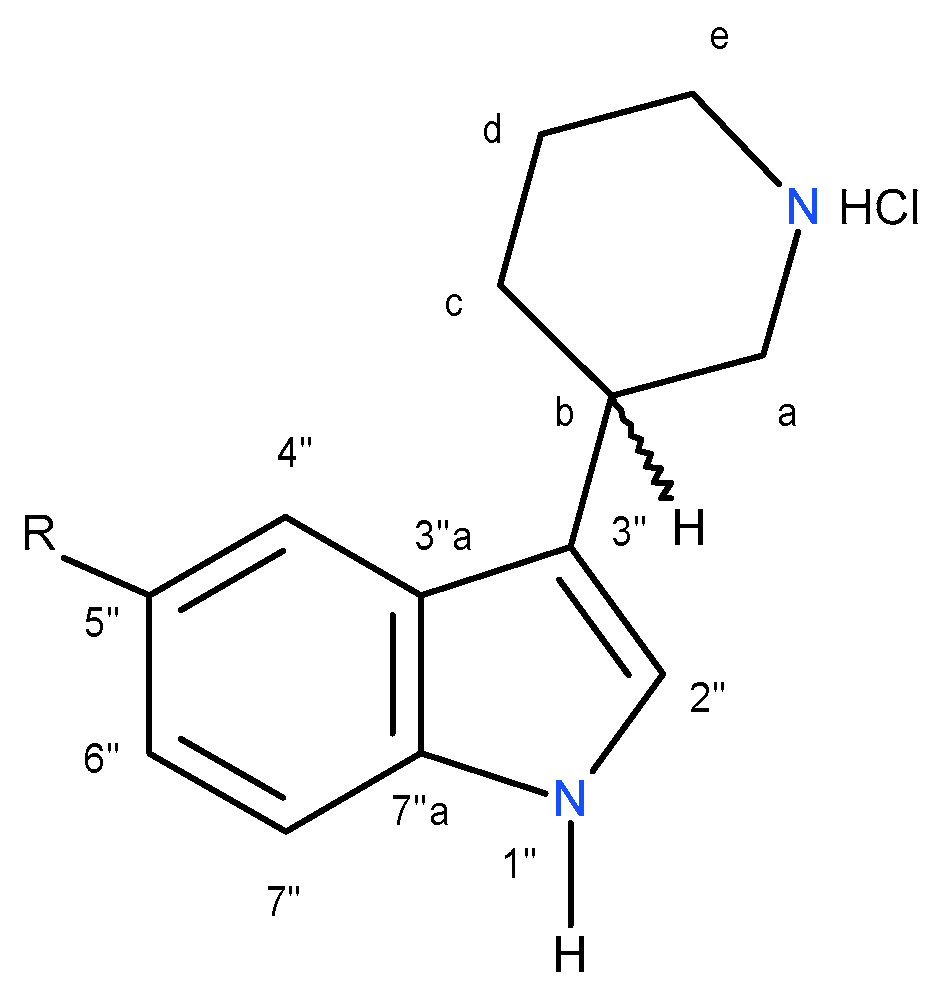
3.3.2. General Procedure for the Synthesis of Diasteromers (3R,2S)-2a, (3S,2S)-3a, (3R,2R)-4a, (3S,2R)-5a, (3R,2R)-6b, (3S,2R)-7b, (3R,2R)-8c and (3S,2R)-9c
A suitable 3- (piperidin-3-yl) -1H-indole
1a-c (0.01 m) derivative, chiral reagent
(R)-I or
(S)-II (0.01 m), and potassium carbonate (0.011 m) were added to 100 mL of acetonitrile. The mixture was stirred at 45 °C; reaction times are given in
Table 2. The post-reaction mixture was concentrated dry at 45 °C under vacuum. The residue was pre-purified on a silica gel column using an eluent CH
2Cl
2/MeOH/TEA 98:2:0.1. A mixture of
(3R,2S)-2a and
(3S,2S)-3a diastereomers was separated by semipreparative HPLC. The remaining diastereomers were separated on a silica gel column using 50:50 acetone/cyclohexane eluent.
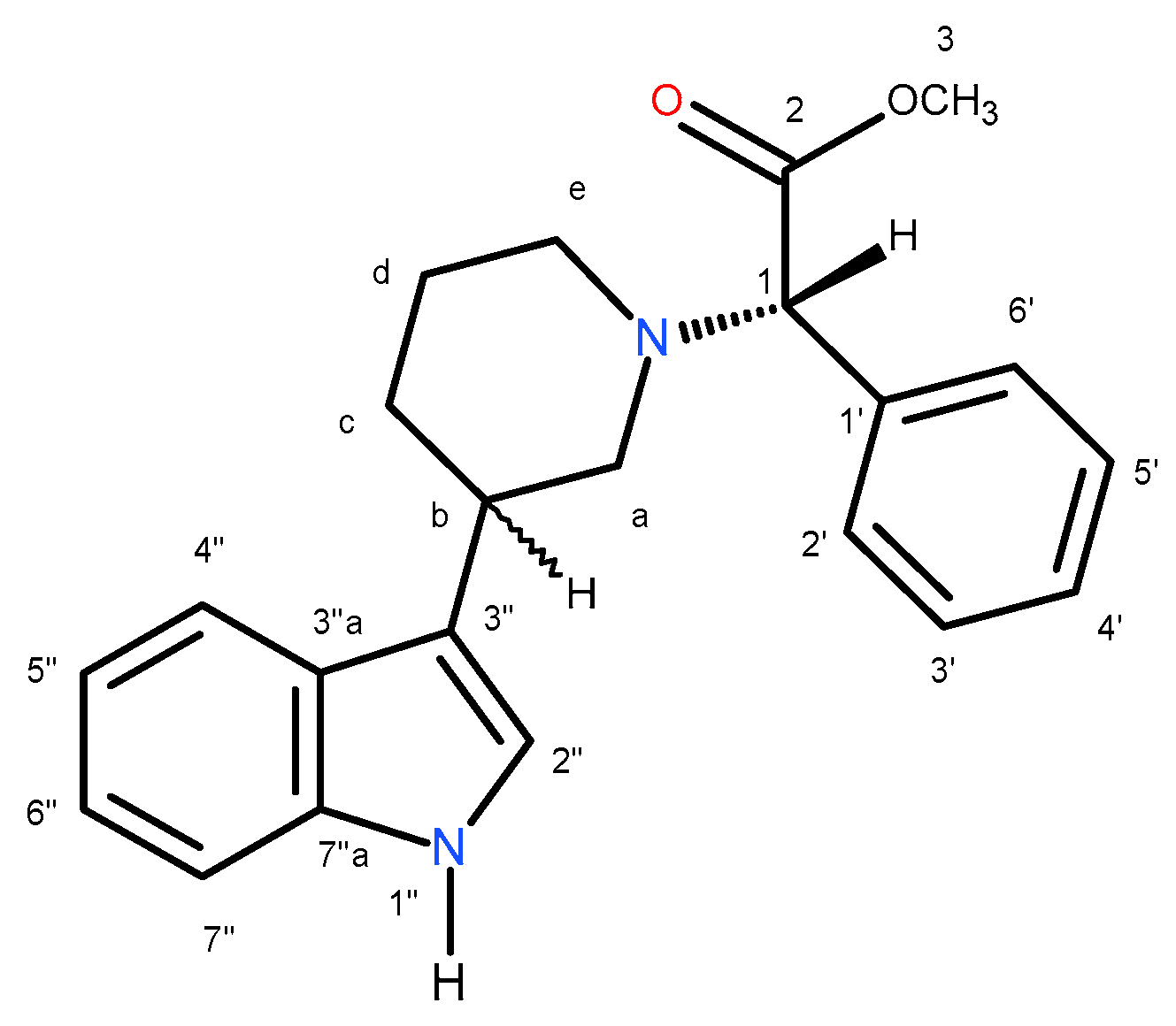
Table 1 lists dr for mixtures; in
Table 2, m.p., [α] and dr for diastereomers are given. Numbering system, which was used in NMR spectra interpretation of compounds
(3R,2S)-2a and
(3S, 2S)-3a is shown in
Figure 6.
Methyl (2S)-2-[(3R)-3-(1H-indol-3-yl)piperidin-1-yl-2-phenylacetate (3R,2S)-2a
1H NMR (500 MHz, CDCl3): δ 8.00 (NH, [1H], bs), 7.52 (C4″H, [1H], dd, 3J = 8.0, 4J = 1.0), 7.45 (C2′H,C6′H, [2H], dt, 3J = 7.0, 4J = 1.5), 7.22–7.33 (C3′H,C4′H,C5′H,C7″H, [4H], m), 7.13 (C6″H, [1H], m, 3J1 = 8.0, 3J2 = 7.0, 4J = 1.0), 7.05 (C5″H, [1H], m, 3J1 = 8.0, 3J2 = 7.0, 4J = 1.0), 6.98 (C2″H, [1H], bs), 410 (C1H, [1H], s), 3.68 (OCH3, [3H], s), 3.22 (CbH(A), [1H], tt, 3JA-A = 11.0, 3JA-E = 3.5), 3.09 (CaH(E),[1H], m), 2.93 (CeH(E), [1H], pd), 2.27 (CeH(A), [1H], td), 2.06 (CaH(A), CcH(E), [2H], m), 1.73–1.90 (CdH2, [2H], m) 1.55 (CcH(A), [1H], m).
