Blood Plasma Proteome: A Meta-Analysis of the Results of Protein Quantification in Human Blood by Targeted Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
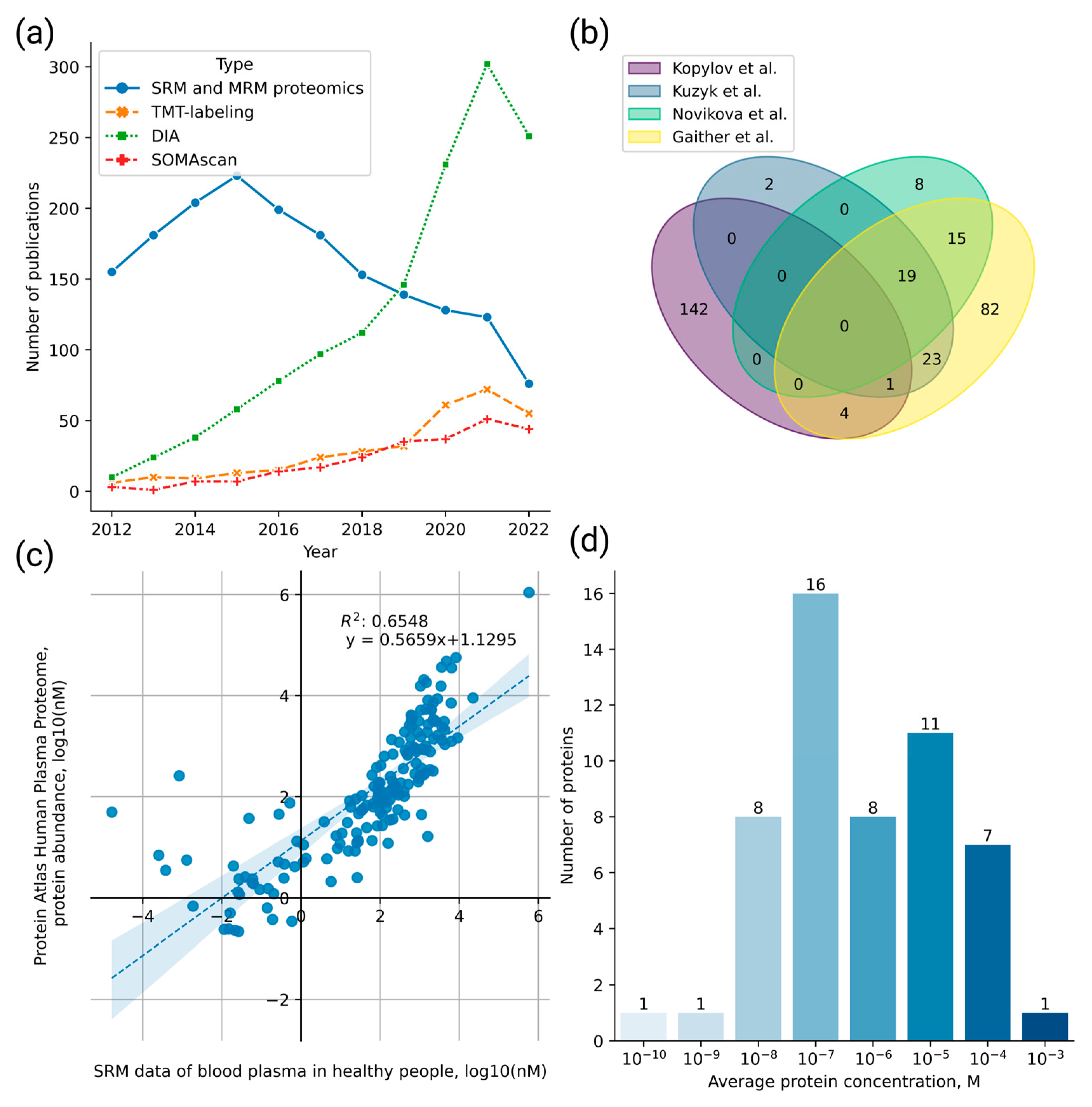
2.1. Meta-Analysis of the SRM Data for Plasma from Healthy Volunteers
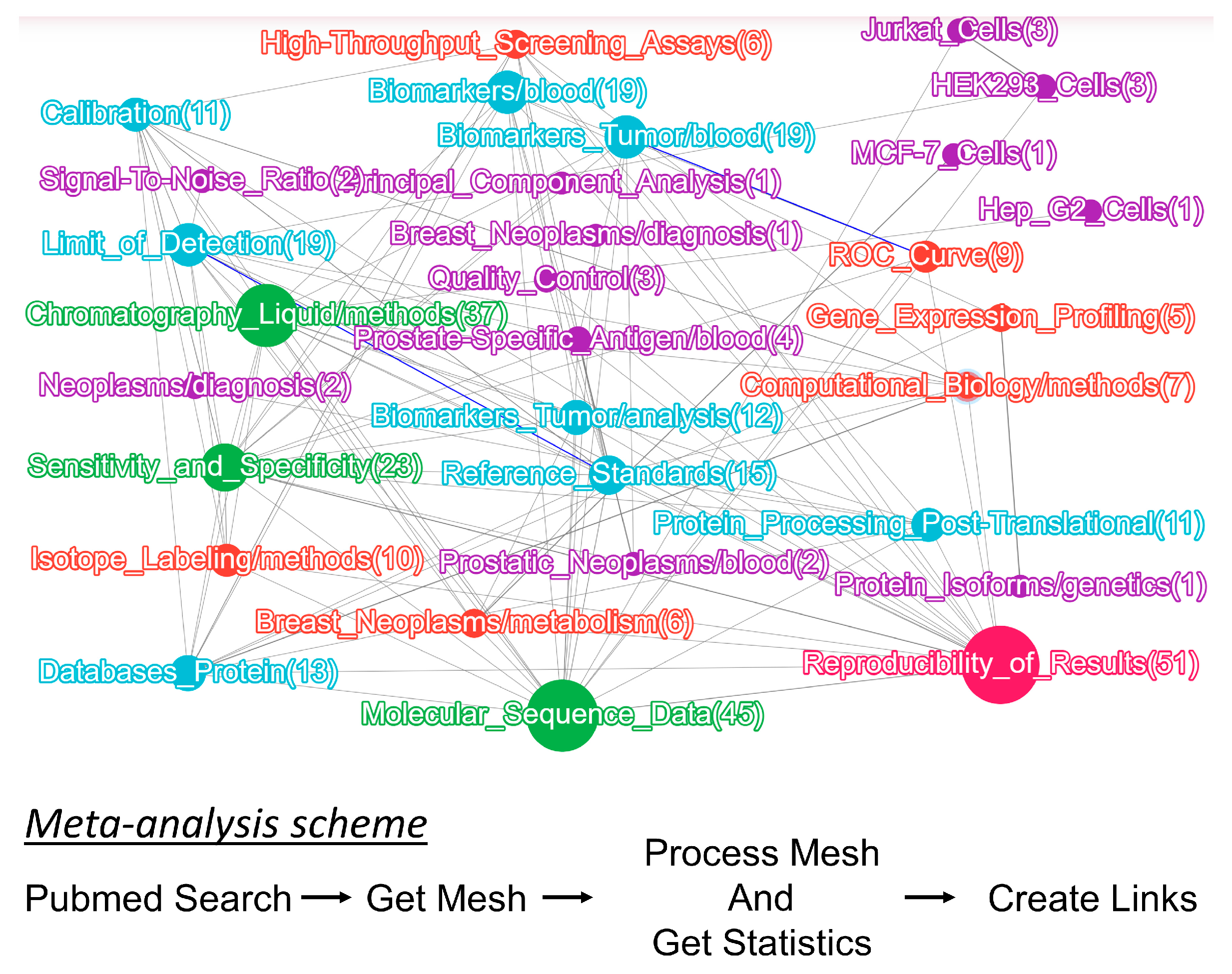
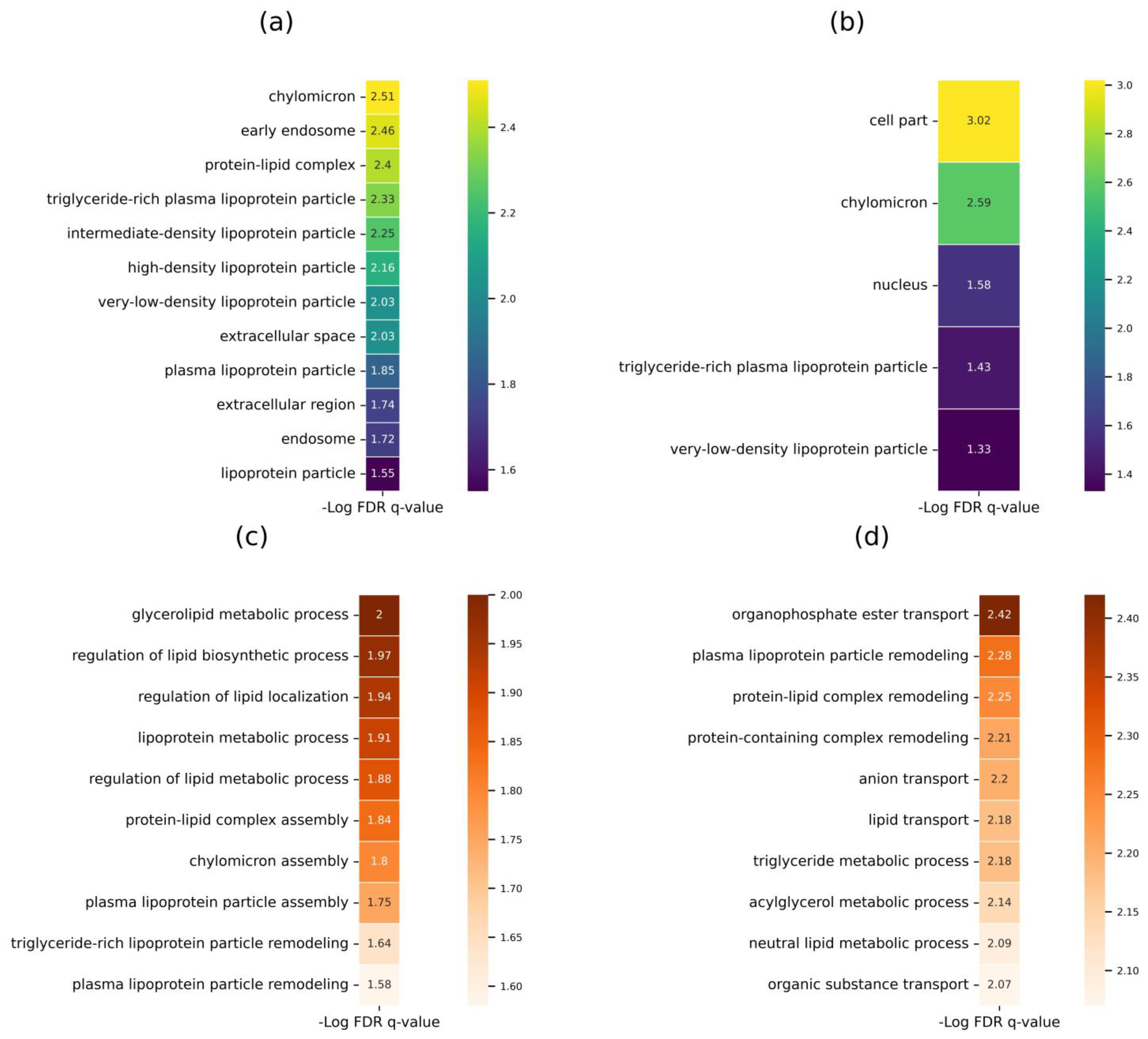
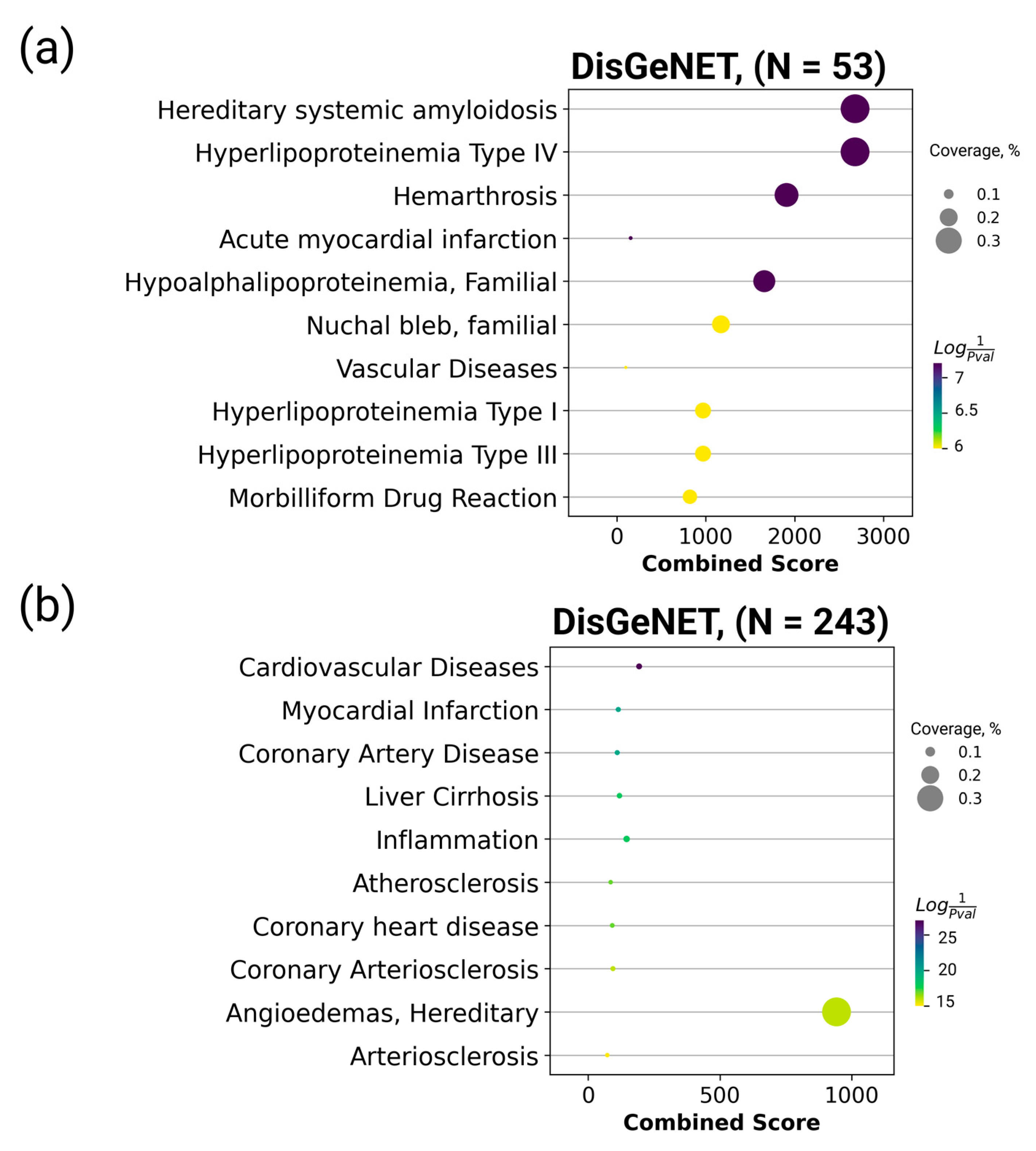
2.2. Panel for Monitoring Human Health Status and Potential Biomarker Selection
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skates, S.J.; Gillette, M.A.; LaBaer, J.; Carr, S.A.; Anderson, L.; Liebler, D.C.; Ransohoff, D.; Rifai, N.; Kondratovich, M.; Težak, Ž.; et