Higher Circulating Levels of Neutrophils and Basophils Are Linked to Myopic Retinopathy
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. Circulating Immune Cells in Myopic Patients and Controls
2.3. Correlation between Immune Cell Parameters and Axial Length (AL), Myopic Diopter, Myopic Duration, Age of Myopic Onset
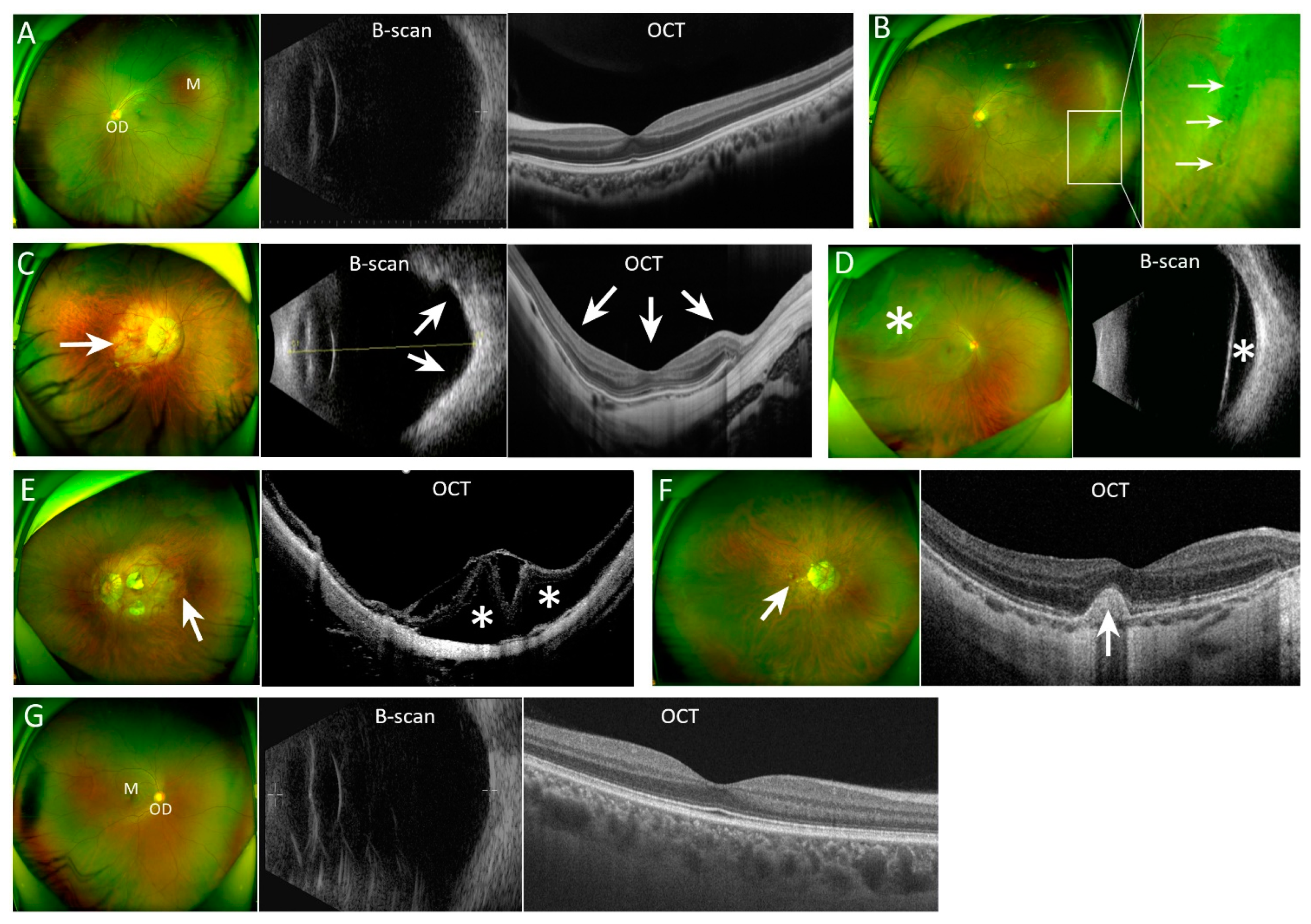
2.4. Circulating Immune Cells in Subgroups of Myopic Patients and Controls
3. Discussion
4. Materials and Methods
4.1. Clinical Data Collection
4.2. Blood Test
4.3. Ocular Information
4.4. Demographic Information
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Dong, L.; Kang, Y.; Li, Y.; Wei, W.; Jonas, J. Prevalence and time trends of myopa in children and adolescents in china:A systemic review and Meta-analysis. Retina 2020, 40, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Liu, Y.; Ma, I.; Su, C.; Lin, C.; Lin, L.; Hsiao, C.; Wang, I. Evolution of the Prevalence of Myopia among Taiwanese Schoolchildren: A Review of Survey Data from 1983 through 2017. Ophthalmology 2021, 128, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Choy, B.; You, Q.; Zhu, M.; Lai, J.; Ng, A.; Wong, I. Prevalence and associations of myopia in Hong Kong primary school students. Jpn. J. Ophthalmol. 2020, 64, 437–449. [Google Scholar] [CrossRef]
- Nakao, S.; Miyake, M.; Hosoda, Y.; Nakano, E.; Mori, Y.; Takahashi, A.; Ooto, S.; Tamura, H.; Tabara, Y.; Yamashiro, K.; et al. Myopia Prevalence and Ocular Biometry Features in a General Japanese Population: The Nagahama Study. Ophthalmology 2021, 128, 522–531. [Google Scholar] [CrossRef]
- Matsumura, S.; Lanca, C.; Htoon, H.; Brennan, N.; Tan, C.; Kathrani, B.; Chia, A.; Tan, D.; Sabanayagam, C.; Saw, S. Annual Myopia Progression and Subsequent 2-Year Myopia Progression in Singaporean Children. Transl. Vis. Sci. Technol. 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Jang, J.; Yang, H.; Hwang, J.; Park, S. Prevalence and risk factors of myopia in adult Korean population: Korea national health and nutrition examination survey 2013–2014 (KNHANES VI). PLoS ONE 2019, 14, e0211204. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.S.; Ferris, L.M.; Santamaria, J.; Mehta, A.; Musto, M.; Apsey, D.; Legault, G.L. Prevalence of Myopia in Newly Enlisted Airmen at Joint Base San Antonio. Clin. Ophthalmol. 2020, 14, 133–137. [Google Scholar] [CrossRef]
