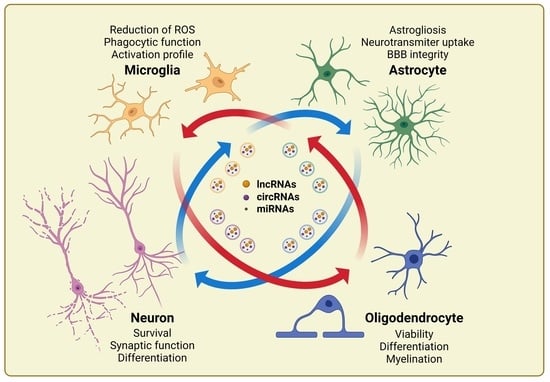
Neuronal and Glial Communication via Non-Coding RNAs: Messages in Extracellular Vesicles
Abstract
1. Introduction
2. Effect of NSC-Derived ncRNAs on Neurogenesis
3. Role of Neuronal EV-Derived ncRNAs in Neuron-to-Glia Communication
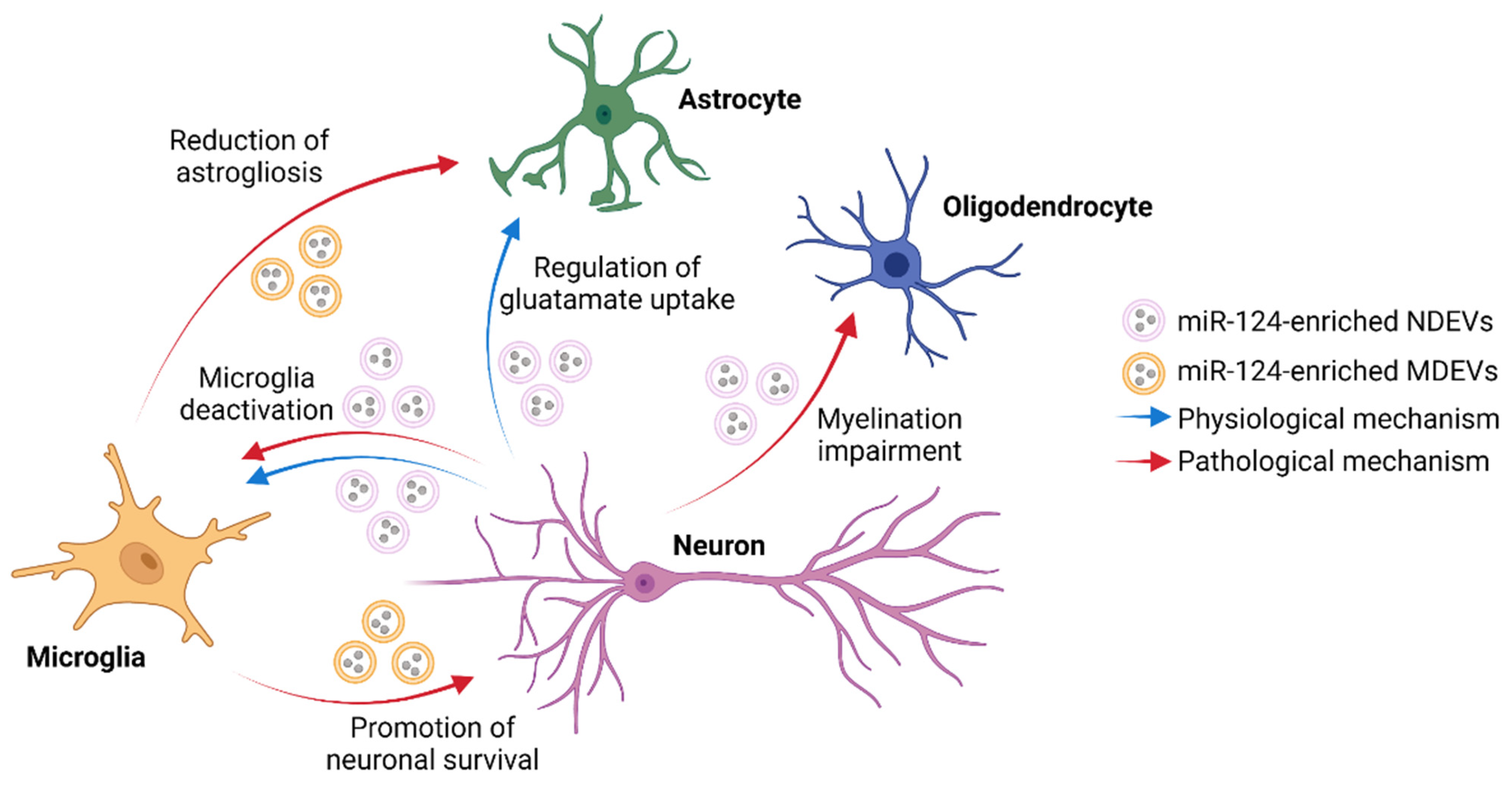
3.1. Effect of Neuron-Derived ncRNAs on Microglia Functions
| Source | Recipient Cells | Neuronal ncRNAs | ncRNA Targets | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Mouse cortical neurons | Mouse microglia | miR-124-3p | CEBPα-PU.1 and NFκB | Microglia deactivation in the normal CNS | [28] |
| Mouse neurons | Mouse microglia | miR-124-3p | MYH9 and NFκB | Suppressing microglia activation in SCI | [30] |
| Rat PC12 cells | Mouse microglial BV2 cell line | miR-9-5p | SOCS2 | Promotion of the M1 phenotype in MDD | [31] |
| Mouse cortical neurons | Mouse cortical astrocytes | miR-124-3p | GLT1-binding miRs, CREB | Regulation of synaptic functions in CNS | [32] |
| Mouse NSC-34 neuronal cell line | Mouse cortical astrocytes | miR-218-5p | GLT1 | Alteration of glutamate uptake and astrogliosis | [33] |
| Rat cortical neurons | Rat cortical astrocytes | miR-181c-3p | CXCL1 | Reduction of neuroinflammation | [34] |
| Mouse CATH.a neuronal cell line | Mouse C8-D1A astrocytic cell line | lncRNA H19 | miR-18a/VEGF axis | Alteration of BBB permeability | [35] |
3.2. Effect of Neuron-Derived ncRNAs on Astrocyte Functions
3.3. Effect of Neuron-Derived ncRNAs on Oligodendrocytes
4. Role of Glial EV-Derived ncRNAs in Cell-to-Cell Communication
4.1. Effect of Microglial EV-Derived ncRNAs on Neuroinflammation
4.2. Effect of Astrocytic EV-Derived ncRNAs on Neuroinflammation
4.3. Effect of Oligodendroglial EV-Derived ncRNAs on Neuroinflammation
| Source | Recipient Cells | ncRNAs | ncRNA TARGETS | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Rat hippocampal and cortical microglia | Rat hippocampal neurons | miR-146a-5p miR-181a miR-223-3p | Nlg1, GluR2, and GluN2B | Synaptic strength impairment | [59] |
| BV-2 microglial cell line | Rat cortical oligodendrocytes | miR-23a-5p | Olig3 | Survival and maturation under oxygen-glucose deprivation (OGD), and white matter repair after mouse tMCAO ischemic model | [60] |
| BV-2 microglial cell line | Mouse cortical neurons | miR-124-3p | PDE4B | Inhibited neuroinflammation in s scratch-injury model | [46] |
| BV-2 microglial cell line | Mouse cortical astrocytes | miR-124 | STAT3 | Reduction of glial scar under oxygen-glucose deprivation, and reduction of infarct volume after mouse tMCAO ischemic model | [61] |
| Microglia derived EVs from rat serum | Neurons from rat hippocampal slices | miR-146a-5p circANKS1B | KLF4 and CDKL5 | Suppression of neurogenesis, and synaptic impairment in chronic unpredictable mild stress (CUMS) model | [62] |
| BV-2 microglial cell line | HT22 mouse hippocampal neuronal cell line, mouse neurons | miR-124-3p | Rela (p65) | Inhibition of neurodegeneration and improvement of cognitive function in repetitive mild traumatic brain injury (rmTBI) | [49] |
| BV-2 microglial cell line | Mouse cortical neurons | miR-137 | Notch1 | Increased viability and reduction of apoptosis after mouse transient middle cerebral artery occlusion (tMCAO) ischemic model | [65] |
