Genetic Mapping of Behavioral Traits Using the Collaborative Cross Resource
Abstract
1. Introduction
2. Results
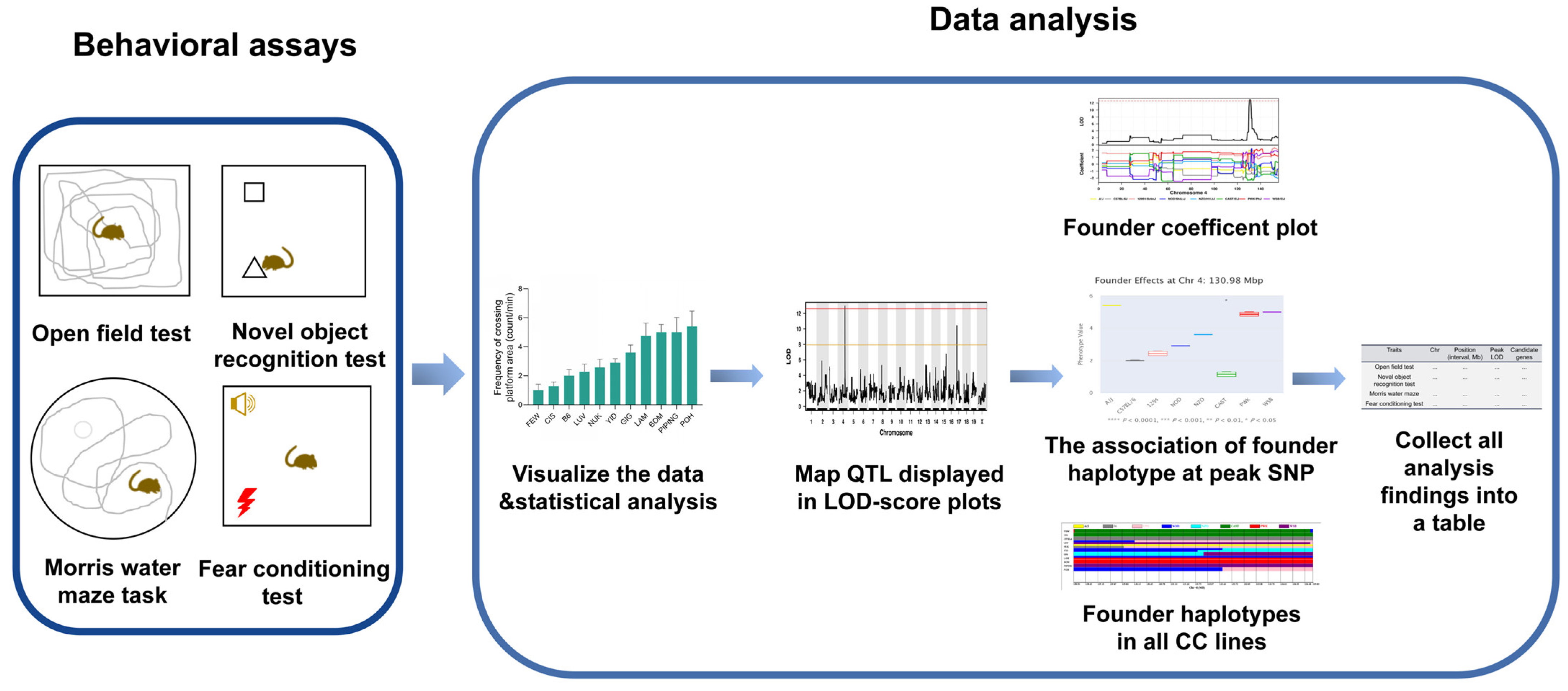
2.1. CC Behavioral Study Workflow
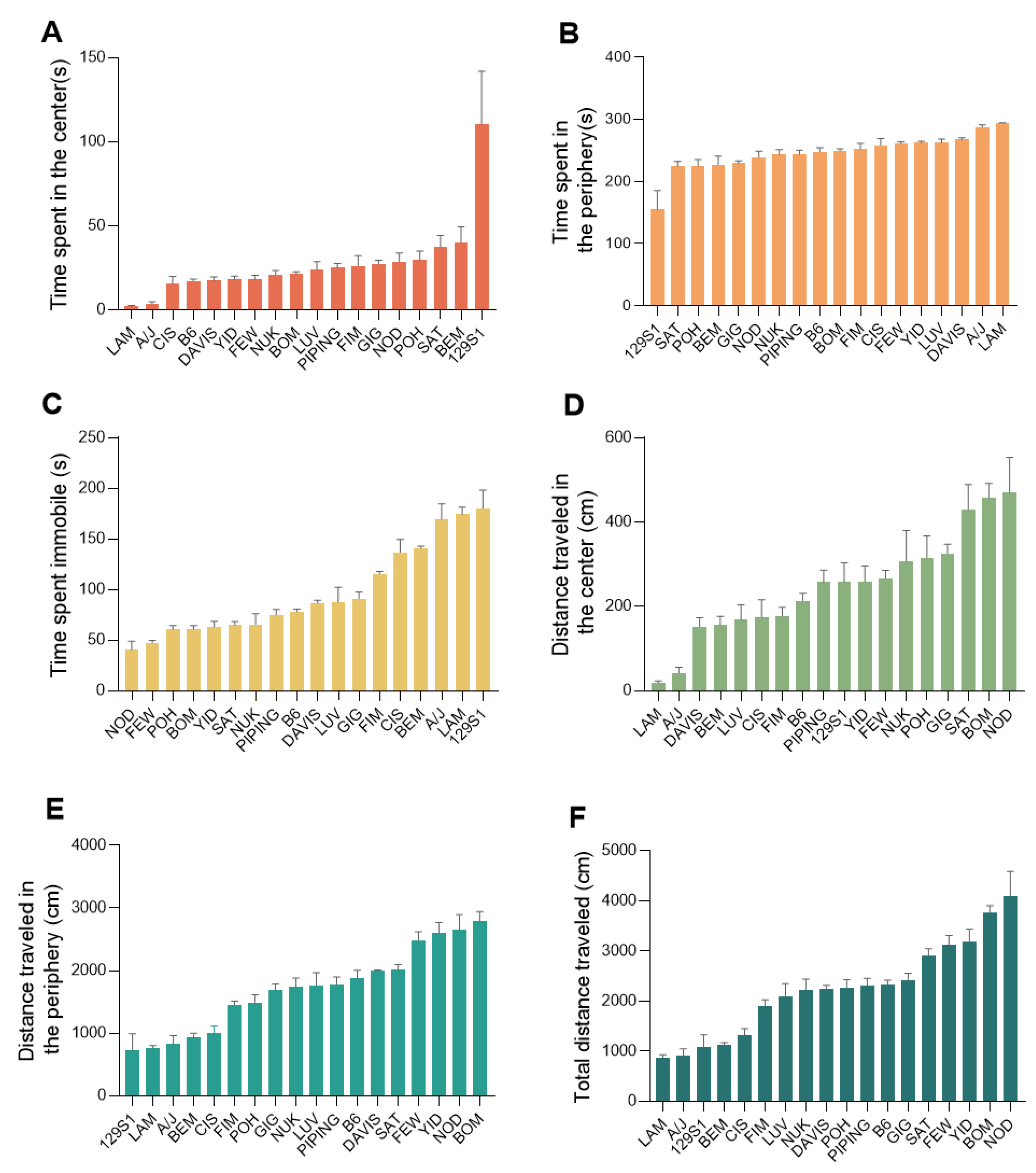
2.2. Open Field Test Displays a High Phenotypic Diversity across the CC Strains
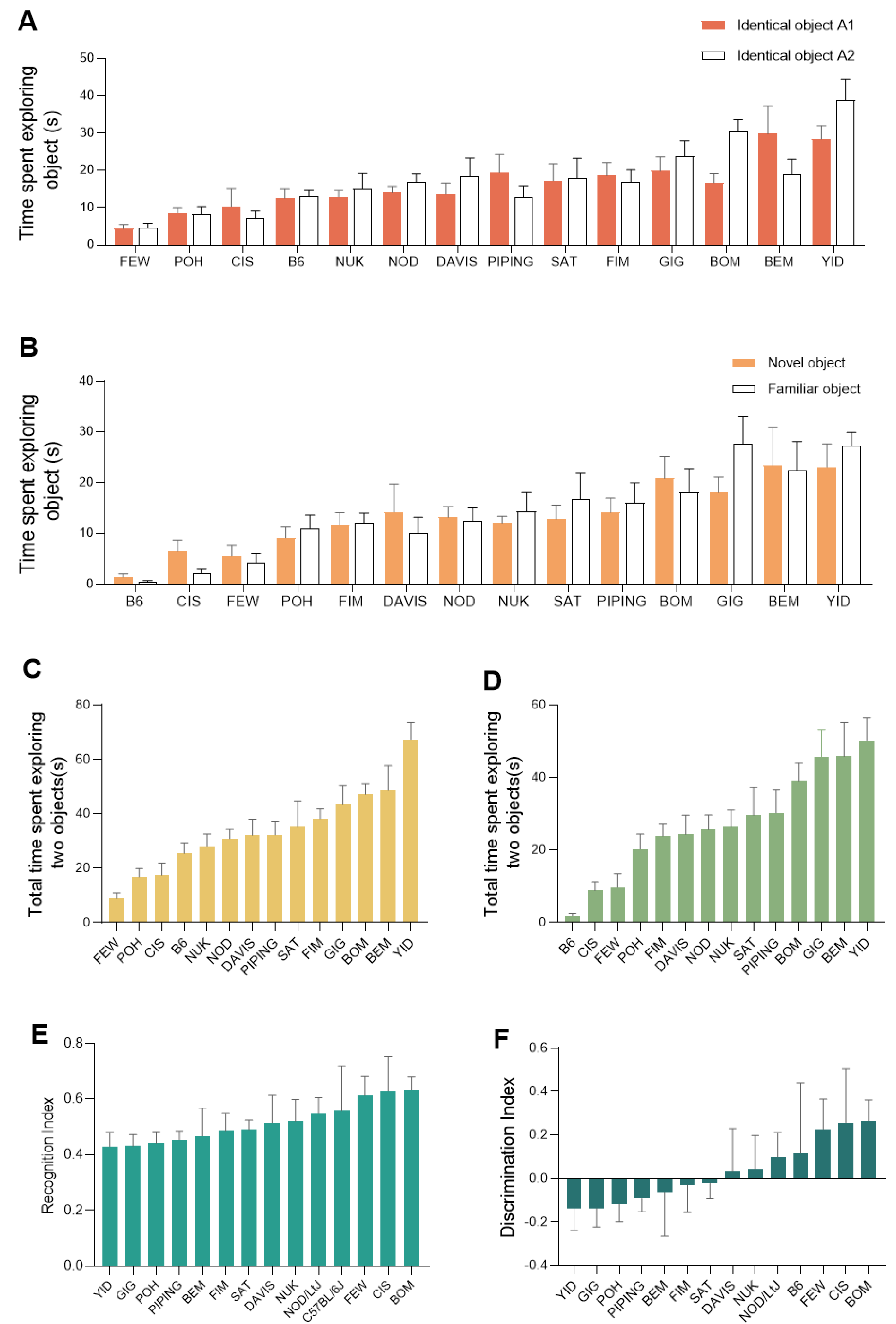
2.3. Extensive Variation of Novel Object Recognition Test Traits among the CC Population
2.4. MWM Task Traits Are Widely Diverse in the CC Population
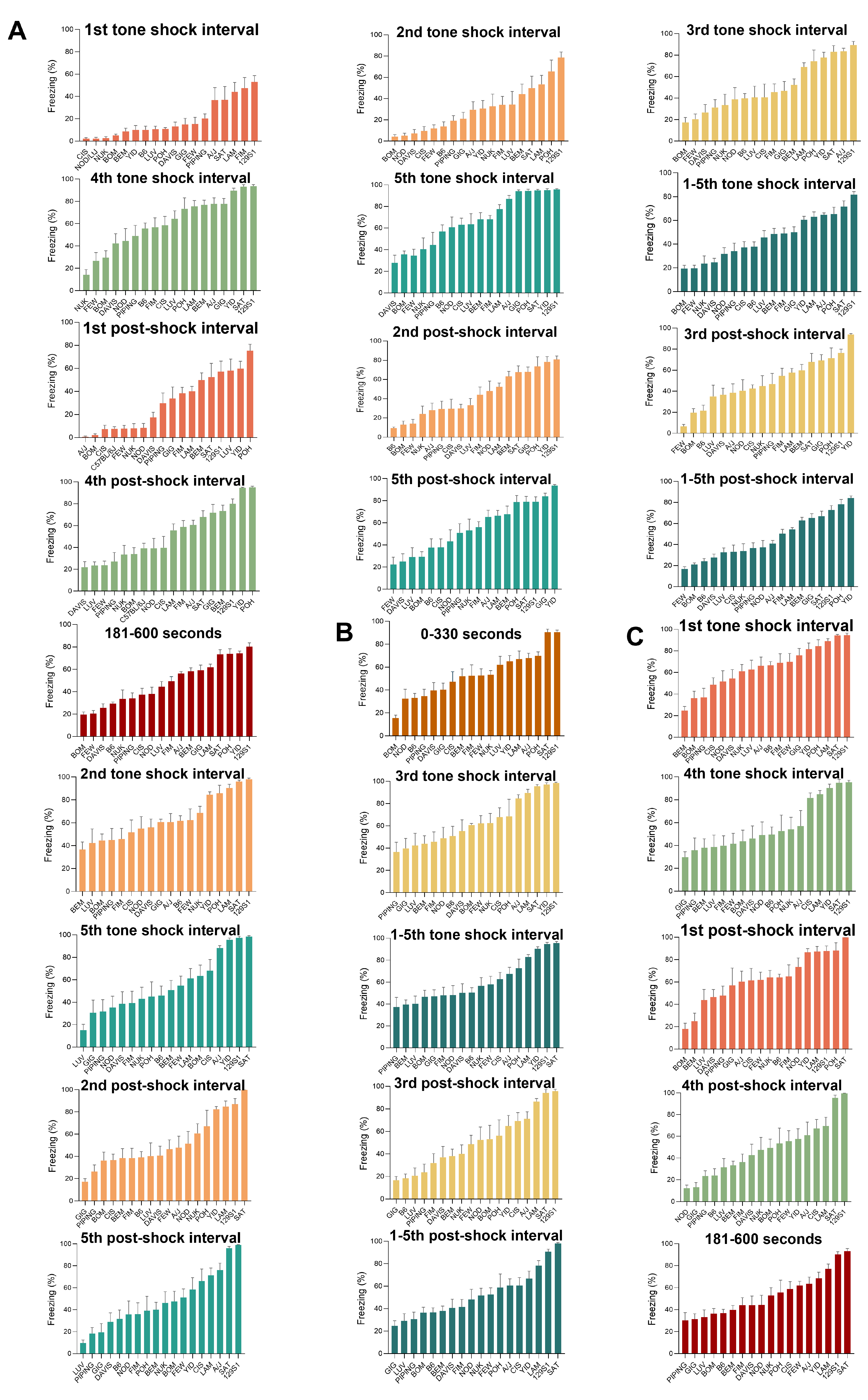
2.5. Fear Conditioning Performance Covers a Wide Range in the CC Population
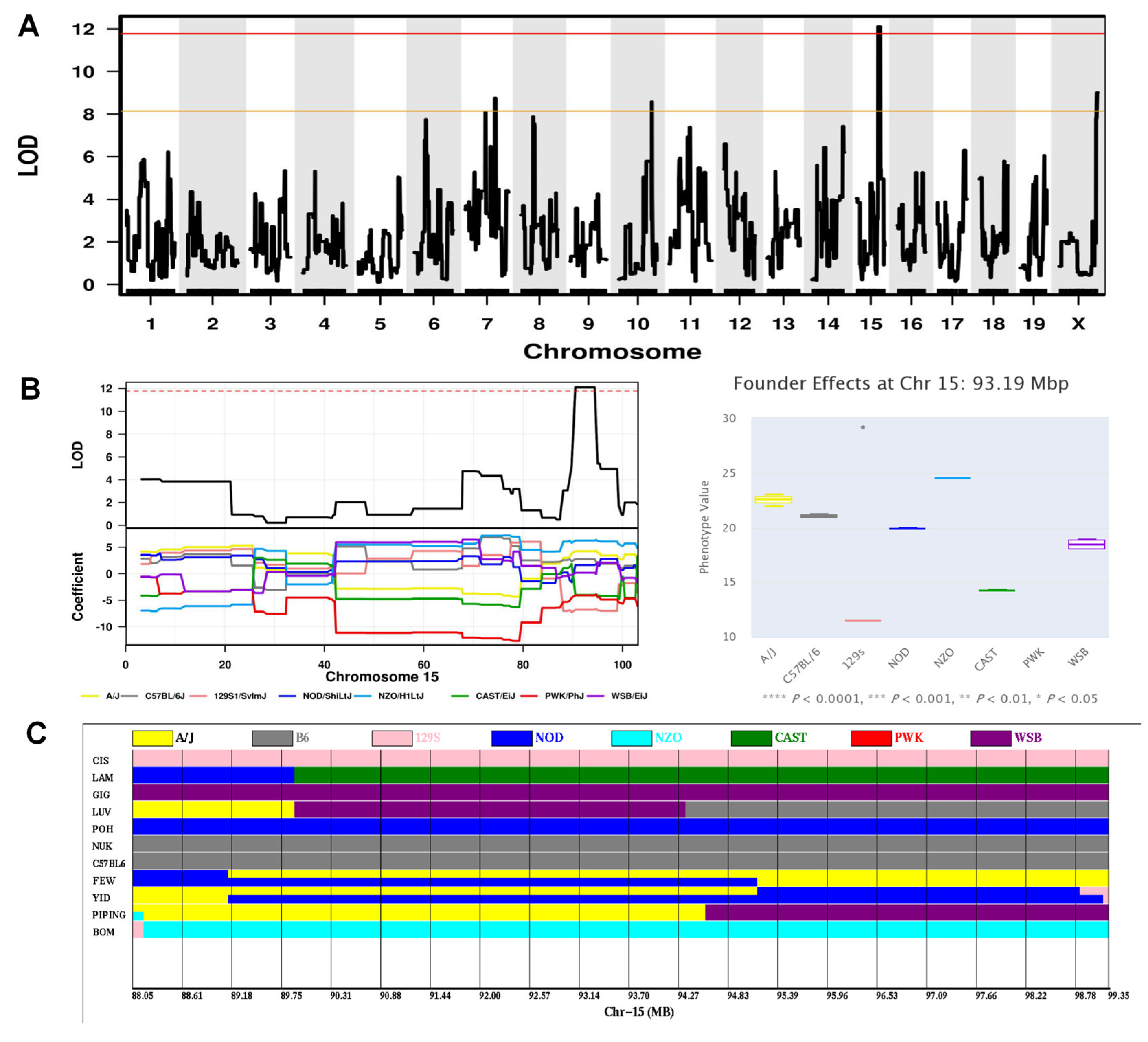
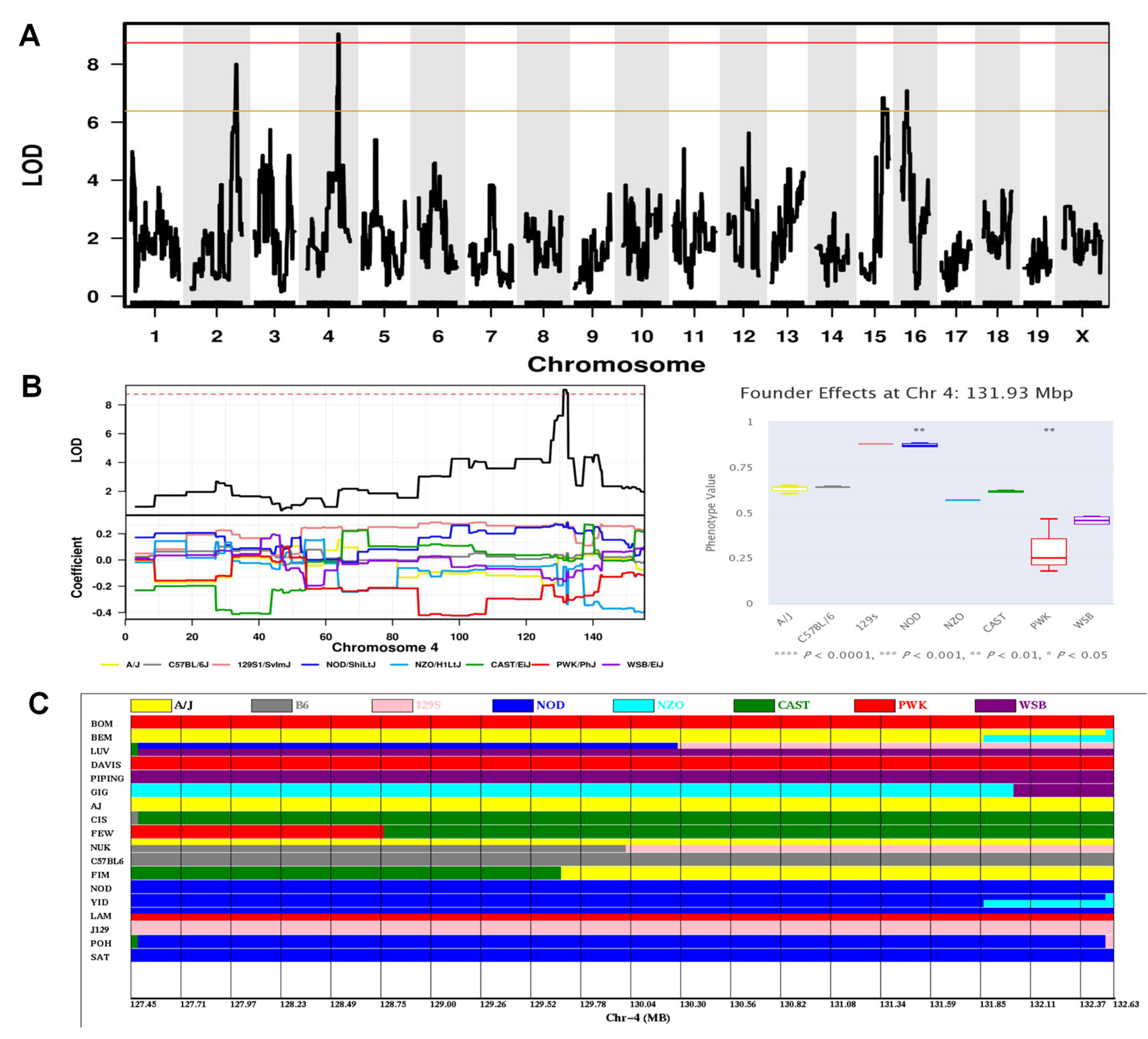
2.6. Genome-Wide Association Analysis for Behavioral Phenotypes Identified Significant QTLs
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. General Behavioral Testing Procedures
4.2.1. Open Field Test
4.2.2. Novel Object Recognition Test
4.2.3. MWM Task
4.2.4. Fear Conditioning Test
4.3. Data Analysis
4.4. QTL Mapping
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simmel, E.C.; Eleftheriou, B.E. Multivariate and behavior genetic analysis of avoidance of complex visual stimuli and activity in recombinant inbred strains of mice. Behav. Genet. 1977, 7, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; López-Granero, C.; Ferrer, B.; Tinkov, A.A.; Skalny, A.V.; Paoliello, M.M.B.; Aschner, M. BXD Recombinant Inbred Mice as a Model to Study Neurotoxicity. Biomolecules 2021, 11, 1762. [Google Scholar] [CrossRef] [PubMed]
- Philip, V.M.; Duvvuru, S.; Gomero, B.; Ansah, T.A.; Blaha, C.D.; Cook, M.N.; Hamre, K.M.; Lariviere, W.R.; Matthews, D.B.; Mittleman, G.; et al. High-throughput behavioral phenotyping in the expanded panel of BXD recombinant inbred strains. Genes Brain Behav. 2010, 9, 129–159. [Google Scholar] [CrossRef] [PubMed]
- Chesler, E.J. Out of the bottleneck: The Diversity Outcross and Collaborative Cross mouse populations in behavioral genetics research. Mamm. Genome 2014, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.W.; Williams, E.G. Resources for Systems Genetics. Methods Mol. Biol. 2017, 1488, 3–29. [Google Scholar]
- Churchill, G.A.; Airey, D.C.; Allayee, H.; Angel, J.M.; Attie, A.D.; Beatty, J.; Beavis, W.D.; Belknap, J.K.; Bennett, B.; Berrettini, W.; et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 2004, 36, 1133–1137. [Google Scholar]
- Morahan, G. The genetic reference population “Collaborative Cross” is a powerful resource for genetic discoveries and understanding complex genetic traits. Chin. J. Comp. Med. 2016, 26, 1–10. [Google Scholar]
- Roberts, A.; Pardo-Manuel de Villena, F.; Wang, W.; McMillan, L.; Threadgill, D.W. The polymorphism architecture of mouse genetic resources elucidated using genome-wide resequencing data: Implications for QTL discovery and systems genetics. Mamm. Genome 2007, 18, 473–481. [Google Scholar] [CrossRef]
- Leist, S.R.; Baric, R.S. Giving the Genes a Shuffle: Using Natural Variation to Understand Host Genetic Contributions to Viral Infections. Trends Genet. 2018, 34, 777–789. [Google Scholar] [CrossRef]
- Krištić, J.; Zaytseva, O.O.; Ram, R.; Nguyen, Q.; Novokmet, M.; Vučković, F.; Vilaj, M.; Trbojević-Akmačić, I.; Pezer, M.; Davern, K.M.; et al. Profiling and genetic control of the murine immunoglobulin G glycome. Nat. Chem. Biol. 2018, 14, 516–524. [Google Scholar] [CrossRef]
- Yang, C.H.; Mangiafico, S.P.; Waibel, M.; Loudovaris, T.; Loh, K.; Thomas, H.E.; Morahan, G.; Andrikopoulos, S. E2f8 and Dlg2 genes have independent effects on impaired insulin secretion associated with hyperglycaemia. Diabetologia 2020, 63, 1333–1348. [Google Scholar] [CrossRef]
- Salimova, E.; Nowak, K.J.; Estrada, A.C.; Furtado, M.B.; McNamara, E.; Nguyen, Q.; Balmer, L.; Preuss, C.; Holmes, J.W.; Ramialison, M.; et al. Variable outcomes of human heart attack recapitulated in genetically diverse mice. NPJ Regen. Med. 2019, 4, 5. [Google Scholar] [CrossRef]