- Hopf, S.; Korb, C.; Nickels, S.; Schulz, A.; Münzel, T.; Wild, P.; Michal, M.; Schmidtmann, I.; Lackner, K.; Pfeiffer, N.; et al. Prevalence of myopic maculopathy in the German population: Results from the Gutenberg health study. Br. J. Ophthalmol. 2020, 104, 1254–1259. [Google Scholar] [CrossRef]
- Bikbov, M.; Gilmanshin, T.; Kazakbaeva, G.; Zainullin, R.; Rakhimova, E.; Rusakova, I.; Bolshakova, N.; Safiullina, K.; Zaynetdinov, A.; Zinatullin, A.; et al. Prevalence of Myopic Maculopathy Among Adults in a Russian Population. JAMA Netw. Open 2020, 3, e200567. [Google Scholar] [CrossRef]
- Holden, B.; Fricke, T.; Wilson, D.; Jong, M.; Naidoo, K.; Sankaridurg, P.; Wong, T.; Naduvilath, T.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Leveziel, N.; Marillet, S.; Dufour, Q.; Lichtwitz, O.; Bentaleb, Y.; Pelen, F.; Ingrand, P.; Bourne, R. Prevalence of macular complications related to myopia—Results of a multicenter evaluation of myopic patients in eye clinics in France. Acta Ophthalmol. 2020, 98, e245–e251. [Google Scholar] [CrossRef] [PubMed]
- Haarman, A.; Tedja, M.; Brussee, C.; Enthoven, C.; van Rijn, G.; Vingerling, J.; Keunen, J.; Boon, C.; Geerards, A.; Luyten, G.; et al. Prevalence of Myopic Macular Features in Dutch Individuals of European Ancestry with High Myopia. JAMA Ophthalmol. 2022, 140, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lam, C.; Yap, M. Prevalence of myopia-related retinal changes among 12–18 year old Hong Kong Chinese high myopes. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2013, 33, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Vongphanit, J.; Mitchell, P.; Wang, J. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology 2002, 109, 704–711. [Google Scholar] [CrossRef]
- Wei, C.C.; Kung, Y.J.; Chen, C.S.; Chang, C.Y.; Lin, C.J.; Tien, P.T.; Chang, H.Y.; Chen, H.J.; Huang, Y.S.; Lin, H.J.; et al. Allergic Conjunctivitis-induced Retinal Inflammation Promotes Myopia Progression. EBioMedicine 2018, 28, 274–286. [Google Scholar] [CrossRef]
- Lin, H.J.; Wei, C.C.; Chang, C.Y.; Chen, T.H.; Hsu, Y.A.; Hsieh, Y.C.; Chen, H.J.; Wan, L. Role of Chronic Inflammation in Myopia Progression: Clinical Evidence and Experimental Validation. EBioMedicine 2016, 10, 269–281. [Google Scholar] [CrossRef]
- Herbort, C.P.; FMER, F.; Neri, M.P.P. Myopia and Inflammation. J. Ophthalmic Vis. Res. 2011, 6, 270–283. [Google Scholar]