| BV-2 microglial cell line | HT22 mouse hippocampal neuronal cell line, mouse neurons | miR-124-3p | FIP200 | Inhibition of autophagy | [68] |
| Rat cortical astrocytes | Mouse hippocampal and cortical neurons | miR-125a-5p miR-16-5p | NTRK3 | Reduction of dendritic growth and complexity in Parkinson’s disease (PD) model | [70] |
| Rat cortical astrocytes | Rat dopaminergic neurons | miR-34a | Bcl-2 | Enhanced vulnerability of dopaminergic neurons to neurotoxin in PD model | [73] |
| Human induced astrocytes | Mouse Hb9-GFP motor neurons | miR-494-3p | Semaphorin 3A | Sustainment of survival and protection of neurites | [74] |
| Mouse cortical astrocytes | HT22 mouse hippocampal neuronal cell line | miR-190b | ATG7 | Reduction of apoptosis and inhibition of autophagy after OGD | [75] |
| Human astrocytic cell line A172, mouse whole brain astrocytes | BV-2 microglial cell line, Mouse cortical microglia | lincRNA-Cox2 | Toll-like receptors and NFκB | Restores phagocytic activity in vitro and in mice treated with morphine | [78] |
| Human cortical astrocytes | Human neurons | NKILA | miR-195, NFκB and NLRX1 | Increases cell proliferation; attenuation of apoptosis and injury in mice submitted to TBI | [77] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernaus, A.; Blanco, S.; Sevilla, A. Glia Crosstalk in Neuroinflammatory Diseases. Front. Cell Neurosci. 2020, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.J.; Simkins, T.J.; Emery, B. Neuron-Oligodendrocyte Interactions in the Structure and Integrity of Axons. Front. Cell Dev. Biol. 2021, 9, 653101. [Google Scholar] [CrossRef] [PubMed]
- Marangon, D.; Caporale, N.; Boccazzi, M.; Abbracchio, M.P.; Testa, G.; Lecca, D. Novel in vitro Experimental Approaches to Study Myelination and Remyelination in the Central Nervous System. Front. Cell Neurosci. 2021, 15, 748849. [Google Scholar] [CrossRef] [PubMed]
- Angelini, J.; Marangon, D.; Raffaele, S.; Lecca, D.; Abbracchio, M.P. The Distribution of GPR17-Expressing Cells Correlates with White Matter Inflammation Status in Brain Tissues of Multiple Sclerosis Patients. Int. J. Mol. Sci. 2021, 22, 4574. [Google Scholar] [CrossRef]
- Marangon, D.; Raffaele, S.; Fumagalli, M.; Lecca, D. MicroRNAs change the games in central nervous system pharmacology. Biochem. Pharm. 2019, 168, 162–172. [Google Scholar] [CrossRef]
- Policarpo, R.; Sierksma, A.; De Strooper, B.; d’Ydewalle, C. From Junk to Function: LncRNAs in CNS Health and Disease. Front. Mol. Neurosci. 2021, 14, 714768. [Google Scholar] [CrossRef]
- Li, M.L.; Wang, W.; Jin, Z.B. Circular RNAs in the Central Nervous System. Front. Mol. Biosci. 2021, 8, 629593. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Teng, P.; Ku, L.; Chen, L.; Feng, Y.; Yao, B. Accurate identification of circRNA landscape and complexity reveals their pivotal roles in human oligodendroglia differentiation. Genome Biol. 2022, 23, 48. [Google Scholar] [CrossRef]
- Militello, G.; Weirick, T.; John, D.; Doring, C.; Dimmeler, S.; Uchida, S. Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief. Bioinform. 2017, 18, 780–788. [Google Scholar] [CrossRef]
- Huo, L.; Du, X.; Li, X.; Liu, S.; Xu, Y. The Emerging Role of Neural Cell-Derived Exosomes in Intercellular Communication in Health and Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 738442. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, M.L.; Baer, C.; Burdet, F.; Maderna, C.; Gilfillan, G.D.; Lyle, R.; Ibberson, M.; De Palma, M. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep. 2014, 8, 1432–1446. [Google Scholar] [CrossRef] [PubMed]
