Peptide-Based Materials That Exploit Metal Coordination
Abstract
1. Introduction
- Amino acids without chelating sidechains, e.g., Phe or Leu, can coordinate metals through their backbone amides and their ammonium and carboxylate termini;
- Those with hydrophilic sidechains containing a Lewis base, e.g., Cys or His, can chelate metals not only through these functional groups, but also through their backbone or termini, so that metal ions act as bridges to enable intermolecular cross-linking;
- Those with chelating ionizable sidechains, e.g., Asp or Lys, can chelate metal ions either through these sidechains, or through their backbone and termini [4];
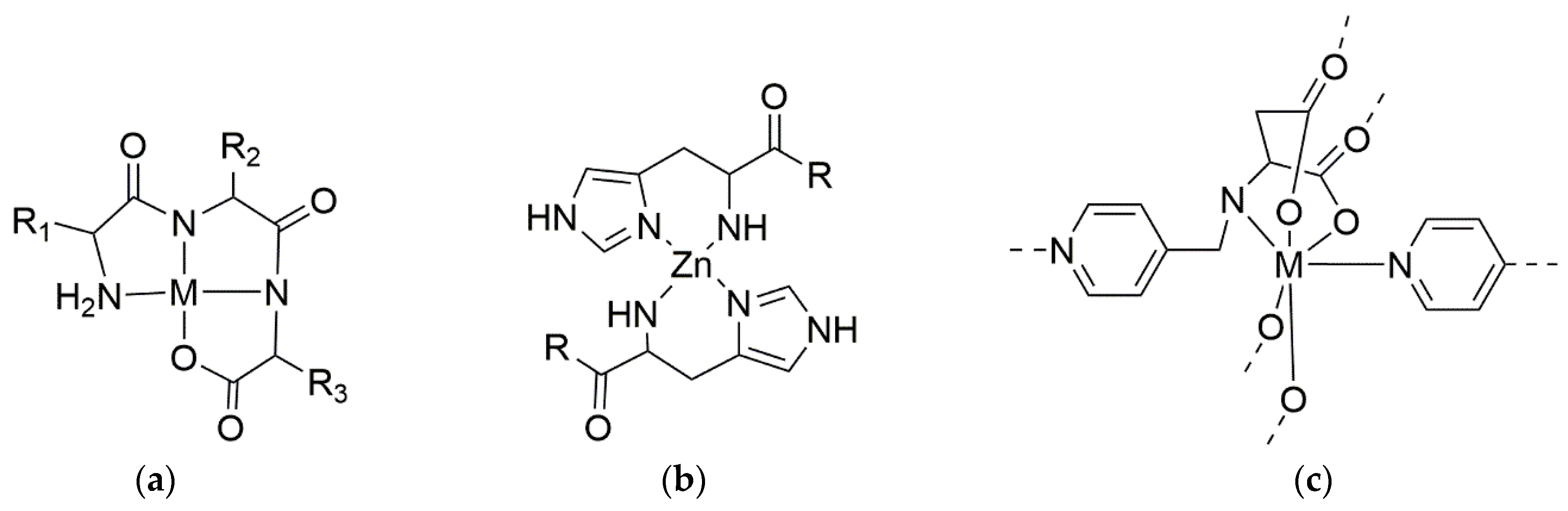
2. Metal–Peptide Materials in Medicine
2.1. Minimalistic Systems Based on Dipeptides Interacting with Metal Ions for Drug Delivery
2.2. Longer Peptides Interacting with Metal Ions for Drug Delivery
2.3. Peptides and Metal Ions for Tissue Regeneration
2.4. Peptides and Metal Ions for Wound Healing
2.5. Peptides and Metal Ions for Antimicrobial Materials
2.6. Amyloid Beta (Aβ) Fibrillation Inhibitors Based on Metal–Ion–Peptide Nanostructures
3. Metal–Peptide Materials for Environmental Remediation
4. Metal–Peptide Materials for Sensing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References and Note
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed]
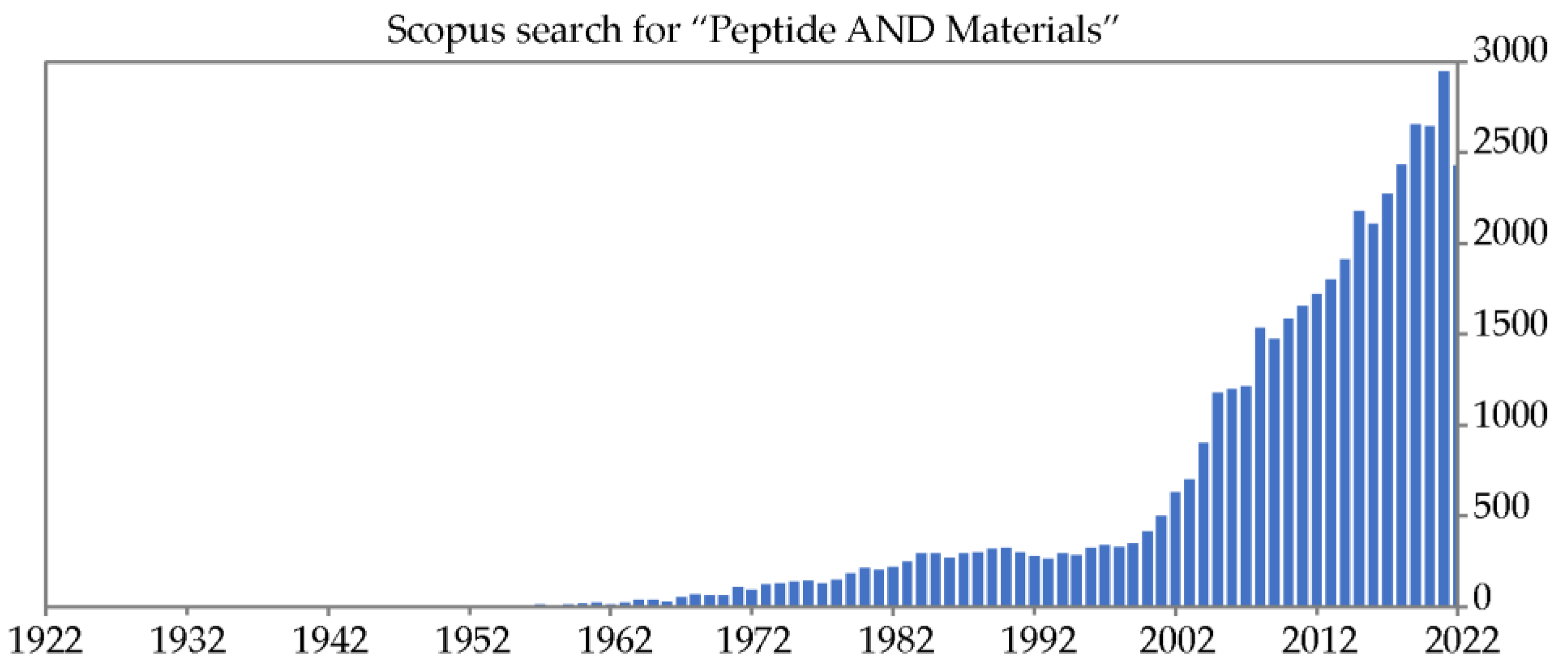
- Scopus search on 04-11-22 for “Peptide AND Materials” on title, abstract, or keywords.