- Yi, Q.; Wang, Y.; Chen, L.; Li, W.; Shen, Y.; Jin, Y.; Yang, J.; Wang, Y.; Yuan, J.; Cheng, L. Implication of inflammatory cytokines in the aqueous humour for management of macular diseases. Acta Ophthalmol. 2020, 98, e309–e315. [Google Scholar] [CrossRef]
- Xue, M.; Ke, Y.; Ren, X.; Zhou, L.; Liu, J.; Zhang, X.; Shao, X.; Li, X. Proteomic analysis of aqueous humor in patients with pathologic myopia. J. Proteom. 2021, 234, 104088. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M. Immune response in retinal degenerative diseases—Time to rethink? Prog. Neurobiol. 2022, 219, 102350. [Google Scholar] [CrossRef]
- Tedja, M.; Wojciechowski, R.; Hysi, P.; Eriksson, N.; Furlotte, N.; Verhoeven, V.; Iglesias, A.; Meester-Smoor, M.; Tompson, S.; Fan, Q.; et al. Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat. Genet. 2018, 50, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, X.; Liu, J.; Liu, H.; Xu, H.; Yang, Z. RNA-Seq Analysis Reveals an Essential Role of the Tyrosine Metabolic Pathway and Inflammation in Myopia-Induced Retinal Degeneration in Guinea Pigs. Int. J. Mol. Sci. 2021, 22, 12598. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Xie, S.; Igarashi-Yokoi, T.; Watanabe, T.; Uramoto, K.; Takahashi, H.; Nakao, N.; Yoshida, T.; Fang, Y.; Ohno-Matsui, K. Continued Increase of Axial Length and Its Risk Factors in Adults With High Myopia. JAMA Ophthalmol. 2021, 139, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.; Sabanayagam, C.; Cheung, Y.; Chia, A.; Valenzuela, R.; Tan, D.; Wong, T.; Cheng, C.; Saw, S. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2016, 36, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Ye, J.; Wang, M.; Shen, M.; Huang, S.; Xue, A.; Lin, J.; Fan, Y.; Wang, J.; Lu, F.; Shao, Y. Deep Retinal Capillary Plexus Decreasing Correlated With the Outer Retinal Layer Alteration and Visual Acuity Impairment in Pathological Myopia. Investig. Ophthalmol. Vis. Sci. 2020, 61, 45. [Google Scholar] [CrossRef]
- Chen, D.; Koh, V.; Tan, M.; Tan, C.; Nah, G.; Shen, L.; Bhargava, M.; Cheng, C.; Zhao, P.; Wong, T.; et al. Peripheral retinal changes in highly myopic young Asian eyes. Acta Ophthalmol. 2018, 96, e846–e851. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Jonas, J. Posterior staphyloma in pathologic myopia. Prog. Retin. Eye Res. 2019, 70, 99–109. [Google Scholar] [CrossRef]