13C NMR (125 MHz, CDCl3): δ 172.4 (C2, s), 136.1 (C7″a, s), 136.1 (C1′, s), 128.8 (C2′,C6′, s), 128.2 (C3′,C5′, s), 128.2 (C4′, s), 126.7 (C3″a, s), 121.8 (C6″, s), 120.4 (C2″, s), 119.5 (C3″, s), 119.1 (C5″, s), 119.0 (C4″, s), 111.1 (C7″, s),74.5 (C1, s), 58.1 (Ca, s), 52.2 (Ce, s), 52.0 (OC3H3, s), 33.8 (Cb, s), 31.1 (Cc, s), 25.4 (Cd, s).
ESI-HRMS m/z: Calcd for C22H25N2O2 [M+H]+ 349.19160. Found: 349.19136
Methyl (2S)-2-[(3S)-3-(1H-indol-3-yl)piperidin-1-yl]-2-phenylacetate (3S,2S)-3a (Figure 7)
1H NMR (500 MHz, CDCl3): δ 8.00 (NH, [1H], bs), 7.63 (C4″H, [1H], d, 3J = 8.0), 7.46 (C2′H,C6′H, [2H], dt, 3J = 8.0, 4J = 2.0), 7.28–7.38 (C3′H, C4′H, C5′H, C7″H, [4H], m), 7.17 (C6″H, [1H], m, 3J1 = 8.5, 3J2 = 7.0, 4J = 1.0), 7.09 (C5″H, [1H], m, 3J1 = 7.5, 3J2 = 7.0, 4J = 1.0), 7.00 (C2″H, [1H], d, 3J = 2.5), 4.09 (C1H, [1H], s), 3.66 (OCH3, [3H], s), 3.22–3.32 (CbH (A), CaH(E), [2H], m, 3JA-A = 11.0), 2.83 (CeH(E), [1H], pd), 2.26 (CaH(A), [1H], t, 2J = 3JA-A = 10.5), 2.07 (CcH(E), [1H], m), 1.98 (CeH(A), [1H], td), 1.68–1.86 (CdH(A),CdH(E), [2H], m), 1.53 (CcH(A), [1H], m).
13C NMR (125 MHz, CDCl3): δ 172.4 (C2, s), 136.2 (C7″a, s), 136.1 (C1′, s), 128.9 (C2′,C6′, s), 128.5 (C3′,C5′, s), 128.2 (C4′, s), 126.7 (C3″a, s), 121.9 (C6″, s), 120.3 (C2″, s), 119.6 (C3″, s), 119.2 (C5″, s), 119.2 (C4″, s), 111.1 (C7″, s), 74.6 (C1, s), 58.9 (Ca, s), 52.0 (OCH3, s), 51.6 (Ce, s), 33.9 (Cb, s), 31.3 (Cc, s), 25.5 (Cd, s).
ESI-HRMS m/z: Calcd for C22H25N2O2 [M+H]+ 349.19160. Found: 349.19136
Crystal data for (3S,2S)-3a. Formula C22H24N2O2; Mw 348.43. Crystal system orthorhombic, space group P212121, unit cell dimensions a = 9.023(2) Å, b = 11.615(3) Å, c = 18.937(4) Å, V = 1984.6(8) Å3, Z = 4, Dcalc = 1.166 g/cm3, μ = 0.594 mm−1, F(000) = 744. θ range 4.66 to 73.946°; reflections collected/independent 7902/3718 [R(int) = 0.0203]. Goodness-of-fit on F2 1.046; final R indices [I > 2σ(I)]: R1 = 0.0356, wR2 = 0.0866; R indices (all data) R1 = 0.0422, wR2 = 0.0950, GOOF = 1.046. CCDC No 2085108.
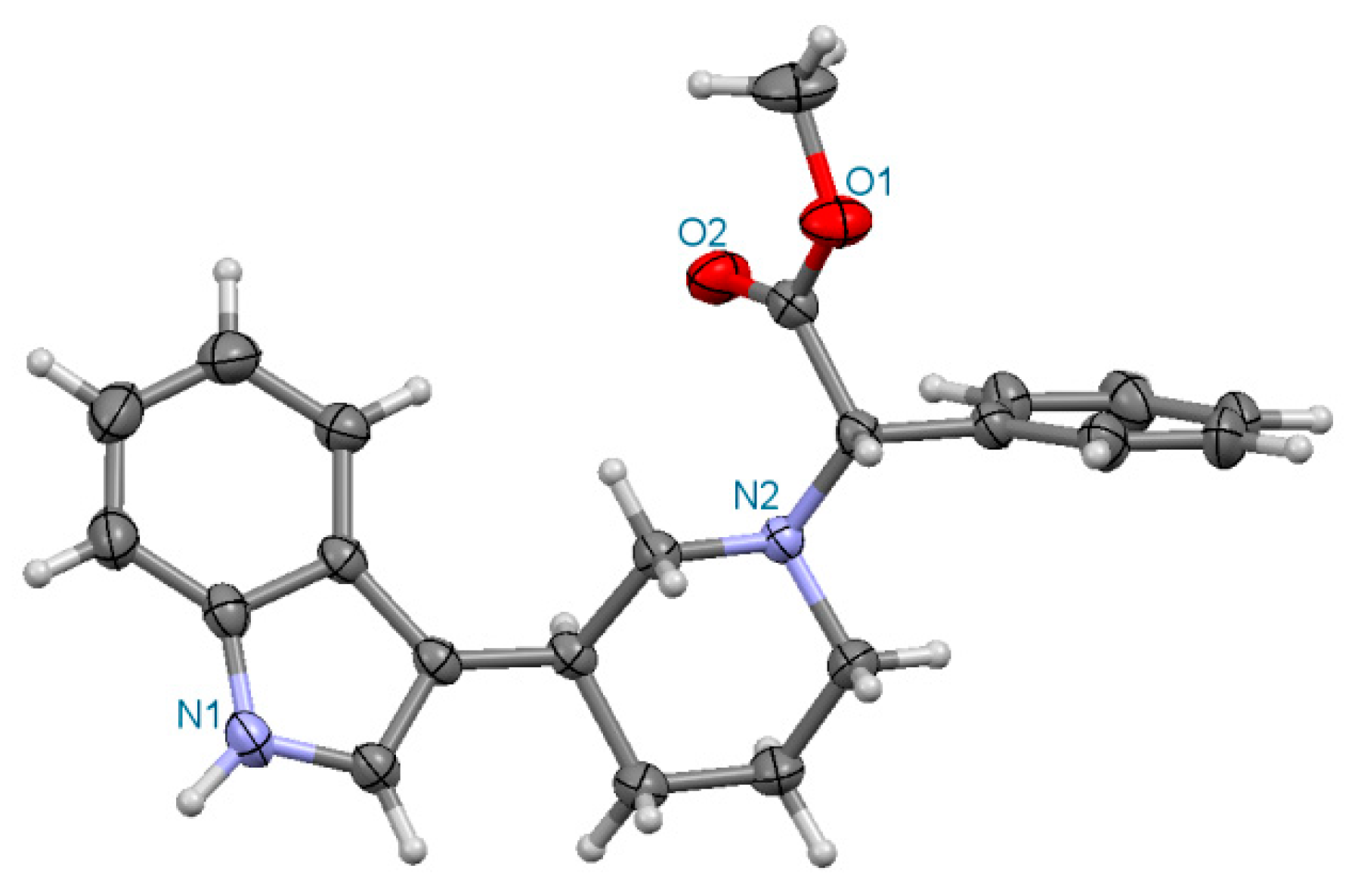
Figure 7.
Perspective drawing of the molecule (3S,2S)-3a.
Figure 7.
Perspective drawing of the molecule (3S,2S)-3a.