- Maldonado, N.; Amo-Ochoa, P. Advances and novel perspectives on colloids, hydrogels, and aerogels based on coordination bonds with biological interest ligands. Nanomaterials 2021, 11, 1865. [Google Scholar] [CrossRef] [PubMed]
- Sóvágó, I.; Kállay, C.; Várnagy, K. Peptides as complexing agents: Factors influencing the structure and thermodynamic stability of peptide complexes. Coord. Chem. Rev. 2012, 256, 2225–2233. [Google Scholar] [CrossRef]
- Zou, R.; Wang, Q.; Wu, J.; Wu, J.; Schmuck, C.; Tian, H. Peptide self-assembly triggered by metal ions. Chem. Soc. Rev. 2015, 44, 5200–5219. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, N.; Hou, C.-Y.; Han, X.-X.; Liu, C.-H.; Xing, Y.-H.; Bai, F.-Y.; Sun, L.-X. Transition metal complexes constructed by pyridine–amino acid: Fluorescence sensing and catalytic properties. Transit. Met. Chem. 2020, 45, 423–433. [Google Scholar] [CrossRef]
- Monger, L.J.; Razinkov, D.; Bjornsson, R.; Suman, S.G. Synthesis, characterization, and reaction studies of Pd(II) tripeptide complexes. Molecules 2021, 26, 5169. [Google Scholar] [CrossRef]
- Förster, M.; Vahrenkamp, H. Zinc complexes of histidine-containing di- and tripeptides. Chem. Ber. 1995, 128, 541–550. [Google Scholar] [CrossRef]
- Xia, Y.; Xue, B.; Qin, M.; Cao, Y.; Li, Y.; Wang, W. Printable fluorescent hydrogels based on self-assembling peptides. Sci. Rep. 2017, 7, 9691. [Google Scholar] [CrossRef]
- Shao, T.; Falcone, N.; Kraatz, H.-B. Supramolecular peptide gels: Influencing properties by metal ion coordination and their wide-ranging applications. ACS Omega 2020, 5, 1312–1317. [Google Scholar] [CrossRef]
- Khare, E.; Holten-Andersen, N.; Buehler, M.J. Transition-metal coordinate bonds for bioinspired macromolecules with tunable mechanical properties. Nat. Rev. Mater. 2021, 6, 421–436. [Google Scholar] [CrossRef]
- Bellotto, O.; Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Peptide gelators to template inorganic nanoparticle formation. Gels 2021, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Merg, A.D.; Boatz, J.C.; Mandal, A.; Zhao, G.; Mokashi-Punekar, S.; Liu, C.; Wang, X.; Zhang, P.; van der Wel, P.C.A.; Rosi, N.L. Peptide-directed assembly of single-helical gold nanoparticle superstructures exhibiting intense chiroptical activity. J. Am. Chem. Soc. 2016, 138, 13655–13663. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Zhang, J.; Hai, X.; Yan, Y.; Song, W.; Bi, S. Recent advances in templated synthesis of metal nanoclusters and their applications in biosensing, bioimaging and theranostics. Biosens. Bioelectron. 2021, 176, 112898. [Google Scholar] [CrossRef] [PubMed]
- Rozhin, P.; Melchionna, M.; Fornasiero, P.; Marchesan, S. Nanostructured ceria: Biomolecular templates and (bio)applications. Nanomaterials 2021, 11, 2259. [Google Scholar] [CrossRef] [PubMed]
- Saif, B.; Yang, P. Metal–protein hybrid materials with desired functions and potential applications. ACS Appl. Bio Mater. 2021, 4, 1156–1177. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, K.; Yoo, S.H.; Lee, H.-S.; Kwon, S. Crystalline metal-peptide networks: Structures, applications, and future outlook. ChemBioChem 2022, e202200448. [Google Scholar] [CrossRef]
- Jahović, I.; Zou, Y.-Q.; Adorinni, S.; Nitschke, J.R.; Marchesan, S. Cages meet gels: Smart materials with dual porosity. Matter 2021, 4, 2123–2140. [Google Scholar] [CrossRef]
- Dey, S.; Misra, R.; Saseendran, A.; Pahan, S.; Gopi, H.N. Metal-coordinated supramolecular polymers from the minimalistic hybrid peptide foldamers. Angew. Chem. Int. Ed. 2021, 60, 9863–9868. [Google Scholar] [CrossRef]
- Kieffer, M.; Garcia, A.M.; Haynes, C.J.E.; Kralj, S.; Iglesias, D.; Nitschke, J.R.; Marchesan, S. Embedding and positioning of two FeII4L4 cages in supramolecular tripeptide gels for selective chemical segregation. Angew. Chem. Int. Ed. 2019, 58, 7982–7986. [Google Scholar] [CrossRef]
- Falcone, N.; Kraatz, H.-B. Supramolecular assembly of peptide and metallopeptide gelators and their stimuli-responsive properties in biomedical applications. Chem. Eur. J. 2018, 24, 14316–14328. [Google Scholar] [CrossRef]
- McEwen, H.; Du, E.Y.; Mata, J.P.; Thordarson, P.; Martin, A.D. Tuning hydrogels through metal-based gelation triggers. J. Mater. Chem. B 2017, 5, 9412–9417. [Google Scholar] [CrossRef] [PubMed]
- Metrano, A.J.; Chinn, A.J.; Shugrue, C.R.; Stone, E.A.; Kim, B.; Miller, S.J. Asymmetric catalysis mediated by synthetic peptides, version 2.0: Expansion of scope and mechanisms. Chem. Rev. 2020, 120, 11479–11615. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Gong, H.; Ren, X.; Yan, X. Supramolecular nanozymes based on peptide self-assembly for biomimetic catalysis. Nano Today 2021, 41, 101295. [Google Scholar] [CrossRef]
- Arad, E.; Jelinek, R. Catalytic amyloids. Trends Chem. 2022, 4, 907–917. [Google Scholar] [CrossRef]
- Carvalho, S.; Peralta Reis, D.Q.; Pereira, S.V.; Kalafatovic, D.; Pina, A.S. Catalytic peptides: The challenge between simplicity and functionality. Isr. J. Chem. 2022, 62, e202200029. [Google Scholar] [CrossRef]
