Understanding the Biophysical Interaction of LTX-315 with Tumoral Model Membranes
Abstract
1. Introduction
2. Results
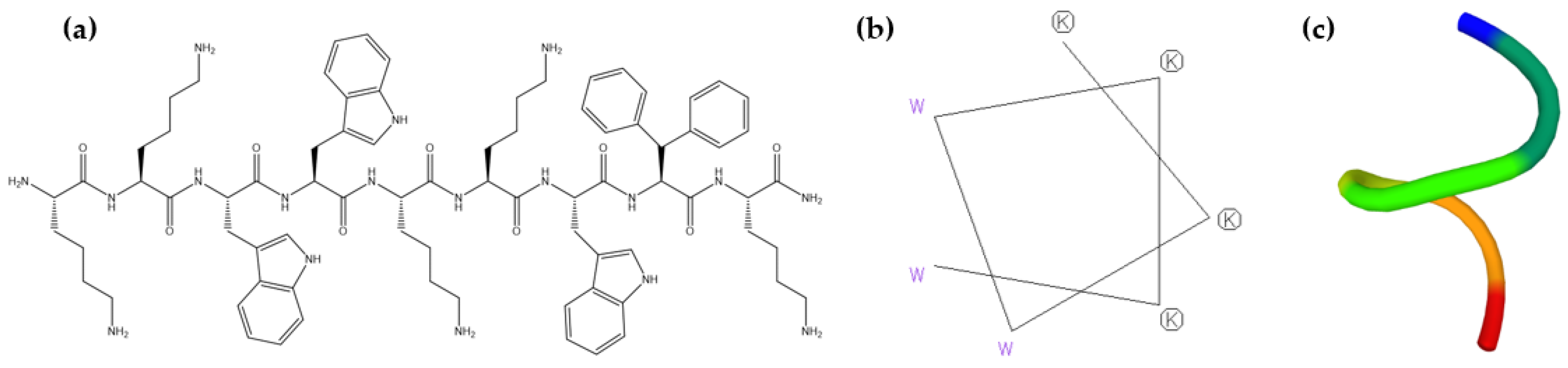
2.1. Prediction of LTX-315 Structure
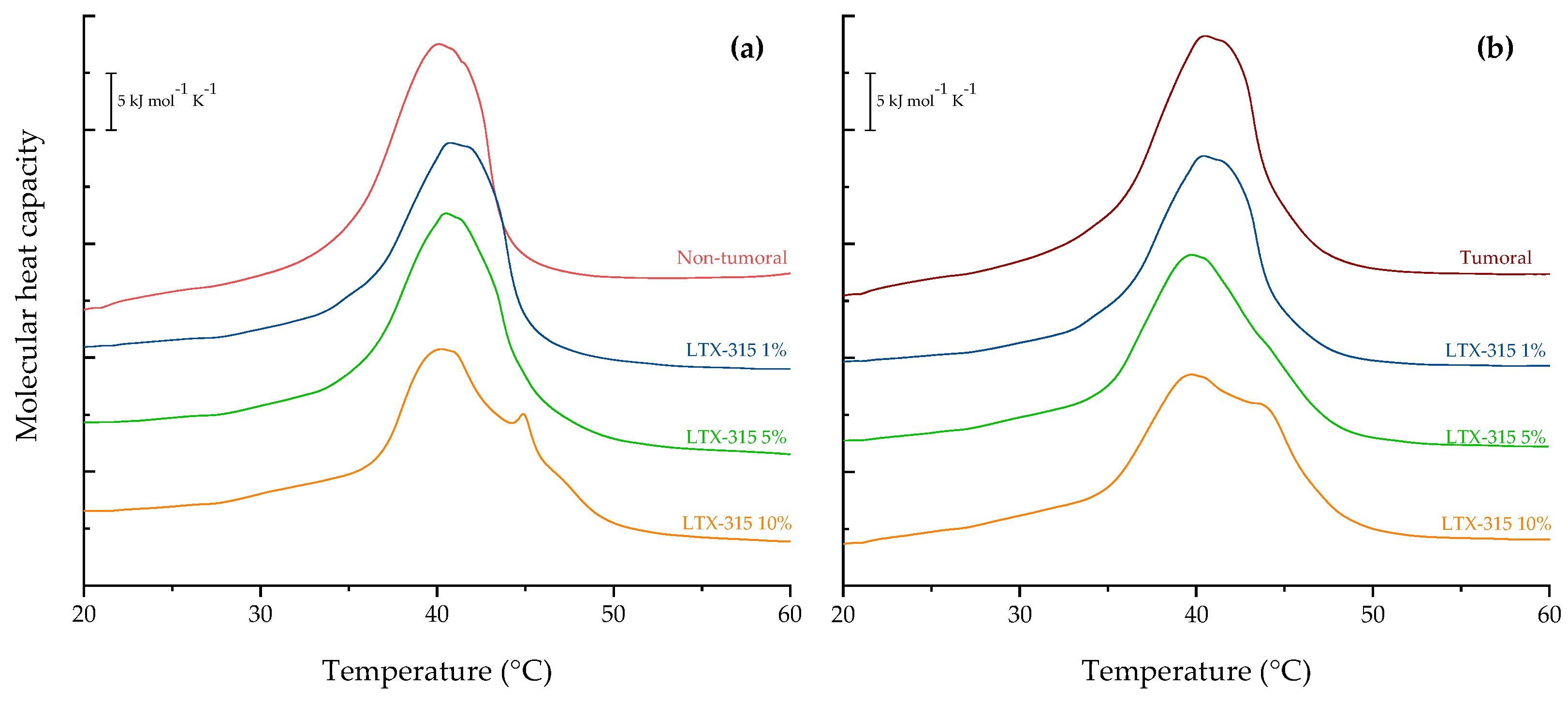
2.2. Differential Scanning Calorimetry (DSC) of Tumoral and Non-Tumoral Model Membranes
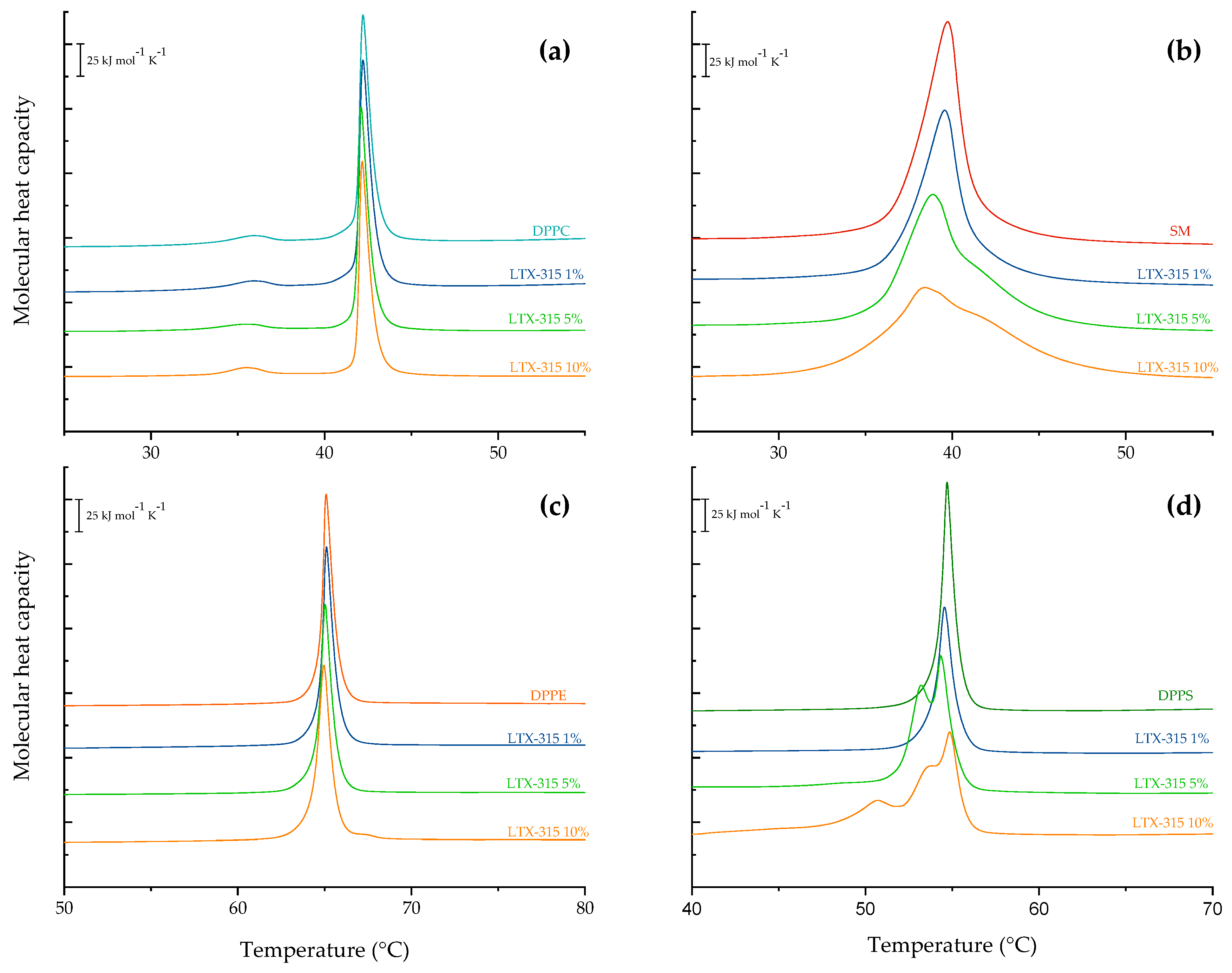
2.3. Phase Transition Measurements
2.4. Determination of the Secondary Structure of LTX-315
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Prediction of LTX-315 Structure
4.3. Differential Scanning Calorimetry (DSC) of Tumoral and Non-Tumoral Model Membranes
4.4. Infrared Spectroscopy Experiments
4.5. Determination of the Secondary Structure of LTX-315
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Beuerman, R.W.; Dua, H.S.; Lakshminarayanan, R.; Mohammed, I. Strategies in translating the therapeutic potentials of host defense peptides. Front. Immunol. 2020, 11, 983. [Google Scholar] [CrossRef]
- Dostert, M.; Pedraz, L.; Hancock, R.E. Host Defense Peptides: Multifront Attack on Biofilms. In Antibiofilm Strategies; Springer: Berlin/Heidelberg, Germany, 2022; pp. 299–323. [Google Scholar]
- Do, N.; Weindl, G.; Grohmann, L.; Salwiczek, M.; Koksch, B.; Korting, H.C.; Schäfer-Korting, M. Cationic membrane-active peptides–anticancer and antifungal activity as well as penetration into human skin. Exp. Dermatol. 2014, 23, 326–331. [Google Scholar] [CrossRef]
- Hegedüs, N.; Marx, F. Antifungal proteins: More than antimicrobials? Fungal Biol. Rev. 2013, 26, 132–145. [Google Scholar] [CrossRef]