- Brown, D.; Mazade, R.; Clarkson-Townsend, D.; Hogan, K.; Datta Roy, P.; Pardue, M. Candidate pathways for retina to scleral signaling in refractive eye growth. Exp. Eye Res. 2022, 219, 109071. [Google Scholar] [CrossRef]
- Xu, H.; Dawson, R.; Forrester, J.; Liversidge, J. Identification of novel dendritic cell populations in normal mouse retina. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1701–1710. [Google Scholar] [CrossRef]
- Li, S.; Cao, W.; Han, J.; Tang, B.; Sun, X. The diagnostic value of white blood cell, neutrophil, neutrophil-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio in patients with primary angle closure glaucoma. Oncotarget 2017, 8, 68984–68995. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, N.; Daglioglu, M.; Ilhan, O.; Coskun, M.; Tuzcu, E.; Kahraman, H.; Keskin, U. Assessment of Neutrophil/Lymphocyte Ratio in Patients with Age-related Macular Degeneration. Ocul. Immunol. Inflamm. 2015, 23, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Obasanmi, G.; Lois, N.; Armstrong, D.; Lavery, N.; Hombrebueno, J.; Lynch, A.; Wright, D.; Chen, M.; Xu, H. Circulating Leukocyte Alterations and the Development/Progression of Diabetic Retinopathy in Type 1 Diabetic Patients—A Pilot Study. Curr. Eye Res. 2020, 45, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, P.; Rogan, M.; Greene, C.; Boxio, R.; Poiriert, T.; O’Mahony, M.; Belaaouaj, A.; O’Neill, S.; Taggart, C.; McElvaney, N. Neutrophil elastase up-regulates cathepsin B and matrix metalloprotease-2 expression. J. Immunol. 2007, 178, 5871–5878. [Google Scholar] [CrossRef]
- Binet, F.; Cagnone, G.; Crespo-Garcia, S.; Hata, M.; Neault, M.; Dejda, A.; Wilson, A.; Buscarlet, M.; Mawambo, G.; Howard, J.; et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 2020, 369, eaay5356. [Google Scholar] [CrossRef]
- Semple, J.; Italiano, J.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Cheung, C.; Arnold, J.; Holz, F.; Park, K.; Lai, T.; Larsen, M.; Mitchell, P.; Ohno-Matsui, K.; Chen, S.; Wolf, S.; et al. Myopic Choroidal Neovascularization: Review, Guidance, and Consensus Statement on Management. Ophthalmology 2017, 124, 1690–1711. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Ikuno, Y.; Lai, T.; Gemmy Cheung, C. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog. Retin. Eye Res. 2018, 63, 92–106. [Google Scholar] [CrossRef]
- Miyake, K.; Ito, J.; Karasuyama, H. Role of Basophils in a Broad Spectrum of Disorders. Front. Immunol. 2022, 13, 902494. [Google Scholar] [CrossRef]