- Chatterjee, A.; Reja, A.; Pal, S.; Das, D. Systems chemistry of peptide-assemblies for biochemical transformations. Chem. Soc. Rev. 2022, 51, 3047–3070. [Google Scholar] [CrossRef] [PubMed]
- Firipis, K.; Nisbet, D.R.; Franks, S.J.; Kapsa, R.M.I.; Pirogova, E.; Williams, R.J.; Quigley, A. Enhancing peptide biomaterials for biofabrication. Polymers 2021, 13, 2590. [Google Scholar] [CrossRef]
- Li, T.; Lu, X.M.; Zhang, M.R.; Hu, K.; Li, Z. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioactive Mater. 2022, 11, 268–282. [Google Scholar] [CrossRef]
- Lee, S.; Trinh, T.H.T.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.B.; Ryou, C. Self-assembling peptides and their application in the treatment of diseases. Int. J. Mol. Sci. 2019, 20, 5850. [Google Scholar] [CrossRef] [PubMed]
- La Manna, S.; Di Natale, C.; Onesto, V.; Marasco, D. Self-assembling peptides: From design to biomedical applications. Int. J. Mol. Sci. 2021, 22, 12662. [Google Scholar] [CrossRef]
- Yu, Y.; Mok, B.Y.L.; Loh, X.J.; Tan, Y.N. Rational design of biomolecular templates for synthesizing multifunctional noble metal nanoclusters toward personalized theranostic applications. Adv. Health Mater. 2016, 5, 1844–1859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, N.; Fu, B.; Huang, F.; Liu, J. Self-assembling peptide-based nanodrug delivery systems. Biomater. Sci. 2019, 7, 4888–4911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ai, S.; Yang, Z.; Li, X. Peptide-based supramolecular hydrogels for local drug delivery. Adv. Drug Deliv. Rev. 2021, 174, 482–503. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Rosa, E.; Accardo, A.; Morelli, G. Peptide-based hydrogels as delivery systems for doxorubicin. J. Pept. Sci. 2022, 28, e3301. [Google Scholar] [CrossRef]
- Contini, A.; Erba, E.; Bondavalli, V.; Barbiroli, A.; Gelmi, M.L.; Romanelli, A. Morpholino-based peptide oligomers: Synthesis and DNA binding properties. Biochem. Biophys. Res. Commun. 2021, 549, 8–13. [Google Scholar] [CrossRef]
- Zeng, X.Z.; An, H.W.; Wang, H. Chemical reactions trigger peptide self-assembly in vivo for tumor therapy. ChemMedChem 2021, 16, 2452–2458. [Google Scholar] [CrossRef]
- Conibear, A.C.; Schmid, A.; Kamalov, M.; Becker, C.F.W.; Bello, C. Recent advances in peptide-based approaches for cancer treatment. Curr. Med. Chem. 2020, 27, 1174–1205. [Google Scholar] [CrossRef]
- De Rosa, L.; Di Stasi, R.; D’Andrea, L.D. Pro-angiogenic peptides in biomedicine. Arch. Biochem. Biophys. 2018, 660, 72–86. [Google Scholar] [CrossRef]
- Diaferia, C.; Gianolio, E.; Accardo, A. Peptide-based building blocks as structural elements for supramolecular Gd-containing MRI contrast agents. J. Pept. Sci. 2019, 25, e3157. [Google Scholar] [CrossRef]
- Gallo, E.; Rosa, E.; Diaferia, C.; Rossi, F.; Tesauro, D.; Accardo, A. Systematic overview of soft materials as a novel frontier for mri contrast agents. RSC Adv. 2020, 10, 27064–27080. [Google Scholar] [CrossRef]
- Li, L.L.; Qiao, Z.Y.; Wang, L.; Wang, H. Programmable construction of peptide-based materials in living subjects: From modular design and morphological control to theranostics. Adv. Mater. 2019, 31, e1804971. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Ferrão, R.; Palma, P.; Patricio, T.; Parreira, P.; Anes, E.; Tonda-Turo, C.; Martins, M.C.L.; Alves, N.; Ferreira, L. Antimicrobial peptide-based materials: Opportunities and challenges. J. Mater. Chem. B 2022, 10, 2384–2429. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-based hydrogels: New materials for biosensing and biomedical applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef] [PubMed]
- Adedeji Olulana, A.F.; Soler, M.A.; Lotteri, M.; Vondracek, H.; Casalis, L.; Marasco, D.; Castronovo, M.; Fortuna, S. Computational evolution of beta-2-microglobulin binding peptides for nanopatterned surface sensors. Int. J. Mol. Sci. 2021, 22, 812. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Lindstrom, A.R.; Lin, T.Y.; Lam, K.S.; Li, Y. Peptide-based materials for cancer immunotherapy. Theranostics 2019, 9, 7807–7825. [Google Scholar] [CrossRef]
- O’Neill, C.L.; Shrimali, P.C.; Clapacs, Z.P.; Files, M.A.; Rudra, J.S. Peptide-based supramolecular vaccine systems. Acta Biomater. 2021, 133, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, N.; AL, S.; Chandran, A.; Sadanandan, S. Recent advances in peptides-based stimuli-responsive materials for biomedical and therapeutic applications: A review. Mol. Pharm. 2022, 19, 1999–2021. [Google Scholar] [CrossRef]
- Martin, A.D.; Thordarson, P. Beyond Fmoc: A review of aromatic peptide capping groups. J. Mater. Chem. B 2020, 8, 863–877. [Google Scholar] [CrossRef]
- Tao, K.; Levin, A.; Adler-Abramovich, L.; Gazit, E. Fmoc-modified amino acids and short peptides: Simple bio-inspired building blocks for the fabrication of functional materials. Chem. Soc. Rev. 2016, 45, 3935–3953. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, H.; Roy, S. Inducing differential self-assembling behavior in ultrashort peptide hydrogelators using simple metal salts. Biomacromolecules 2019, 20, 2610–2624. [Google Scholar] [CrossRef]