- Arias, M.; Haney, E.F.; Hilchie, A.L.; Corcoran, J.A.; Hyndman, M.E.; Hancock, R.E.; Vogel, H.J. Selective anticancer activity of synthetic peptides derived from the host defence peptide tritrpticin. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183228. [Google Scholar] [CrossRef]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635. [Google Scholar] [CrossRef]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef]
- Hancock, R.E.; Alford, M.A.; Haney, E.F. Antibiofilm activity of host defence peptides: Complexity provides opportunities. Nat. Rev. Microbiol. 2021, 19, 786–797. [Google Scholar] [CrossRef]
- Haug, B.E.; Camilio, K.A.; Eliassen, L.T.; Stensen, W.; Svendsen, J.S.; Berg, K.; Mortensen, B.; Serin, G.; Mirjolet, J.-F.; Bichat, F. Discovery of a 9-Mer Cationic Peptide (LTX-315) as a Potential First in Class Oncolytic Peptide; ACS Publications: Washington, DC, USA, 2016. [Google Scholar]
- Sveinbjørnsson, B.; Camilio, K.A.; Haug, B.E.; Rekdal, Ø. LTX-315: A first-in-class oncolytic peptide that reprograms the tumor microenvironment. Future Med. Chem. 2017, 9, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Eike, L.-M.; Yang, N.; Rekdal, Ø.; Sveinbjørnsson, B. The oncolytic peptide LTX-315 induces cell death and DAMP release by mitochondria distortion in human melanoma cells. Oncotarget 2015, 6, 34910. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Monberg, T.; Albieri, B.; Sundvold, V.; Rekdal, O.; Junker, N.; Svane, I.M. LTX-315 and Adoptive Cell Therapy Using Tumor-Infiltrating Lymphocytes in Patients with Metastatic Soft Tissue Sarcoma; American Society of Clinical Oncology: Alexandria, VA, USA, 2022. [Google Scholar]
- Camilio, K.A.; Wang, M.