, Y.S.; Haynes, C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef]
- Di Pasqua, A.J.; Sharma, K.K.; Shi, Y.L.; Toms, B.B.; Ouellette, W.; Dabrowiak, J.C.; Asefa, T. Cytotoxicity of mesoporous silica nanomaterials. J. Inorg. Biochem. 2008, 102, 1416–1423. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Weber, F.J.; Sikkema, J.; Keweloh, H.; de Bont, J.A. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994, 12, 409–415. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.; Alberda, M.; Hoekstra, F.A.; Smid, E.J. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 2000, 174, 233–238. [Google Scholar] [CrossRef]
- Brandao Melo, A.D.; Figueiredo Amaral, A.; Schaefer, G.; Bittencourt Luciano, F.; de Andrade, C.; Batista Costa, L.; Rostagno, M.H. Antimicrobial effect against different bacterial strains and bacterial adaptation to essential oils used as feed additives. Can. J. Vet. Res. 2015, 79, 285–289. [Google Scholar]
- Goy, R.C.; Morais, S.T.; Assis, O.B. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Ngamviriyavong, P.; Thananuson, A.; Pankongadisak, P.; Tanjak, P.; Janvikul, W. Antibacterial hydrogels from chitosan derivates. J. Met. Mater. Miner. 2010, 20, 113–117. [Google Scholar]
- Rehman, F.; Ahmed, K.; Rahim, A.; Muhammad, N.; Tariq, S.; Azhar, U.; Khan, A.J.; Us Sama, Z.; Volpe, P.L.; Airoldi, C. Organo-bridged silsesquioxane incorporated mesoporous silica as a carrier for the controlled delivery of ibuprofen and fluorouracil. J. Mol. Liq. 2018, 258, 319–326. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, J.; Gou, K.; Guo, Y.; Xu, X.; Li, S.; Li, H. Amino functionalized mesoporous silica with twisted rod-like shapes: Synthetic design, in vitro and in vivo evaluation for ibuprofen delivery. Micropor. Mesopor. Mater. 2020, 294, 109896. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Z.; Wang, J.; Shen, J.; Hao, Q.; Li, Y.; Yang, J.; Niu, Y.; Xiao, Z.; Chen, L.; et al. Preparation of mesoporous silica nanoparticle with tunable pore diameters for encapsulating and slowly releasing eugenol. Chin. Chem. Lett. 2021, 32, 1755–1758. [Google Scholar] [CrossRef]
- dos Santos Paglione, I.; Galindo, M.V.; de Medeiros JA, S.; Yamashita, F.; Alvim, I.D.; Ferreira Grosso, C.R.; Sakanaka, L.S.; Shirai, M.A. Comparative study of the properties of soy protein concentrate films containing free and encapsulated oregano essential oil. Food Packag. Shelf Life. 2019, 22, 100419. [Google Scholar] [CrossRef]
- Chen, S.; Wu, M.; Wang, C.; Yan, S.; Lu, P.; Wang, S. Developed chitosan/oregano essential oil biocomposite packaging film enhanced by cellulose nanofibril. Polymers 2020, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Antibacterial and antioxidant properties of hydroxypropyl methylcellulose-based active composite films incorporating oregano essential oil nanoemulsions. LWT 2019, 106, 164–171. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef]
- López, D.N.; Galante, M.; Robson, M.; Boeris, V.; Spelzini, D. Amaranth, quinoa and chia protein isolates: Physicochemical and structural properties. Int. J. Biol. Macromol. 2018, 109, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bats, I.; di Pierro, P.; Villalonga-Santana, R.; Garcia-Almendarez, B.; Porta, R. Bioactive mesoporous silica nanocomposite films obtained from native and transglutaminase-crosslinked bitter vetch proteins. Food Hydrocoll. 2018, 82, 106–115. [Google Scholar] [CrossRef]
- Sabbah, M.; Esposito, M. Insight into zeta potential measurements in biopolymer film preparation. J. Biotechnol. Biomater. 2016, 6, 2–4. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging: A review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Moreira Gonçalves, S.; Gomes Motta, J.F.; Ribeiro-Santos, R.; Hidalgo Chávez, D.W.; Ramos De Melo, N. Functional and antimicrobial properties of cellulose acetate films incorporated with sweet fennel essential oil and plasticizers. CRFS 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, R.; León, A. Antimicrobial, mechanical and barrier properties of triticale protein films incorporated with oregano essential oil. Food Biosci. 2013, 1, 2–9. [Google Scholar] [CrossRef]