- Ji, W.; Yuan, C.; Zilberzwige-Tal, S.; Xing, R.; Chakraborty, P.; Tao, K.; Gilead, S.; Yan, X.; Gazit, E. Metal-ion modulated structural transformation of amyloid-like dipeptide supramolecular self-assembly. ACS Nano 2019, 13, 7300–7309. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska, J.; De la Flor, S.; Kozlowska, J. From supramolecular hydrogels to multifunctional carriers for biologically active substances. Int. J. Mol. Sci. 2021, 22, 7402. [Google Scholar] [CrossRef] [PubMed]
- Pal, V.K.; Roy, S. Cooperative metal ion coordination to the short self-assembling peptide promotes hydrogelation and cellular proliferation. Macromol. Biosci. 2022, 22, 2100462. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Chen, H.; Liu, T.; Zhu, J.; Yu, M.; Yuan, Q. Poly(L-cysteine) peptide amphiphile derivatives containing disulfide bonds: Synthesis, self-assembly-induced β-sheet nanostructures, pH/reduction dual response, and drug release. Biomacromolecules 2021, 22, 5374–5381. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.A.; Eckrich, J.; Wiesmann, N.; Kuczelinis, F.; Sun, W.; Zeng, X.; Weber, B.; Wu, S.; Bings, N.H.; Strieth, S.; et al. Photocleavable core cross-linked polymeric micelles of polypept(o)ides and ruthenium(II) complexes. J. Mater. Chem. B 2021, 9, 8211–8223. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Zhai, Z.; Yang, L.; Tang, X.; Zhao, L.; Xu, K.; Zhong, W. Double-crosslinked nanocomposite hydrogels for temporal control of drug dosing in combination therapy. Acta Biomater. 2020, 106, 278–288. [Google Scholar] [CrossRef]
- Zhai, Z.; Xu, K.; Mei, L.; Wu, C.; Liu, J.; Liu, Z.; Wan, L.; Zhong, W. Co-assembled supramolecular hydrogels of cell adhesive peptide and alginate for rapid hemostasis and efficacious wound healing. Soft Matter 2019, 15, 8603–8610. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Chang, R.; Duan, H.-Z.; Chen, Y.-X. Metal ion and light sequentially induced sol–gel–sol transition of a responsive peptide-hydrogel. Soft Matter 2020, 16, 7652–7658. [Google Scholar] [CrossRef]
- Marin, D.; Marchesan, S. Self-assembled peptide nanostructures for ECM biomimicry. Nanomaterials 2022, 12, 2147. [Google Scholar] [CrossRef]
- Zeng, L.; Song, M.; Gu, J.; Xu, Z.; Xue, B.; Li, Y.; Cao, Y. A highly stretchable, tough, fast self-healing hydrogel based on peptide⁻metal ion coordination. Biomimetics 2019, 4, 36. [Google Scholar] [CrossRef]
- Tunn, I.; Harrington, M.J.; Blank, K.G. Bioinspired histidine⁻Zn2+ coordination for tuning the mechanical properties of self-healing coiled coil cross-linked hydrogels. Biomimetics 2019, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Lee, H.J.; Son, S.; Kim, H.; Kim, J.; Jeong, B. Iron ion-releasing polypeptide thermogel for neuronal differentiation of mesenchymal stem cells. Biomacromolecules 2020, 21, 143–151. [Google Scholar] [CrossRef]
- Paladini, F.; Meikle, S.T.; Cooper, I.R.; Lacey, J.; Perugini, V.; Santin, M. Silver-doped self-assembling di-phenylalanine hydrogels as wound dressing biomaterials. J. Mater. Sci. 2013, 24, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Lackovic, M.; Lalatovic, J.; Mougharbel, A.S.; Kortz, U.; Krstic, D.Z. Polyoxometalates in biomedicine: Update and overview. Curr. Med. Chem. 2020, 27, 362–379. [Google Scholar] [CrossRef]
- Li, X.; Zheng, T.; Liu, X.; Du, Z.; Xie, X.; Li, B.; Wu, L.; Li, W. Coassembly of short peptide and polyoxometalate into complex coacervate adapted for ph and metal ion-triggered underwater adhesion. Langmuir 2019, 35, 4995–5003. [Google Scholar] [CrossRef] [PubMed]
- Colomina-Alfaro, L.; Marchesan, S.; Stamboulis, A.; Bandiera, A. Smart tools for antimicrobial peptides expression and application: The elastic perspective. Biotechnol. Bioeng. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bellotto, O.; Semeraro, S.; Bandiera, A.; Tramer, F.; Pavan, N.; Marchesan, S. Polymer conjugates of antimicrobial peptides (AMPs) with D-amino acids (D-aa): State of the art and future opportunities. Pharmaceutics 2022, 14, 446. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Lavendomme, R.; Kralj, S.; Kurbasic, M.; Bellotto, O.; Cringoli, M.C.; Semeraro, S.; Bandiera, A.; De Zorzi, R.; Marchesan, S. Self-assembly of an amino acid derivative into an antimicrobial hydrogel biomaterial. Chem. Eur. J. 2020, 26, 1880–1886. [Google Scholar] [CrossRef]
- Nowak, M.G.; Skwarecki, A.S.; Milewska, M.J. Amino acid based antimicrobial agents—Synthesis and properties. ChemMedChem 2021, 16, 3513–3544. [Google Scholar] [CrossRef]
- Qian, Y.; Altamimi, A.; Yates, S.A.; Sarkar, S.; Cochran, M.; Zhou, M.; Levi-Polyachenko, N.; Matson, J.B. H2S-releasing amphiphilic dipeptide hydrogels are potent S. aureus biofilm disruptors. Biomater. Sci. 2020, 8, 2564–2576. [Google Scholar] [CrossRef]