- Zeng, E.Z.; Chen, I.; Chen, X.; Yuan, X. Exosomal MicroRNAs as Novel Cell-Free Therapeutics in Tissue Engineering and Regenerative Medicine. Biomedicines 2022, 10, 2485. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Jean-Toussaint, R.; Sacan, A.; Ajit, S.K. Differential RNA packaging into small extracellular vesicles by neurons and astrocytes. Cell Commun. Signal. 2021, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef]
- Gauthier, S.A.; Perez-Gonzalez, R.; Sharma, A.; Huang, F.K.; Alldred, M.J.; Pawlik, M.; Kaur, G.; Ginsberg, S.D.; Neubert, T.A.; Levy, E. Enhanced exosome secretion in Down syndrome brain—A protective mechanism to alleviate neuronal endosomal abnormalities. Acta Neuropathol. Commun. 2017, 5, 65. [Google Scholar] [CrossRef]
- Papadopoulos, V.E.; Nikolopoulou, G.; Antoniadou, I.; Karachaliou, A.; Arianoglou, G.; Emmanouilidou, E.; Sardi, S.P.; Stefanis, L.; Vekrellis, K. Modulation of beta-glucocerebrosidase increases alpha-synuclein secretion and exosome release in mouse models of Parkinson’s disease. Hum. Mol. Genet. 2018, 27, 1696–1710. [Google Scholar] [CrossRef]
- Song, Z.; Xu, Y.; Deng, W.; Zhang, L.; Zhu, H.; Yu, P.; Qu, Y.; Zhao, W.; Han, Y.; Qin, C. Brain Derived Exosomes Are a Double-Edged Sword in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 79. [Google Scholar] [CrossRef]
- Verderio, C.; Muzio, L.; Turola, E.; Bergami, A.; Novellino, L.; Ruffini, F.; Riganti, L.; Corradini, I.; Francolini, M.; Garzetti, L.; et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 2012, 72, 610–624. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, P.; Zhang, Z.; Wu, M. Insights into Exosomal Non-Coding RNAs Sorting Mechanism and Clinical Application. Front. Oncol. 2021, 11, 664904. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, M.; Chen, B.; Calin, G.A. Exosomal lncRNAs as new players in cell-to-cell communication. Transl. Cancer Res. 2018, 7, S243–S252. [Google Scholar] [CrossRef] [PubMed]
- Ottoboni, L.; von Wunster, B.; Martino, G. Therapeutic Plasticity of Neural Stem Cells. Front. Neurol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Upadhya, R.; Madhu, L.N.; Attaluri, S.; Gitai, D.L.G.; Pinson, M.R.; Kodali, M.; Shetty, G.; Zanirati, G.; Kumar, S.; Shuai, B.; et al. Extracellular vesicles from human iPSC-derived neural stem cells: miRNA and protein signatures, and anti-inflammatory and neurogenic properties. J. Extracell Vesicles 2020, 9, 1809064. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shan, B.; Cheng, X.; He, H.; Qin, J.; Zhao, H.; Tian, M.; Zhang, X.; Jin, G. circRNA Acbd6 promotes neural stem cell differentiation into cholinergic neurons via the miR-320-5p-Osbpl2 axis. J. Biol. Chem. 2022, 298, 101828. [Google Scholar] [CrossRef]
- Yuan, P.; Ding, L.; Chen, H.; Wang, Y.; Li, C.; Zhao, S.; Yang, X.; Ma, Y.; Zhu, J.; Qi, X.; et al. Neural Stem Cell-Derived Exosomes Regulate Neural Stem Cell Differentiation Through miR-9-Hes1 Axis. Front. Cell Dev. Biol. 2021, 9, 601600. [Google Scholar] [CrossRef] [PubMed]