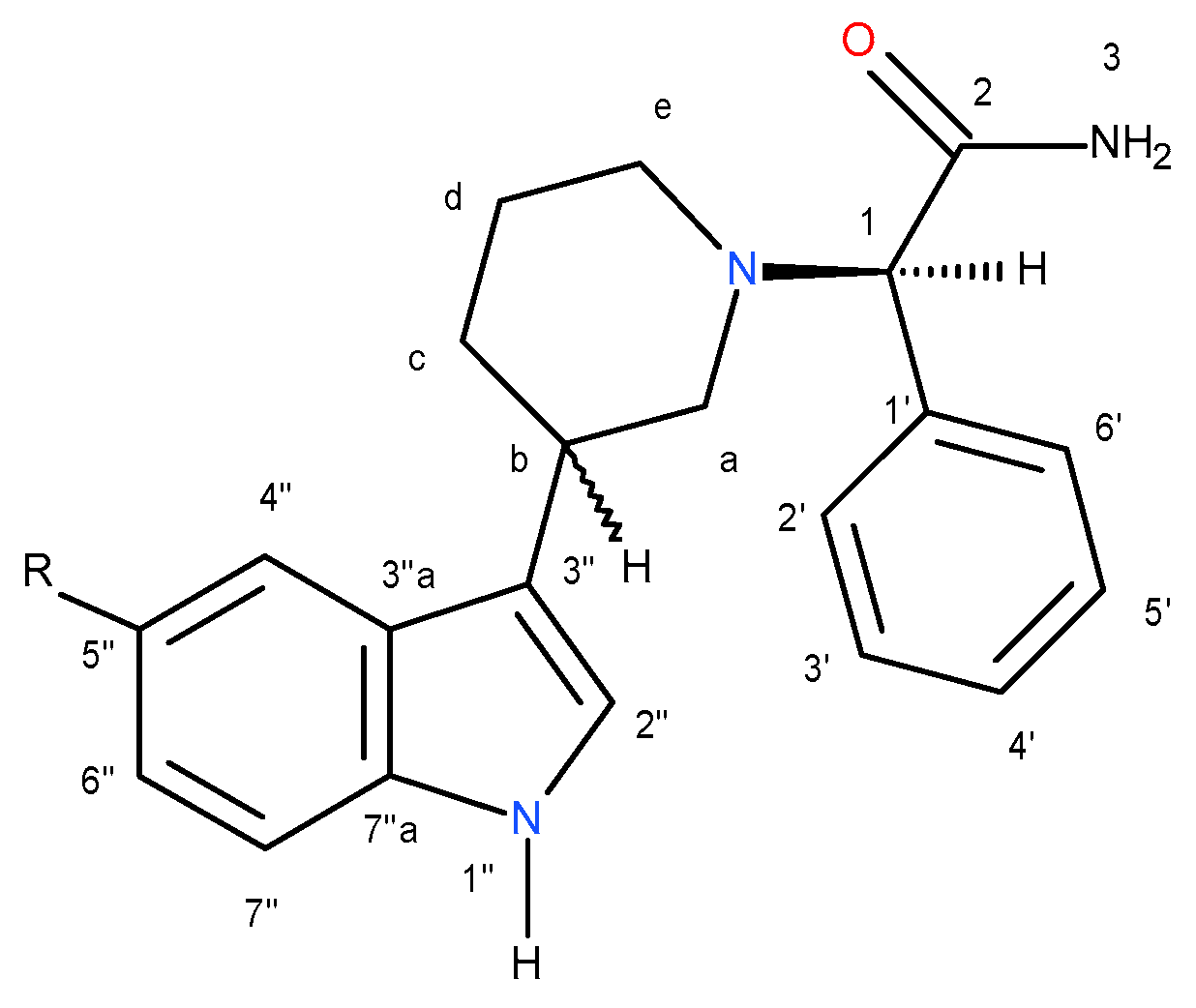
Numbering system, which was used in NMR spectra interpretation of compounds
(3R,2R)-4a,
(3S,2R)-
5a,
(3R,2R)-
6b,
(3S,2R)-
7b,
(3R,2R)-
8c and
(3S,2R)-
9c is shown in
Figure 8.
(2R)-2-[(3R)-3-(1H-indol-3-yl)piperidin-1-yl]-2-phenylacetamide (3R,2R)-4a
1H NMR (500 MHz, CDCl3): δ 8.11 (N1″H, [1H], bs), 7.59 (C4″H, [1H], 4d, 3J = 8.0), 7.28–7.36 (C2′-6′H,C7″H, [6H], m), 7.21 (N3H(2), [1H], d, 2J = 4.5), 7.17 (C6″H, [1H], m, 3J1 = 8.0, 3J2 = 7.0, 4J = 1.0), 7.09 (C5″H, [1H], m, 3J1 = 7.5, 3J2 = 7.0, 4J = 1.0), 6.91 (C2″H, [1H], d, 3J = 2.0), 5.83 (N3H(1), [1H], d, 2J = 4.0), 3.97 (C1H, [1H], s), 3.34 (CaH(E), [1H], pd), 3.20 (CbH, [1H], tt, 3JA-A = 11.0, 3JA-E = 5.0), 2.78 (CeH(E), [1H], pd), 2.26 (CaH(A), [1H], t, 2J = 3JA-A = 11.0), 2.09 (CcH(E), [1H], m), 1.85 (CeH(A), [1H], td, 2J = 3JA-A = 11.0, 3JA-E = 3.0), 1.66–1.77 (CdH(E),CdH(A), [2H], m), 1.47 (CcH(A), [1H], kd, 2J = 3JA-A = 11.0, 3JA-E = 5.0).
13C-NMR (125 MHz, CDCl3): δ 174.8 (C2, s), 136.2 (C7″a, s), 135.3 (C1′, s), 129.2 (C2′,C6′, s), 128.4 (C3′,C5′, s), 128.1 (C4′, s), 126.6 (C3″a, s), 122.0 (C6″, s), 120.1 (C2″, s), 119.2 (C5″, s), 119.0 (C3″, s), 118.9 (C4″, s), 111.3 (C7″, s), 75.9 (C1, s), 60.4 (Ca, s), 50.6 (Ce, s), 34.4 (Cb, s), 31.1 (Cc, s), 25.9 (Cd, s).
ESI-HRMS m/z: Calcd for C21H24N3O [M+H]+ 334.19193. Found: 334.19148
(2R)-2-[(3S)-3-(1H-indol-3-yl)piperidin-1-yl]-2-phenylacetamide (3S,2R)-5a
1H-NMR (500 MHz, CDCl3): δ 8.05 (N1″H, [1H], bs), 7.50 (C4″H, [1H], d, 3J = 8.0), 7.26–7.35 (C2′H,C3′H,C5′H,C6′H,C7″H, [5H], m), 7.23 (C4′H, [1H], tt, 3J = 7.0, 4J = 1.5), ~7,14 (N3H(2), [1H] bs*), 7.14 (C6″H, [1H], m, 3J1 = 8.0, 3J2 = 7.0, 4J = 1.0), 7.05 (C5″H, [1H], m, 3J1 = 7.5, 3J2 = 7.5, 4J = 1.0), 6.83 (C2″H, [1H], d, 3J = 2.0), 5.87 (N3H(1), [1H], d, 2J = 2.5), 3.95 (C1H, [1H], s), 3.09 (CaH(E), CbH, CeH(E), [3H], m), 2.25 (CeH(A), [1H], m), 2.08 (CcH(E), [1H], m), 1.70–1.92 (CaH(A), CdH(E), CdH(A), [3H], m), 1.47 (CcH(A), [1H], 4d, 2J = 3JA-A = 11.5, 3JA-E = 5.5).
13C-NMR (125 MHz, CDCl3): δ 174.9 (C2, s), 136.1 (C7″a, s), 135.3 (C1′, s), 129.0 (C2′,C6′, s), 128.5 (C3′, C5′, s), 128.1 (C4′, s), 126.6 (C3″a, s), 121.9 (C6″, s), 120.0 (C2″, s), 119.2 (C3″, s), 119.2 (C5″, s), 118.8 (C4″, s), 111.2 (C7″, s), 76.1 (C1, s), 57.0 (Ca, s), 54.0 (Ce, s), 34.4 (Cb, s), 31.0 (Cc, s), 26.1 (Cd, s).
ESI-HRMS m/z: Calcd for C21H24N3O [M+H]+ 334.19193. Found: 334.19147
(2R)-2-[(3R)-3-(5-fluoro-1H-indol-3-yl)piperidin-1-yl]-2-phenylacetamide (3R,2R)-6b
1H-NMR (500 MHz, CDCl3): δ 8.13 (N1″H, [1H], bs), 7.28–7.38 (C2′-6′H, [5H], m), 7.24 (C7″H, [1H], dd, 3J = 8.5, 4JH-F = 4.5), 7.20 (C4″H, [1H], dd, 3JH-F = 9.5, 4J = 2.5), ~7.18 (N3H(2), [1H], bs), 6.97 (C2″H, [1H], d, 3J = 2.5), 6.92 (C6″H, [1H], td, 3J = 9.0, 4J = 2.5), 5.72 (N3H(1), [1H], d 3J = 4.5), 3.97 (C1H, [1H], s), 3.30 (CaH(E), [1H], pd), 3.12 (CbH, [1H], tt, 3JA-A = 11.5, 3JA-E = 3.5), 2.79 (CeH(E), [1H], pd), 2.24 (CaH(A), [1H], t, 2J = 3JA-A = 11.5), 2.07 (CcH(E), [1H], m), 1.86 (CeH(A), [1H], td, 2J = 3JA-A = 11.0, 3JA-E = 3.0), 1.66–1.78 (CdH2, [2H], m), 1.43 (CcH(A), [1H], kd, 2J = 3JA-A = 11.5, 3JA-E = 5.0).
13C-NMR (125 MHz, CDCl3): δ 174.6 (C2, s), 157.6 (C5″, d, 1J = 234.5), 135.3 (C1′, s), 132.7 (C7″a, s), 129.2 (C2′, C6′, s), 128.5 (C3′, C5′, s), 128.2 (C4′, s), 127.0 (C3″a, d, 3J = 9.6), 121.9 (C2″, s), 119.2 (C3″, d, 4J = 4.6), 111.9 (C7″, d, 3J = 9.6), 110.4 (C6″, d, 2J = 26.4), 103.8 (C4″, d, 2J = 23.4), 75.9 (C1, s), 60.1 (Ca, s), 50.7 (Ce, s), 34.4 (Cb, s), 31.1 (Cc,s), 25.8 (Cd, s).