- Deng, Y.; Bi, M.; Delerue, F.; Forrest, S.L.; Chan, G.; van der Hoven, J.; van Hummel, A.; Feiten, A.F.; Lee, S.; Martinez-Valbuena, I.; et al. Loss of LAMP5 interneurons drives neuronal network dysfunction in Alzheimer’s disease. Acta Neuropathol. 2022, 144, 637–650. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Langley, S.A.; Zhou, Y.X.; Jen, K.Y.; Sun, Q.; Brislawn, C.; Rojas, C.M.; Wahl, K.L.; Wang, T.; et al. Diverse tumour susceptibility in Collaborative Cross mice: Identification of a new mouse model for human gastric tumourigenesis. Gut 2019, 68, 1942–1952. [Google Scholar] [CrossRef]
- Ferguson, B.; Handoko, H.Y.; Mukhopadhyay, P.; Chitsazan, A.; Balmer, L.; Morahan, G.; Walker, G.J. Different genetic mechanisms mediate spontaneous versus UVR-induced malignant melanoma. eLife 2019, 8, e42424. [Google Scholar] [CrossRef]
- Karkar, L.; Abu-Toamih Atamni, H.J.; Milhem, A.; Houri-Haddad, Y.; Iraqi, F.A. Assessing the host genetic background effects on type 2 diabetes and obesity development in response to mixed-oral bacteria and high-fat diet using the collaborative cross mouse model. Anim. Model Exp. Med. 2020, 3, 152–159. [Google Scholar] [CrossRef]
- Rasmussen, A.L.; Okumura, A.; Ferris, M.T.; Green, R.; Feldmann, F.; Kelly, S.M.; Scott, D.P.; Safronetz, D.; Haddock, E.; LaCasse, R.; et al. Host genetic diversity enables Ebola hemorrhagic fever pathogenesis and resistance. Science 2014, 346, 987–991. [Google Scholar] [CrossRef]
- Oldstone, M.B.A.; Ware, B.C.; Horton, L.E.; Welch, M.J.; Aiolfi, R.; Zarpellon, A.; Ruggeri, Z.M.; Sullivan, B.M. Lymphocytic choriomeningitis virus Clone 13 infection causes either persistence or acute death dependent on IFN-1, cytotoxic T lymphocytes (CTLs), and host genetics. Proc. Natl. Acad. Sci. USA 2018, 115, E7814–E7823. [Google Scholar] [CrossRef]
- Durrant, C.; Tayem, H.; Yalcin, B.; Cleak, J.; Goodstadt, L.; de Villena, F.P.; Mott, R.; Iraqi, F.A. Collaborative Cross mice and their power to map host susceptibility to Aspergillus fumigatus infection. Genome Res. 2011, 21, 1239–1248. [Google Scholar] [CrossRef]
- Lawley, K.S.; Rech, R.R.; Perez Gomez, A.A.; Hopkins, L.; Han, G.; Amstalden, K.; Welsh, C.J.; Young, C.R.; Jones-Hall, Y.; Threadgill, D.W.; et al. Viral Clearance and Neuroinflammation in Acute TMEV Infection Vary by Host Genetic Background. Int. J. Mol. Sci. 2022, 23, 10482. [Google Scholar] [CrossRef]
- Jo, Y.; Balmer, L.; Lee, B.; Shim, J.A.; Ali, L.A.; Morahan, G.; Hong, C. Variants of innate CD8(+) T cells are associated with Grip2 and Klf15 genes. Cell. Mol. Immunol. 2020, 17, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Philip, V.M.; Sokoloff, G.; Ackert-Bicknell, C.L.; Striz, M.; Branstetter, L.; Beckmann, M.A.; Spence, J.S.; Jackson, B.L.; Galloway, L.D.; Barker, P.; et al. Genetic analysis in the Collaborative Cross breeding population. Genome Res. 2011, 21, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.H.; Langley, S.A.; Huang, Y.; Hang, M.; Bouchard, K.E.; Celniker, S.E.; Brown, J.B.; Jansson, J.K.; Karpen, G.H.; Snijders, A.M. Identification of genetic factors that modify motor performance and body weight using Collaborative Cross mice. Sci. Rep. 2015, 5, 16247. [Google Scholar] [CrossRef] [PubMed]
- Molenhuis, R.T.; Bruining, H.; Brandt, M.J.V.; van Soldt, P.E.; Abu-Toamih Atamni, H.J.; Burbach, J.P.H.; Iraqi, F.A.; Mott, R.F.; Kas, M.J.H. Modeling the quantitative nature of neurodevelopmental disorders using Collaborative Cross mice. Mol. Autism 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, Y.; Celniker, S.E.; Xia, Y.; Mao, J.H.; Snijders, A.M.; Chang, H. Gut microbiome partially mediates and coordinates the effects of genetics on anxiety-like behavior in Collaborative Cross mice. Sci. Rep. 2021, 11, 270. [Google Scholar] [CrossRef]
- Mao, J.H.; Kim, Y.M.; Zhou, Y.X.; Hu, D.; Zhong, C.; Chang, H.; Brislawn, C.J.; Fansler, S.; Langley, S.; Wang, Y.; et al. Genetic and metabolic links between the murine microbiome and memory. Microbiome 2020, 8, 53. [Google Scholar] [CrossRef]
- Maquet, P. The role of sleep in learning and memory. Science 2001, 294, 1048–1052. [Google Scholar] [CrossRef]
- Meeusen, R.; Decroix, L. Nutritional Supplements and the Brain. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 200–211. [Google Scholar] [CrossRef]
