A Novel Biocidal Nanocomposite: Spherical Silica with Silver Ions Anchored at the Surface
Abstract
1. Introduction
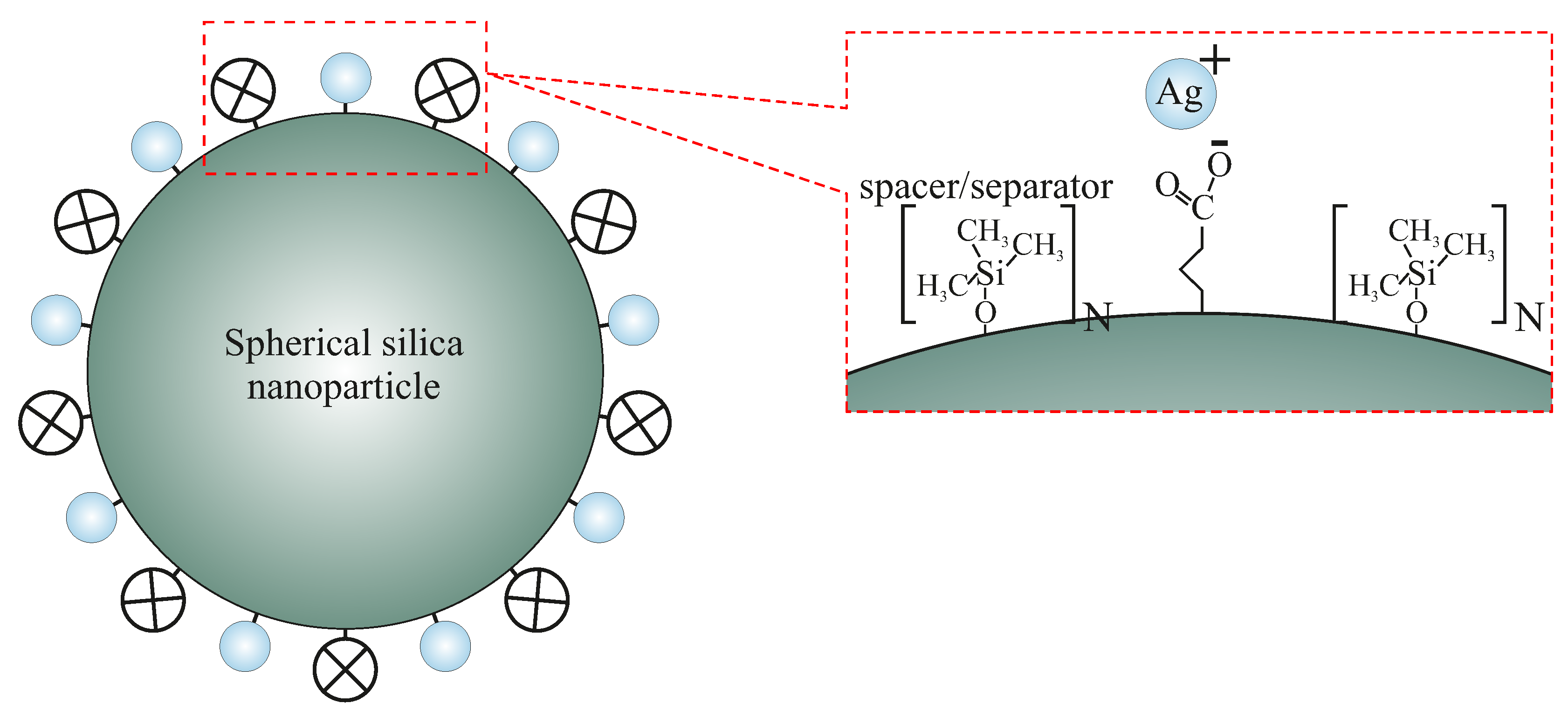
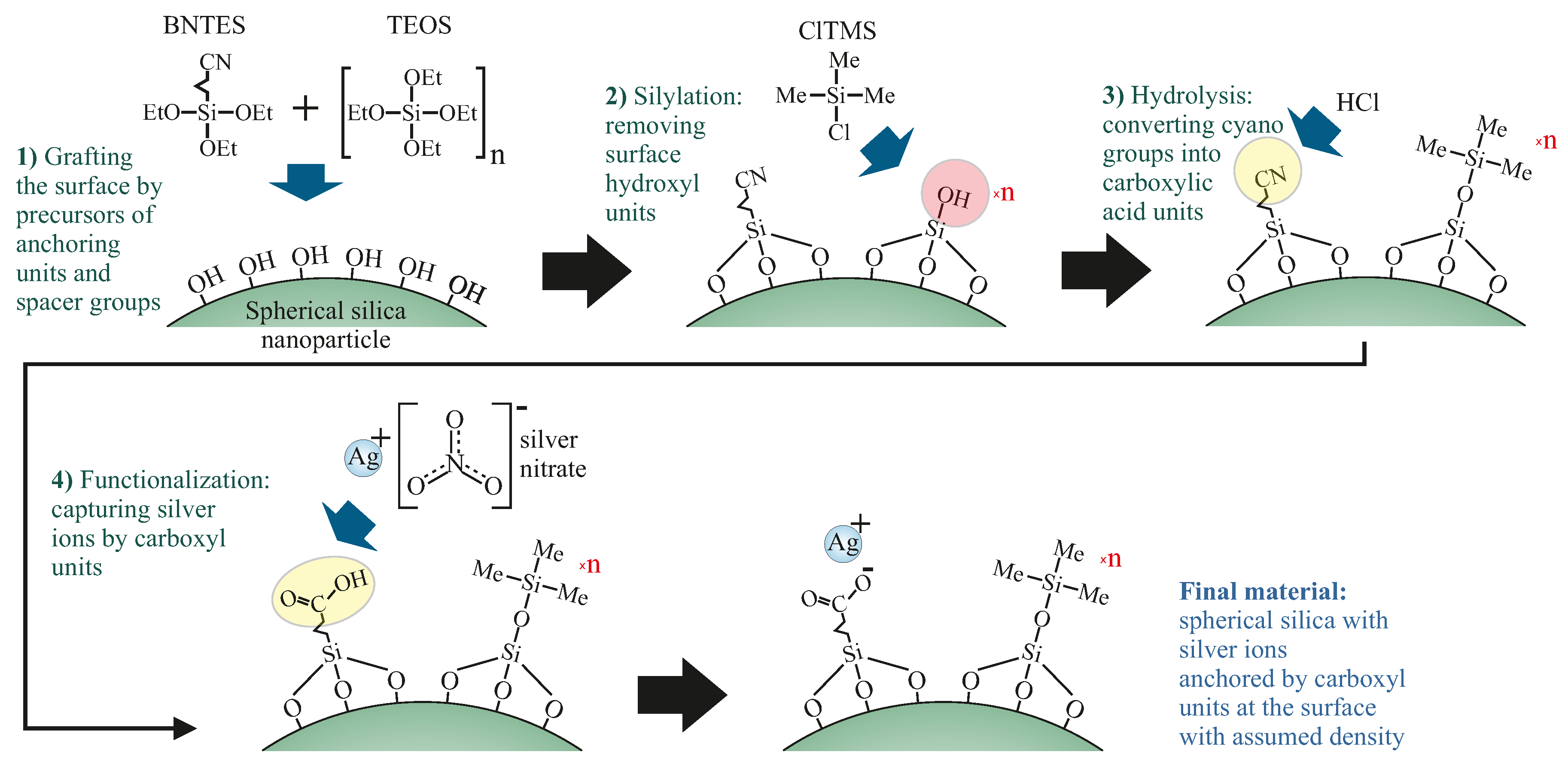
- SilS-COOAg 1—spherical silica functionalized with silver ions, containing one spacer unit per single functional group (the highest concentration of silver among all the samples).
- SilS-COOAg 6—spherical silica functionalized with silver ions, containing six spacer units per single functional group.
- SilS-COOAg 12—spherical silica functionalized with silver ions, containing 12 spacer units per single functional group (the lowest concentration of silver among all functionalized samples).