-Y.; Mauseth, B.; Waagene, S.; Kvalheim, G.; Rekdal, Ø.; Sveinbjørnsson, B.; Mælandsmo, G.M. Combining the oncolytic peptide LTX-315 with doxorubicin demonstrates therapeutic potential in a triple-negative breast cancer model. Breast Cancer Res. 2019, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Wennerberg, E.; Hensler, M.; Buqué, A.; Kraynak, J.; Fucikova, J.; Zhou, X.K.; Sveinbjørnsson, B.; Rekdal, Ø.; Demaria, S. LTX-315-enabled, radiotherapy-boosted immunotherapeutic control of breast cancer by NK cells. Oncoimmunology 2021, 10, 1962592. [Google Scholar] [CrossRef] [PubMed]
- Camilio, K.A.; Berge, G.; Ravuri, C.S.; Rekdal, Ø.; Sveinbjørnsson, B. Complete regression and systemic protective immune responses obtained in B16 melanomas after treatment with LTX-315. Cancer Immunol. Immunother. 2014, 63, 601–613. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=LTX-315&term=&cntry=&state=&city=&dist (accessed on 4 October 2022).
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application. Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Ran, S.; Downes, A.; Thorpe, P.E. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002, 62, 6132–6140. [Google Scholar]
- Doktorova, M.; Symons, J.L.; Levental, I. Structural and functional consequences of reversible lipid asymmetry in living membranes. Nat. Chem. Biol. 2020, 16, 1321–1330. [Google Scholar] [CrossRef]
- Zwaal, R.; Comfurius, P.; Bevers, E. Surface exposure of phosphatidylserine in pathological cells. Cell. Mol. Life Sci. 2005, 62, 971–988. [Google Scholar] [CrossRef]
- Riedl, S.; Rinner, B.; Asslaber, M.; Schaider, H.; Walzer, S.; Novak, A.; Lohner, K.; Zweytick, D. In search of a novel target—Phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Trinidad-Calderón, P.A.; Varela-Chinchilla, C.D.; García-Lara, S. Natural Peptides Inducing Cancer Cell Death: Mechanisms and Properties of Specific Candidates for Cancer Therapeutics. Molecules 2021, 26, 7453. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial peptides as anticancer agents: Functional properties and biological activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef] [PubMed]