- Saadouli, I.; Zendah El Euch, I.; Trabelsi, E.; Mosbah, A.; Redissi, A.; Ferjani, R.; Fhoula, I.; Cherif, A.; Sabatier, J.-M.; Sewald, N.; et al. Isolation, characterization and chemical synthesis of large spectrum antimicrobial cyclic dipeptide (L-leu-L-pro) from Streptomyces misionensis V16R3Y1 bacteria extracts. A novel 1H NMR metabolomic approach. Antibiotics 2020, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, L.; Sun, S.; Chang, A.; Dai, X.; Li, H.; Wang, Y.; Zhu, H. A cyclic dipeptide from marine fungus Penicillium chrysogenum DXY-1 exhibits anti-quorum sensing activity. ACS Omega 2021, 6, 7693–7700. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Aaghaz, S.; Shenmar, K.; Jain, R. Short antimicrobial peptides. Recent Pat. Anti-Infect. Drug Discov. 2018, 13, 12–52. [Google Scholar] [CrossRef] [PubMed]
- Rosetti, B.; Scarel, E.; Colomina-Alfaro, L.; Adorinni, S.; Pierri, G.; Bellotto, O.; Mamprin, K.; Polentarutti, M.; Bandiera, A.; Tedesco, C.; et al. Self-assembly of homo- and hetero-chiral cyclodipeptides into supramolecular polymers towards antimicrobial gels. Polymers 2022, 14, 4554. [Google Scholar] [CrossRef]
- Carpa, R.; Remizovschi, A.; Culda, C.A.; Butiuc-Keul, A.L. Inherent and composite hydrogels as promising materials to limit antimicrobial resistance. Gels 2022, 8, 70. [Google Scholar] [CrossRef]
- Masimen, M.A.A.; Harun, N.A.; Maulidiani, M.; Ismail, W.I.W. Overcoming methicillin-resistance Staphylococcus aureus (MRSA) using antimicrobial peptides-silver nanoparticles. Antibiotics 2022, 11, 951. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Bashiri, S.; Yuan, Y.; Ziora, Z.M.; Nabil, O.; Masuda, K.; Khongkow, M.; Rimsueb, N.; Cabral, H.; Ruktanonchai, U.; et al. Antimicrobial activity enhancers: Towards smart delivery of antimicrobial agents. Antibiotics 2022, 11, 412. [Google Scholar] [CrossRef]
- Birkett, M.; Dover, L.; Cherian Lukose, C.; Wasy Zia, A.; Tambuwala, M.M.; Serrano-Aroca, Á. Recent advances in metal-based antimicrobial coatings for high-touch surfaces. Int. J. Mol. Sci. 2022, 23, 1162. [Google Scholar] [CrossRef]
- Mishra, A.; Pradhan, D.; Halder, J.; Biswasroy, P.; Rai, V.K.; Dubey, D.; Kar, B.; Ghosh, G.; Rath, G. Metal nanoparticles against multi-drug-resistance bacteria. J. Inorg. Biochem. 2022, 237, 111938. [Google Scholar] [CrossRef]
- Song, J.; Yuan, C.; Jiao, T.; Xing, R.; Yang, M.; Adams, D.J.; Yan, X. Multifunctional antimicrobial biometallohydrogels based on amino acid coordinated self-assembly. Small 2020, 16, 1907309. [Google Scholar] [CrossRef]
- D’Souza, A.; Yoon, J.H.; Beaman, H.; Gosavi, P.; Lengyel-Zhand, Z.; Sternisha, A.; Centola, G.; Marshall, L.R.; Wehrman, M.D.; Schultz, K.M.; et al. Nine-residue peptide self-assembles in the presence of silver to produce a self-healing, cytocompatible, antimicrobial hydrogel. ACS Appl. Mater. Interfaces 2020, 12, 17091–17099. [Google Scholar] [CrossRef]
- Schnaider, L.; Toprakcioglu, Z.; Ezra, A.; Liu, X.; Bychenko, D.; Levin, A.; Gazit, E.; Knowles, T.P.J. Biocompatible hybrid organic/inorganic microhydrogels promote bacterial adherence and eradication in vitro and in vivo. Nano Lett. 2020, 20, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Iudin, D.; Vasilieva, M.; Knyazeva, E.; Korzhikov-Vlakh, V.; Demyanova, E.; Lavrentieva, A.; Skorik, Y.; Korzhikova-Vlakh, E. Hybrid nanoparticles and composite hydrogel systems for delivery of peptide antibiotics. Int. J. Mol. Sci. 2022, 23, 2771. [Google Scholar] [CrossRef] [PubMed]
- Baghdasaryan, A.; Grillo, R.; Roy Bhattacharya, S.; Sharma, M.; Reginato, E.; Theraulaz, H.; Dolamic, I.; Dadras, M.; Rudaz, S.; Varesio, E.; et al. Facile synthesis, size-separation, characterization, and antimicrobial properties of thiolated copper clusters. ACS Appl. Nano Mater. 2018, 1, 4258–4267. [Google Scholar] [CrossRef]
- Gatto, E.; Toniolo, C.; Venanzi, M. Peptide self-assembled nanostructures: From models to therapeutic peptides. Nanomaterials 2022, 12, 466. [Google Scholar] [CrossRef]
- Taş, K.; Volta, B.D.; Lindner, C.; El Bounkari, O.; Hille, K.; Tian, Y.; Puig-Bosch, X.; Ballmann, M.; Hornung, S.; Ortner, M.; et al. Designed peptides as nanomolar cross-amyloid inhibitors acting via supramolecular nanofiber co-assembly. Nat. Commun. 2022, 13, 5004. [Google Scholar] [CrossRef]
- Gao, K.X.; Zhou, Z.; Yao, L.; Wang, S.; Zhang, Y.; Zou, Q.; Ma, L.X.; Wang, H.X. Aspartic acid-assisted size-controllable synthesis of nanoscale spherical covalent organic frameworks with chiral interfaces for inhibiting amyloid-β fibrillation. ACS Appl. Bio Mater. 2022, 5, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Sigurdsson, E.M.; Morelli, L.; Asok Kumar, R.; Castaño, E.M.; Frangione, B. Β-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: Implications for alzheimer’s therapy. Nat. Med. 1998, 4, 822–826. [Google Scholar] [CrossRef]
- Garcia, A.M.; Melchionna, M.; Bellotto, O.; Kralj, S.; Semeraro, S.; Parisi, E.; Iglesias, D.; D’Andrea, P.; De Zorzi, R.; Vargiu, A.V.; et al. Nanoscale assembly of functional peptides with divergent programming elements. ACS Nano 2021, 15, 3015–3025. [Google Scholar] [CrossRef]
- Fasae, K.D.; Abolaji, A.O.; Faloye, T.R.; Odunsi, A.Y.; Oyetayo, B.O.; Enya, J.I.; Rotimi, J.A.; Akinyemi, R.O.; Whitworth, A.J.; Aschner, M. Metallobiology and therapeutic chelation of biometals (copper, zinc and iron) in Alzheimer’s disease: Limitations, and current and future perspectives. J. Trace Elem. Med. Biol. 2021, 67, 126779. [Google Scholar] [CrossRef]