- Chivet, M.; Javalet, C.; Laulagnier, K.; Blot, B.; Hemming, F.J.; Sadoul, R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J. Extracell. Vesicles 2014, 3, 24722. [Google Scholar] [CrossRef]
- Veremeyko, T.; Kuznetsova, I.S.; Dukhinova, M.; Yung, A.W.Y.; Kopeikina, E.; Barteneva, N.S.; Ponomarev, E.D. Neuronal extracellular microRNAs miR-124 and miR-9 mediate cell-cell communication between neurons and microglia. J. Neurosci. Res. 2019, 97, 162–184. [Google Scholar] [CrossRef]
- Ponomarev, E.D.; Veremeyko, T.; Barteneva, N.; Krichevsky, A.M.; Weiner, H.L. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat. Med. 2011, 17, 64–70. [Google Scholar] [CrossRef]
- Jiang, D.; Gong, F.; Ge, X.; Lv, C.; Huang, C.; Feng, S.; Zhou, Z.; Rong, Y.; Wang, J.; Ji, C.; et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J. Nanobiotechnol. 2020, 18, 105. [Google Scholar] [CrossRef]
- Xian, X.; Cai, L.L.; Li, Y.; Wang, R.C.; Xu, Y.H.; Chen, Y.J.; Xie, Y.H.; Zhu, X.L.; Li, Y.F. Neuron secrete exosomes containing miR-9-5p to promote polarization of M1 microglia in depression. J. Nanobiotechnol. 2022, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Yelick, J.; Jin, S.; Tian, Y.; Chiang, M.S.R.; Higashimori, H.; Brown, E.; Jarvis, R.; Yang, Y. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 2019, 10, 4136. [Google Scholar] [CrossRef] [PubMed]
- Hoye, M.L.; Regan, M.R.; Jensen, L.A.; Lake, A.M.; Reddy, L.V.; Vidensky, S.; Richard, J.P.; Maragakis, N.J.; Rothstein, J.D.; Dougherty, J.D.; et al. Motor neuron-derived microRNAs cause astrocyte dysfunction in amyotrophic lateral sclerosis. Brain 2018, 141, 2561–2575. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Chen, R.; Miao, J.; Wang, L.; Cui, L.; Ji, H.; Liu, Y. Cortical Neuron-Derived Exosomal MicroRNA-181c-3p Inhibits Neuroinflammation by Downregulating CXCL1 in Astrocytes of a Rat Model with Ischemic Brain Injury. Neuroimmunomodulation 2019, 26, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, B.; Sun, R.; Chen, Y.; Feng, J. Exosome-transported Long Non-coding Ribonucleic Acid H19 Induces Blood-brain Barrier Disruption in Cerebral Ischemic Stroke Via the H19/micro Ribonucleic Acid-18a/Vascular Endothelial Growth factor Axis. Neuroscience 2022, 500, 41–51. [Google Scholar] [CrossRef]
- Krichevsky, A.M.; Sonntag, K.C.; Isacson, O.; Kosik, K.S. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells 2006, 24, 857–864. [Google Scholar] [CrossRef]
- Liu, G.; Abraham, E. MicroRNAs in immune response and macrophage polarization. Arter. Thromb. Vasc. Biol. 2013, 33, 170–177. [Google Scholar] [CrossRef]
- Ferraiuolo, L.; Shaw, P.J. Lost in translation: MicroRNAs mediate pathological cross-talk between motor neurons and astrocytes. Brain 2018, 141, 2534–2536. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, L.Y.; Li, Y.J.; Hong, Z.; Wei, W.S. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. J. Neuroinflammation 2012, 9, 211. [Google Scholar] [CrossRef]