- Bobone, S.; Piazzon, A.; Orioni, B.; Pedersen, J.Z.; Nan, Y.H.; Hahm, K.S.; Shin, S.Y.; Stella, L. The thin line between cell-penetrating and antimicrobial peptides: The case of Pep-1 and Pep-1-K. J. Pept. Sci. 2011, 17, 335–341. [Google Scholar] [CrossRef]
- Hadianamrei, R.; Tomeh, M.A.; Brown, S.; Wang, J.; Zhao, X. Rationally designed short cationic α-helical peptides with selective anticancer activity. J. Colloid Interface Sci. 2022, 607, 488–501. [Google Scholar] [CrossRef]
- Spicer, J.; Marabelle, A. Safety, Antitumor Activity, and T-cell Responses in a Dose-Ranging Phase I Trial of the Oncolytic Peptide LTX-315 in Patients with Solid Tumors. Clin. Cancer Res. 2021, 27, 2755–2763. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Utsugi, T.; Schroit, A.J.; Connor, J.; Bucana, C.D.; Fidler, I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991, 51, 3062–3066. [Google Scholar]
- Luchini, A.; Vitiello, G. Mimicking the mammalian plasma membrane: An overview of lipid membrane models for biophysical studies. Biomimetics 2020, 6, 3. [Google Scholar] [CrossRef]
- Gong, Z.; Ikonomova, S.P.; Karlsson, A.J. Secondary structure of cell-penetrating peptides during interaction with fungal cells. Protein Sci. 2018, 27, 702–713. [Google Scholar] [CrossRef]
- Hädicke, A.; Blume, A. Binding of cationic model peptides (KX) 4K to anionic lipid bilayers: Lipid headgroup size influences secondary structure of bound peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Camilio, K.A.; Rekdal, Ø.; Sveinbjörnsson, B. LTX-315 (Oncopore™) A short synthetic anticancer peptide and novel immunotherapeutic agent. Oncoimmunology 2014, 3, e29181. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Mansour, S.C.; Hancock, R.E. Antimicrobial peptides: An introduction. In Antimicrobial Peptides; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–22. [Google Scholar]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- Çetinel, Z.Ö.; Bilge, D. The effects of miltefosine on the structure and dynamics of DPPC and DPPS liposomes mimicking normal and cancer cell membranes: FTIR and DSC studies. J. Mol. Liq. 2022, 356, 119041. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N. Calorimetric and spectroscopic studies of the polymorphic phase behavior of a homologous series of n-saturated 1,2-diacyl phosphatidylethanolamines. Biophys. J. 1993, 64, 1081–1096. [Google Scholar] [CrossRef]
- Pohle, W.; Gauger, D.R.; Dornberger, U.; Birch-Hirschfeld, E.; Selle, C.; Rupprecht, A.; Bohl, M. Hydration of biological molecules: Lipids versus nucleic acids. Biopolymers 2002, 67, 499–503. [Google Scholar] [CrossRef]
- Hübner, W.; Blume, A. Interactions at the lipid-water interface. Chem. Phys. Lipids 1998, 96, 99–123. [Google Scholar] [CrossRef]
- Selle, C.; Pohle, W. Fourier transform infrared spectroscopy as a probe for the study of the hydration of lipid self-assemblies. II. Water binding versus phase transitions. Biospectroscopy 1998, 4, 281–294. [Google Scholar] [CrossRef]
- Milhaud, J. New insights into water-phospholipid model membrane interactions. Biochim. Biophys. Acta 2004, 1663, 19–51. [Google Scholar] [CrossRef]
- Miller, I.R.; Bach, D.; Wachtel, E.J.; Eisenstein, M. Interrelation between hydration and interheadgroup interaction in phospholipids. Bioelectrochemistry 2002, 58, 193–196. [Google Scholar] [CrossRef]