- Atarés, L.; De Jesús, C.; Talens, P.; Chiralt, A. Characterization of SPI-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 99, 384–391. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing Origanum vulgare L. essential oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.; Sobral PD, A.; Menegalli, F. Effect of drying conditions and plasticizer type on some physical and mechanical properties of amaranth flour films. LWT 2013, 50, 392–400. [Google Scholar] [CrossRef]
- Haris, P.I.; Severcan, F. FTIR spectroscopic characterization of protein structure in aqueous and non-aqueous media. J. Mol. Catal. 1999, 7, 207–221. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Fernandez-Saiz, P.; Ocio, M.J. Using ATR-FTIR spectroscopy to design active antimicrobial food packaging structures based on high molecular weight chitosan polysaccharide. J. Agric. Food Chem. 2007, 55, 2554–2562. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.; de la Caba, K. Characterization and antimicrobial analysis of chitosan-based films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Zhang, B.; Liu, J.; Luo, Z.; Cai, K. Redox-responsive nanocarrier based on Heparin end-capped mesoporous silica nanoparticles for targeted tumor therapy in vitro and in vivo. Langmuir 2014, 30, 7867–7877. [Google Scholar] [CrossRef]
- Alahmadi, S.M.; Mohamad, S.; Maah, M.J. Synthesis and characterization of mesoporous silica functionalized with Calix[4]arene derivatives. Int. J. Mol. Sci. 2012, 13, 13726–13736. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, D.; Shen, J.; Wang, Q. A review of mesoporous silica nanoparticle delivery systems in chemo-based combination cancer therapies. Front. Chem. 2020, 8, 598722. [Google Scholar] [CrossRef] [PubMed]
- Jaspal, M.H.; Ijaz, M.; Haq, H.A.U.; Yar, M.K.; Asghar, B.; Manzoor, A.; Badar, I.H.; Ullah, S.; Islam, M.S.; Hussain, J. Effect of oregano essential oil or lactic acid treatments combined with air and modified atmosphere packaging on the quality and storage properties of chicken breast meat. LWT 2021, 146, 111459. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kontominas, M.G. Modified atmosphere packaging of foods. In Encyclopedia of Food Microbiology; Robinson, R.K., Batt, C.A., Patel, P.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 1012–1016. [Google Scholar]
- Comi, G. Spoilage of Meat and Fish. In The Microbiological Quality of Food; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publ. Imprint of Elsevier: Cambridgeshire, UK; Cambridge, MA, USA; Kidlington, UK, 2017; pp. 179–210. [Google Scholar]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou MA, R.; Venkitanarayanan, K. Spoilage bacteria and meat quality. In Meat Quality Analysis; Biswas, A.K., Mandal, P.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 307–334. [Google Scholar]
- Samelis, J. Managing microbial spoilage in the meat industry. In Food Spoilage Microorganisms; Blackburn, C.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 213–286. [Google Scholar]
- Hosseini, M.; Razavi, S.; Mousavi, M. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process. Preserv. 2009, 33, 727–743. [Google Scholar] [CrossRef]
- Azarifar, M.; Ghanbarzadeh, B.; Sowti Khiabani, M.; Akhondzadeh Basti, A.; Abdulkhani, A. The effects of gelatin-CMC films incorporated with chitin nanofiber and Trachyspermum ammi essential oil on the shelf life characteristics of refrigerated raw beef. Int. J. Food Microbiol. 2020, 318, 108493. [Google Scholar] [CrossRef]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical composition, extraction sources and action mechanisms of essential oils: Natural preservative and limitations of use in meat products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Munekata, P.E.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- Norma Oficial Mexicana NOM-194-SSA1-2004; Productos y servicios. Especificaciones Sanitarias en Los Establecimientos Dedicados al Sacrificio y Faenado de Animales Para Abasto, Almacenamiento, Transporte y Expendio. Especificaciones Sanitarias de Productos. Secretaría de Salud: Ciudad de México, Mexico.