- Karasuyama, H.; Miyake, K.; Yoshikawa, S.; Yamanishi, Y. Multifaceted roles of basophils in health and disease. J. Allergy Clin. Immunol. 2018, 142, 370–380. [Google Scholar] [CrossRef] [PubMed]
| Participant Characteristics | Control n = 129 | Myopia n = 393 | Subgroups of Myopia | |||||
|---|---|---|---|---|---|---|---|---|
| SHM n = 96 | mPRD n = 60 | PS n = 58 | mRRD n = 86 | MM n = 37 | mCNV n = 55 | |||
| Age, Median (IQR) a | 43 (33–54) | 31 (23–46)** | 24 (21–30) ** | 24 (20–30) ** | 27 (24–34) ** | 40 (31–51) | 56 (51–68) ** | 50 (36–59) * |
| Female (%) b | 58.91 | 61.73 | 69.79 | 63.33 | 79.31 ** | 36.05 ** | 70.27 | 61.82 |
| History of allergic disease (%) b | 17.05 | 15.24 | 12.00 | 13.95 | 25.00 | 17.54 | 15.79 | 8.57 |
| History of drug allergy (%) b | 1.55 | 4.42 | 1.04 | 6.66 | 0.00 | 4.69 | 10.71 * | 9.09 * |
| History of hypertension/diabetes (%) b | 20.90 | 8.90** | 0.00** | 0.00** | 0.00** | 11.60 | 32.40 | 23.60 |
| Surgical history (%) b | 27.13 | 25.20 | 15.63* | 15.00 | 22.41 | 36.20 | 43.33 | 32.73 |
| BMI (Mean ± SD) c | 23.72 ± 3.63 | 22.94 ± 3.60 | 22.64 ± 5.27 | 21.27 ± 3.86** | 20.40 ± 2.78** | 23.42 ± 3.26 | 23.45 ± 3.01 | 23.77 ± 3.29 |
| Blood Immune Cells LSM ± SE | Control n = 129 | Myopia n = 392 | p Value |
|---|---|---|---|
| WBC 109/L | 6.21 ± 0.16 | 6.07 ± 0.12 | 0.41 |
| Neutrophils (%) | 56.24 ± 0.88 | 58.76 ± 0.66 | 0.005 |
| Lymphocytes (%) | 34.85 ±0.81 | 32.91 ± 0.61 | 0.02 |
| Monocytes (%) | 5.88 ±0.15 | 5.48 ± 0.11 | 0.008 |
| Eosinophils (%) | 2.45 ±0.18 | 2.20 ±0.13 | 0.16 |
| Basophils (%) | 0.57 ±0.03 | 0.67 ± 0.03 | 0.007 |
| Neutrophils (109/L) | 3.54 ±0.13 | 3.63 ±0.10 | 0.53 |
| Lymphocytes (109/L) | 2.11 ± 0.06 | 1.94 ± 0.04 | 0.004 |
| Monocytes (109/L) | 0.37 ± 0.04 | 0.35 ±0.03 | 0.65 |
| Eosinophils (109/L) | 0.19 ± 0.01 | 0.15 ± 0.01 | 0.002 |
| Basophils (109/L) | 0.05 ±0.00 | 0.04 ± 0.00 | 0.28 |
| Platelets (109/L) | 225.70 ±5.35 | 213.50 ±4.03 | 0.02 |
| PLR | 111.29 ± 4.28 | 117.84 ±3.23 | 0.13 |
| LMR | 6.28 ±0.20 | 6.27 ± 0.15 | 0.98 |
| NLR | 1.74 ±0.11 | 2.02 ±0.08 | 0.01 |
| Blood Immune Cells | AL n = 415 | Myopic Diopter n = 482 | Myopia Duration n = 311 | Age of Myopic Onset n = 311 | ||||
|---|---|---|---|---|---|---|---|---|
| r | p a | r | p a | r | p b | r | p b | |
| WBC 109/L | 0.018 | 0.716 | 0.028 | 0.525 | −0.026 | 0.648 | −0.006 | 0.912 |