- Ganesan, S.J.; Schneider, J.P.; Blumenthal, R.; Matysiak, S. Characterization of Anticancer Peptides in Membrane Disruption. Biophys. J. 2013, 104 (Suppl. S1), 597a. [Google Scholar] [CrossRef][Green Version]
- Luan, X.; Wu, Y.; Shen, Y.-W.; Zhang, H.; Zhou, Y.-D.; Chen, H.-Z.; Nagle, D.G.; Zhang, W.-D. Cytotoxic and antitumor peptides as novel chemotherapeutics. Nat. Prod. Rep. 2021, 38, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.J.; Sut, T.N.; Tan, S.W.; Yoon, B.K.; Jackman, J.A. Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge. Int. J. Mol. Sci. 2022, 23, 10558. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: Classification, mechanism of action, reconstruction and modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Vunnam, S.; Juvvadi, P.; Merrifield, R. Synthesis and antibacterial action of cecropin and proline-arginine-rich peptides from pig intestine. J. Pept. Res. 1997, 49, 59–66. [Google Scholar] [CrossRef]
- Jo, H.; Lee, D.; Go, C.; Jang, Y.; Bae, S.; Agura, T.; Hong, J.; Kang, D.; Kim, Y.; Kang, J.S. Alloferon Affects the Chemosensitivity of Pancreatic Cancer by Regulating the Expression of SLC6A14. Biomedicines 2022, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Kuczer, M.; Midak-Siewirska, A.; Zahorska, R.; Łuczak, M.; Konopińska, D. Further studies on the antiviral activity of alloferon and its analogues. J. Pept. Sci. 2011, 17, 715–719. [Google Scholar] [CrossRef]
- Lewis, R.N.; Mannock, D.A.; McElhaney, R.N. Differential Scanning Calorimetry (DSC) for Lipids and Lipid Membranes; Wiley Encyclopedia of Chemical Biology: Hoboken, NJ, USA, 2008; pp. 1–11. [Google Scholar]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan-and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1184–1202. [Google Scholar] [CrossRef]