- Chaves, S.; Várnagy, K.; Santos, M.A. Recent multi-target approaches on the development of anti- Alzheimer’s agents integrating metal chelation activity. Curr. Med. Chem. 2021, 28, 7247–7277. [Google Scholar] [CrossRef] [PubMed]
- Benoit, S.L.; Maier, R.J. The nickel-chelator dimethylglyoxime inhibits human amyloid beta peptide in vitro aggregation. Sci. Rep. 2021, 11, 6622. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, Y.L.; Liu, X.Z.; Shen, P.; Zheng, Y.G.; Lan, X.R.; Lu, C.B.; Wang, J.Z. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodeg. 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.G.; Ruehl, C.L.; Morse, S.V.; Simon, M.; Rakers, V.; Watts, H.; Aprile, F.A.; Choi, J.J.; Vilar, R. Modulation of amyloid-β aggregation by metal complexes with a dual binding mode and their delivery across the blood-brain barrier using focused ultrasound. Chem. Sci. 2021, 12, 9485–9493. [Google Scholar] [CrossRef]
- Iscen, A.; Brue, C.R.; Roberts, K.F.; Kim, J.; Schatz, G.C.; Meade, T.J. Inhibition of amyloid-β aggregation by cobalt(III) Schiff base complexes: A computational and experimental approach. J. Am. Chem. Soc. 2019, 141, 16685–16695. [Google Scholar] [CrossRef] [PubMed]
- La Manna, S.; Leone, M.; Iacobucci, I.; Annuziata, A.; Di Natale, C.; Lagreca, E.; Malfitano, A.M.; Ruffo, F.; Merlino, A.; Monti, M.; et al. Glucosyl platinum(II) complexes inhibit aggregation of the C-terminal region of the Aβ peptide. Inorg. Chem. 2022, 61, 3540–3552. [Google Scholar] [CrossRef]
- Manna, S.; Florio, D.; Iacobucci, I.; Napolitano, F.; Benedictis, I.; Malfitano, A.M.; Monti, M.; Ravera, M.; Gabano, E.; Marasco, D. A comparative study of the effects of platinum (II) complexes on β-amyloid aggregation: Potential neurodrug applications. Int. J. Mol. Sci. 2021, 22, 3015. [Google Scholar] [CrossRef]
- Florio, D.; Malfitano, A.M.; Di Somma, S.; Mügge, C.; Weigand, W.; Ferraro, G.; Iacobucci, I.; Monti, M.; Morelli, G.; Merlino, A.; et al. Platinum(II) O,S complexes inhibit the aggregation of amyloid model systems. Int. J. Mol. Sci. 2019, 20, 829. [Google Scholar] [CrossRef]
- Cali, M.P.; Pereira, L.M.B.; Teodoro, M.D.; Sellani, T.A.; Rodrigues, E.G.; Carlos, R.M. Comparison of Aβ (1-40, 1-28, 11-22, and 29-40) aggregation processes and inhibition of toxic species generated in early stages of aggregation by a water-soluble ruthenium complex. J. Inorg. Biochem. 2021, 215, 111314. [Google Scholar] [CrossRef]
- Son, G.; Lee, B.I.; Chung, Y.J.; Park, C.B. Light-triggered dissociation of self-assembled β-amyloid aggregates into small, nontoxic fragments by ruthenium (II) complex. Acta Biomater. 2018, 67, 147–155. [Google Scholar] [CrossRef]
- Vyas, N.A.; Singh, S.B.; Kumbhar, A.S.; Ranade, D.S.; Walke, G.R.; Kulkarni, P.P.; Jani, V.; Sonavane, U.B.; Joshi, R.R.; Rapole, S. Acetylcholinesterase and Aβ aggregation inhibition by heterometallic ruthenium(II)-platinum(II) polypyridyl complexes. Inorg. Chem. 2018, 57, 7524–7535. [Google Scholar] [CrossRef]
- Peng, Y.B.; Tao, C.; Tan, C.P.; Zhao, P. Inhibition of Aβ peptide aggregation by ruthenium(II) polypyridyl complexes through copper chelation. J. Inorg. Biochem. 2021, 224, 111591. [Google Scholar] [CrossRef]
- Okafor, M.; Gonzalez, P.; Ronot, P.; El Masoudi, I.; Boos, A.; Ory, S.; Chasserot-Golaz, S.; Gasman, S.; Raibaut, L.; Hureau, C.; et al. Development of Cu(II)-specific peptide shuttles capable of preventing Cu-amyloid beta toxicity and importing bioavailable Cu into cells. Chem. Sci. 2022, 13, 11829–11840. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dong, X.; Sun, Y. D-enantiomeric rthlvffark-NH2: A potent multifunctional decapeptide inhibiting Cu2+-mediated amyloid β-protein aggregation and remodeling Cu2+-mediated amyloid β aggregates. ACS Chem. Neurosci. 2019, 10, 1390–1401. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Dong, X.; Sun, Y. Conjugation of rthlvffark to human lysozyme creates a potent multifunctional modulator for Cu2+-mediated amyloid β-protein aggregation and cytotoxicity. J. Mater. Chem. B 2020, 8, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dong, X.; Sun, Y. Carnosine-lvffark-NH2 conjugate: A moderate chelator but potent inhibitor of Cu2+-mediated amyloid β-protein aggregation. ACS Chem. Neurosci. 2018, 9, 2689–2700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, C.; Dong, X.Y.; Zheng, J.; Sun, Y. Design of nonapeptide lvffarkhh: A bifunctional agent against Cu2+ -mediated amyloid β-protein aggregation and cytotoxicity. J. Mol. Recognit. 2018, 31, e2697. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, H.; Dong, X.; Liu, F.; Sun, Y. Rthlvffark-NH2: A potent and selective modulator on Cu2+-mediated amyloid-β protein aggregation and cytotoxicity. J. Inorg. Biochem. 2018, 181, 56–64. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Zhong, M.; Zhao, P.; Guo, C.; Li, Y.; Xu, H.; Wang, T.; Gao, H. A novel Cu(II)-binding peptide identified by phage display inhibits Cu2+-mediated Aβ aggregation. Int. J. Mol. Sci. 2021, 22, 6842. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, M.; Zhao, P.; Zhang, X.; Li, Y.; Wang, X.; Sun, J.; Lan, W.; Sun, H.; Wang, Z.; et al. Screening a specific Zn(II)-binding peptide for improving the cognitive decline of alzheimer’s disease in app/ps1 transgenic mice by inhibiting Zn2+-mediated amyloid protein aggregation and neurotoxicity. Biomater. Sci. 2019, 7, 5197–5210. [Google Scholar] [CrossRef]