al. Statistical design for biospecimen cohort size in proteomics-based biomarker discovery and verification studies. J. Proteome Res. 2013, 12, 5383–5394. [Google Scholar] [CrossRef] [PubMed]
- Candia, J.; Cheung, F.; Kotliarov, Y.; Fantoni, G.; Sellers, B.; Griesman, T.; Huang, J.; Stuccio, S.; Zingone, A.; Ryan, B.M.; et al. Assessment of variability in the somascan assay. Sci. Rep. 2017, 7, 14248. [Google Scholar] [CrossRef]
- Cui, M.; Cheng, C.; Zhang, L. High-throughput proteomics: A methodological mini-review. Lab. Investig. 2022. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.L.; Anderson, N.G.; Pearson, T.W.; Borchers, C.H.; Paulovich, A.G.; Patterson, S.D.; Gillette, M.; Aebersold, R.; Carr, S.A. A human proteome detection and quantitation project. Mol. Cell Proteom. 2009, 8, 883–886. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Eng, J.K.; Zhang, H.; King, N.L.; Nesvizhskii, A.I.; Lin, B.; Lee, H.; Yi, E.C.; Ossola, R.; Aebersold, R. Human Plasma PeptideAtlas. Proteomics 2005, 5, 3497–3500. [Google Scholar] [CrossRef] [PubMed]
- Farrah, T.; Deutsch, E.W.; Omenn, G.S.; Campbell, D.S.; Sun, Z.; Bletz, J.A.; Mallick, P.; Katz, J.E.; Malmström, J.; Ossola, R.; et al. A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol. Cell Proteom. 2011, 10, M110.006353. [Google Scholar] [CrossRef] [PubMed]
- Archakov, A.; Lisitsa, A.; Ponomarenko, E.; Zgoda, V. Recent advances in proteomic profiling of human blood: Clinical scope. Expert Rev. Proteom. 2015, 12, 111–113. [Google Scholar] [CrossRef][Green Version]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/humanproteome/blood+protein/proteins+detected+in+ms (accessed on 17 October 2022).