- Niemelä, P.; Hyvönen, M.T.; Vattulainen, I. Structure and dynamics of sphingomyelin bilayer: Insight gained through systematic comparison to phosphatidylcholine. Biophys. J. 2004, 87, 2976–2989. [Google Scholar] [CrossRef]
- Shen, Y.; Maupetit, J.; Derreumaux, P.; Tufféry, P. Improved PEP-FOLD approach for peptide and miniprotein structure prediction. J. Chem. Theory Comput. 2014, 10, 4745–4758. [Google Scholar] [CrossRef] [PubMed]
- Thévenet, P.; Shen, Y.; Maupetit, J.; Guyon, F.; Derreumaux, P.; Tuffery, P. PEP-FOLD: An updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012, 40, W288–W293. [Google Scholar] [CrossRef] [PubMed]
- Almarwani, B.; Phambu, E.N.; Alexander, C.; Nguyen, H.A.T.; Phambu, N.; Sunda-Meya, A. Vesicles mimicking normal and cancer cell membranes exhibit differential responses to the cell-penetrating peptide Pep-1. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.; Gilbert, G.E.; Shi, J.; Silvius, J.; Kapus, A.; Grinstein, S. Membrane phosphatidylserine regulates surface charge and protein localization. Science 2008, 319, 210–213. [Google Scholar] [CrossRef]
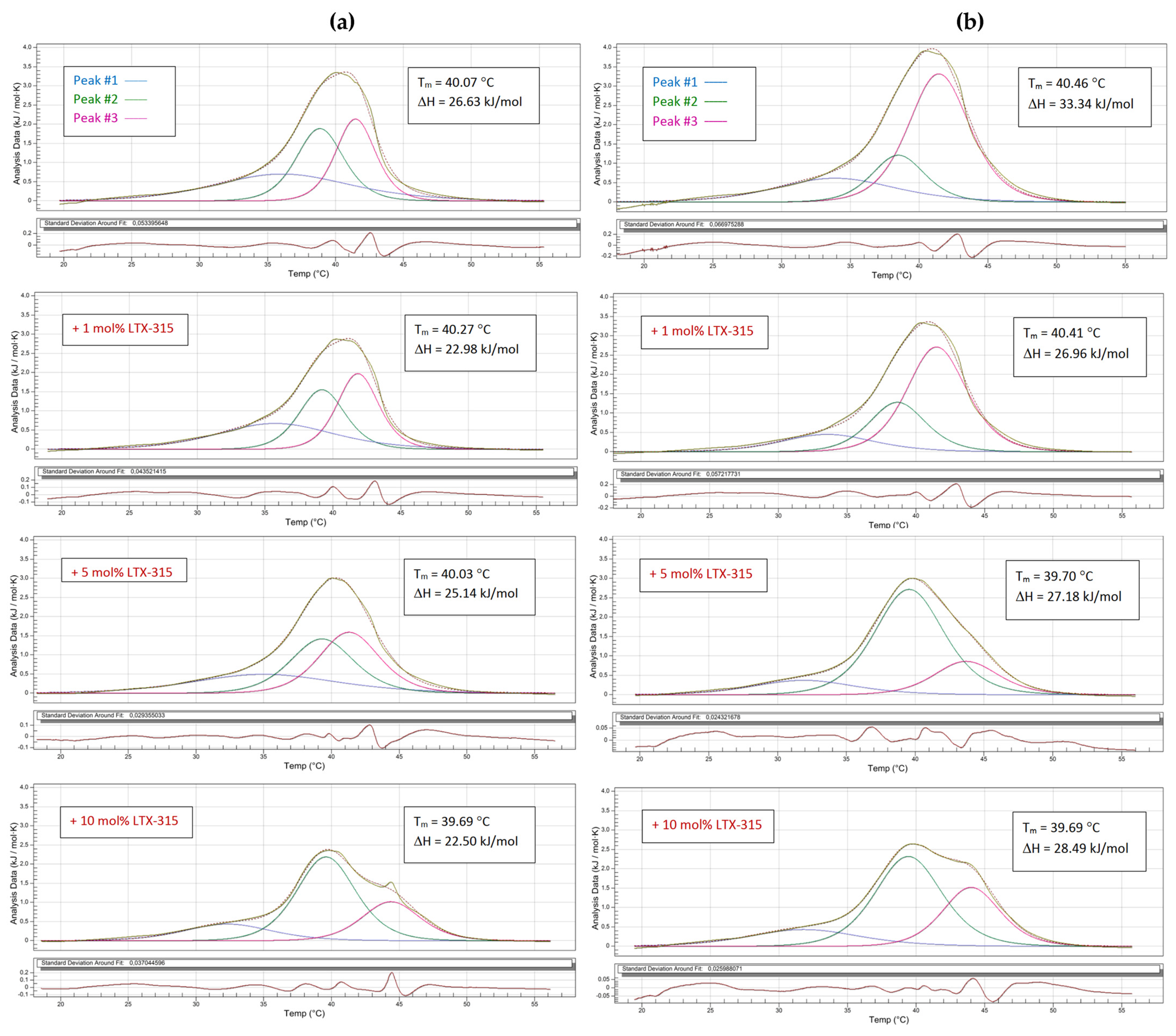
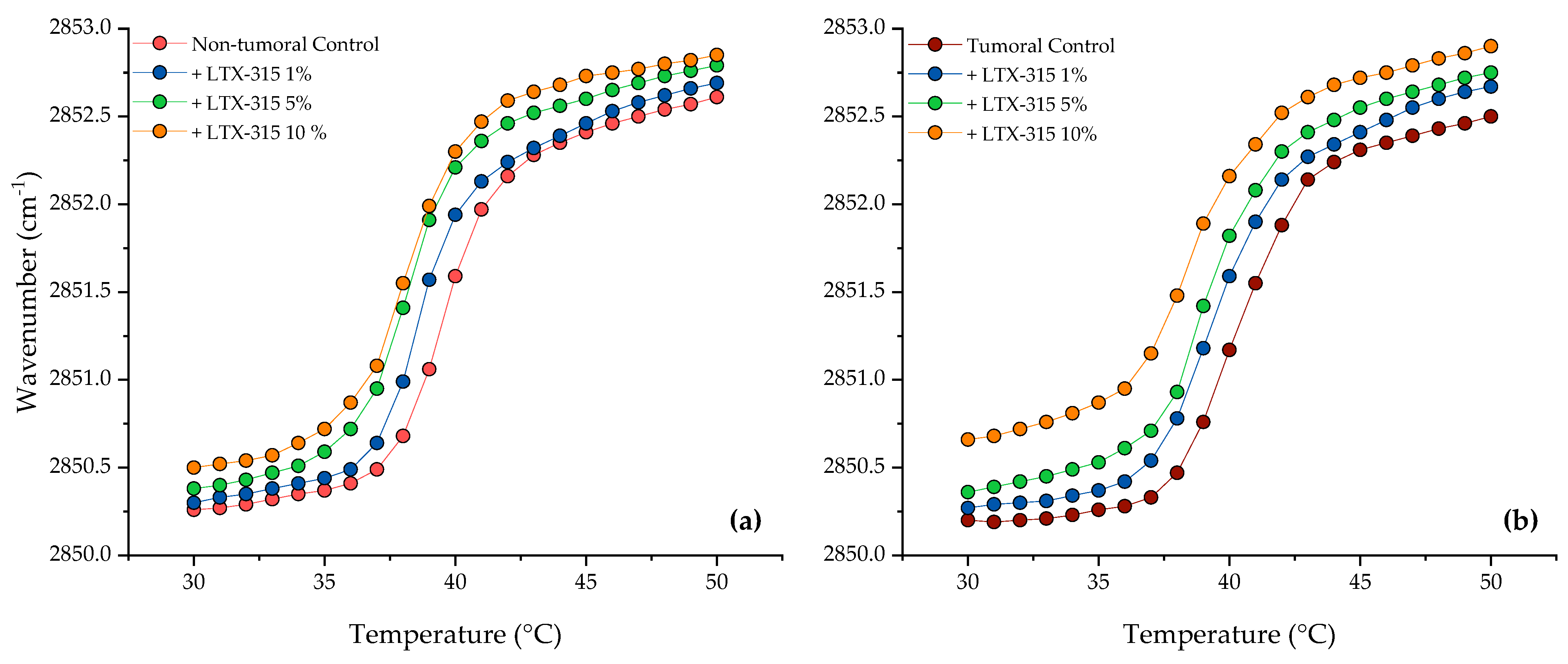
| Peak #1 | Peak #2 | Peak #3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | ∆T1/2 (°C) | Cpmax (kJ mol−1 K−1) | ∆H (kJ mol−1) | T (°C) | ∆T1/2 (°C) | Cpmax (kJ mol−1 K−1) | ∆H (kJ mol−1) | T (°C) | ∆T1/2 (°C) | Cpmax (kJ mol−1 K−1) | ∆H (kJ mol−1) | |
| Non-tumoral | 36.20 | 14.69 | 0.69 | 9.48 | 38.86 | 3.98 | 1.88 | 8.02 | 41.48 | 3.37 | 2.13 | 8.39 |
| +1 mol% LTX-315 | 35.84 | 11.22 | 0.69 | 7.69 | 39.20 | 3.57 | 1.80 | 7.10 | 41.84 | 3.16 | 2.24 | 8.07 |
| +5 mol% LTX-315 | 35.14 | 18.56 | 0.76 | 7.24 | 39.32 | 3.88 | 2,02 | 8.79 | 41.32 | 4.79 | 1.84 | 9.39 |