ESI-HRMS m/z: Calcd for C21H23FN3O [M+H]+ 352.18251. Found: 352.18202
(2R)-2-[(3S)-3-(5-fluoro-1H-indol-3-yl)piperidin-1-yl]-2-phenylacetamide (3S, 2R)-7b
1H-NMR (500 MHz, CDCl3): δ 8.07 (N1″H, [1H], bs), 7.28–7.35 (C2′, 3′, 5′, 6′H, [4H], m), 7.25 (C4′H, [1H], tt, 3J = 8.5), 7.19 (C7″H, [1H], dd, 3J = 9.0, 4JH-F = 4.5), ~7.12 (N3H(2), [1H], bs), 7.11 (C4″H, [1H], dd, 3JH-F = 9.5, 4J = 2.5), 6.88 (C2″H, C6″H, [2H], m), 5.75 (N3H(1), [1H], d, 3J = 3.0), 3.94 (C1H, [1H], s), 3.09 (CaH(E), [1H], pd), 2.98–3.07 (CbF, CeH(E), [2H], m), 2.24 (CeH(A), [1H], td, 2J = 3JA-A = 11.5, 3JA-E = 3.5), 2.07 (CcH(E), [1H], m), 1.76–1.90 (CaH(A), CdH2, [3H], m), 1.46 (CcH(A), [1H], kd, 2J = 3JA-A = 11.5, 3JA-E = 5.0).
13C-NMR (125 MHz, CDCl3): δ 174.8 (C2, s), 157.5 (C5″, d, 1J = 234.3), 135.4 (C1′, s), 132.6 (C7″a, s), 128.9 (C2′, C6′, s), 128.5 (C3′, C5′, s), 128.2 (C4′, s), 126.9 (C3″a, d, 3J = 9.6), 121.9 (C2″, s), 119.3 (C3″, d, 4J = 4.8), 111.8 (C7″, d, 3J = 9.8), 110.3 (C6″, d, 2J = 26.3), 103.7 (C4″, d, 2J = 23.5), 76.1 (C1, s), 56.8 (Ca, s), 54.0 (Ce, s), 34.4 (Cb, s), 31.0 (Cc, s), 26.1 (Cd, s).
ESI-HRMS m/z: Calcd for C21H23FN3O [M+H]+ 352.18251. Found: 352.18197
(2R)-2-[(3R)-3-(5-methoxy-1H-indol-3-yl)piperidin-1-yl]-2-phenylacetamide (3R,2R)-8c
1H-NMR (500 MHz, CDCl3): δ 7.98 (N1″H, [1H], bs), 7.29–7.39 (C2′-6′H, [5H], m), 7.24 (C7″H, [1H], d, 3J = 9.0), 7.19 (N3H(2), [1H], bs), 7.04 (C4″H, [1H], d, 4J = 2.5), 6.93 (C2″H, [1H], d, 3J = 2.5), 6.86 (C6″H, [1H], dd, 3J = 9.0, 4J = 2.5), 5.75 (N3H(1), [1H], d, 2J = 4.0), 3.97 (C1H, [1H], s), 3.86 (OCH3, [3H], s), 3.53 (CaH(E), [1H], pd), 3.15 (CbH, [1H], tt, 2J = 3JA-A = 15.0), 2.79 (CeH(E), [1H], pd), 2.25 (CaH(A), [1H], t, 2J = 3JA-A = 11.0), 2.09 (CcH(E), [1H],m), 1.85 (CeH(A), [1H], m), 1.65–1.80 (CdH, [2H], m), 1.44 (CcH(A), [1H], kd, 2J = 3JA-A = 11.5, 3JA-E = 5.0).
13C-NMR (125 MHz, CDCl3): δ 174.5 (C2, s), 153.9 (C5″, s), 135.3 (C1′, s), 131.4 (C7″a, s), 129.2 (C2′, C6′, s), 128.5 (C3′, C5′, s), 128.1 (C4′, s), 127.0 (C3″a, s), 120.9 (C2″, s), 119.0 (C3″, s), 112.1 (C7″, s), 112.0 (C6″, s), 101.1 (C4″, s), 75.9 (C1, s), 60.2 (Ca, s), 56.1 (OCH3, s), 50.7 (Ce, s), 34.5 (Cb, s), 31.1 (Cc, s), 26.0 (Cd, s).
ESI-HRMS m/z: Calcd for C22H26N3O2 [M+H]+ 364.20250. Found: 364.20180
(2R)-2-[(3S)-3-(5-methoxy-1H-indol-3-yl)piperidin-1-yl]-2-phenylacetamide (3S,2R)-9c
1H-NMR(500 MHz, CDCl3): δ 7.84 (N1″H, [1H], bs), 7.36 (C2′H, C6′H, [2H], dt, 3J = 7.0, 4J = 1.5), 7.30 (C3′H, C5′H, [2H], tt, 3J = 7.0, 4J = 1.5), 7.26 (C4′H, [1H], tt, 3J = 7.0, 4J = 1.5), 7.19 (C7″H, [1H], dd, 3J = 8.5, 5J = 0.5), 7.09 (N3H(2), [1H], bs), 6.89 (C4″H, [1H], d, 4J = 2.5), 6.85 (C2′H, [1H], d, 3J = 2.5), 6.81 (C6″H, [1H], dd, 3J = 8.5, 4J = 2.5), 5.55 (N3H(1), [1H], bs), 3.93 (C1H, [1H], s), 3.81 (OCH3), [3H], s), 3.03–3.16 (CaH(E),CeH(E),CbH, [3H], m), 2.24 (CeH(A), [1H], td, 2J = 3JA-A = 11.0, 3JA-E = 3.5), 2.06–2.14 (Cc(E), [1H], m), 1.78–1.91 (CaH(A), CdH2, [3H], m), 1.50 (CcH(A), [1H], kd, 2J = 3JA-A = 11.5, 3JA-E = 5.0).
13C-NMR (125 MHz, CDCl3): δ 175.0 (C2, s), 153.7 (C5″, s), 135.6 (C1′, s), 131.2 (C7″a, s), 128.9 (C2′, C6′, s), 128.5 (C3′, C5′, s), 128.1 (C4′, s), 126.9 (C3″a, s), 120.9 (C2″, s), 118.7 (C3″, s), 112.2 (C7″, s), 111.9 (C6″, s), 100.6 (C4″, s), 76.2 (C1, s), 57.0 (Ca, s), 55.9 (OCH3, s), 54.0 (Ce, s), 34.3 (Cb, s), 30.8 (Cc, s), 26.0 (Cd, s).
ESI-HRMS m/z: Calcd for C22H26N3O2 [M+H]+ 364.20250. Found: 364.20188
3.3.3. General Procedure for the Synthesis of Chiral (R) and (S) 3-(piperidin-3-yl) -1H-indol derivatives (R)-10a-c and (S)-11a-c
An ampoule with the appropriate diastereomer (0.014m), Pd/C 10% 0.3 g catalyst, and 180 mL methanol were placed in the autoclave. The hydrogenolysis process was carried out at 30 °C under the pressure of 1–3 atm. The mixture was blended for 8 h. The catalyst was filtered off from the postreaction mixture and the filtrate was cooled down; after that, it was acidified with a methanolic HCl solution to a pH of about 2 and concentrated at 45 °C under vacuum until dry. After concentrating solutions of
(R)-10a or (S)-11a compounds to dryness, 5 mL of absolute EtOH and Et2O were added to the remaining part to obtain a cloudy structure in the solutions, which were then placed in a refrigerator. As for the
(R)-10-b, (S)-11-b enantiomers, after concentration to dryness, 10 mL of acetone was added to the residue, which was then placed in the refrigerator. In the case of
(R)-10c and
(S)-11c compounds, the solutions were concentrated to a volume of 10 mL and placed in a refrigerator, and the isolated crystals
(R)-12-c or
(S)-13c were filtered off. Then, 20 mL of acetone was added to the filtrate and placed in a refrigerator to obtain salt
(R)-10c and
(S)-11c. The reaction yield, melting temp, er, and [α] are given in
Table 3. Numbering system, which was used in NMR spectra interpretation of compounds
(R)-10a-c and
(S)-11a-c is shown in
Figure 9.
(3R)-3-(piperidin-3-yl)-1H-indole hydrochloride (R)-10a (Figure 10)
1H-NMR (500 MHz, D2O): δ 7.70 (C4″H, [1H], dd, 3J = 8.0), 7.53 (C7″H, [1H], m, 3J = 7.0, 4J = 0.5), 7.27 (C6″H, [1H], m), 7.21 (C2″H, [1H], s), 7.18 (C5″H, [1H], m), 3.57 (CaH(E), [1H], m), 3.47 (CeH(E), [1H], m), 3.29 (CbH(A), [1H], tt, 3JA-A = 12.0, 3JA-E = 4.0), 2.98 (CaH(A), CeH(A), [2H], m), 2.14 (CcH(E), [1H], m), 2.04 (CdH(E), [1H], m), 1.89 (CdH(A), [1H], m), 1.74 (CcH(A), [1H], m).
13C-NMR (125 MHz, D2O): δ 135.7 (C7″a, s), 124.9 (C3″a, s), 121.8 (C6″, s), 121.6 (C2″, s), 119.0 (C5″, s), 118.0 (C4″, s), 114.4 (C3″, s), 111.7 (C7″, s), 48.3 (Ca,s), 43.7 (Ce, s), 30.6 (Cb,s), 28.3 (Cc, s), 21.9 (Cd, s).