- Schreurs, B.G. The effects of cholesterol on learning and memory. Neurosci. Biobehav. Rev. 2010, 34, 1366–1379. [Google Scholar] [CrossRef]
- Torregrossa, M.M.; Corlett, P.R.; Taylor, J.R. Aberrant learning and memory in addiction. Neurobiol. Learn. Mem. 2011, 96, 609–623. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Lipp, H.P.; Wolfer, D.P. Genetically modified mice and cognition. Curr. Opin. Neurobiol. 1998, 8, 272–280. [Google Scholar] [CrossRef]
- Wolfer, D.P.; Stagljar-Bozicevic, M.; Errington, M.L.; Lipp, H.P. Spatial Memory and Learning in Transgenic Mice: Fact or Artifact? News Physiol. Sci. 1998, 13, 118–123. [Google Scholar] [CrossRef]
- Wolfer, D.P.; Lipp, H.P. Dissecting the behaviour of transgenic mice: Is it the mutation, the genetic background, or the environment? Exp. Physiol. 2000, 85, 627–634. [Google Scholar] [CrossRef]
- Clapcote, S.J.; Roder, J.C. Survey of embryonic stem cell line source strains in the water maze reveals superior reversal learning of 129S6/SvEvTac mice. Behav. Brain Res. 2004, 152, 35–48. [Google Scholar] [CrossRef]
- Ram, R.; Mehta, M.; Balmer, L.; Gatti, D.M.; Morahan, G. Rapid identification of major-effect genes using the collaborative cross. Genetics 2014, 198, 75–86. [Google Scholar] [CrossRef]
- Ram, R.; Morahan, G. Complex Trait Analyses of the Collaborative Cross: Tools and Databases. Methods Mol. Biol. 2017, 1488, 121–129. [Google Scholar]
- Keane, T.M.; Goodstadt, L.; Danecek, P.; White, M.A.; Wong, K.; Yalcin, B.; Heger, A.; Agam, A.; Slater, G.; Goodson, M.; et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 2011, 477, 289–294. [Google Scholar] [CrossRef]
- Ho, M.; Post, C.M.; Donahue, L.R.; Lidov, H.G.; Bronson, R.T.; Goolsby, H.; Watkins, S.C.; Cox, G.A.; Brown, R.H., Jr. Disruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum. Mol. Genet. 2004, 13, 1999–2010. [Google Scholar] [CrossRef]
- Uaesoontrachoon, K.; Cha, H.J.; Ampong, B.; Sali, A.; Vandermeulen, J.; Wei, B.; Creeden, B.; Huynh, T.; Quinn, J.; Tatem, K.; et al. The effects of MyD88 deficiency on disease phenotype in dysferlin-deficient A/J mice: Role of endogenous TLR ligands. J. Pathol. 2013, 231, 199–209. [Google Scholar] [CrossRef]
- Wenzel, K.; Zabojszcza, J.; Carl, M.; Taubert, S.; Lass, A.; Harris, C.L.; Ho, M.; Schulz, H.; Hummel, O.; Hubner, N.; et al. Increased susceptibility to complement attack due to down-regulation of decay-accelerating factor/CD55 in dysferlin-deficient muscular dystrophy. J. Immunol. 2005, 175, 6219–6225. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Izawa, T.; Kuwamura, M.; Yamate, J. The distribution and characterization of skeletal muscle lesions in dysferlin-deficient SJL and A/J mice. Exp. Toxicol. Pathol. 2010, 62, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P.; Maillet, M.; Roche, J.A.; Sargent, M.A.; McNally, E.M.; Bloch, R.J.; Molkentin, J.D. Genetic manipulation of dysferlin expression in skeletal muscle: Novel insights into muscular dystrophy. Am. J. Pathol. 2009, 175, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Escobar, H.; Schöwel, V.; Spuler, S.; Marg, A.; Izsvák, Z. Full-length Dysferlin Transfer by the Hyperactive Sleeping Beauty Transposase Restores Dysferlin-deficient Muscle. Mol. Ther. Nucleic Acids 2016, 5, e277. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Aoki, M.; Illa, I.; Wu, C.; Fardeau, M.; Angelini, C.; Serrano, C.; Urtizberea, J.A.; Hentati, F.; Hamida, M.B.; et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat. Genet. 1998, 20, 31–36. [Google Scholar] [CrossRef]
- Bashir, R.; Britton, S.; Strachan, T.; Keers, S.; Vafiadaki, E.; Lako, M.; Richard, I.; Marchand, S.; Bourg, N.; Argov, Z.; et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat. Genet. 1998, 20, 37–42. [Google Scholar] [CrossRef]
- Matsuda, C.; Aoki, M.; Hayashi, Y.K.; Ho, M.F.; Arahata, K.; Brown, R.H., Jr. Dysferlin is a surface membrane-associated protein that is absent in Miyoshi myopathy. Neurology 1999, 53, 1119–1122. [Google Scholar] [CrossRef]
- Bansal, D.; Campbell, K.P. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 2004, 14, 206–213. [Google Scholar] [CrossRef]