- Jaerve, A.; Muller, H.W. Chemokines in CNS injury and repair. Cell Tissue Res. 2012, 349, 229–248. [Google Scholar] [CrossRef]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Shang, W.; Nie, Q.; Li, T.; Li, S. Long non-coding RNA H19 suppresses retinoblastoma progression via counteracting miR-17-92 cluster. J. Cell Biochem. 2018, 119, 3497–3509. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Santos, T.; Amar, A.; Tahara, S.M.; Chen, T.C.; Giannotta, S.L.; Hofman, F.M. MicroRNA-18a improves human cerebral arteriovenous malformation endothelial cell function. Stroke 2014, 45, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Lecca, D.; Marangon, D.; Coppolino, G.T.; Mendez, A.M.; Finardi, A.; Costa, G.D.; Martinelli, V.; Furlan, R.; Abbracchio, M.P. MiR-125a-3p timely inhibits oligodendroglial maturation and is pathologically up-regulated in human multiple sclerosis. Sci. Rep. 2016, 6, 34503. [Google Scholar] [CrossRef] [PubMed]
- Marangon, D.; Abbracchio, M.P.; Lecca, D. Pathway-Focused Profiling of Oligodendrocytes Over-Expressing miR-125a-3p Reveals Alteration of Wnt and Cell-to-Cell Signaling. Cell Mol. Neurobiol. 2021, 41, 105–114. [Google Scholar] [CrossRef]
- Marangon, D.; Boda, E.; Parolisi, R.; Negri, C.; Giorgi, C.; Montarolo, F.; Perga, S.; Bertolotto, A.; Buffo, A.; Abbracchio, M.P.; et al. In vivo silencing of miR-125a-3p promotes myelin repair in models of white matter demyelination. Glia 2020, 68, 2001–2014. [Google Scholar] [CrossRef]
- Bellingham, S.A.; Coleman, B.M.; Hill, A.F. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012, 40, 10937–10949. [Google Scholar] [CrossRef]
- Marcuzzo, S.; Bonanno, S.; Kapetis, D.; Barzago, C.; Cavalcante, P.; D’Alessandro, S.; Mantegazza, R.; Bernasconi, P. Up-regulation of neural and cell cycle-related microRNAs in brain of amyotrophic lateral sclerosis mice at late disease stage. Mol. Brain 2015, 8, 5. [Google Scholar] [CrossRef]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef]
- Lau, P.; Verrier, J.D.; Nielsen, J.A.; Johnson, K.R.; Notterpek, L.; Hudson, L.D. Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J. Neurosci. 2008, 28, 11720–11730. [Google Scholar] [CrossRef]
- Yang, L.; Cui, H.; Cao, T. Negative regulation of miRNA-9 on oligodendrocyte lineage gene 1 during hypoxic-ischemic brain damage. Neural Regen. Res. 2014, 9, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Chomyk, A.M.; Chang, A.; Ribaudo, M.V.; Deckard, S.A.; Doud, M.K.; Edberg, D.D.; Bai, B.; Li, M.; Baranzini, S.E.; et al. Hippocampal demyelination and memory dysfunction are associated with increased levels of the neuronal microRNA miR-124 and reduced AMPA receptors. Ann. Neurol. 2013, 73, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pang, Y.; Feng, W.; Jin, Y.; Chen, S.; Ding, S.; Wang, Z.; Zou, Y.; Li, Y.; Wang, T.; et al. miR-124 regulates early isolation-induced social abnormalities via inhibiting myelinogenesis in the medial prefrontal cortex. Cell Mol. Life Sci. 2022, 79, 507. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Chomyk, A.; Song, P.; Parker, N.; Deckard, S.; Trapp, B.D.; Pimplikar, S.W.; Dutta, R. Decrease in levels of the evolutionarily conserved microRNA miR-124 affects oligodendrocyte numbers in Zebrafish, Danio rerio. Invert. Neurosci. 2015, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Stevens, B. The “quad-partite” synapse: Microglia-synapse interactions in the developing and mature CNS. Glia 2013, 61, 24–36. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharm. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Guo, M.; Hao, Y.; Feng, Y.; Li, H.; Mao, Y.; Dong, Q.; Cui, M. Microglial Exosomes in Neurodegenerative Disease. Front. Mol. Neurosci. 2021, 14, 630808. [Google Scholar] [CrossRef]