ESI-HRMS m/z: Calcd for C13H17N2 [M+H]+ 201.13917. Found: 201.13874
HPLC separation method: LUX Cellulose-3 5 µm, 250 × 4.6 mm column; T = 35 °C; phase A: 0.05% diisopropylamine in ethanol; phase B: hexane; flow: 1 mL/min, 40% of phase B, isocratic elution; detection at 220 nm.
Crystal data for orthorhombic polymorph of (R)-10a. Formula C13H17N2Cl, Mw = 236.74. Crystal system orthorhombic at 293 K, space group P212121, unit cell dimensions a = 6.173(2) Å, b = 10.788(3) Å, c = 18.982(4) Å, V = 1264.1(6) Å3, Z = 4, Dcalc = 1.244 g/cm3, μ = 2.457 mm−1, F(000) = 504. θ range for data collection 4.66 to 76.14; reflections collected/independent 8343/2605 [R(int) = 0.0285]. Goodness-of-fit on F2 1.266, final R indices [I > 2σ(I)] R1 = 0.0346, wR2 = 0.0882; R indices (all data) R1 = 0.0415, wR2 = 0.0939. CCDC No. 2085101.
Crystal data for monoclinic polymorph of
(R)-10a. Formula C
13H
17N
2Cl, M
w = 236.74. Crystal system monoclinic at 120K, space group
P2
1, unit cell dimensions a = 6.091(1) Å, b = 18.884(3) Å, c = 10.795(2) Å, β = 90.57(2)°, V = 1241.6(4) Å
3, Z = 4, D
calc = 1.266 g/cm
3, μ = 2.502 mm
−1, F(000) = 504. θ range for data collection 4.66 to 73.28°; reflections collected/independent 3220/2645 [R(int) = 0.019]. Goodness-of-fit on F
2 1.202; final R indices [I > 2σ(I)] R1 = 0.0416, wR2 = 0.0891, R indices (all data) R1 = 0.0503, wR2 = 0.1111. CCDC No. 2085100.
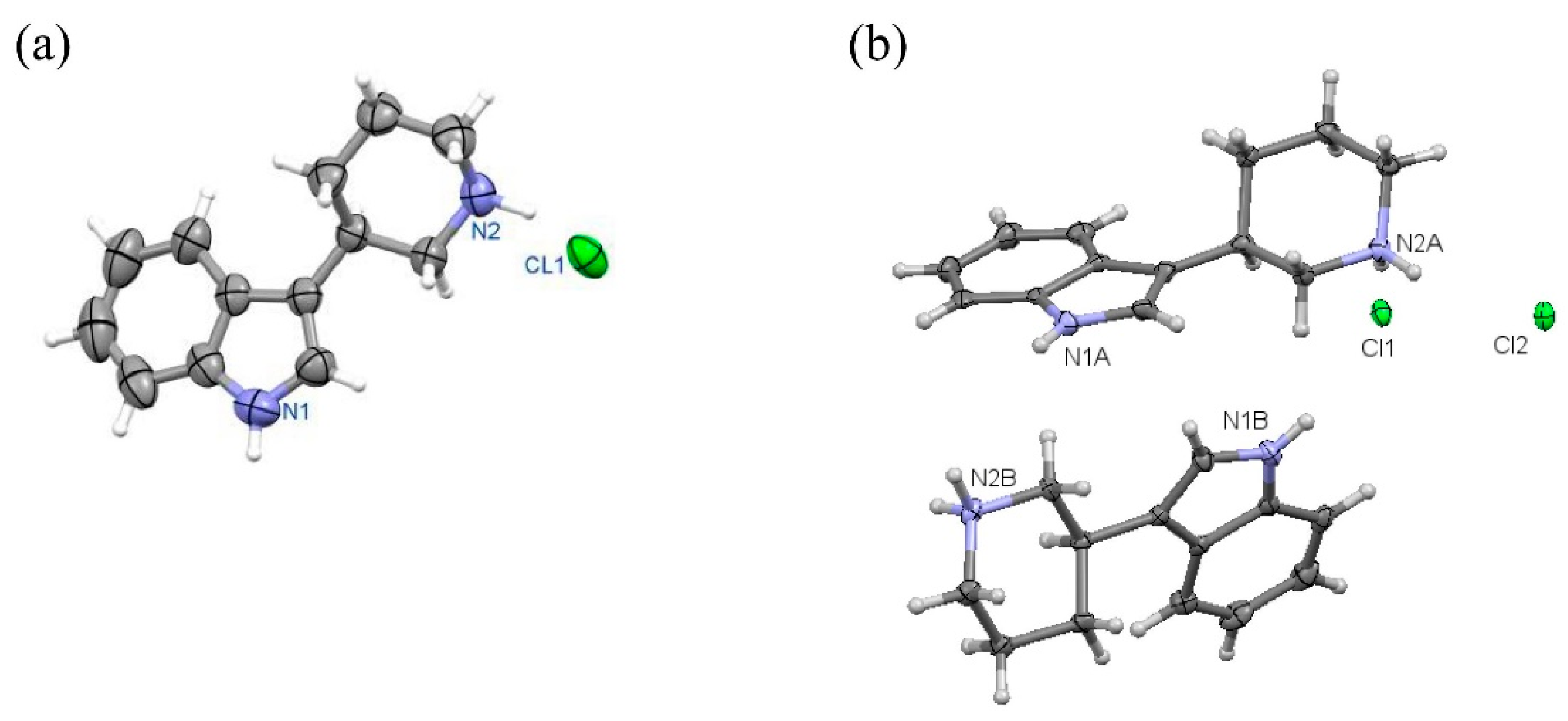
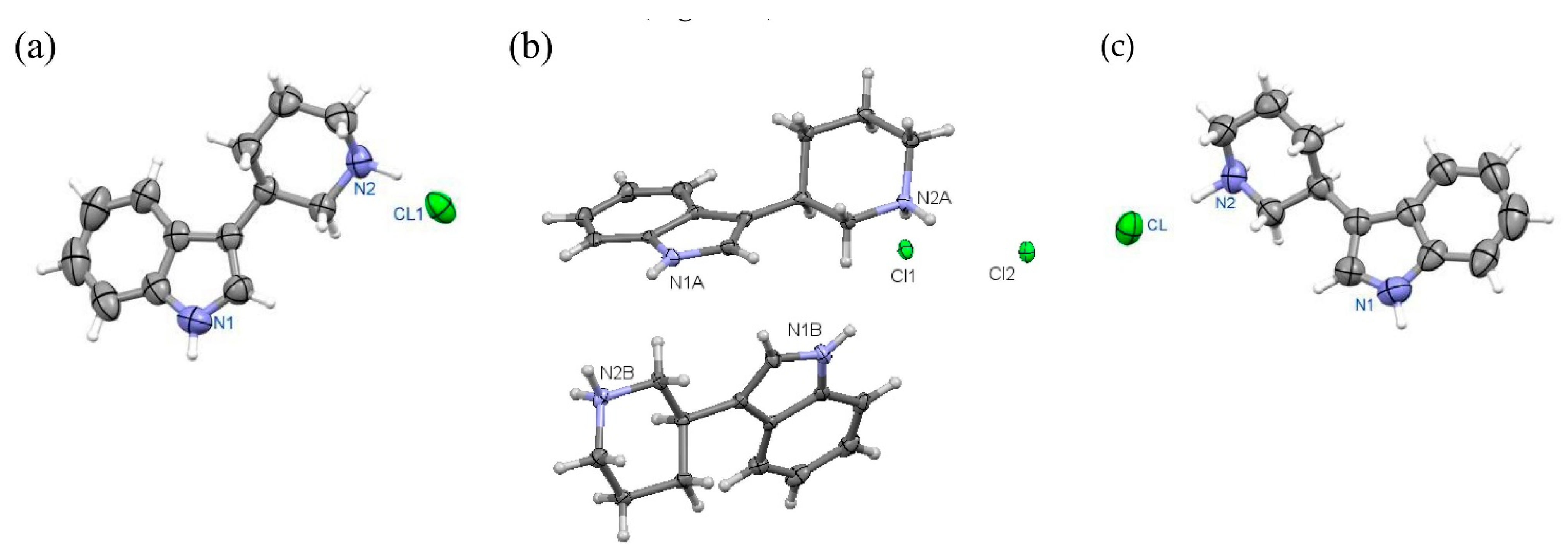
Figure 10.
Perspective view of molecules: (R)-10a in orthorhombic (a) and monoclinic (b) polymorph.
Figure 10.
Perspective view of molecules: (R)-10a in orthorhombic (a) and monoclinic (b) polymorph.
(3S)-3-(piperidin-3-yl)-1H-indole hydrochloride (S)-11a (Figure 11)
1H-NMR (500 MHz, D2O): δ 7.70 (C4″H, [1H], dd, 3J = 8.0), 7.53 (C7″H, [1H], m, 3J = 7.0, 4J = 0.5), 7.27 (C6″H, [1H], m), 7.20 (C2″H, [1H], s), 7.18 (C5″H, [1H], m), 3.57 (CaH(E), [1H], m), 3.47 (CeH(E), [1H], m), 3.28 (CbH(A), [1H], tt, 3JA-A = 12.0, 3JA-E = 4.0), 2.99 (CaH(A), CeH(A), [2H], m), 2.14 (CcH(E), [1H], m), 2.05 (CdH(E), [1H], m), 1.89 (CdH(A), [1H], m), 1.76 (CcH(A), [1H], m).