2. Results and Discussion
2.1. Transmission Electron Microscopy Imaging
2.2. Antimicrobial Tests
3. Materials and Methods
3.1. Synthesis of Silica Nanoparticles Functionalized Silver Ions
3.2. Characterization Methods
3.3. Microorganisms and Media
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TEOS | tetraethyl orthosilicate |
| BNTES | butyronitrile triethoxysilane |
| ClTMS | trimethylsilyl chloride |
| Fpg | formamidopyrimidine-DNA glycosylase |
| MIC | minimal inhibitory concentration |
| MBC | minimum bactericidal concentration |
| LPS | lipopolysaccharides |
References
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Bone, A.J.; Colman, B.P.; Gondikas, A.P.; Newton, K.M.; Harrold, K.H.; Cory, R.M.; Unrine, J.M.; Klaine, S.J.; Matson, C.W.; Di Giulio, R.T. Biotic and abiotic interactions in aquatic microcosms determine fate and toxicity of Ag nanoparticles: Part 2—Toxicity and Ag speciation. Environ. Sci. Technol. 2012, 46, 6925–6933. [Google Scholar] [CrossRef] [PubMed]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef]
- Yu, S.J.; Yin, Y.G.; Liu, J.F. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Panyala, N.R.; Peña-Méndez, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef]
- Banach, M.; Pulit-Prociak, J. Synthesis, characteristics, and biocidal activity of silver nanoparticles. In Fabrication and Self-Assembly of Nanobiomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 367–399. [Google Scholar]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Khurana, C.; Sharma, P.; Pandey, O.; Chudasama, B. Synergistic effect of metal nanoparticles on the antimicrobial activities of antibiotics against biorecycling microbes. J. Mater. Sci. Technol. 2016, 32, 524–532. [Google Scholar] [CrossRef]
- Khurana, C.; Vala, A.K.; Andhariya, N.; Pandey, O.; Chudasama, B. Antibacterial activity of silver: The role of hydrodynamic particle size at nanoscale. J. Biomed. Mater. Res. Part A 2014, 102, 3361–3368. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Jannathul Firdhouse, M.; Lalitha, P. Biocidal potential of biosynthesized silver nanoparticles against fungal threats. J. Nanostruct. Chem. 2015, 5, 25–33. [Google Scholar] [CrossRef]
- Laskowska, M.; Pastukh, O.; Fedorchuk, A.; Schabikowski, M.; Kowalczyk, P.; Zalasiński, M.; Laskowski, Ł. Nanostructured Silica with Anchoring Units: The 2D Solid Solvent for Molecules and Metal Ions. Int. J. Mol. Sci. 2020, 21, 8137. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M.; Oyama, M.; Kityk, I.; Marszalek, M.; Dulski, M.; Laskowski, L. Surface functionalization by silver-containing molecules with controlled distribution of functionalities. Appl. Surf. Sci. 2019, 481, 433–436. [Google Scholar] [CrossRef]
- Gnatowski, A.; Jelonkiewicz, J.; Laskowski, Ł.; Laskowska, M. Influence of the copper-containing SBA-15 silica fillers on the mechanical properties of high density polyethylene. J. Nanomater. 2016, 2016, 3291719. [Google Scholar] [CrossRef]