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’Arrigo, G.; Parolisi, R.; De Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: A new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018, 135, 529–550. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Song, Y.; Pan, J.J.; Jiang, Y.; Shi, X.; Liu, C.; Ma, Y.; Luo, L.; Mamtilahun, M.; et al. M2 microglia-derived extracellular vesicles promote white matter repair and functional recovery via miR-23a-5p after cerebral ischemia in mice. Theranostics 2022, 12, 3553–3573. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; He, T.; Wen, R.; Li, Y.; Chen, T.; Huang, S.; Wang, Y.; Tang, Y.; Shen, F.; et al. M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics 2021, 11, 1232–1248. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, Y.; Lan, T.; Wang, W.; Long, Y.; Yu, S.Y. Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Molecules 2022, 30, 1300–1314. [Google Scholar] [CrossRef]
- Leschik, J.; Lutz, B.; Gentile, A. Stress-Related Dysfunction of Adult Hippocampal Neurogenesis-An Attempt for Understanding Resilience? Int. J. Mol. Sci. 2021, 22, 7339. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018, 32, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cai, G.; Liu, K.; Zhuang, Z.; Jia, K.; Pei, S.; Wang, X.; Wang, H.; Xu, S.; Cui, C.; et al. Microglia exosomal miRNA-137 attenuates ischemic brain injury through targeting Notch1. Aging 2021, 13, 4079–4095. [Google Scholar] [CrossRef]
- Arumugam, T.V.; Baik, S.H.; Balaganapathy, P.; Sobey, C.G.; Mattson, M.P.; Jo, D.G. Notch signaling and neuronal death in stroke. Prog. Neurobiol. 2018, 165–167, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Guo, M.; Hu, T.; Li, W.; Huang, S.; Yin, Z.; Li, Y.; Chen, F.; Zhu, L.; Kang, C.; et al. Increased Microglial Exosomal miR-124-3p Alleviates Neurodegeneration and Improves Cognitive Outcome after rmTBI. Molecules 2020, 28, 503–522. [Google Scholar] [CrossRef]
- Li, D.; Huang, S.; Yin, Z.; Zhu, J.; Ge, X.; Han, Z.; Tan, J.; Zhang, S.; Zhao, J.; Chen, F.; et al. Increases in miR-124-3p in Microglial Exosomes Confer Neuroprotective Effects by Targeting FIP200-Mediated Neuronal Autophagy Following Traumatic Brain Injury. Neurochem. Res. 2019, 44, 1903–1923. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Chaudhuri, A.D.; Dastgheyb, R.M.; Yoo, S.W.; Trout, A.; Talbot, C.C., Jr.; Hao, H.; Witwer, K.W.; Haughey, N.J. TNFalpha and IL-1beta modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 2018, 9, 363. [Google Scholar] [CrossRef]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes From Astrocyte Processes: Signaling to Neurons. Front. Pharm. 2019, 10, 1452. [Google Scholar] [CrossRef] [PubMed]
- Datta Chaudhuri, A.; Dasgheyb, R.M.; DeVine, L.R.; Bi, H.; Cole, R.N.; Haughey, N.J. Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia 2020, 68, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Sun, Q.; Xiao, H.; Zhang, C.; Li, L. Secreted miR-34a in astrocytic shedding vesicles enhanced the vulnerability of dopaminergic neurons to neurotoxins by targeting Bcl-2. Protein Cell 2015, 6, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Varcianna, A.; Myszczynska, M.A.; Castelli, L.M.; O’Neill, B.; Kim, Y.; Talbot, J.; Nyberg, S.; Nyamali, I.; Heath, P.R.; Stopford, M.J.; et al. Micro-RNAs secreted through astrocyte-derived extracellular vesicles cause neuronal network degeneration in C9orf72 ALS. EBioMedicine 2019, 40, 626–635. [Google Scholar] [CrossRef]