13C-NMR (125 MHz, D2O): δ 135.7 (C7″a, s), 124.9 (C3″a, s), 121.8 (C6″, s), 121.6 (C2″, s), 119.0 (C5″, s), 118.0 (C4″, s), 114.4 (C3″, s), 111.7 (C7″, s), 48.3 (Ca,s), 43.7 (Ce, s), 30.6 (Cb,s), 28.3 (Cc, s), 21.9 (Cd, s).
ESI-HRMS m/z: Calcd for C13H17N2 [M+H]+ 201.13917. Found: 201.13874
HPLC separation method: LUX Cellulose-3 5 µm, 250 × 4.6 mm column; T = 35 °C; phase A: 0.05% diisopropylamine in ethanol; phase B: hexane; flow: 1 mL/min, 40% of phase B, isocratic elution; detection at 220 nm.
Crystal data for
(S)-11a. Formula C
13H
17N
2Cl, M
w = 236.74. Crystal system orthorhombic, space group
P2
12
12
1, unit cell dimensions a = 6.181(1) Å, b = 10.795(2) Å, c = 18.967(3) Å, V = 1265.6(4) Å
3, Z = 4, D
calc = 1.242 g/cm
3, μ = 2.455 mm
−1, F(000) = 504. θ range for data collection 4.66 to 73.28°; reflections collected/independent 3248/2162 [R(int) = 0.0263]. Goodness-of-fit on F
2 1.116; final R indices [I > 2σ(I)] R1 = 0.0427, wR2 = 0.0878; R indices (all data) R1 = 0.0576, wR2 = 0.1138. CCDC No. 2085102.
Figure 11.
Perspective view of molecule (S)-11a.
Figure 11.
Perspective view of molecule (S)-11a.
(3R)-5-fluoro-3-(piperidin-3-yl)-1H-indole hydrochloride (R)-10b (Figure 12)
1H-NMR (500 MHz, D2O): δ 7.43 (C7″H, [1H], 4d, 3J = 9.0, 4JH-F = 5.0, 5J = 0.5), 7.34 (C4″H, [1H], 4d, 3JH-F = 10.0, 4J = 2.5, 5J = 0.5), 7.24 (C2″H, [1H], s), 7.02 (C6″H, [1H], 8d, 3J = 9.5, 3JH-F = 8.0, 4J = 2.5, 5J = 0.5), 3.56 (CaH(E). [1H], m), 3.49 (CeH(E), [1H], m), 3.22 (CbH, [1H], tt, 3JA-A = 12.0, 3JA-E = 3.5), 3.01 (CeH(A), [1H], td, 2J = 3JA-A = 13.0, 3JA-E = 3.5), 2.98 (CaH(A), [1H], t, 2J = 3JA-A = 12.5), 2.01–2.14 (CcH(E),CdH(E), [2H], m), 1.83–1.95 (CdH(A), [1H], m), 1.72 (CcH(A), [1H], kd, 2J = 3JA-A = 12.0, 3JA-E = 4.0).
13C-NMR (125 MHz, D2O): δ 156.9 (C5″, d, 1J = 231.9), 132.3 (C3″, s), 125.1 (C3″a, d, 3J = 9.9), 123.3 (C2″, s), 114.6 (C7″a, d, 4J = 4.8), 112.6 (C7″, d, 3J = 9.9), 109.8 (C6″, d, 2J = 26.4), 102.5 (C4″, d, 2J = 23.8), 48.2 (Ca, s), 43.7 (Ce, s), 30.6 (Cb, s), 28.2 (Cc, s), 21.9 (Cd, s).
ESI-HRMS m/z: Calcd for C13H16FN2 [M+H]+ 219.12975. Found: 219.12935
HPLC separation method: LUX Cellulose-3 5 µm, 250 × 4.6 mm column; T = 35 °C; phase A: 0.05% diisopropylamine in ethanol/methanol 80/20; phase B: hexane; flow: 0.5 mL/min, 92% of phase B, isocratic elution; detection at 220 nm.
Crystal data for (R)-10b. Formula C13H16FN2Cl; Mw 254.73. Crystal system orthorhombic, space group P212121, unit cell dimensions a = 5.510(1) Å, b = 11.905(2) Å, c = 19.148(3) Å, V = 1256.0(4) Å3, Z = 4, Dcalc = 1.347 g/cm3, μ = 2.631 mm−1, F(000) = 536. θ range for data collection 4.37 to 76.33°; reflections collected/independent 8559/2577 [R(int) = 0.0341]. Goodness-of-fit on F2 1.156; final R indices [I > 2σ(I)] R1 = 0.0308, wR2 = 0.0715, R indices (all data) R1 = 0.0385, wR2 = 0.0964. CCDC No. 2085104.
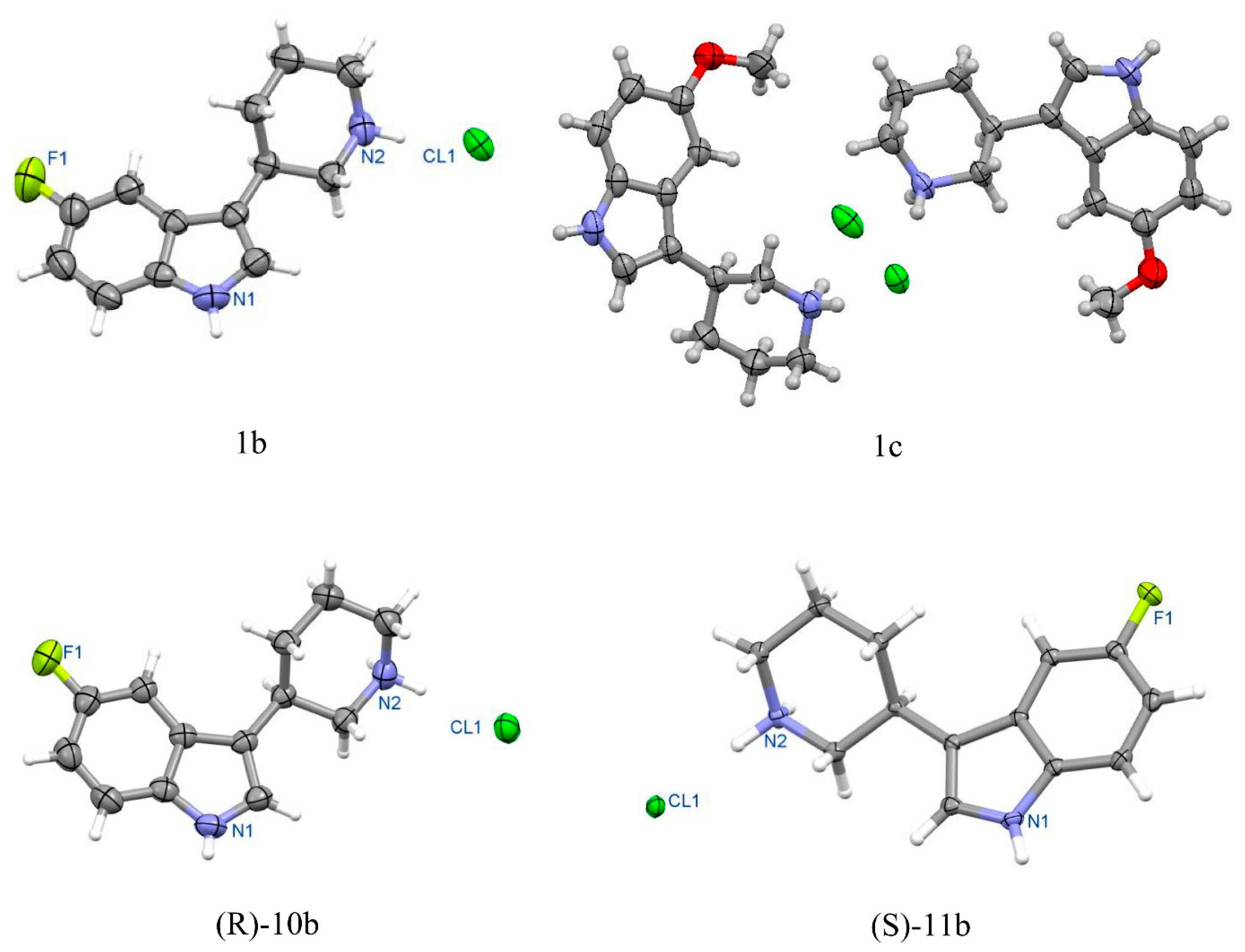
Crystal data for racemic
1b. Formula C
13H
16FN
2Cl; M
w 254.73. Crystal system monoclinic, space group
P2
1/
c, unit cell dimensions a = 10.736(3) Å, b = 6.117(2) Å, c = 20.151(4) Å, β = 97.84(3)°, V = 1311.0(6) Å
3, Z = 4, D
calc = 1.291 g/cm
3, μ = 2.521 mm
−1, F(000) = 536. θ range 4.16 to 73.53°; reflections collected/independent 4524/2572 [R(int) = 0.0244]. Goodness-of-fit on F
2 1.067; final R indices [I > 2σ(I)]: R1 = 0.0456, wR2 = 0.1161; R indices (all data) R1 = 0.0536, wR2 = 0.1254. CCDC No. 2085103. See
Figure 4, Perspective view of molecule 1b in racemic crystal.