- Sabri, B.A.; Meenaloshini, S.; Abreeza, N.; Abed, A.N. A review study on coupling agents used as ceramic fillers modifiers for dental applications. Mater. Today Proc. 2021. In Press. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Potiyaraj, P. A critical review on the utilization of various reinforcement modifiers in filled rubber composites. J. Elastomers Plast. 2020, 52, 167–193. [Google Scholar] [CrossRef]
- Xanthos, M. Functional Fillers for Plastics; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Wang, Y.; Zhu, M.; Zhu, X. Functional fillers for dental resin composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Wan, Z.; Yang, X.; Cai, Q. Dental resin composites with improved antibacterial and mineralization properties via incorporating zinc/strontium-doped hydroxyapatite as functional fillers. Biomed. Mater. 2022, 17, 045002. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Borkowski, A.; Czerwonka, G.; Cłapa, T.; Cieśla, J.; Misiewicz, A.; Borowiec, M.; Szala, M. The microbial toxicity of quaternary ammonium ionic liquids is dependent on the type of lipopolysaccharide. J. Mol. Liq. 2018, 266, 540–547. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Wilk, M.; Parul, P.; Szymczak, M.; Kramkowski, K.; Raj, S.; Skiba, G.; Sulejczak, D.; Kleczkowska, P.; Ostaszewski, R. The Synthesis and Evaluation of Aminocoumarin Peptidomimetics as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials 2021, 14, 5725. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Trzepizur, D.; Szymczak, M.; Skiba, G.; Kramkowski, K.; Ostaszewski, R. 1, 2-Diarylethanols—A New Class of Compounds That Are Toxic to E. coli K12, R2–R4 Strains. Materials 2021, 14, 1025. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Gawdzik, B.; Trzepizur, D.; Szymczak, M.; Skiba, G.; Raj, S.; Kramkowski, K.; Lizut, R.; Ostaszewski, R. δ-Lactones—A New Class of Compounds That Are Toxic to E. coli K12 and R2–R4 Strains. Materials 2021, 14, 2956. [Google Scholar] [CrossRef] [PubMed]
- Sahrawat, P.; Kowalczyk, P.; Koszelewski, D.; Szymczak, M.; Kramkowski, K.; Wypych, A.; Ostaszewski, R. Influence of Open Chain and Cyclic Structure of Peptidomimetics on Antibacterial Activity in E. coli Strains. Molecules 2022, 27, 3633. [Google Scholar] [CrossRef] [PubMed]
- Brodzka, A.; Kowalczyk, P.; Trzepizur, D.; Koszelewski, D.; Kramkowski, K.; Szymczak, M.; Wypych, A.; Lizut, R.; Ostaszewski, R. The Synthesis and Evaluation of Diethyl Benzylphosphonates as Potential Antimicrobial Agents. Molecules 2022, 27, 6865. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin-and fish oil-loaded spongosome and cubosome nanoparticles with neuroprotective potential against H2O2-induced oxidative stress in differentiated human SH-SY5Y cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Barros, C.H.; Fulaz, S.; Stanisic, D.; Tasic, L. Biogenic nanosilver against multidrug-resistant bacteria (MDRB). Antibiotics 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.B.R.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. In Vitro 2017, 38, 179–192. [Google Scholar] [CrossRef]