| Neutrophils (%) | 0.063 | 0.198 | −0.062 | 0.160 | −0.045 | 0.424 | 0.055 | 0.335 |
| Lymphocytes (%) | −0.065 | 0.187 | 0.057 | 0.197 | 0.027 | 0.631 | −0.015 | 0.793 |
| Monocytes (%) | −0.052 | 0.289 | 0.056 | 0.202 | −0.048 | 0.402 | −0.106 | 0.061 |
| Eosinophils (%) | 0.013 | 0.796 | 0.021 | 0.636 | 0.126 | 0.027 | −0.043 | 0.445 |
| Basophils (%) | 0.019 | 0.695 | −0.09 | 0.039 | 0.134 | 0.018 | −0.099 | 0.083 |
| Neutrophils (109/L) | 0.051 | 0.304 | −0.012 | 0.779 | −0.031 | 0.585 | 0.019 | 0.735 |
| Lymphocytes (109/L) | −0.062 | 0.208 | 0.086 | 0.049 | −0.029 | 0.606 | −0.047 | 0.408 |
| Monocytes (109/L) | −0.044 | 0.372 | 0.028 | 0.521 | −0.043 | 0.445 | −0.09 | 0.111 |
| Eosinophils (109/L) | 0.005 | 0.917 | 0.081 | 0.066 | 0.112 | 0.049 | −0.067 | 0.236 |
| Basophils (109/L) | −0.046 | 0.345 | 0.041 | 0.355 | 0.072 | 0.203 | −0.109 | 0.054 |
| Platelet (109/L) | −0.027 | 0.575 | 0.093 | 0.034 | −0.139 | 0.014 | −0.096 | 0.090 |
| PLR | 0.044 | 0.373 | −0.044 | 0.313 | −0.048 | 0.398 | −0.052 | 0.363 |
| LMR | −0.009 | 0.856 | 0.006 | 0.885 | 0.053 | 0.354 | 0.053 | 0.351 |
| NLR | 0.104 | 0.035 | −0.089 | 0.043 | −0.031 | 0.588 | 0.024 | 0.672 |
| Blood Immune Cells (LSM Difference (95% CI)) | SHM n = 96 | mPRD n = 60 | PS n = 58 | mRRD n = 86 | MM n = 37 | mCNV n = 55 |
|---|---|---|---|---|---|---|
| WBC 109/L | −0.44 (−0.91, 0.03) | −0.35 (−0.88, 0.17) | −0.46 (−0.98, 0.05) | 0.10 (−0.33, 0.53) | −0.30 (−0.90, 0.29) | 0.31 (−0.19, 0.81) |
| Neutrophils (%) | 4.58 (2.08, 7.08) ** | 5.38 (2.58, 8.18) ** | 5.80 (3.05, 8.55) ** | 1.19 (−1.10, 3.49) | −0.95 (−4.14, 2.25) | 1.15 (−1.52, 3.82) |
| Lymphocytes (%) | −3.26 (−5.59, −0.92) ** | −4.17 (−6.78, −1.56) ** | −4.40 (−6.97, −1.83) ** | −0.98 (−3.13, 1.16) | 0.57 (−2.42, 3.55) | −0.96 (−3.46, 1.53) |
| Monocytes (%) | −0.51 (−0.94, −0.09) * | −0.69 (−1.17, −0.21) ** | −0.61 (−1.08, −0.13) * | −0.35 (−0.74, 0.05) | 0.05 (−0.50, 0.59) | −0.38 (−0.84, 0.07) |
| Eosinophils (%) | −0.76 (−1.26, −0.25) ** | −0.67 (−1.24, −0.11) * | −0.88 (−1.43, −0.32) ** | 0.09 (−0.37, 0.55) | 0.21 (−0.43, 0.86) | 0.08 (−0.46, 0.62) |
| Basophils (%) | 0.08 (−0.02, 0.18) | 0.10 (−0.01, 0.21) | 0.09 (−0.01, 0.20) | 0.04 (−0.05, 0.13) | 0.08 (−0.04, 0.21) | 0.18 (0.07, 0.28) ** |
| Neutrophils (109/L) | 0.02 (−0.36, 0.41) | 0.13 (−0.30, 0.55) | 0.11 (−0.31, 0.53) | 0.14 (−0.21, 0.49) | −0.23 (−0.72, 0.26) | 0.27 (−0.14, 0.68) |