- Picotti, P.; Rinner, O.; Stallmach, R.; Dautel, F.; Farrah, T.; Domon, B.; Wenschuh, H.; Aebersold, R. High-throughput generation of selected reaction-monitoring assays for proteins and proteomes. Nat. Methods 2010, 7, 43–46. [Google Scholar] [CrossRef]
- Kopylov, A.T.; Zgoda, V.G.; Lisitsa, A.V.; Archakov, A.I. Combined use of irreversible binding and MRM technology for low- and ultralow copy-number protein detection and quantitation. Proteomics 2013, 13, 727–742. [Google Scholar] [CrossRef]
- Vavilov, N.E.; Zgoda, V.G.; Tikhonova, O.V.; Farafonova, T.E.; Shushkova, N.A.; Novikova, S.E.; Yarygin, K.N.; Radko, S.P.; Ilgisonis, E.V.; Ponomarenko, E.A.; et al. Proteomic analysis of chr 18 proteins using 2D fractionation. J. Proteome Res. 2020, 19, 4901–4906. [Google Scholar] [CrossRef]
- Vavilov, N.E.; Ilgisonis, E.V.; Lisitsa, A.V.; Ponomarenko, E.A.; Farafonova, T.E.; Tikhonova, O.V.; Zgoda, V.G.; Archakov, A.I. Deep proteomic dataset of human liver samples obtained by two-dimensional sample fractionation coupled with tandem mass spectrometry. Data Brief 2022, 42, 108055. [Google Scholar] [CrossRef] [PubMed]
- Vavilov, N.; Ilgisonis, E.; Lisitsa, A.; Ponomarenko, E.; Farafonova, T.; Tikhonova, O.; Zgoda, V.; Archakov, A. Number of detected proteins as the function of the sensitivity of proteomic technology in human liver cells. Curr. Protein Pept. Sci. 2022. [Google Scholar] [CrossRef]
- Hüttenhain, R.; Soste, M.; Selevsek, N.; Röst, H.; Sethi, A.; Carapito, C.; Farrah, T.; Deutsch, E.W.; Kusebauch, U.; Moritz, R.L.; et al. Reproducible quantification of cancer-associated proteins in body fluids using targeted proteomics. Sci. Transl. Med. 2012, 4, 142ra94. [Google Scholar] [CrossRef] [PubMed]
- Domanski, D.; Percy, A.J.; Yang, J.; Chambers, A.G.; Hill, J.S.; Freue, G.V.C.; Borchers, C.H. MRM-based multiplexed quantitation of 67 putative cardiovascular disease biomarkers in human plasma. Proteomics 2012, 12, 1222–1243. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Girod, M.; Fonbonne, C.; Salvador, A.; Clément, Y.; Lantéri, P.; Amouyel, P.; Lambert, J.C.; Lemoine, J. Total ApoE and ApoE4 isoform assays in an Alzheimer’s disease case-control study by targeted mass spectrometry (n = 669): A pilot assay for methionine-containing proteotypic peptides. Mol. Cell Proteom. 2012, 11, 1389–1403. [Google Scholar] [CrossRef] [PubMed]
- Kliuchnikova, A.A.; Goncharov, A.O.; Levitsky, L.I.; Pyatnitskiy, M.A.; Novikova, S.E.; Kuznetsova, K.G.; Ivanov, M.V.; Ilina, I.Y.; Farafonova, T.E.; Zgoda, V.G.; et al. Proteome-Wide Analysis of ADAR-Mediated Messenger RNA Editing during Fruit Fly Ontogeny. J. Proteome Res. 2020, 19, 4046–4060. [Google Scholar] [CrossRef] [PubMed]
- Gaither, C.; Popp, R.; Mohammed, Y.; Borchers, C.H. Determination of the concentration range for 267 proteins from 21 lots of commercial human plasma using highly multiplexed multiple reaction monitoring mass spectrometry. Analyst 2020, 145, 3634–3644. [Google Scholar] [CrossRef] [PubMed]
- Balashova, E.E.; Lokhov, P.G.; Ponomarenko, E.A.; Markin, S.S.; Lisitsa, A.V.; Archakov, A.I. Metabolomic diagnostics and human digital image. Per. Med. 2019, 16, 133–144. [Google Scholar] [CrossRef]
- Geyer, P.E.; Voytik, E.; Treit, P.V.; Doll, S.; Kleinhempel, A.; Niu, L.; Müller, J.B.; Buchholtz, M.-L.; Bader, J.M.; Teupser, D.; et al. Plasma Proteome Profiling to detect and avoid sample-related biases in biomarker studies. EMBO Mol. Med. 2019, 11, e10427. [Google Scholar] [CrossRef]
- Tarbeeva, S.; Lyamtseva, E.; Lisitsa, A.; Kozlova, A.; Ponomarenko, E.; Ilgisonis, E. Scanbious: Survey for obesity genes using pubmed abstracts and disgenet. J. Pers. Med. 2021, 11, 246. [Google Scholar] [CrossRef]