- Verkhovskii, R.; Kozlova, A.; Atkin, V.; Kamyshinsky, R.; Shulgina, T.; Nechaeva, O. Physical properties and cytotoxicity of silver nanoparticles under different polymeric stabilizers. Heliyon 2019, 5, e01305. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Mosleh, A.M.; Abd Elghany, A.; Shams-Eldin, E.; Serea, E.S.A.; Ali, S.A.; Shalan, A.E. Coated silver nanoparticles: Synthesis, cytotoxicity, and optical properties. RSC Adv. 2019, 9, 20118–20136. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555. [Google Scholar] [CrossRef]
- Samuel, M.S.; Jose, S.; Selvarajan, E.; Mathimani, T.; Pugazhendhi, A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; Application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. B Biol. 2020, 202, 111642. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Barnard, R.T.; Blaskovich, M.; Ziora, Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, A.; Kowalczyk, P.; Czerwonka, G.; Cieśla, J.; Cłapa, T.; Misiewicz, A.; Szala, M.; Drabik, M. Interaction of quaternary ammonium ionic liquids with bacterial membranes—Studies with Escherichia coli R1–R4-type lipopolysaccharides. J. Mol. Liq. 2017, 246, 282–289. [Google Scholar] [CrossRef]
- Wernicki, A.; Puchalski, A.; Urban-Chmiel, R.; Dec, M.; Stegierska, D.; Dudzic, A.; Wojcik, A. Antimicrobial properties of gold, silver, copper and platinum nanoparticles against selected microorganisms isolated from cases of mastitis in cattle. Med. Weter. 2014, 70, 564–567. [Google Scholar]
- Gamboa, S.M.; Rojas, E.; Martínez, V.; Vega-Baudrit, J. Synthesis and characterization of silver nanoparticles and their application as an antibacterial agent. Int. J. Biosens. Bioelectron. 2019, 5, 166–173. [Google Scholar]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist. Updat. 2017, 33–35, 1–22. [Google Scholar] [CrossRef]
- Vila Domínguez, A.; Ayerbe Algaba, R.; Miró Canturri, A.; Rodríguez Villodres, Á.; Smani, Y. Antibacterial activity of colloidal silver against gram-negative and gram-positive bacteria. Antibiotics 2020, 9, 36. [Google Scholar] [CrossRef]
- Prasetyo, B.C.; Sugiharti, R.J.; Mahendra, I.; Halimah, I.; Widyasar, E.M.; Rusminah, N.; Mustika, I. Evaluation of Silver Nanoparticles Addition in Periodontal Dressing for Wound Tissue Healing by 99mTc-ciprofloxacin. J. Young Pharm. 2019, 11, 17. [Google Scholar] [CrossRef]
- Borum, A.E.; Güneş, E. Antibacterial effect of different concentrations of silver nanoparticles. Pak. Vet. J. 2018, 38, 321–324. [Google Scholar] [CrossRef]
- Tăbăran, A.F.; Matea, C.T.; Mocan, T.; Tăbăran, A.; Mihaiu, M.; Iancu, C.; Mocan, L. Silver nanoparticles for the therapy of tuberculosis. Int. J. Nanomed. 2020, 15, 2231. [Google Scholar] [CrossRef]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Kim, T.G.; An, G.S.; Han, J.S.; Hur, J.U.; Park, B.G.; Choi, S.C. Synthesis of size controlled spherical silica nanoparticles via sol-gel process within hydrophilic solvent. J. Korean Ceram. Soc. 2017, 54, 49–54. [Google Scholar] [CrossRef]