| +10 mol% LTX-315 | 32.35 | 9.38 | 0.43 | 3.92 | 39.61 | 4.69 | 2.45 | 12.87 | 44.40 | 5.20 | 1.01 | 5.85 |
| Tumoral | 33.98 | 8.26 | 0.87 | 7.20 | 38.51 | 3.57 | 1.47 | 19.78 | 41.46 | 5.00 | 3.31 | 6.26 |
| +1 mol% LTX-315 | 33.62 | 8.77 | 0.45 | 4.15 | 38.68 | 4.90 | 1.28 | 15.95 | 41.53 | 5.10 | 2.71 | 7.10 |
| +5 mol%LTX-315 | 31.95 | 11.22 | 0.38 | 3.72 | 39.58 | 6.32 | 2.71 | 18.40 | 43.71 | 6.12 | 0.87 | 5.36 |
| +10 mol%LTX-315 | 31.87 | 11.42 | 0.38 | 4.81 | 39.46 | 5.71 | 2.33 | 14.97 | 44.03 | 4.90 | 1.51 | 8.32 |
| LTX-315 (mol%) | Tm (°C) | |
|---|---|---|
| Non-Tumoral | Tumoral | |
| 0 | 39.7 | 40.4 |
| 1.0 | 38.4 | 39.7 |
| 5.0 | 38.3 | 39.4 |
| 10.0 | 38.3 | 38.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaiss-Luna, M.C.; Jemioła-Rzemińska, M.; Strzałka, K.; Manrique-Moreno, M. Understanding the Biophysical Interaction of LTX-315 with Tumoral Model Membranes. Int. J. Mol. Sci. 2023, 24, 581. https://doi.org/10.3390/ijms24010581
Klaiss-Luna MC, Jemioła-Rzemińska M, Strzałka K, Manrique-Moreno M. Understanding the Biophysical Interaction of LTX-315 with Tumoral Model Membranes. International Journal of Molecular Sciences. 2023; 24(1):581. https://doi.org/10.3390/ijms24010581
Chicago/Turabian StyleKlaiss-Luna, Maria C., Małgorzata Jemioła-Rzemińska, Kazimierz Strzałka, and Marcela Manrique-Moreno. 2023. "Understanding the Biophysical Interaction of LTX-315 with Tumoral Model Membranes" International Journal of Molecular Sciences 24, no. 1: 581. https://doi.org/10.3390/ijms24010581
APA StyleKlaiss-Luna, M. C., Jemioła-Rzemińska, M., Strzałka, K., & Manrique-Moreno, M. (2023). Understanding the Biophysical Interaction of LTX-315 with Tumoral Model Membranes. International Journal of Molecular Sciences, 24(1), 581. https://doi.org/10.3390/ijms24010581