- Kumar, A.; Gangadharan, B.; Zitzmann, N. Multiple reaction monitoring and multiple reaction monitoring cubed based assays for the quantitation of apolipoprotein F. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1033–1034, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Teng, Y.; Chen, K.; Liu, S.; Wei, C.; Wang, B.; Yuan, G.; Zhang, R.; Liu, X.; Guo, R. Determination of salbutamol in human plasma and urine using liquid chromatography coupled to tandem mass spectrometry and its pharmacokinetic study. Biomed. Chromatogr. 2012, 26, 1176–1182. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, J.; Yu, J.; Wu, X.; Shi, Y.; Tsai, C.-Y. A liquid chromatography-tandem mass spectrometry assay for the determination of nemonoxacin (TG-873870), a novel nonfluorinated quinolone, in human plasma and urine and its application to a single-dose pharmacokinetic study in healthy Chinese volunteers. Biomed. Chromatogr. 2012, 26, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.S.; Sharma, N.; Patel, M.C.; Patel, B.N.; Shrivastav, P.S.; Sanyal, M. Application of a rapid and sensitive liquid chromatography-tandem mass spectrometry method for determination of bumetanide in human plasma for a bioequivalence study. J. Pharm. Biomed. Anal. 2012, 66, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Yadav, M.; Winter, S.; Guttikar, S.; Patel, D.; Mills, M.; Shrivastav, P.S. Enantiomeric separation of verapamil and its active metabolite, norverapamil, and simultaneous quantification in human plasma by LC-ESI-MS-MS. J. Chromatogr. Sci. 2012, 50, 839–848. [Google Scholar] [CrossRef]
- Archakov, A.I.; Aseev, A.L.; Bykov, V.A.; Grigoriev, A.I.; Govorun, V.M.; Ilgisonis, E.V.; Ivanov, Y.D.; Ivanov, V.T.; Kiseleva, O.I.; Kopylov, A.T.; et al. Challenges of the Human Proteome Project: 10-Year Experience of the Russian Consortium. J. Proteome Res. 2019, 18, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson-Bunch, C.; Sanford, J.A.; Hansen, J.R.; Gritsenko, M.A.; Rodland, K.D.; Piehowski, P.D.; Qian, W.-J.; Adkins, J.N. Assessment of TMT Labeling Efficiency in Large-Scale Quantitative Proteomics: The Critical Effect of Sample pH. ACS Omega 2021, 6, 12660–12666. [Google Scholar] [CrossRef] [PubMed]
- Stopfer, L.E.; Mesfin, J.M.; Joughin, B.A.; Lauffenburger, D.A.; White, F.M. Multiplexed relative and absolute quantitative immunopeptidomics reveals MHC I repertoire alterations induced by CDK4/6 inhibition. Nat. Commun. 2020, 11, 2760. [Google Scholar] [CrossRef]
- Joshi, A.; Mayr, M. In Aptamers They Trust: The Caveats of the SOMAscan Biomarker Discovery Platform from SomaLogic. Circulation 2018, 138, 2482–2485. [Google Scholar] [CrossRef]
- Brown, L.M. Quantitative shotgun proteomics with data-independent acquisition and traveling wave ion mobility spectrometry: A versatile tool in the life sciences. Adv. Exp. Med. Biol. 2014, 806, 79–91. [Google Scholar] [CrossRef]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef] [PubMed]
- Novikova, S.E.; Farafonova, T.E.; Tikhonova, O.V.; Shushkova, N.A.; Pyatnitsky, M.A.; Zgoda, V.G.; Ponomarenko, E.A.; Lisitsa, A.V.; Grigoryev, A.I.; Tutelyan, V.A.; et al. Mass-spectrometric MRM analysis of FDA-verified proteins in the blood plasma of healthy volunteers. Biomed. Khim. 2020, 66, 294–316. [Google Scholar] [CrossRef] [PubMed]
- Candia, J.; Daya, G.N.; Tanaka, T.; Ferrucci, L.; Walker, K.A. Assessment of variability in the plasma 7k SomaScan proteomics assay. Sci Rep. 2022, 12, 17147. [Google Scholar] [CrossRef]