Figure 12.
View of molecular structure of enantiomeric fluoro- derivative (R)-10b.
Figure 12.
View of molecular structure of enantiomeric fluoro- derivative (R)-10b.
(3S)-5-fluoro-3-(piperidin-3-yl)-1H-indole hydrochloride (S)-11b (Figure 13)
1H-NMR (500 MHz, D2O): δ 7.44 (C7″H, [1H], 4d, 3J = 9.0, 4JH-F = 5.0, 5J = 0.5), 7.35 (C4″H, [1H], 4d, 3JH-F = 10.0, 4J = 2.5, 5J = 0.5), 7.24 (C2″H, [1H], s), 7.03 (C6″H, [1H], 8d, 3J = 9.5, 3JH-F = 8.0, 4J = 2.5, pJ = 0.5), 3.56 (CaH(E). [1H], m), 3.49 (CeH(E), [1H], m), 3.22 (CbH, [1H], tt, 3JA-A = 12.0, 3JA-E = 3.5), 3.01 (CeH(A), [1H], td, 2J = 3JA-A = 13.0, 3JA-E = 3.5), 2.98 (CaH(A), [1H], t, 2J = 3JA-A = 12.5), 2.01–2.14 (CcH(E),CdH(E), [2H], m), 1.83–1.95 (CdH(A), [1H], m), 1.72 (CcH(A), [1H], kd, 2J = 3JA-A = 12.0, 3JA-E = 4.0).
13C-NMR (125 MHz, D2O): δ 156.9 (C5″, d, 1J = 231.9), 132.3 (C3″, s), 125.1 (C3″a, d, 3J = 9.9), 123.3 (C2″, s), 114.6 (C7″a, d, 4J = 4.8), 112.6 (C7″, d, 3J = 9.9), 109.8 (C6″, d, 2J = 26.4), 102.5 (C4″, d, 2J = 23.8), 48.2 (Ca, s), 43.7 (Ce, s), 30.6 (Cb, s), 28.2 (Cc, s), 21.9 (Cd, s).
ESI-HRMS m/z: Calcd for C13H16FN2 [M+H]+ 219.12975. Found: 219.12936
HPLC separation method: LUX Cellulose-3 5 µm, 250 × 4.6 mm column; T = 35 °C; phase A: 0.05% diisopropylamine in ethanol/methanol 80/20; phase B: hexane; flow: 0.5 mL/min, 92% of phase B, isocratic elution; detection at 220 nm.
Crystal data for (S)-11b. Formula C13H16N2FCl; Mw 254.73. Crystal system orthorhombic, space group P212121, unit cell dimensions a = 5.499(1) Å, b = 11.719(2) Å, c = 19.103(3) Å, V = 1231.1(4) Å3, Z = 4, Dcalc = 1.374 g/cm3, μ = 2.685 mm−1, F(000) = 536. θ range for data collection 4.43 to 76.35; reflections collected/independent 8080/2514 [R(int) = 0.0352]. Goodness-of-fit on F2 1.136; final R indices [I > 2σ(I)] R1 = 0.0277, wR2 = 0.0648, R indices (all data) R1 = 0.0365, wR2 = 0.0764. CCDC No. 2085105.
Figure 13.
View of molecular structure of enantiomeric fluoro- derivative (S)-11b.
Figure 13.
View of molecular structure of enantiomeric fluoro- derivative (S)-11b.
(3R)-5-methoxy-3-(piperidin-3-yl)-1H-indole hydrochloride (R)-10c
1H-NMR (500 MHz, D2O): δ 7.43 (C7″H, [1H], dd, 3J = 9.0, 4J = 0.5), 7.23 (C2″H, [1H], s), 7.18 (C4″H, [1H], d, 4J = 2.5), 6.93 (C6″H, [1H], dd, 3J = 9.0, 4J = 2.5), 3.90 (OCH3, [3H], s), 3.63 (CaH(E), [1H], m), 3.51 (CeH(E), [1H], m), 3.32 (CbH, [1H], tt, 3JA-A = 12.0, 3JA-E = 4.0), 3.08 (CaH(A), [1H], t, 2J = 3JA-A = 12.5), 3.06 (CeH(A), [1H], td, 2J = 3JA-A = 13.0, 3JA-E = 3.0), 2.22 (CcH(E), [1H], m), 2.09 (CdH(E), [1H], m), 1.93 (CdH(A), [1H], m), 1.81 (CcH(A), [1H], kd, 2J = 3JA-A = 13.0, 3JA-E = 3.5).
13C-NMR (125 MHz, D2O): δ 153.0 (C5″, s), 131.6 (C7″a, s), 125.7 (C3″a, s), 123.0 (C2″, s), 114.8 (C3″, s), 113.1 (C6″, s), 112.0 (C7″, s), 100.9 (C4″, s), 56.3 (OCH3, 2s), 48.7 (Ca, s), 44.2 (Ce, s), 31.0 (Cb, s), 28.9 (Cc, s), 22.4 (Cd, s).
ESI-HRMS m/z: Calcd for C14H19N2O [M+H]+ 231.14973. Found: 231.14941
HPLC separation method: LUX Cellulose-3 5 µm, 250 × 4.6 mm column; T = 35 °C; phase A: 0.05% diisopropylamine in ethanol; phase B: hexane; flow: 1 mL/min, 80% of phase B, isocratic elution; detection at 220 nm.
(3S)-5-methoxy-3-(piperidin-3-yl)-1H-indole hydrochloride (S)-11c
1H-NMR (500 MHz, D2O): δ 7.46 (C7″H, [1H], d, 3J = 9.0), 7.27 (C2″H, [1H], s), 7.22 (C4″H, [1H], d, 4J = 2.5), 6.96 (C6″H, [1H], dd, 3J = 9.0, 4J = 2.5), 3.90 (OCH3, [3H], s), 3.63 (CaH(E), [1H], m), 3.51 (CeH(E), [1H], m), 3.32 (CbH, [1H], tt, 3JA-A = 12.0, 3JA-E = 4.0), 3.08 (CaH(A), [1H], t, 2J = 3JA-A = 12.5), 3.06 (CeH(A), [1H], td, 2J = 3JA-A = 13.0, 3JA-E = 3.0), 2.22 (CcH(E), [1H], m), 2.09 (CdH(E), [1H], m), 1.93 (CdH(A), [1H], m), 1.81 (CcH(A), [1H], kd, 2J = 3JA-A = 13.0, 3JA-E = 3.5).
13C-NMR (125 MHz, D2O): δ 153.0 (C5″, s), 131.6 (C7″a, s), 125.7 (C3″a, s), 123.0 (C2″, s), 114.8 (C3″, s), 113.1 (C6″, s), 112.0 (C7″, s), 100.9 (C4″, s), 56.3 and 56.3 (OCH3, 2s*), 48.7 (Ca, s), 44.2 (Ce, s), 31.0 (Cb, s), 28.9 (Cc, s), 22.4 (Cd, s).
ESI-HRMS m/z: Calcd for C14H19N2O [M+H]+ 231.14973. Found: 231.14931
HPLC separation method: LUX Cellulose-3 5 µm, 250 × 4.6 mm column; T = 35 °C; phase A: 0.05% diisopropylamine in ethanol; phase B: hexane; flow: 1 mL/min, 80% of phase B, isocratic elution; detection at 220 nm.
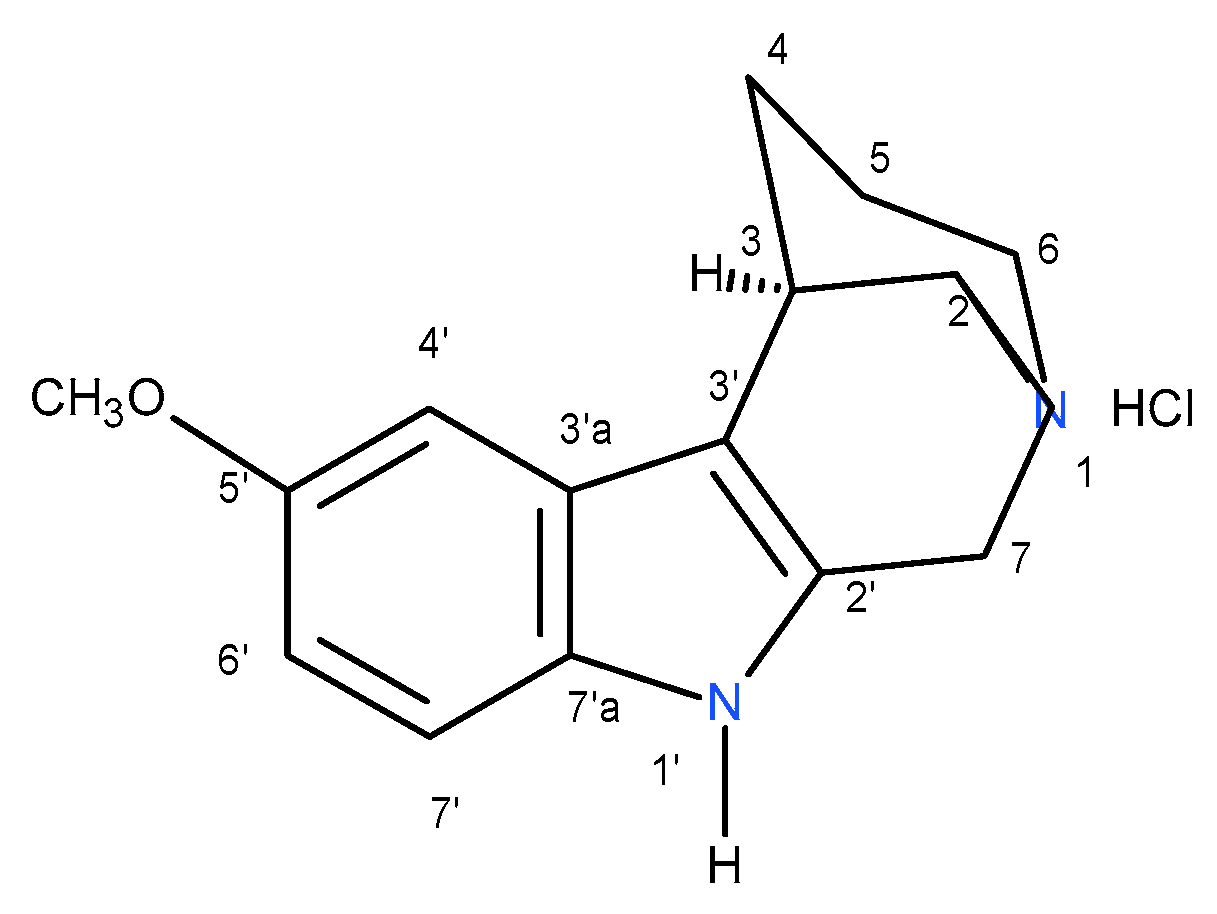
Numbering system for NMR spectra interpretation of compounds (R)-
12c and (S)-
13c is shown in
Figure 14.