- De la Calle, I.; Menta, M.; Séby, F. Current trends and challenges in sample preparation for metallic nanoparticles analysis in daily products and environmental samples: A review. Spectrochim. Acta Part B At. Spectrosc. 2016, 125, 66–96. [Google Scholar] [CrossRef]
- Maciejewska, A.; Kaszowska, M.; Jachymek, W.; Lugowski, C.; Lukasiewicz, J. Lipopolysaccharide-linked Enterobacterial Common Antigen (ECALPS) Occurs in Rough Strains of Escherichia coli R1, R2, and R4. Int. J. Mol. Sci. 2020, 21, 6038. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Madej, A.; Szymczak, M.; Ostaszewski, R. α-Amidoamids as New Replacements of Antibiotics—Research on the Chosen K12, R2–R4 E. coli Strains. Materials 2020, 13, 5169. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Koszelewski, D.; Gawdzik, B.; Samsonowicz-Górski, J.; Kramkowski, K.; Wypych, A.; Lizut, R.; Ostaszewski, R. Promiscuous Lipase-Catalyzed Markovnikov Addition of H-Phosphites to Vinyl Esters for the Synthesis of Cytotoxic α-Acyloxy Phosphonate Derivatives. Materials 2022, 15, 1975. [Google Scholar] [CrossRef]
- Samsonowicz-Górski, J.; Koszelewski, D.; Kowalczyk, P.; Śmigielski, P.; Hrunyk, A.; Kramkowski, K.; Wypych, A.; Szymczak, M.; Lizut, R.; Ostaszewski, R. Promiscuous Lipase-Catalyzed Knoevenagel–Phospha–Michael Reaction for the Synthesis of Antimicrobial β-Phosphono Malonates. Int. J. Mol. Sci. 2022, 23, 8819. [Google Scholar] [CrossRef]


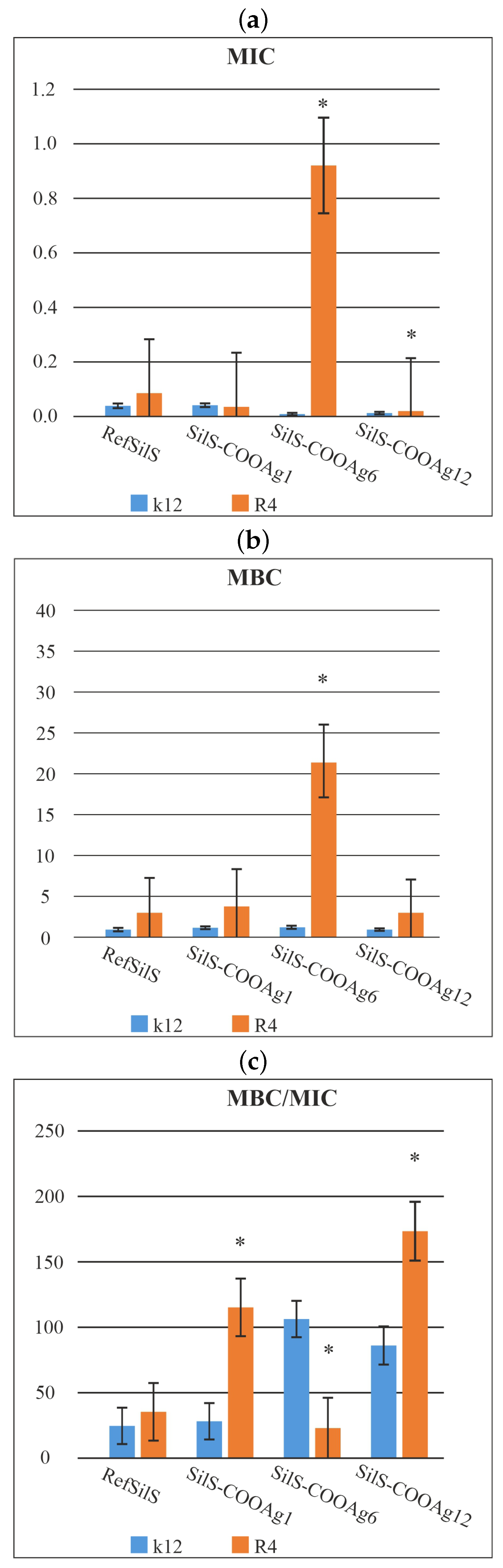
| Name of Sample | SilS-COOAg1 | SilS-COOAg6 | SilS-COOAg12 | Type of Test |
|---|---|---|---|---|
| k12 | * | * | MIC | |
| R4 | * | * | MIC | |
| k12 | * | MBC | ||
| R4 | * | MBC | ||
| k12 | * | * | * | MBC/MIC |
| R4 | * | * | * | MBC/MIC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowska, M.; Kowalczyk, P.; Karczmarska, A.; Kramkowski, K.; Wrzosek, K.; Laskowski, Ł. A Novel Biocidal Nanocomposite: Spherical Silica with Silver Ions Anchored at the Surface. Int. J. Mol. Sci. 2023, 24, 545. https://doi.org/10.3390/ijms24010545
Laskowska M, Kowalczyk P, Karczmarska A, Kramkowski K, Wrzosek K, Laskowski Ł. A Novel Biocidal Nanocomposite: Spherical Silica with Silver Ions Anchored at the Surface. International Journal of Molecular Sciences. 2023; 24(1):545. https://doi.org/10.3390/ijms24010545
Chicago/Turabian StyleLaskowska, Magdalena, Paweł Kowalczyk, Agnieszka Karczmarska, Karol Kramkowski, Karol Wrzosek, and Łukasz Laskowski. 2023. "A Novel Biocidal Nanocomposite: Spherical Silica with Silver Ions Anchored at the Surface" International Journal of Molecular Sciences 24, no. 1: 545. https://doi.org/10.3390/ijms24010545
APA StyleLaskowska, M., Kowalczyk, P., Karczmarska, A., Kramkowski, K., Wrzosek, K., & Laskowski, Ł. (2023). A Novel Biocidal Nanocomposite: Spherical Silica with Silver Ions Anchored at the Surface. International Journal of Molecular Sciences, 24(1), 545. https://doi.org/10.3390/ijms24010545