- Livy, D.J.; Wahlsten, D. Retarded formation of the hippocampal commissure in embryos from mouse strains lacking a corpus callosum. Hippocampus 1997, 7, 2–14. [Google Scholar] [CrossRef]
- Yeritsyan, N.; Lehmann, K.; Puk, O.; Graw, J.; Löwel, S. Visual capabilities and cortical maps in BALB/c mice. Eur. J. Neurosci. 2012, 36, 2801–2811. [Google Scholar] [CrossRef]
- Roffler-Tarlov, S.; Liu, J.H.; Naumova, E.N.; Bernal-Ayala, M.M.; Mason, C.A. L-Dopa and the albino riddle: Content of L-Dopa in the developing retina of pigmented and albino mice. PLoS ONE 2013, 8, e57184. [Google Scholar] [CrossRef]
- Errijgers, V.; Van Dam, D.; Gantois, I.; Van Ginneken, C.J.; Grossman, A.W.; D’Hooge, R.; De Deyn, P.P.; Kooy, R.F. FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes Brain Behav. 2007, 6, 552–557. [Google Scholar] [CrossRef]
- Balkema, G.W.; Dräger, U.C. Impaired visual thresholds in hypopigmented animals. Vis. Neurosci. 1991, 6, 577–585. [Google Scholar] [CrossRef]
- Kim, E.J.; Monje, F.J.; Li, L.; Höger, H.; Pollak, D.D.; Lubec, G. Alzheimer’s disease risk factor lymphocyte-specific protein tyrosine kinase regulates long-term synaptic strengthening, spatial learning and memory. Cell. Mol. Life Sci. 2013, 70, 743–759. [Google Scholar] [CrossRef]
- Zhong, W.; Yamagata, H.D.; Taguchi, K.; Akatsu, H.; Kamino, K.; Yamamoto, T.; Kosaka, K.; Takeda, M.; Kondo, I.; Miki, T. Lymphocyte-specific protein tyrosine kinase is a novel risk gene for Alzheimer disease. J. Neurol. Sci. 2005, 238, 53–57. [Google Scholar] [CrossRef]
- Kaksonen, M.; Pavlov, I.; Võikar, V.; Lauri, S.E.; Hienola, A.; Riekki, R.; Lakso, M.; Taira, T.; Rauvala, H. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol. Cell. Neurosci. 2002, 21, 158–172. [Google Scholar] [CrossRef]
- Hudák, A.; Letoha, A.; Vizler, C.; Letoha, T. Syndecan-3 as a Novel Biomarker in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 63407. [Google Scholar] [CrossRef]
- Lu, G.L.; Lee, C.H.; Chiou, L.C. Orexin A induces bidirectional modulation of synaptic plasticity: Inhibiting long-term potentiation and preventing depotentiation. Neuropharmacology 2016, 107, 168–180. [Google Scholar] [CrossRef]
- Soya, S.; Shoji, H.; Hasegawa, E.; Hondo, M.; Miyakawa, T.; Yanagisawa, M.; Mieda, M.; Sakurai, T. Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation. J. Neurosci. 2013, 33, 14549–14557. [Google Scholar] [CrossRef]
- Akbari, E.; Naghdi, N.; Motamedi, F. The selective orexin 1 receptor antagonist SB-334867-A impairs acquisition and consolidation but not retrieval of spatial memory in Morris water maze. Peptides 2007, 28, 650–656. [Google Scholar] [CrossRef]
- Akbari, E.; Naghdi, N.; Motamedi, F. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav. Brain Res. 2006, 173, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Yang, H.; Hu, B.; Wang, S.; Chen, M.; Cohen, D.S.; Chen, H.S.; Jarrell, J.T.; Carpenter, K.A.; Rosin, E.R.; et al. Identification and Analysis of Alzheimer’s Candidate Genes by an Amplitude Deviation Algorithm. J. Alzheimer’s Dis. Park. 2019, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, J. Immune-Related Hub Genes and the Competitive Endogenous RNA Network in Alzheimer’s Disease. J. Alzheimers Dis. 2020, 77, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Gao, L.; Stamm, S.; Andreadis, A. An SRp75/hnRNPG complex interacting with hnRNPE2 regulates the 5′ splice site of tau exon 10, whose misregulation causes frontotemporal dementia. Gene 2011, 485, 130–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, Y.; Almeida, S.; Gao, F.B. TBK1 haploinsufficiency in ALS and FTD compromises membrane trafficking. Acta Neuropathol. 2021, 142, 217–221. [Google Scholar] [CrossRef]
- Cho, H.M.; Kim, J.Y.; Kim, H.; Sun, W. Phosphatase and actin regulator 4 is associated with intermediate filaments in adult neural stem cells and their progenitor astrocytes. Histochem. Cell Biol. 2014, 142, 411–419. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, S.Y.; Moon, Y.; Kim, H.J.; Chin, J.H.; Kim, H.; Sun, W. Different expression patterns of Phactr family members in normal and injured mouse brain. Neuroscience 2012, 221, 37–46. [Google Scholar] [CrossRef]