- Kopylov, A.T.; Ponomarenko, E.A.; Ilgisonis, E.V.; Pyatnitskiy, M.A.; Lisitsa, A.V.; Poverennaya, E.V.; Kiseleva, O.I.; Farafonova, T.E.; Tikhonova, O.V.; Zavialova, M.G.; et al. 200+ protein concentrations in healthy human blood plasma: Targeted quantitative SRM SIS screening of chromosomes 18, 13, Y, and the mitochondrial chromosome encoded proteome. J. Proteome Res. 2019, 18, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Raffield, L.M.; Dang, H.; Pratte, K.A.; Jacobson, S.; Gillenwater, L.A.; Ampleford, E.; Barjaktarevic, I.; Basta, P.; Clish, C.B.; Comellas, A.P.; et al. NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium. Comparison of Proteomic Assessment Methods in Multiple Cohort Studies. Proteomics. 2020, 20, e1900278. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.V.; Bubis, J.A.; Gorshkov, V.; Tarasova, I.A.; Levitsky, L.I.; Lobas, A.A.; Solovyeva, E.M.; Pridatchenko, M.L.; Kjeldsen, F.; Gorshkov, M.V. DirectMS1: MS/MS-Free Identification of 1000 Proteins of Cellular Proteomes in 5 Minutes. Anal Chem. 2020, 92, 4326–4333. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, A.; Kopylov, A.; Lisitsa, A.; Archakov, A. Manual method of visually identifying candidate signals for a targeted peptide. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1083, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, M.A.; Smith, D.; Yang, J.; Cross, T.J.; Jackson, A.M.; Hardie, D.B.; Anderson, N.L.; Borchers, C.H. Multiple reaction monitoring-based, multiplexed, absolute quantitation of 45 proteins in human plasma. Mol. Cell Proteomics 2009, 8, 1860–1877. [Google Scholar] [CrossRef]
- Piñero, J.; Saüch, J.; Sanz, F.; Furlong, L.I. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 2021, 19, 2960–2967. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Kiseleva, O.I.; Romashova, Y.A.; Moskaleva, N.E.; Petushkova, N.A.; Teryaeva, N.B.; Belyaev, A.Y.; Lisitsa, A.V. Plasma preparation to measure FDA-approved protein markers by selected reaction monitoring. Clin. Transl. Med. 2015, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.; Pan, Y.-Y.; Kopylov, A.T.; Zgoda, V.; Ma, M.-C.; Wang, C.-H.; Su, W.-C.; Lai, W.-W.; Cheng, P.-N.; Liao, P.-C. Assessment of Serological Early Biomarker Candidates for Lung Adenocarcinoma by using Multiple Reaction Monitoring-Mass Spectrometry. Proteomics Clin. Appl. 2020, 14, e1900095. [Google Scholar] [CrossRef] [PubMed]
- Arandjelovic, S.; Dragojlovic, N.; Li, X.; Myers, R.R.; Campana, W.M.; Gonias, S.L. A derivative of the plasma protease inhibitor alpha(2)-macroglobulin regulates the response to peripheral nerve injury. J. Neurochem. 2007, 103, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.R.; Varma, S.; An, Y.; Hohman, T.J.; Seddighi, S.; Casanova, R.; Beri, A.; Dammer, E.B.; Seyfried, N.T.; Pletnikova, O.; et al. Alpha-2 macroglobulin in Alzheimer’s disease: A marker of neuronal injury through the RCAN1 pathway. Mol. Psychiatry 2017, 22, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Texel, S.J.; Xu, X.; Harris, Z.L. Ceruloplasmin in neurodegenerative diseases. Biochem. Soc. Trans. 2008, 36, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Vassiliev, V.; Harris, Z.L.; Zatta, P. Ceruloplasmin in neurodegenerative diseases. Brain Res. Brain Res. Rev. 2005, 49, 633–640. [Google Scholar] [CrossRef]
- Yang, J.; Tan, D.; Asch, H.L.; Swede, H.; Bepler, G.; Geradts, J.; Moysich, K.B. Prognostic significance of gelsolin expression level and variability in non-small cell lung cancer. Lung Cancer 2004, 46, 29–42. [Google Scholar] [CrossRef]
- Ajona, D.; Castaño, Z.; Garayoa, M.; Zudaire, E.; Pajares, M.J.; Martinez, A.; Cuttitta, F.; Montuenga, L.M.; Pio, R. Expression of complement factor H by lung cancer cells: Effects on the activation of the alternative pathway of complement. Cancer Res. 2004, 64, 6310–6318. [Google Scholar] [CrossRef]
- Cui, T.; Chen, Y.; Knösel, T.; Yang, L.; Zöller, K.; Galler, K.; Berndt, A.; Mihlan, M.; Zipfel, P.F.; Petersen, I. Human complement factor H is a novel diagnostic marker for lung adenocarcinoma. Int. J. Oncol. 2011, 39, 161–168. [Google Scholar] [CrossRef]