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-derived exosomes transfer miR-190b to inhibit oxygen and glucose deprivation-induced autophagy and neuronal apoptosis. Cell Cycle 2020, 19, 906–917. [Google Scholar] [CrossRef]
- Jovicic, A.; Gitler, A.D. Distinct repertoires of microRNAs present in mouse astrocytes compared to astrocyte-secreted exosomes. PLoS ONE 2017, 12, e0171418. [Google Scholar] [CrossRef]
- He, B.; Chen, W.; Zeng, J.; Tong, W.; Zheng, P. Long noncoding RNA NKILA transferred by astrocyte-derived extracellular vesicles protects against neuronal injury by upregulating NLRX1 through binding to mir-195 in traumatic brain injury. Aging 2021, 13, 8127–8145. [Google Scholar] [CrossRef]
- Hu, G.; Liao, K.; Niu, F.; Yang, L.; Dallon, B.W.; Callen, S.; Tian, C.; Shu, J.; Cui, J.; Sun, Z.; et al. Astrocyte EV-Induced lincRNA-Cox2 Regulates Microglial Phagocytosis: Implications for Morphine-Mediated Neurodegeneration. Mol. Nucleic Acids 2018, 13, 450–463. [Google Scholar] [CrossRef]
- Carpenter, S.; Aiello, D.; Atianand, M.K.; Ricci, E.P.; Gandhi, P.; Hall, L.L.; Byron, M.; Monks, B.; Henry-Bezy, M.; Lawrence, J.B.; et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science 2013, 341, 789–792. [Google Scholar] [CrossRef]
- Robinson, E.K.; Worthington, A.; Poscablo, D.; Shapleigh, B.; Salih, M.M.; Halasz, H.; Seninge, L.; Mosqueira, B.; Smaliy, V.; Forsberg, E.C.; et al. lincRNA-Cox2 Functions to Regulate Inflammation in Alveolar Macrophages during Acute Lung Injury. J. Immunol. 2022, 208, 1886–1900. [Google Scholar] [CrossRef]
- Korner, S.; Boselt, S.; Wichmann, K.; Thau-Habermann, N.; Zapf, A.; Knippenberg, S.; Dengler, R.; Petri, S. The Axon Guidance Protein Semaphorin 3A Is Increased in the Motor Cortex of Patients With Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2016, 75, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeis, C.; Frohlich, D.; Kuo, W.P.; Amphornrat, J.; Thilemann, S.; Saab, A.S.; Kirchhoff, F.; Mobius, W.; Goebbels, S.; Nave, K.A.; et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013, 11, e1001604. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, B.; Wuyts, C.; Sisto, A.; Pintelon, I.; Timmermans, J.P.; Somers, V.; Timmerman, V.; Hellings, N.; Irobi, J. Oligodendroglia-derived extracellular vesicles activate autophagy via LC3B/BAG3 to protect against oxidative stress with an enhanced effect for HSPB8 enriched vesicles. Cell Commun. Signal. 2022, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Rasouli, J.; Boehm, A.; Zhang, W.; Xiao, D.; Ishikawa, L.L.W.; Thome, R.; Li, X.; Hwang, D.; Porazzi, P.; et al. Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci. Transl. Med. 2020, 12, eaba0599. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wang, J.; Lu, Y.; Deng, Y.; Zhao, C.; Xu, L.; Chen, Y.; Hu, Y.C.; Zhou, W.; Lu, Q.R. lncRNA Functional Networks in Oligodendrocytes Reveal Stage-Specific Myelination Control by an lncOL1/Suz12 Complex in the CNS. Neuron 2017, 93, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Moyano, A.L.; Ma, Z.; Deng, Y.; Lin, Y.; Zhao, C.; Zhang, L.; Jiang, M.; He, X.; Ma, Z.; et al. miR-219 Cooperates with miR-338 in Myelination and Promotes Myelin Repair in the CNS. Dev. Cell 2017, 40, e565. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Ong, W.; Wang, K.; Wang, M.; Nizetic, D.; Chew, S.Y. Effects of miR-219/miR-338 on microglia and astrocyte behaviors and astrocyte-oligodendrocyte precursor cell interactions. Neural Regen Res. 2020, 15, 739–747. [Google Scholar] [CrossRef]