| Lymphocytes (109/L) | −0.35 (−0.52, −0.19) ** | −0.36 (−0.55, −0.18) ** | −0.44 (−0.62, −0.25) ** | −0.03 (−0.18, 0.12) | −0.07 (−0.28, 0.14) | 0.03 (−0.15, 0.20) |
| Monocytes (109/L) | 0.02 (−0.11, 0.15) | −0.08 (−0.22, 0.07) | −0.08 (−0.22, 0.06) | −0.02 (−0.14, 0.10) | −0.00 (−0.17, 0.16) | −0.01 (−0.14, 0.13) |
| Eosinophils (109/L) | −0.08 (−0.12, −0.04) ** | −0.07 (−0.12, −0.03) ** | −0.08 (−0.13, −0.04) ** | −0.02 (−0.06, 0.02) | −0.03 (−0.08, 0.02) | −0.01 (−0.06, 0.03) |
| Basophils (109/L) | −0.00 (−0.02, 0.01) | −0.00 (−0.02, 0.01) | −0.00 (−0.02, 0.01) | −0.01 (−0.02, 0.00) | −0.01 (−0.02, 0.01) | 0.00 (−0.01, 0.01) |
| Platelets (109/L) | −5.62 (−21.03, 9.79) | −18.58 (−35.84, −1.32) * | −18.46 (−35.43, −1.49) * | −17.27 (−31.44, −3.09) * | −5.24 (−24.96, 14.48) | −9.59 (−26.07, 6.89) |
| PLR | 16.39 (4.10, 28.68) ** | 10.14 (−3.62, 23.91) | 15.54 (2.01, 29.07) * | −0.51 (−11.81, 10.79) | 1.97 (−13.76, 17.69) | 2.04 (−11.10, 15.19) |
| LMR | −0.21 (−0.79, 0.38) | −0.26 (−0.91, 0.40) | −0.21 (−0.85, 0.43) | 0.22 (−0.32, 0.75) | −0.04 (−0.79, 0.71) | 0.22 (−0.41, 0.84) |
| NLR | 0.34 (0.03, 0.65) * | 0.44 (0.10, 0.79) * | 0.48 (0.14, 0.82) ** | 0.29 (0.01, 0.58) * | −0.06 (−0.46, 0.33) | 0.22 (−0.11, 0.55) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Pan, W.; Peng, T.; Zeng, L.; Li, X.; Chen, Z.; Yang, Z.; Xu, H. Higher Circulating Levels of Neutrophils and Basophils Are Linked to Myopic Retinopathy. Int. J. Mol. Sci. 2023, 24, 80. https://doi.org/10.3390/ijms24010080
Qi J, Pan W, Peng T, Zeng L, Li X, Chen Z, Yang Z, Xu H. Higher Circulating Levels of Neutrophils and Basophils Are Linked to Myopic Retinopathy. International Journal of Molecular Sciences. 2023; 24(1):80. https://doi.org/10.3390/ijms24010080
Chicago/Turabian StyleQi, Jinyan, Wei Pan, Ting Peng, Ling Zeng, Xiaoning Li, Zhongping Chen, Zhikuan Yang, and Heping Xu. 2023. "Higher Circulating Levels of Neutrophils and Basophils Are Linked to Myopic Retinopathy" International Journal of Molecular Sciences 24, no. 1: 80. https://doi.org/10.3390/ijms24010080
APA StyleQi, J., Pan, W., Peng, T., Zeng, L., Li, X., Chen, Z., Yang, Z., & Xu, H. (2023). Higher Circulating Levels of Neutrophils and Basophils Are Linked to Myopic Retinopathy. International Journal of Molecular Sciences, 24(1), 80. https://doi.org/10.3390/ijms24010080