- Eden, E.; Navon, R.; Steinfeld, I.; Lipson, D.; Yakhini, Z. GOrilla: A tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinform. 2009, 10, 48. [Google Scholar] [CrossRef]
| Method Feature | SOMAscan | SRM |
|---|---|---|
| Volume of crude serum/plasma, µL | 15–50 [2,32] | 2.5–5 [33] |
| Sample preparation | Not needed | Tryptic digestion |
| Special reagent | DNA-based aptamers | SIS peptides |
| Multiplexity of protein analytes | 813–7288 [2,32,34] | 111–329 [33,35] |
| Quantification type | Relative [36] | Absolute |
| Direct/indirect protein quantification | DNA serves as an intermediary | Peptide amino acid sequence serves as an intermediary |
| Sensitivity | 10−14–10−3 [32] | 10−16–10−3 [34,35] |
| Dynamic range in serum/plasma, orders of magnitude | 8–10 [34] | 4–8 [34,35] |
| High throughput array multiplexing | Yes | No |
| Structure analysis, analysis of SAP, isoforms, etc. | No | Yes [17] |
| Protein Name | Gene Name | Log 10 (Average Concentration, fM, M × 10−15) | ||
|---|---|---|---|---|
| Healthy Human Plasma | Neurological Diseases (n = 19, Kiseleva et al., Clin Trans Med, 2015) * | Lung Adenocarcinoma (n = 102, Wu et al., Proteomics Clin Appl, 2020) | ||
| Alpha-1-antitrypsin | A1AT | 10.0 | 9.5 | |
| Alpha-2-macroglobulin | A2MG | 10.2 | 8.5 | |
| Apolipoprotein A-I | APOA1 | 10.6 | 9.1 | |
| Ceruloplasmin | CERU | 9.1 | 6.5 | |
| Complement C3 | CO3 | 9.2 | 8.4 | |
| Cystatin-C | CST3 | 7.7 | 7 | |
| Fibrinogen alpha chain | FIBA | 10.3 | 8.9 | |
| Haptoglobin | HPT | 9.9 | 9 | |
| Hemopexin | HEMO | 9.4 | 8.3 | |
| Insulin-like growth factor-binding protein 3 | IGFBP3 | 7.4 | 7 | |
| Plasma protease C1 inhibitor | IC1 | 8.6 | 7.5 | |
| Platelet basic protein | CXCL7 | 8.0 | 6.8 | |
| Serotransferrin | TRFE | 10.3 | 9.3 | |
| Serum albumin * | ALB | 11.8 | 10 | |
| Transthyretin | TTR | 9.9 | 8.5 | |
| von Willebrand factor | VWF | 7.8 | 7 | |
| Complement factor H | CFH | 9.0 | 5.8 | |
| Desmoglein-2 | DSG2 | 7.0 | 6.4 | |
| Gelsolin, isoform 1 | GSN | 9.2 | 6.8 | |
| Lambda-crystallin homolog | CRYL1 | 6.6 | 6.2 | |
| Lumican | LUM | 8.5 | 6.7 | |
| Mucin-16 | MUC16 | 7.7 | 5.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kliuchnikova, A.A.; Novikova, S.E.; Ilgisonis, E.V.; Kiseleva, O.I.; Poverennaya, E.V.; Zgoda, V.G.; Moshkovskii, S.A.; Poroikov, V.V.; Lisitsa, A.V.; Archakov, A.I.; et al. Blood Plasma Proteome: A Meta-Analysis of the Results of Protein Quantification in Human Blood by Targeted Mass Spectrometry. Int. J. Mol. Sci. 2023, 24, 769. https://doi.org/10.3390/ijms24010769
Kliuchnikova AA, Novikova SE, Ilgisonis EV, Kiseleva OI, Poverennaya EV, Zgoda VG, Moshkovskii SA, Poroikov VV, Lisitsa AV, Archakov AI, et al. Blood Plasma Proteome: A Meta-Analysis of the Results of Protein Quantification in Human Blood by Targeted Mass Spectrometry. International Journal of Molecular Sciences. 2023; 24(1):769. https://doi.org/10.3390/ijms24010769
Chicago/Turabian StyleKliuchnikova, Anna A., Svetlana E. Novikova, Ekaterina V. Ilgisonis, Olga I. Kiseleva, Ekaterina V. Poverennaya, Victor G. Zgoda, Sergei A. Moshkovskii, Vladimir V. Poroikov, Andrey V. Lisitsa, Alexander I. Archakov, and et al. 2023. "Blood Plasma Proteome: A Meta-Analysis of the Results of Protein Quantification in Human Blood by Targeted Mass Spectrometry" International Journal of Molecular Sciences 24, no. 1: 769. https://doi.org/10.3390/ijms24010769
APA StyleKliuchnikova, A. A., Novikova, S. E., Ilgisonis, E. V., Kiseleva, O. I., Poverennaya, E. V., Zgoda, V. G., Moshkovskii, S. A., Poroikov, V. V., Lisitsa, A. V., Archakov, A. I., & Ponomarenko, E. A. (2023). Blood Plasma Proteome: A Meta-Analysis of the Results of Protein Quantification in Human Blood by Targeted Mass Spectrometry. International Journal of Molecular Sciences, 24(1), 769. https://doi.org/10.3390/ijms24010769