- Florentinus-Mefailoski, A.; Bowden, P.; Scheltens, P.; Killestein, J.; Teunissen, C.; Marshall, J.G. The plasma peptides of Alzheimer’s disease. Clin. Proteom. 2021, 18, 17. [Google Scholar] [CrossRef]
- Bahari-Javan, S.; Maddalena, A.; Kerimoglu, C.; Wittnam, J.; Held, T.; Bähr, M.; Burkhardt, S.; Delalle, I.; Kügler, S.; Fischer, A.; et al. HDAC1 regulates fear extinction in mice. J. Neurosci. 2012, 32, 5062–5073. [Google Scholar] [CrossRef]
- Zhang, H.L.; Han, W.; Du, Y.Q.; Zhao, B.; Yang, P.; Yin, D.M. SRC3 acetylates calmodulin in the mouse brain to regulate synaptic plasticity and fear learning. J. Biol. Chem. 2021, 297, 101044. [Google Scholar] [CrossRef]
- Papassotiropoulos, A.; Stefanova, E.; Vogler, C.; Gschwind, L.; Ackermann, S.; Spalek, K.; Rasch, B.; Heck, A.; Aerni, A.; Hanser, E.; et al. A genome-wide survey and functional brain imaging study identify CTNNBL1 as a memory-related gene. Mol. Psychiatry 2013, 18, 255–263. [Google Scholar] [CrossRef]
- Dey, K.K.; Wang, H.; Niu, M.; Bai, B.; Wang, X.; Li, Y.; Cho, J.H.; Tan, H.; Mishra, A.; High, A.A.; et al. Deep undepleted human serum proteome profiling toward biomarker discovery for Alzheimer’s disease. Clin. Proteom. 2019, 16, 16. [Google Scholar] [CrossRef]
- Palmer, C.L.; Lim, W.; Hastie, P.G.; Toward, M.; Korolchuk, V.I.; Burbidge, S.A.; Banting, G.; Collingridge, G.L.; Isaac, J.T.; Henley, J.M. Hippocalcin functions as a calcium sensor in hippocampal LTD. Neuron 2005, 47, 487–494. [Google Scholar] [CrossRef]
- Kobayashi, M.; Masaki, T.; Hori, K.; Masuo, Y.; Miyamoto, M.; Tsubokawa, H.; Noguchi, H.; Nomura, M.; Takamatsu, K. Hippocalcin-deficient mice display a defect in cAMP response element-binding protein activation associated with impaired spatial and associative memory. Neuroscience 2005, 133, 471–484. [Google Scholar] [CrossRef]
- Sandusky-Beltran, L.A.; Kovalenko, A.; Placides, D.S.; Ratnasamy, K.; Ma, C.; Hunt, J.B., Jr.; Liang, H.; Calahatian, J.I.T.; Michalski, C.; Fahnestock, M.; et al. Aberrant AZIN2 and polyamine metabolism precipitates tau neuropathology. J. Clin. Investig. 2021, 131, e126299. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, K.J.; Park, P.; Lee, C.; Kim, M.J.; Han, D.H.; Kim, J.I.; Kim, S.; Lee, H.R.; Lee, Y.; et al. Neur1 and Neur2 are required for hippocampus-dependent spatial memory and synaptic plasticity. Hippocampus 2020, 30, 1158–1166. [Google Scholar] [CrossRef]
- Katoh, H.; Negishi, M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 2003, 424, 461–464. [Google Scholar] [CrossRef]
- Baudry, M.; Bi, X. Learning and memory: An emergent property of cell motility. Neurobiol. Learn. Mem. 2013, 104, 64–72. [Google Scholar] [CrossRef]
- Roberts, R.O.; Kang, Y.N.; Hu, C.; Moser, C.D.; Wang, S.; Moore, M.J.; Graham, R.P.; Lai, J.P.; Petersen, R.C.; Roberts, L.R. Decreased Expression of Sulfatase 2 in the Brains of Alzheimer’s Disease Patients: Implications for Regulation of Neuronal Cell Signaling. J. Alzheimer’s Dis. Rep. 2017, 1, 115–124. [Google Scholar] [CrossRef]
- Kvarnung, M.; Nilsson, D.; Lindstrand, A.; Korenke, G.C.; Chiang, S.C.; Blennow, E.; Bergmann, M.; Stödberg, T.; Mäkitie, O.; Anderlid, B.M.; et al. A novel intellectual disability syndrome caused by GPI anchor deficiency due to homozygous mutations in PIGT. J. Med. Genet. 2013, 50, 521–528. [Google Scholar] [CrossRef]
- Ots, H.D.; Tracz, J.A.; Vinokuroff, K.E.; Musto, A.E. CD40-CD40L in Neurological Disease. Int. J. Mol. Sci. 2022, 23, 84115. [Google Scholar] [CrossRef] [PubMed]
- Moore, Y.E.; Conway, L.C.; Wobst, H.J.; Brandon, N.J.; Deeb, T.Z.; Moss, S.J. Developmental Regulation of KCC2 Phosphorylation Has Long-Term Impacts on Cognitive Function. Front. Mol. Neurosci. 2019, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Ferando, I.; Faas, G.C.; Mody, I. Diminished KCC2 confounds synapse specificity of LTP during senescence. Nat. Neurosci. 2016, 19, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Moorhouse, A.J.; Cheung, D.L.; Eto, K.; Takeda, I.; Rozenbroek, P.W.; Nabekura, J. Overexpression of neuronal K(+)-Cl(-) co-transporter enhances dendritic spine plasticity and motor learning. J. Physiol. Sci. 2019, 69, 453–463. [Google Scholar] [CrossRef]