- Shamloo, A.; Asadbegi, M.; Khandan, V.; Amanzadi, A. Designing a new multifunctional peptide for metal chelation and Aβ inhibition. Arch. Biochem. Biophys. 2018, 653, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Asadbegi, M.; Shamloo, A. Identification of a novel multifunctional ligand for simultaneous inhibition of amyloid-beta (Aβ(42)) and chelation of zinc metal ion. ACS Chem. Neurosci. 2019, 10, 4619–4632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Sheng, X.; Xie, H.; Zhou, S.; Zhong, M.; Liu, A. Inhibition of alzheimer’s Aβ(1-42) fibrillogenesis and removal of copper ions by polypeptides modified gold nanoparticles. Chem. Biodivers. 2022, 19, e202200342. [Google Scholar] [CrossRef]
- Basu, K.; Nandi, N.; Mondal, B.; Dehsorkhi, A.; Hamley, I.W.; Banerjee, A. Peptide-based ambidextrous bifunctional gelator: Applications in oil spill recovery and removal of toxic organic dyes for waste water management. Interface Focus 2017, 7, 20160128. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Bairagi, D.; Nandi, N.; Hansda, B.; Das, K.S.; Edwards-Gayle, C.J.C.; Castelletto, V.; Hamley, I.W.; Banerjee, A. Peptide-based gel in environmental remediation: Removal of toxic organic dyes and hazardous Pb2+ and Cd2+ ions from wastewater and oil spill recovery. Langmuir 2020, 36, 12942–12953. [Google Scholar] [CrossRef]
- Fortunato, A.; Mba, M. Metal cation triggered peptide hydrogels and their application in food freshness monitoring and dye adsorption. Gels 2021, 7, 85. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Hu, Q.; Wei, T.; Wang, J.; Chen, H.; Wu, C. Temperature-driven metalloprotein-based hybrid hydrogels for selective and reversible removal of cadmium(II) from water. ACS Appl. Mater. Interfaces 2020, 12, 2991–2998. [Google Scholar] [CrossRef]
- Sfragano, P.S.; Moro, G.; Polo, F.; Palchetti, I. The role of peptides in the design of electrochemical biosensors for clinical diagnostics. Biosensors 2021, 11, 246. [Google Scholar] [CrossRef]
- Rozhin, P.; Charitidis, C.; Marchesan, S. Self-assembling peptides and carbon nanomaterials join forces for innovative biomedical applications. Molecules 2021, 26, 4084. [Google Scholar] [CrossRef]
- Gkika, K.S.; Cullinane, D.; Keyes, T.E. Metal peptide conjugates in cell and tissue imaging and biosensing. Top. Curr. Chem. 2022, 380, 30. [Google Scholar] [CrossRef]
- Heaton, I.; Platt, M. Peptide nanocarriers for detection of heavy metal ions using resistive pulse sensing. Anal. Chem. 2019, 91, 11291–11296. [Google Scholar] [CrossRef] [PubMed]
- Schirone, M.; Esposito, L.; D’Onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic amines in meat and meat products: A review of the science and future perspectives. Foods 2022, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Maiti, B.; Bhattacharjee, S.; Bhattacharya, S. Perfluoroarene induces a pentapeptidic hydrotrope into a pH-tolerant hydrogel allowing naked eye sensing of Ca2+ ions. Nanoscale 2019, 11, 2223–2230. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Sun, D.; Li, S.; Deng, Q.; Xin, X. Supramolecular self-assembly of atomically precise silver nanoclusters with chiral peptide for temperature sensing and detection of arginine. Nanomaterials 2022, 12, 424. [Google Scholar] [CrossRef]
- Gupta, D.; Gupta, V.; Nath, D.; Miglani, C.; Mandal, D.; Pal, A. Stimuli-responsive self-assembly disassembly in peptide amphiphiles to endow block-co-fibers and tunable piezoelectric response. ACS Appl. Mater. Interfaces 2022. [Google Scholar] [CrossRef]
- Garifullin, R.; Guler, M.O. Electroactive peptide-based supramolecular polymers. Mater. Today Bio 2021, 10, 100099. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, J.R.; Waigh, T.A. Electronics of peptide- and protein-based biomaterials. Adv. Coll. Interface Sci. 2021, 287, 102319. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, R.; Hesketh, T.; Wang, H.; Martin, A.R.G.; Bowering, D.; Zhang, C.; Hu, C.T.; McPhee, S.A.; Wang, T.; Park, Y.; et al. Mechanistic insights of evaporation-induced actuation in supramolecular crystals. Nat. Mater. 2021, 20, 403–409. [Google Scholar] [CrossRef]
- Newar, R.; Akhtar, N.; Antil, N.; Kumar, A.; Shukla, S.; Begum, W.; Manna, K. Amino Acid-Functionalized Metal-Organic Frameworks for Asymmetric Base–Metal Catalysis. Angew. Chem. Int. Ed. 2021, 60, 10964–10970. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Fujimoto, Y.; Taniguchi, Y.; Miyake, K.; Uchida, Y.; Nishiyama, N. Amino-Acid-Functionalized Metal–Organic Frameworks as Excellent Precursors toward Bifunctional Metal-Free Electrocatalysts. ACS Appl. Energy Mater. 2022, 5, 11091–11097. [Google Scholar]