(R)-5-methoxy-9,12-diazatetracyclo[10.3.1.02,10.03,8]hexadeca-2(10),3(8),4,6-tetraene hydrochloride (R)-12c (Figure 15)
1H-NMR (500 MHz, MeOD): δ 7.27 (C7′H, [1H], dd, 3J = 8.5, 5J = 0.5), 6.99 (C4′H, [1H], d, 4J = 2.5), 6.80 (C6′H, [1H], dd, 3J = 8.5, 4J = 2.5), 4.86 (C7H(2), [1H], d, 2J = 16.5), 4.47 (C7H(1), [1H], 2J = 16.5), 3.81 (OCH3, [3H], s), 3.68 (C2H(2), [1H], dd, 2J = 12.5, 3J = 3.0), 3.49–3.57 (C2H(1),C3H,C6H2, [4H], m), 1.95–2.03 (C4H(2), [1H] m), 1.83–1.90 (C4H(1), [1H], m), 1.60–1.72 (C5H2, [2H], m).
13C-NMR (125MHz, MeOD): δ 155.7 (C5′, s), 133.6 (C7′a, s), 128.0 (C2′, s), 126.5 (C3′a, s), 113.3 (C6′, s), 113.1 (C7′, s), 110.4 (C3′, s), 100.8 (C4′, s), 56.3 (OCH3, s), 55.5 (C6, s), 53.8 (C2, s), 50.5 (C7, s), 26.8 (C4, s), 26.4 (C3, s), 17.5 (C5, s).
ESI-HRMS m/z: Calcd for C15H19N2O [M+H]+ 243,14973. Found: 243,14919
Crystal data for (R)-12c. Formula C15H19N2OCl; Mw 278.77. Crystal system orthorhombic, space group P212121, unit cell dimensions a = 5.670(1) Å, b = 7.534(1) Å, c = 32.732(4) Å, V = 1398.2(4) Å3, Z = 4, Dcalc = 1.324 g/cm3, μ = 2.360 mm−1, F(000) = 592. θ range for data collection 5.41 to 76.46°; reflections collected/independent 9422/2875 [R(int) = 0.0263]. Goodness-of-fit on F2 1.112; final R indices [I > 2σ(I)] R1 = 0.0293, wR2 = 0.0692, R indices (all data) R1 = 0.0357, wR2 = 0.0797. CCDC No. 2085106.
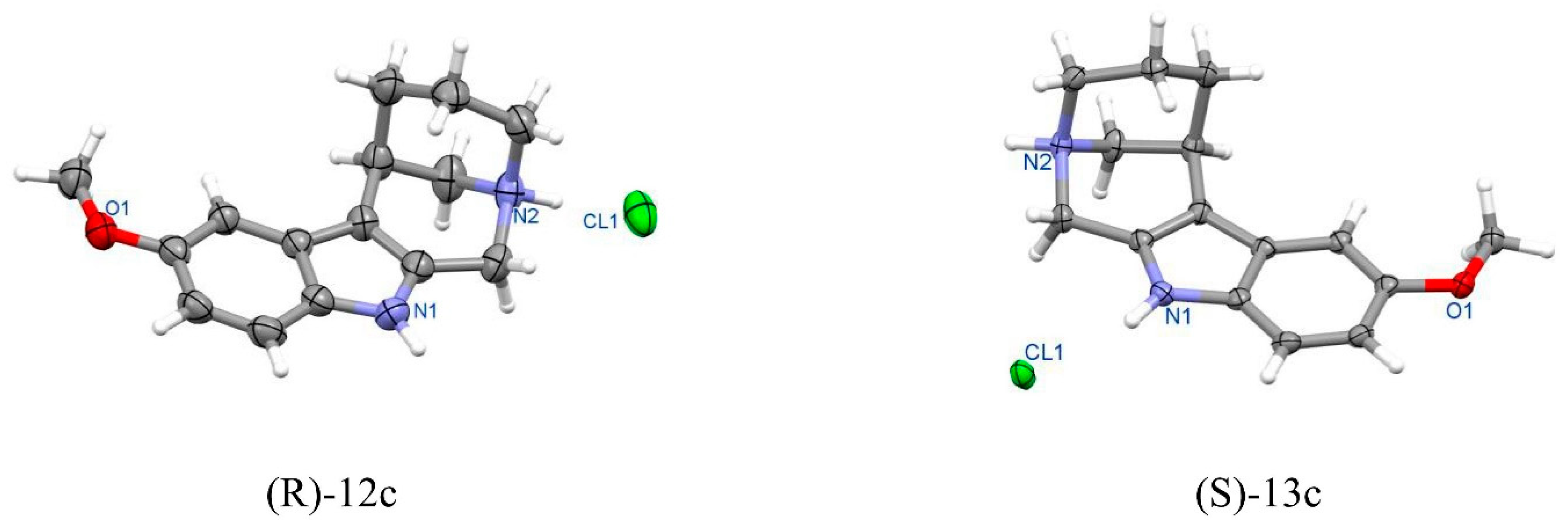
Figure 15.
Perspective view of enantiomeric tetracyclic molecules (R)-12c and (S)-13c.
Figure 15.
Perspective view of enantiomeric tetracyclic molecules (R)-12c and (S)-13c.
(S)-5-methoxy-9,12-diazatetracyclo[10.3.1.02,10.03,8]hexadeca-2(10),3(8),4,6-tetraene hydrochloride (S)-13c (Figure 15)
1H-NMR (500 MHz, MeOD): δ 7.26 (C7′H, [1H], dd, 3J = 8.5, 5J = 0.5), 7.07 (C4′H, [1H], dd, 3J = 2.5, 4J = 0.5), 6.79 (C6′H, [1H], 4d, 3J = 8.5, 4J = 2.5, 5J = 0.5), 3.81 (OCH3, [3H], s), 3.58 (C7H(2), [1H], m), 3.43–3.60 (C7H(1), [1H], m), 3.26–3.35 (C2H(2), [1H], m), 2.57–3.09 (C2H(1),,C6H(1), [2H], m), 2.20 (C6(1), [1H], m, 2.05–2.11 (C4H(2), [1H], m), 1.91–2.02 (C4H(1), [1H], m), 1.81–1.92 (C5H2, [2H], m).
13C-NMR (125MHz, MeOD): δ 155.7 (C5′, s), 133.6 (C7′a, s), 128.0 (C2′, s), 126.5 (C3′a, s), 113.3 (C6′, s), 113.1 (C7′, s), 110.4 (C3′, s), 100.8 (C4′, s), 56.3 (OCH3, s), 55.5 (C6, s), 53.8 (C2, s), 50.5 (C7, s), 26.8 (C4, s), 26.4 (C3, s), 17.5 (C5, s).
ESI-HRMS m/z: Calcd for C15H19N2O [M+H]+ 243.14973. Found: 243.14708
Crystal data for (S)-13c. Formula C15H19N2OCl; Mw 278.77. Crystal system orthorhombic, space group P212121, unit cell dimensions a = 5.583(1) Å, b = 7.486(1) Å, c = 32.589(3) Å,V = 1362.0(3) Å3, Z = 4, Dcalc = 1.359 g/cm3, μ = 2.423 mm−1, F(000) = 592. θ range for data collection 5.43 to 76.64°; reflections collected/independent 19,347/2830 [R(int) = 0.0507]. Goodness-of-fit on F2 1.078; final R indices [I > 2σ(I)] R1 = 0.0269, wR2 = 0.0609, R indices (all data) R1 = 0.0285, wR2 = 0.0621. CCDC No. 2085107.