- Compton, A.G.; Albrecht, D.E.; Seto, J.T.; Cooper, S.T.; Ilkovski, B.; Jones, K.J.; Challis, D.; Mowat, D.; Ranscht, B.; Bahlo, M.; et al. Mutations in contactin-1, a neural adhesion and neuromuscular junction protein, cause a familial form of lethal congenital myopathy. Am. J. Hum. Genet. 2008, 83, 714–724. [Google Scholar] [CrossRef]
- Berglund, E.O.; Murai, K.K.; Fredette, B.; Sekerková, G.; Marturano, B.; Weber, L.; Mugnaini, E.; Ranscht, B. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron 1999, 24, 739–750. [Google Scholar] [CrossRef]
- Falk, J.; Bonnon, C.; Girault, J.A.; Faivre-Sarrailh, C. F3/contactin, a neuronal cell adhesion molecule implicated in axogenesis and myelination. Biol. Cell. 2002, 94, 327–334. [Google Scholar] [CrossRef]
- Bassuk, A.G.; Wallace, R.H.; Buhr, A.; Buller, A.R.; Afawi, Z.; Shimojo, M.; Miyata, S.; Chen, S.; Gonzalez-Alegre, P.; Griesbach, H.L.; et al. A homozygous mutation in human PRICKLE1 causes an autosomal-recessive progressive myoclonus epilepsy-ataxia syndrome. Am. J. Hum. Genet. 2008, 83, 572–581. [Google Scholar] [CrossRef]
- Pergande, M.; Motameny, S.; Özdemir, Ö.; Kreutzer, M.; Wang, H.; Daimagüler, H.S.; Becker, K.; Karakaya, M.; Ehrhardt, H.; Elcioglu, N.; et al. The genomic and clinical landscape of fetal akinesia. Genet. Med. 2020, 22, 511–523. [Google Scholar] [CrossRef]
- Falb, R.J.; Müller, A.J.; Klein, W.; Grimmel, M.; Grasshoff, U.; Spranger, S.; Stöbe, P.; Gauck, D.; Kuechler, A.; Dikow, N.; et al. Bi-allelic loss-of-function variants in KIF21A cause severe fetal akinesia with arthrogryposis multiplex. J. Med. Genet. 2023, 60, 48–56. [Google Scholar] [CrossRef]
- Berwick, D.C.; Heaton, G.R.; Azeggagh, S.; Harvey, K. LRRK2 Biology from structure to dysfunction: Research progresses, but the themes remain the same. Mol. Neurodegener. 2019, 14, 49. [Google Scholar] [CrossRef]
- Santpere, G.; Ferrer, I. LRRK2 and neurodegeneration. Acta Neuropathol. 2009, 117, 227–246. [Google Scholar] [CrossRef]
- Dressel, U.; Bailey, P.J.; Wang, S.C.; Downes, M.; Evans, R.M.; Muscat, G.E. A dynamic role for HDAC7 in MEF2-mediated muscle differentiation. J. Biol. Chem. 2001, 276, 17007–17013. [Google Scholar] [CrossRef]
- Beharry, A.W.; Judge, A.R. Differential expression of HDAC and HAT genes in atrophying skeletal muscle. Muscle Nerve 2015, 52, 1098–1101. [Google Scholar] [CrossRef]
- Meyer, E.; Carss, K.J.; Rankin, J.; Nichols, J.M.; Grozeva, D.; Joseph, A.P.; Mencacci, N.E.; Papandreou, A.; Ng, J.; Barral, S.; et al. Mutations in the histone methyltransferase gene KMT2B cause complex early-onset dystonia. Nat. Genet. 2017, 49, 223–237. [Google Scholar] [CrossRef]
- Qi, J.; Dmochowski, J.M.; Banes, A.N.; Tsuzaki, M.; Bynum, D.; Patterson, M.; Creighton, A.; Gomez, S.; Tech, K.; Cederlund, A.; et al. Differential expression and cellular localization of novel isoforms of the tendon biomarker tenomodulin. J. Appl. Physiol. 2012, 113, 861–871. [Google Scholar] [CrossRef]
- Dex, S.; Alberton, P.; Willkomm, L.; Söllradl, T.; Bago, S.; Milz, S.; Shakibaei, M.; Ignatius, A.; Bloch, W.; Clausen-Schaumann, H.; et al. Tenomodulin is Required for Tendon Endurance Running and Collagen I Fibril Adaptation to Mechanical Load. EBioMedicine 2017, 20, 240–254. [Google Scholar] [CrossRef]
- Friedel, R.H.; Kerjan, G.; Rayburn, H.; Schüller, U.; Sotelo, C.; Tessier-Lavigne, M.; Chédotal, A. Plexin-B2 controls the development of cerebellar granule cells. J. Neurosci. 2007, 27, 3921–3932. [Google Scholar] [CrossRef]
- Saez-Atienzar, S.; Bandres-Ciga, S.; Langston, R.G.; Kim, J.J.; Choi, S.W.; Reynolds, R.H.; Abramzon, Y.; Dewan, R.; Ahmed, S.; Landers, J.E.; et al. Genetic analysis of amyotrophic lateral sclerosis identifies contributing pathways and cell types. Sci. Adv. 2021, 7, eabd9036. [Google Scholar] [CrossRef]
- Nakhro, K.; Park, J.M.; Hong, Y.B.; Park, J.H.; Nam, S.H.; Yoon, B.R.; Yoo, J.H.; Koo, H.; Jung, S.C.; Kim, H.L.; et al. SET binding factor 1 (SBF1) mutation causes Charcot-Marie-Tooth disease type 4B3. Neurology 2013, 81, 165–173. [Google Scholar] [CrossRef]