- Wang, T.; Cai, Q.; Yang, W.J.; Fan, H.H.; Yi, J.F.; Xu, F. MicroRNA-219 alleviates glutamate-induced neurotoxicity in cultured hippocampal neurons by targeting calmodulin-dependent protein kinase II gamma. Neural Regen. Res. 2018, 13, 1216–1224. [Google Scholar] [CrossRef]
- Boscher, E.; Husson, T.; Quenez, O.; Laquerriere, A.; Marguet, F.; Cassinari, K.; Wallon, D.; Martinaud, O.; Charbonnier, C.; Nicolas, G.; et al. Copy Number Variants in miR-138 as a Potential Risk Factor for Early-Onset Alzheimer’s Disease. J. Alzheimers Dis. 2019, 68, 1243–1255. [Google Scholar] [CrossRef]
- Boscher, E.; Goupil, C.; Petry, S.; Keraudren, R.; Loiselle, A.; Planel, E.; Hebert, S.S. MicroRNA-138 Overexpression Alters Abeta42 Levels and Behavior in Wildtype Mice. Front. Neurosci. 2020, 14, 591138. [Google Scholar] [CrossRef]
- Mustapic, M.; Eitan, E.; Werner, J.K., Jr.; Berkowitz, S.T.; Lazaropoulos, M.P.; Tran, J.; Goetzl, E.J.; Kapogiannis, D. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front. Neurosci. 2017, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Picciolini, S.; Gualerzi, A.; Vanna, R.; Sguassero, A.; Gramatica, F.; Bedoni, M.; Masserini, M.; Morasso, C. Detection and Characterization of Different Brain-Derived Subpopulations of Plasma Exosomes by Surface Plasmon Resonance Imaging. Anal. Chem. 2018, 90, 8873–8880. [Google Scholar] [CrossRef] [PubMed]
- Winston, C.N.; Sarsoza, F.; Spencer, B.; Rissman, R.A. Characterizing blood-based, microglial derived exosomes (MDEs) as biomarkers for Alzheimer’s disease. Alzheimer Dement. 2021, 17, e055371. [Google Scholar] [CrossRef]
- Winston, C.N.; Romero, H.K.; Ellisman, M.; Nauss, S.; Julovich, D.A.; Conger, T.; Hall, J.R.; Campana, W.; O’Bryant, S.E.; Nievergelt, C.M.; et al. Assessing Neuronal and Astrocyte Derived Exosomes from Individuals With Mild Traumatic Brain Injury for Markers of Neurodegeneration and Cytotoxic Activity. Front. Neurosci. 2019, 13, 1005. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marangon, D.; Castro e Silva, J.H.; Lecca, D. Neuronal and Glial Communication via Non-Coding RNAs: Messages in Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 470. https://doi.org/10.3390/ijms24010470
Marangon D, Castro e Silva JH, Lecca D. Neuronal and Glial Communication via Non-Coding RNAs: Messages in Extracellular Vesicles. International Journal of Molecular Sciences. 2023; 24(1):470. https://doi.org/10.3390/ijms24010470
Chicago/Turabian StyleMarangon, Davide, Juliana Helena Castro e Silva, and Davide Lecca. 2023. "Neuronal and Glial Communication via Non-Coding RNAs: Messages in Extracellular Vesicles" International Journal of Molecular Sciences 24, no. 1: 470. https://doi.org/10.3390/ijms24010470
APA StyleMarangon, D., Castro e Silva, J. H., & Lecca, D. (2023). Neuronal and Glial Communication via Non-Coding RNAs: Messages in Extracellular Vesicles. International Journal of Molecular Sciences, 24(1), 470. https://doi.org/10.3390/ijms24010470