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, E.; Lira-Moreno, C.Y.; Guerrero-Legarreta, I.; Wild-Padua, G.; di Pierro, P.; García-Almendárez, B.E.; Regalado-González, C. Effect of nanoemulsified and microencapsulated Mexican oregano (Lippia graveolens Kunth) essential oil coatings on quality of fresh pork meat. J. Food Sci. 2017, 82, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Condés, M.C.; Añón, M.C.; Dufresne, A.; Mauri, A.N. Composite and nanocomposite films based on amaranth biopolymers. Food Hydrocoll. 2018, 74, 159–167. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists. Changes in AOAC® official methods of analysis. J. AOAC Int. 1995, 78, 284–288. [Google Scholar] [CrossRef]
- Bravo Cadena, M.; Preston, G.M.; van der Hoorn, R.A.; Townley, H.E.; Thompson, I.P. Species-specific antimicrobial activity of essential oils and enhancement by encapsulation in mesoporous silica nanoparticles. Ind. Crops Prod. 2018, 122, 582–590. [Google Scholar] [CrossRef]
- Ríos-de-Benito, L.F.; Escamilla-García, M.; García-Almendárez, B.; Amaro-Reyes, A.; Di Pierro, P.; Regalado-González, C. Design of an active edible coating based on sodium caseinate, chitosan and oregano essential oil reinforced with silica particles and its application on panela cheese. Coatings 2021, 11, 1212. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic compounds with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol Vitic. 1965, 16, 144–158. [Google Scholar]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- Parish, M.E.; Davidson, P.M. Antimicrobials in Foods. In Methods for evaluation, 2nd ed.; Davidson, P.M., Branen, A.L., Eds.; Marcel Dekker: New York, NY, USA, 1993; pp. 597–615. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; di Pierro, P.; Gunning, P.; Mackie, A.; Porta, R.; Mariniello, L. Characterization of citrus pectin edible films containing transglutaminase-modified phaseolin. Carbohydr. Polym. 2014, 106, 200–208. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-10; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2010.
- Porta, R.; di Pierro, P.; Sabbah, M.; Regalado-Gonzales, C.; Mariniello, L.; Kadivar, M.; Arabestani, A. Blend films of pectin and bitter vetch (Vicia ervilia) proteins: Properties and effect of transglutaminase. Innov. Food Sci. Emerg. Technol. 2016, 36, 245–251. [Google Scholar] [CrossRef]
- ASTM E96-00; Standard test methods for water vapour transmission of material, E96-00. Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2000.
- Escamilla-García, M.; Reyes-Basurto, A.; García-Almendárez, B.; Hernández-Hernández, E.; Calderón-Domínguez, G.; Rossi-Márquez, G.; Regalado-González, C. Modified starch-chitosan edible films: Physicochemical and mechanical characterization. Coatings 2017, 7, 224. [Google Scholar] [CrossRef]
- Emiroğlu, Z.K.; Yemiş, G.P.; Coşkun, B.K.; Candoğan, K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010, 86, 283–288. [Google Scholar] [CrossRef]
- Hunt, M.; King, A. AMSA Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012; pp. 35–44. [Google Scholar]


| AF Protein (%) | API Protein (%) | Yield (%) | Reference |
|---|---|---|---|
| 13.98 ± 0.17 | 61.34 ± 0.45 | 19.71 ± 2.46 | - |
| 14.6 ± 2.1 | 85.42 ± 1.26 | 12.78 | Das et al. [15] |
| - | 85.13 ± 0.01 | - | Cortez-Trejo et al. [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matadamas-Ortiz, A.; Hernández-Hernández, E.; Castaño-Tostado, E.; Amaro-Reyes, A.; García-Almendárez, B.E.; Velazquez, G.; Regalado-González, C. Long-Term Refrigerated Storage of Beef Using an Active Edible Film Reinforced with Mesoporous Silica Nanoparticles Containing Oregano Essential Oil (Lippia graveolens Kunth). Int. J. Mol. Sci. 2023, 24, 92. https://doi.org/10.3390/ijms24010092
Matadamas-Ortiz A, Hernández-Hernández E, Castaño-Tostado E, Amaro-Reyes A, García-Almendárez BE, Velazquez G, Regalado-González C. Long-Term Refrigerated Storage of Beef Using an Active Edible Film Reinforced with Mesoporous Silica Nanoparticles Containing Oregano Essential Oil (Lippia graveolens Kunth). International Journal of Molecular Sciences. 2023; 24(1):92. https://doi.org/10.3390/ijms24010092
Chicago/Turabian StyleMatadamas-Ortiz, Alexis, Elvia Hernández-Hernández, Eduardo Castaño-Tostado, Aldo Amaro-Reyes, Blanca E. García-Almendárez, Gonzalo Velazquez, and Carlos Regalado-González. 2023. "Long-Term Refrigerated Storage of Beef Using an Active Edible Film Reinforced with Mesoporous Silica Nanoparticles Containing Oregano Essential Oil (Lippia graveolens Kunth)" International Journal of Molecular Sciences 24, no. 1: 92. https://doi.org/10.3390/ijms24010092
APA StyleMatadamas-Ortiz, A., Hernández-Hernández, E., Castaño-Tostado, E., Amaro-Reyes, A., García-Almendárez, B. E., Velazquez, G., & Regalado-González, C. (2023). Long-Term Refrigerated Storage of Beef Using an Active Edible Film Reinforced with Mesoporous Silica Nanoparticles Containing Oregano Essential Oil (Lippia graveolens Kunth). International Journal of Molecular Sciences, 24(1), 92. https://doi.org/10.3390/ijms24010092