- Gang, Q.; Bettencourt, C.; Holton, J.; Lovejoy, C.; Chelban, V.; Oconnor, E.; Yuan, Y.; Reilly, M.M.; Hanna, M.; Houlden, H. A novel frameshift deletion in autosomal recessive SBF1-related syndromic neuropathy with necklace fibres. J. Neurol. 2020, 267, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.R.; Claes, K.; Todd, V.; Chaverra, M.; Lefcort, F. NELL2 promotes motor and sensory neuron differentiation and stimulates mitogenesis in DRG in vivo. Dev. Biol. 2004, 270, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Güler, S.; Gül, T.; Güler, Ş.; Haerle, M.C.; Başak, A.N. Early-Onset Parkinson’s Disease: A Novel Deletion Comprising the DJ-1 and TNFRSF9 Genes. Mov. Disord. 2021, 36, 2973–2976. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Palmieri, O.; Corritore, G.; Latiano, T.; Bossa, F.; Scimeca, D.; Biscaglia, G.; Valvano, M.R.; D’Incà, R.; Cucchiara, S.; et al. Association study of a polymorphism in clock gene PERIOD3 and risk of inflammatory bowel disease. Chronobiol. Int. 2012, 29, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.J.; Archer, S.N. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med. Rev. 2010, 14, 151–160. [Google Scholar] [CrossRef]
- Scapoli, C.; Ziliotto, N.; Lunghi, B.; Menegatti, E.; Salvi, F.; Zamboni, P.; Baroni, M.; Mascoli, F.; Bernardi, F.; Marchetti, G. Combination of Genomic and Transcriptomic Approaches Highlights Vascular and Circadian Clock Components in Multiple Sclerosis. Int. J. Mol. Sci. 2021, 23, 10310. [Google Scholar] [CrossRef]
- Long, C.; Grueter, C.E.; Song, K.; Qin, S.; Qi, X.; Kong, Y.M.; Shelton, J.M.; Richardson, J.A.; Zhang, C.L.; Bassel-Duby, R.; et al. Ataxia and Purkinje cell degeneration in mice lacking the CAMTA1 transcription factor. Proc. Natl. Acad. Sci. USA 2014, 111, 11521–11526. [Google Scholar] [CrossRef]
- Thevenon, J.; Lopez, E.; Keren, B.; Heron, D.; Mignot, C.; Altuzarra, C.; Béri-Dexheimer, M.; Bonnet, C.; Magnin, E.; Burglen, L.; et al. Intragenic CAMTA1 rearrangements cause non-progressive congenital ataxia with or without intellectual disability. J. Med. Genet. 2012, 49, 400–408. [Google Scholar] [CrossRef]
- Shinawi, M.; Coorg, R.; Shimony, J.S.; Grange, D.K.; Al-Kateb, H. Intragenic CAMTA1 deletions are associated with a spectrum of neurobehavioral phenotypes. Clin. Genet. 2015, 87, 478–482. [Google Scholar] [CrossRef]
- Wijnen, I.G.M.; Veenstra-Knol, H.E.; Vansenne, F.; Gerkes, E.H.; de Koning, T.; Vos, Y.J.; Tijssen, M.A.J.; Sival, D.; Darin, N.; Vanhoutte, E.K.; et al. De novo variants in CAMTA1 cause a syndrome variably associated with spasticity, ataxia, and intellectual disability. Eur. J. Hum. Genet. 2020, 28, 763–769. [Google Scholar] [CrossRef]
- Dzinovic, I.; Serranová, T.; Prouteau, C.; Colin, E.; Ziegler, A.; Winkelmann, J.; Jech, R.; Zech, M. Myoclonic dystonia phenotype related to a novel calmodulin-binding transcription activator 1 sequence variant. Neurogenetics 2021, 22, 137–141. [Google Scholar] [CrossRef]
- Fogh, I.; Lin, K.; Tiloca, C.; Rooney, J.; Gellera, C.; Diekstra, F.P.; Ratti, A.; Shatunov, A.; van Es, M.A.; Proitsi, P.; et al. Association of a Locus in the CAMTA1 Gene With Survival in Patients With Sporadic Amyotrophic Lateral Sclerosis. JAMA Neurol. 2016, 73, 812–820. [Google Scholar] [CrossRef]
- Ha, S.; Lee, D.; Cho, Y.S.; Chung, C.; Yoo, Y.E.; Kim, J.; Lee, J.; Kim, W.; Kim, H.; Bae, Y.C.; et al. Cerebellar Shank2 Regulates Excitatory Synapse Density, Motor Coordination, and Specific Repetitive and Anxiety-Like Behaviors. J. Neurosci. 2016, 36, 12129–12143. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, Y.; Han, Y.; Li, X.; Lou, H.; Zhu, L.; Zhen, X.; Duan, S. Mice heterozygous for cathepsin D deficiency exhibit mania-related behavior and stress-induced depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 63, 110–118. [Google Scholar] [CrossRef]
- Ziegler, A.N.; Feng, Q.; Chidambaram, S.; Testai, J.M.; Kumari, E.; Rothbard, D.E.; Constancia, M.; Sandovici, I.; Cominski, T.; Pang, K.; et al. Insulin-like Growth Factor II: An Essential Adult Stem Cell Niche Constituent in Brain and Intestine. Stem Cell Rep. 2019, 12, 816–830. [Google Scholar] [CrossRef]