- Tang, H.; Yang, K.; Wang, K.-Y.; Meng, Q.; Wu, F.; Fang, Y.; Wu, X.; Li, Y.; Zhang, W.C.; Luo, Y.; et al. Engineering a homochiral metal–organic framework based on an amino acid for enantioselective separation. Chem. Commun. 2020, 56, 9016–9019. [Google Scholar] [CrossRef] [PubMed]

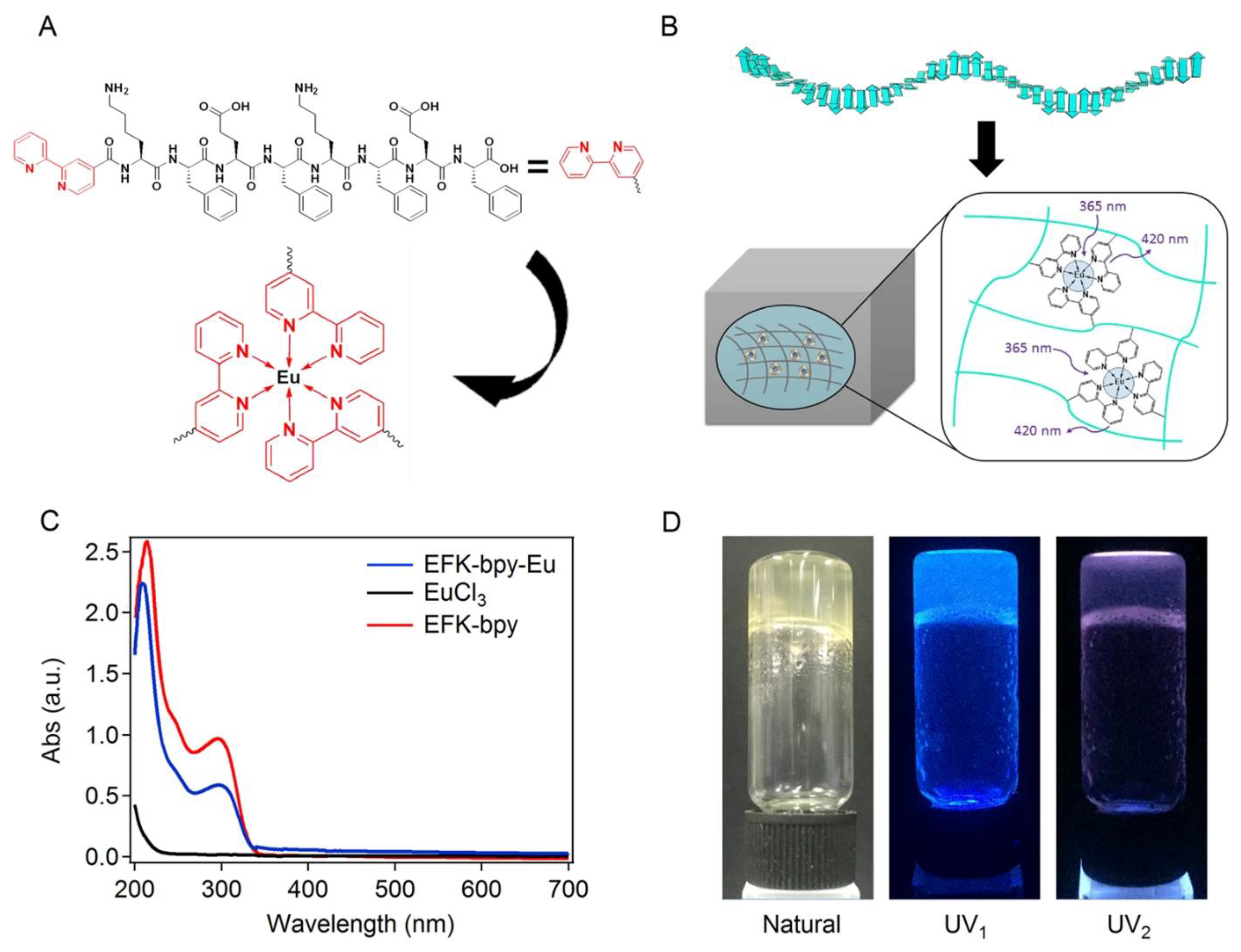
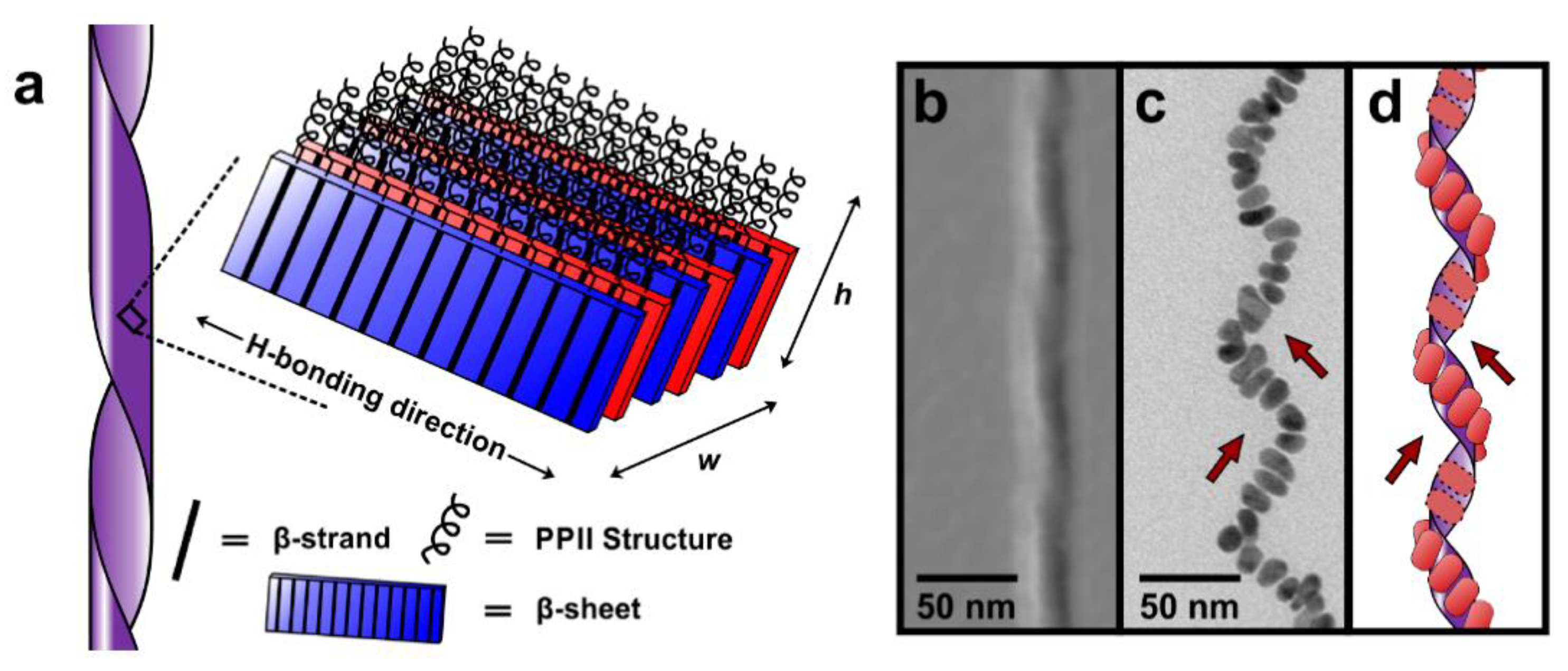
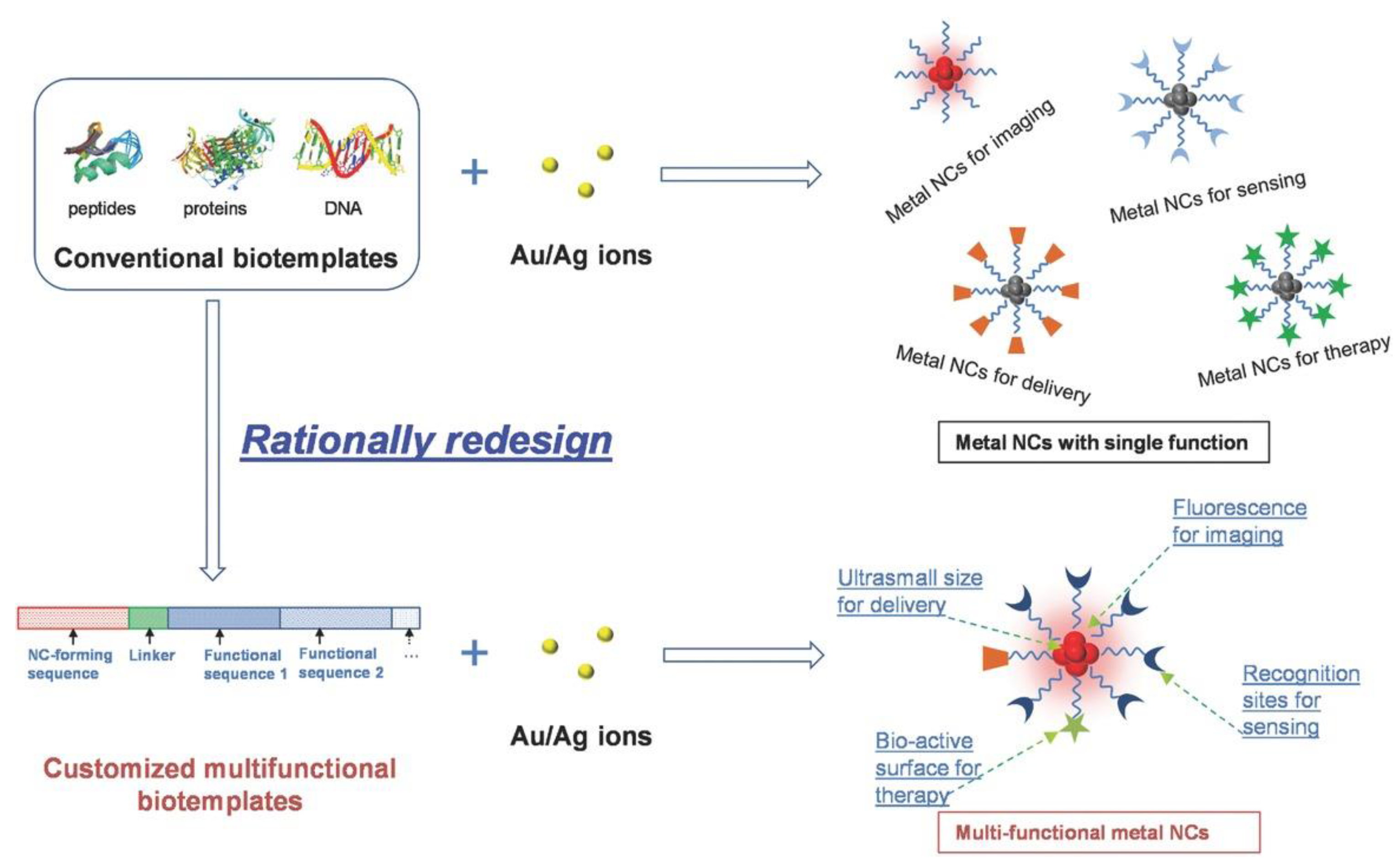
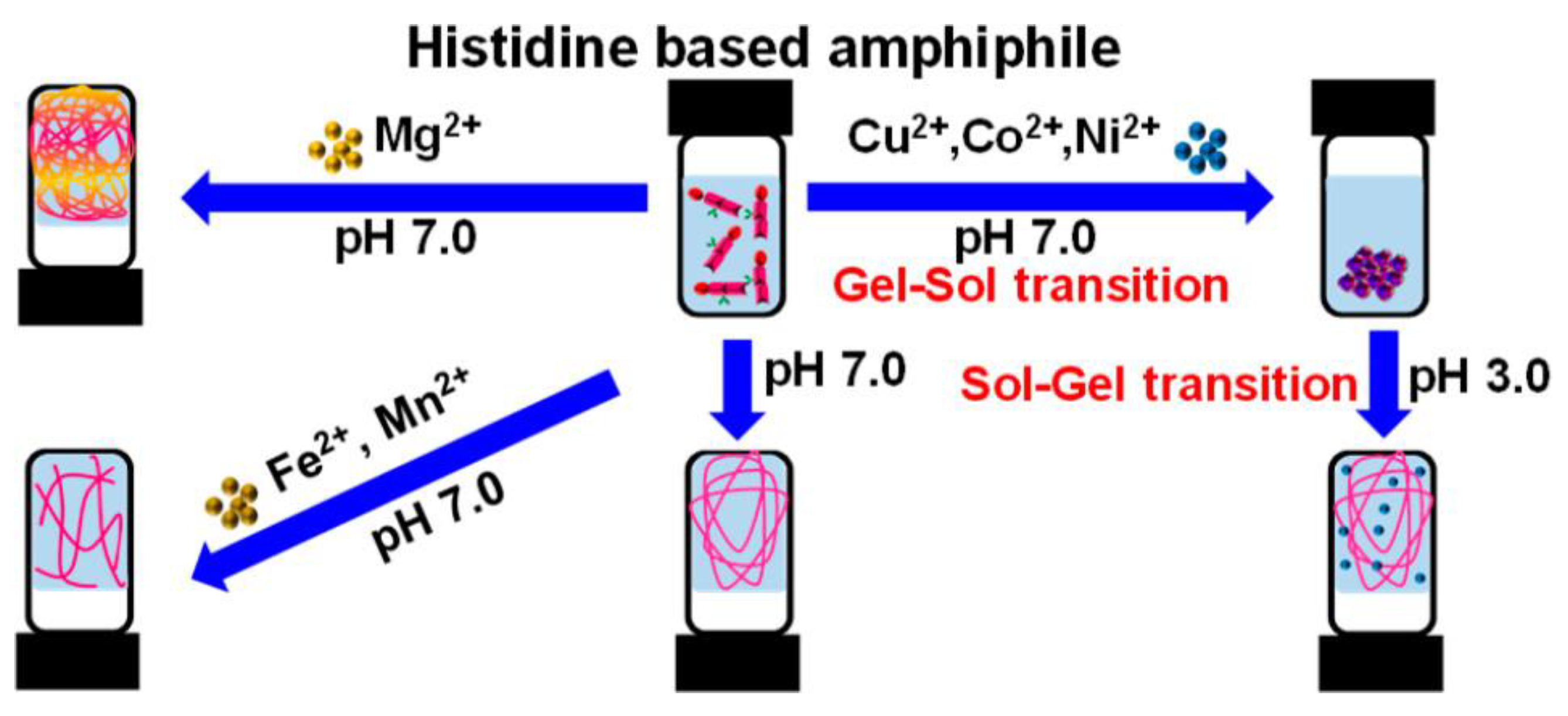

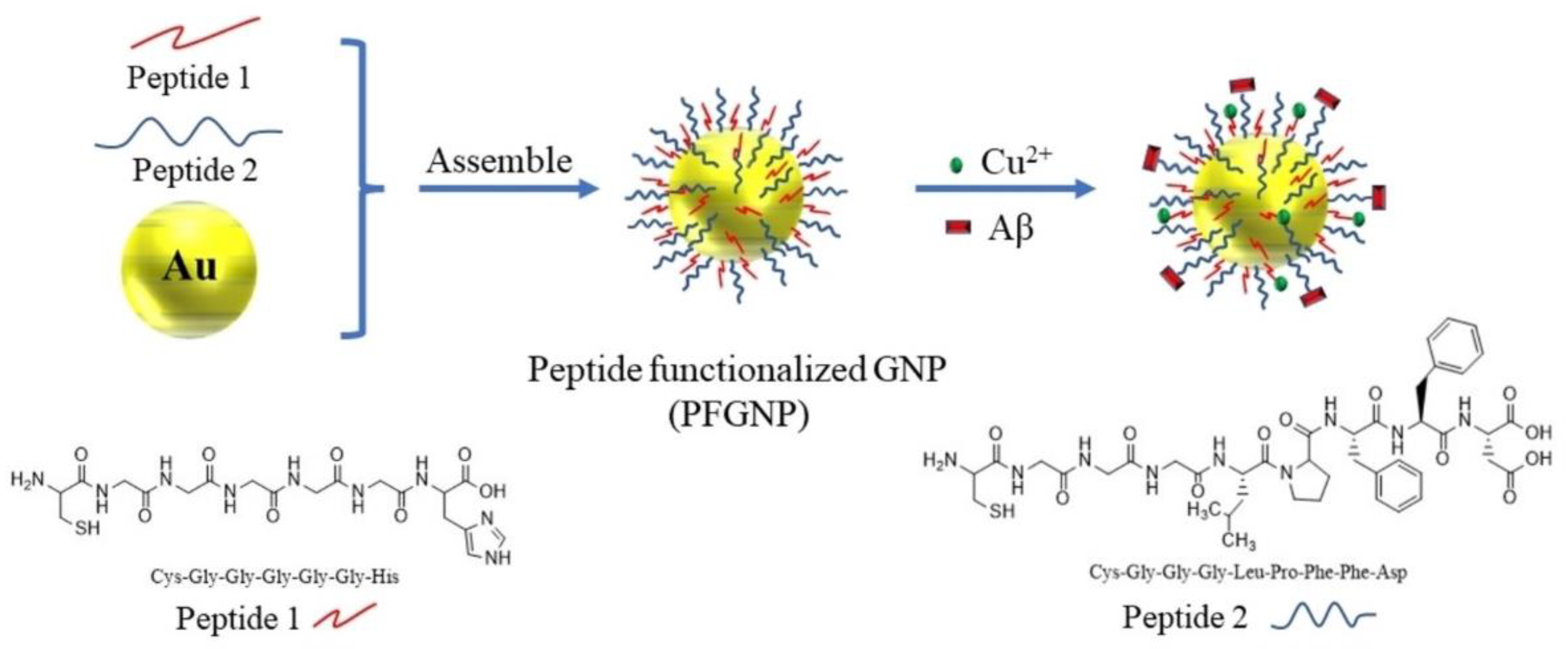

| Metal Ion | Peptide | Material | Application | Ref. |
|---|---|---|---|---|
| Na+ | Fmoc-Phe-Phe | Hydrogel | DNA biochip | [52] |
| K+ | Fmoc-Phe-Phe | Hydrogel | Medicine | [52] |
| Ag+ | Fmoc-Pro | Hydrogel | AM 1/Drug delivery | [81] |
| Fmoc-His | Hydrogel | AM 1/Drug delivery | [81] | |
| Fmoc-Ala | Hydrogel | AM 1/Drug delivery | [81] | |
| Fmoc-Leu | Hydrogel | AM 1/Drug delivery | [81] | |
| (3′-PyA)-Leu-Arg-Leu-Arg-Leu-Arg-Leu-(3′-PyA) | Hydrogel | AM 1 | [82] | |
| Silk fibroin | Nanocomposite | AM 1 | [83] | |
| Poly(Glu)/polymyxin | Nanoparticles | AM 1 | [84] | |
| (D-Asp)5 | Hydrogel | Arg sensing | [125] | |
| Mg2+ | Nap-Phe-Phe-Gly-Asp-Hyp | Hydrogel | Drug delivery | [54] |
| Ca2+ | Nap-Phe-Phe-Gly-Asp-Hyp | Hydrogel | Drug delivery | [54] |
| Poly-Cys amphiphile | Hydrogel | Drug delivery | [55] | |
| Nap-Gly-Phe-Phe-Tyr-Gly-Arg-Gly-Asp-His-His | Hydrogel | Drug delivery | [58] | |
| Fmoc-Phe-Phe-pSerC-(oNB)-PEG | Hydrogel | Drug delivery | [59] | |
| Pyrenyl-Val-Pro-Gly-Lys-Gly | Hydrogel | Ca++ sensing | [124] | |
| Mn2+ | Fmoc-His-Phe | Hydrogel | Drug delivery | [51] |
| Fmoc-His-Leu | Hydrogel | Drug delivery | [51] | |
| Fmoc-His-Val | Hydrogel | Drug delivery | [51] | |
| Gly-His-Lys | Adhesive | Wound healing | [66] | |
| Gly-Phe-Lys | Adhesive | Wound healing | [66] | |
| Gly-Val-Lys | Adhesive | Wound healing | [66] | |
| Fe2+ | Fmoc-His-Phe | Hydrogel | Drug delivery | [51] |
| Fmoc-His-Leu | Hydrogel | Drug delivery | [51] | |
| Fmoc-His-Val | Hydrogel | Drug delivery | [51] | |
| Co2+ | Fmoc-His-Phe | Hydrogel | Drug delivery | [51] |
| Fmoc-His-Leu | Hydrogel | Drug delivery | [51] | |
| Fmoc-His-Val | Hydrogel | Drug delivery | [51] | |
| Gly-His-Lys | Adhesive | Wound healing | [66] | |
| Gly-Phe-Lys | Adhesive | Wound healing | [66] | |
| Gly-Val-Lys | Adhesive | Wound healing | [66] | |
| Fmoc-Phe-Phe-pSerC-(oNB)-PEG | Hydrogel | Drug delivery | [59] | |
| Ni2+ | Fmoc-His-Phe | Hydrogel | Drug delivery | [51] |
| Fmoc-His-Leu | Hydrogel | Drug delivery | [51] | |
| Fmoc-His-Val | Hydrogel | Drug delivery | [51] | |
| Gly-His-Lys | Adhesive | Wound healing | [66] | |
| Gly-Phe-Lys | Adhesive | Wound healing | [66] | |
| Gly-Val-Lys | Adhesive | Wound healing | [66] | |
| Cu2+ | Fmoc-His-Phe | Hydrogel | Drug delivery | [51] |
| Fmoc-His-Leu | Hydrogel | Drug delivery | [51] | |
| Fmoc-His-Val | Hydrogel | Drug delivery | [51] | |
| Fmoc-Phe-Phe | Hydrogel | Drug delivery | [52] | |
| Fmoc-Phe-Phe-pSerC-(oNB)-PEG | Hydrogel | Drug delivery | [59] | |
| Glutathione | Nanoclusters | AM 1 | [85] | |
| Cys-(Gly)5-His, Cys-(Gly)3-Leu-Pro-Phe-Phe-Asp | Nanoparticles | Amyloid inhibition | [114] | |
| Phe-Glu-Phe-Glu-Gly-pyrene | Hydrogel | Amine sensing | [117] | |
| Zn2+ | Fmoc-Phe-Phe | Hydrogel | DNA biochip | [52] |
| Fmoc-Phe-Phe-pSerC-(oNB)-PEG | Hydrogel | Drug delivery | [59] | |
| Phe-Glu-Phe-Glu-Gly-pyrene | Hydrogel | Pollutant capture | [117] | |
| Ru2+ | Poly(Sar)-block-poly(Glu) | Micelles | Drug delivery | [56] |
| Cd2+ | Myristil-Trp-Phe | Hydrogel | Pollutant capture | [116] |
| PNIPAM-CadRP | Hydrogel | Pollutant capture | [118] | |
| Pt2+ | Poly-Cys amphiphile | Hydrogel | Drug delivery | [55] |
| Nap-Phe-Phe-Tyr-Glu-Arg-Gly-Asp | Hydrogel | Drug delivery | [57] | |
| Pb2+ | Myristil-Trp-Phe | Hydrogel | Pollutant capture | [116] |
| Al3+ | Fmoc-Phe-Phe | Nanofibrils/spheres | Medicine | [52] |
| Fe3+ | Fmoc-Phe-Phe | Nanofibrils/spheres | Medicine | [52] |
| PEG-poly-Ala | Hydrogel | Tissue regeneration | [63] | |
| Mo4+ | Gly-His-Lys | Coacervate | Wound healing | [66] |
| Gly-Phe-Lys | Coacervate | Wound healing | [66] | |
| Gly-Val-Lys | Coacervate | Wound healing | [66] | |
| W4+ | Gly-His-Lys | Coacervate | Wound healing | [66] |
| Gly-Phe-Lys | Coacervate | Wound healing | [66] | |
| Gly-Val-Lys | Coacervate | Wound healing | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassan, G.A.; Marchesan, S. Peptide-Based Materials That Exploit Metal Coordination. Int. J. Mol. Sci. 2023, 24, 456. https://doi.org/10.3390/ijms24010456
Bassan GA, Marchesan S. Peptide-Based Materials That Exploit Metal Coordination. International Journal of Molecular Sciences. 2023; 24(1):456. https://doi.org/10.3390/ijms24010456
Chicago/Turabian StyleBassan, Giovanni A., and Silvia Marchesan. 2023. "Peptide-Based Materials That Exploit Metal Coordination" International Journal of Molecular Sciences 24, no. 1: 456. https://doi.org/10.3390/ijms24010456
APA StyleBassan, G. A., & Marchesan, S. (2023). Peptide-Based Materials That Exploit Metal Coordination. International Journal of Molecular Sciences, 24(1), 456. https://doi.org/10.3390/ijms24010456