- Alisch, R.S.; Van Hulle, C.; Chopra, P.; Bhattacharyya, A.; Zhang, S.C.; Davidson, R.J.; Kalin, N.H.; Goldsmith, H.H. A multi-dimensional characterization of anxiety in monozygotic twin pairs reveals susceptibility loci in humans. Transl. Psychiatry 2017, 7, 1282. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Zarrindast, M.R.; Khakpai, F. The Modulatory Role of Dopamine in Anxiety-like Behavior. Arch. Iran. Med. 2015, 18, 591–603. [Google Scholar]
- Meziane, H.; Ouagazzal, A.M.; Aubert, L.; Wietrzych, M.; Krezel, W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: Implications for phenotyping strategies. Genes Brain Behav. 2007, 6, 192–200. [Google Scholar] [CrossRef]
- Laxmi, T.R.; Stork, O.; Pape, H.C. Generalisation of conditioned fear and its behavioural expression in mice. Behav. Brain Res. 2003, 145, 89–98. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Blanchard, D.C. Crouching as an index of fear. J. Comp. Physiol. Psychol. 1969, 67, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Mugwagwa, A.T.; Gadaga, L.L.; Pote, W.; Tagwireyi, D. Antiamnesic Effects of a Hydroethanolic Extract of Crinum macowanii on Scopolamine-Induced Memory Impairment in Mice. J. Neurodegener. Dis. 2015, 2015, 242505. [Google Scholar] [CrossRef] [PubMed][Green Version]


| QTL | Phenotype | Chr | Position (Interval, Mb) | Width (Mb) | Peak LOD | Sig. Level | Best Candidate Genes |
|---|---|---|---|---|---|---|---|
| Sml2 | Frequency of crossing the platform area on the probe trial phase of MWM (spatial memory index) | 4 | 128.5–135.0 | 6.50 | 12.9 | 0.05 | Lck, Ox1r, Sdc3, Ptafr, Srsf4, Stx12, Phactr4 |
| Lal3 | Swim speed on the probe trial phase of MWM (locomotor activity index) | 15 | 88.05–99.35 | 11.3 | 12.1 | 0.05 | Cntn1, Prickle1, Kif21a, Lrrk2, Kmt2b, Hdac7, Senp1,Plxnb2, Sbf1, Nell2 |
| Cfml1 | The percentage of time spent freezing during the first post-shock interval on Day 4 for cued fear conditioning (cued fear memory index) | 4 | 127.45–132.63 | 5.18 | 9.0 | 0.05 | Ak2, Hpca, Phactr4, Sdc3, Azin2, Hdac1 |
| Lal4 | Time spent immobile in an open field during a 5 min test period in the open field test (locomotor activity index) | 4 | 149.98–152.61 | 2.63 | 9.8 | 0.1 | Tnfrsf9, Per3, Camta1 |
| All4 | Total distance traveled in the periphery of an open field during a 5 min test period in the open field test (anxiety level index) | 7 | 149.55–152.51 | 2.96 | 8.3 | 0.1 | Shank2, Ctsd, Igf2, Th |
| Cfml2 | The percentage of time spent freezing during the third post-shock interval on Day 4 for cued fear conditioning & The percentage of time spent freezing during the fifth post-shock interval on Day 4 for cued fear conditioning & The percentage of time spent freezing during five post-shock intervals on Day 4 for cued fear conditioning(cued fear memory index) | 2 | 156.60–170.90 | 14.3 | 8.8 | 0.1 | Src3, Neurl2, Elmo2, Sulf2, Pigt, Ctnnbl1, Slc12a5, Cd40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, W.; Zhang, L.; Zhang, Y.; Sun, X.; Wang, J.; Li, X.; Zhang, L.; Wang, X.; Morahan, G.; Qin, C. Genetic Mapping of Behavioral Traits Using the Collaborative Cross Resource. Int. J. Mol. Sci. 2023, 24, 682. https://doi.org/10.3390/ijms24010682
Xuan W, Zhang L, Zhang Y, Sun X, Wang J, Li X, Zhang L, Wang X, Morahan G, Qin C. Genetic Mapping of Behavioral Traits Using the Collaborative Cross Resource. International Journal of Molecular Sciences. 2023; 24(1):682. https://doi.org/10.3390/ijms24010682
Chicago/Turabian StyleXuan, Wei, Ling Zhang, Yu Zhang, Xiuping Sun, Jue Wang, Xianglei Li, Lingyan Zhang, Xinpei Wang, Grant Morahan, and Chuan Qin. 2023. "Genetic Mapping of Behavioral Traits Using the Collaborative Cross Resource" International Journal of Molecular Sciences 24, no. 1: 682. https://doi.org/10.3390/ijms24010682
APA StyleXuan, W., Zhang, L., Zhang, Y., Sun, X., Wang, J., Li, X., Zhang, L., Wang, X., Morahan, G., & Qin, C. (2023). Genetic Mapping of Behavioral Traits Using the Collaborative Cross Resource. International Journal of Molecular Sciences, 24(1), 682. https://doi.org/10.3390/ijms24010682







