Subcellular Localization of Homomeric TASK3 Channels and Its Presumed Functional Significances in Trigeminal Motoneurons
Abstract
1. Introduction
2. Results
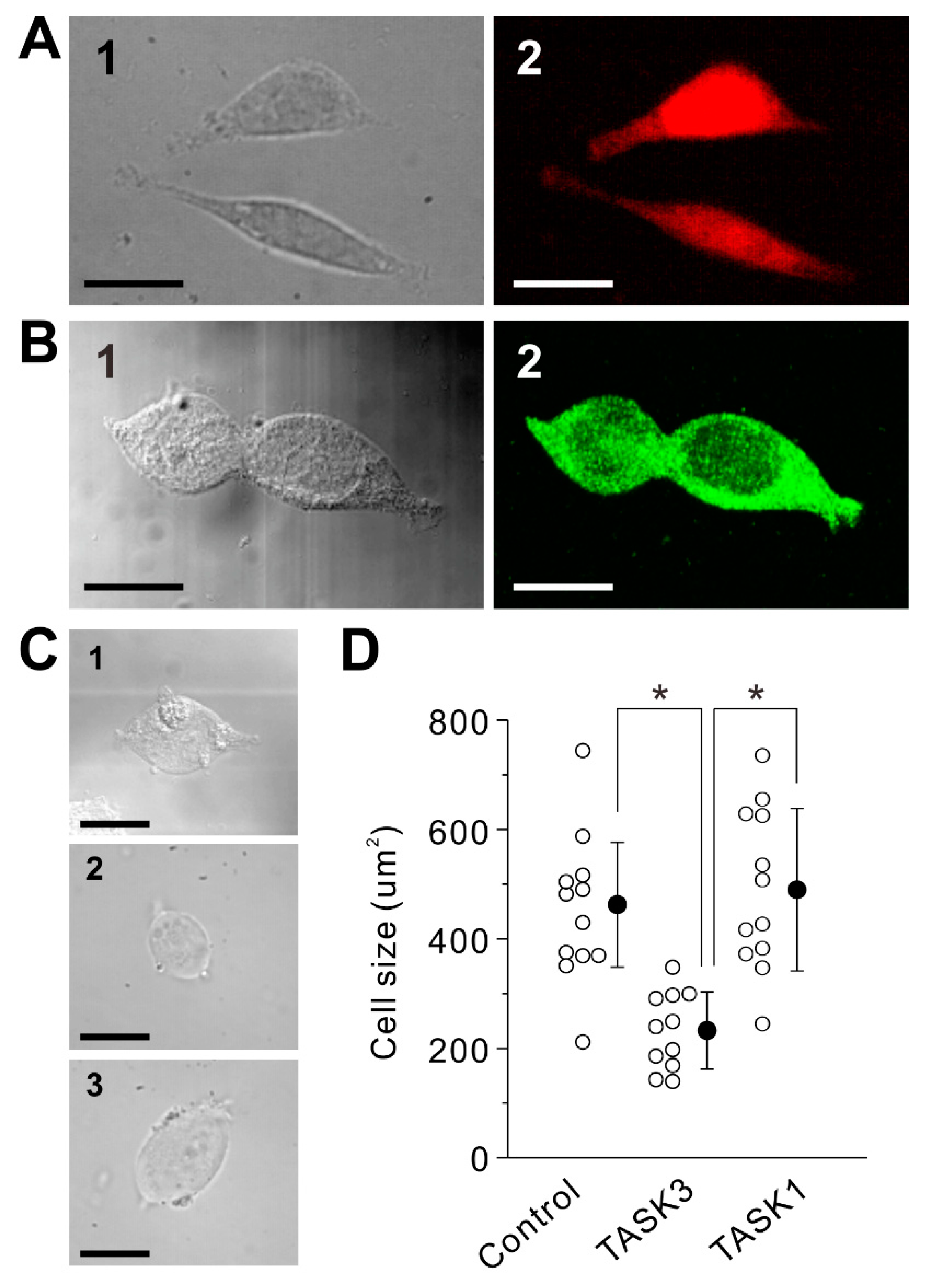
2.1. Transduction of Homomeric TASK3 Channels
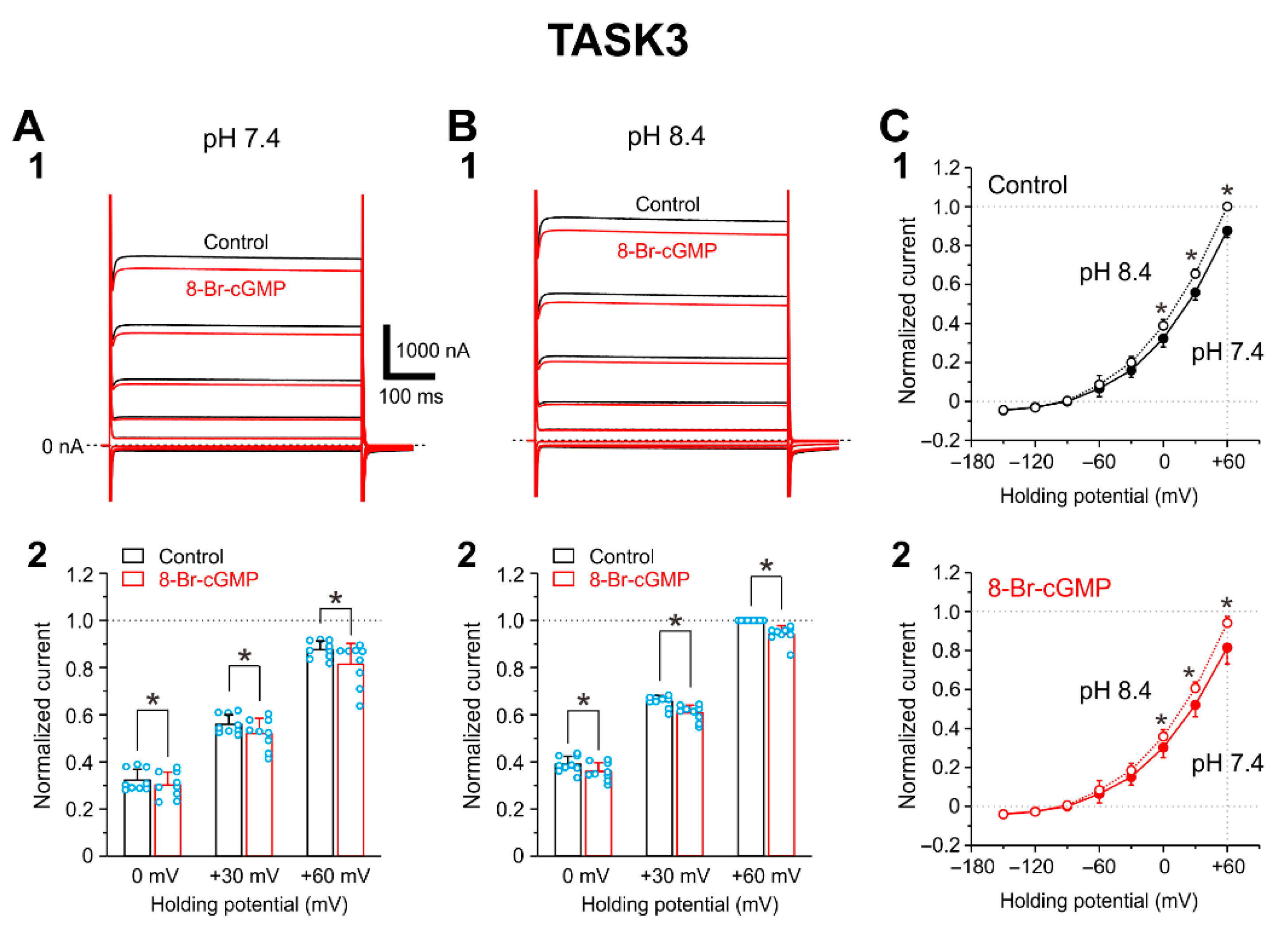
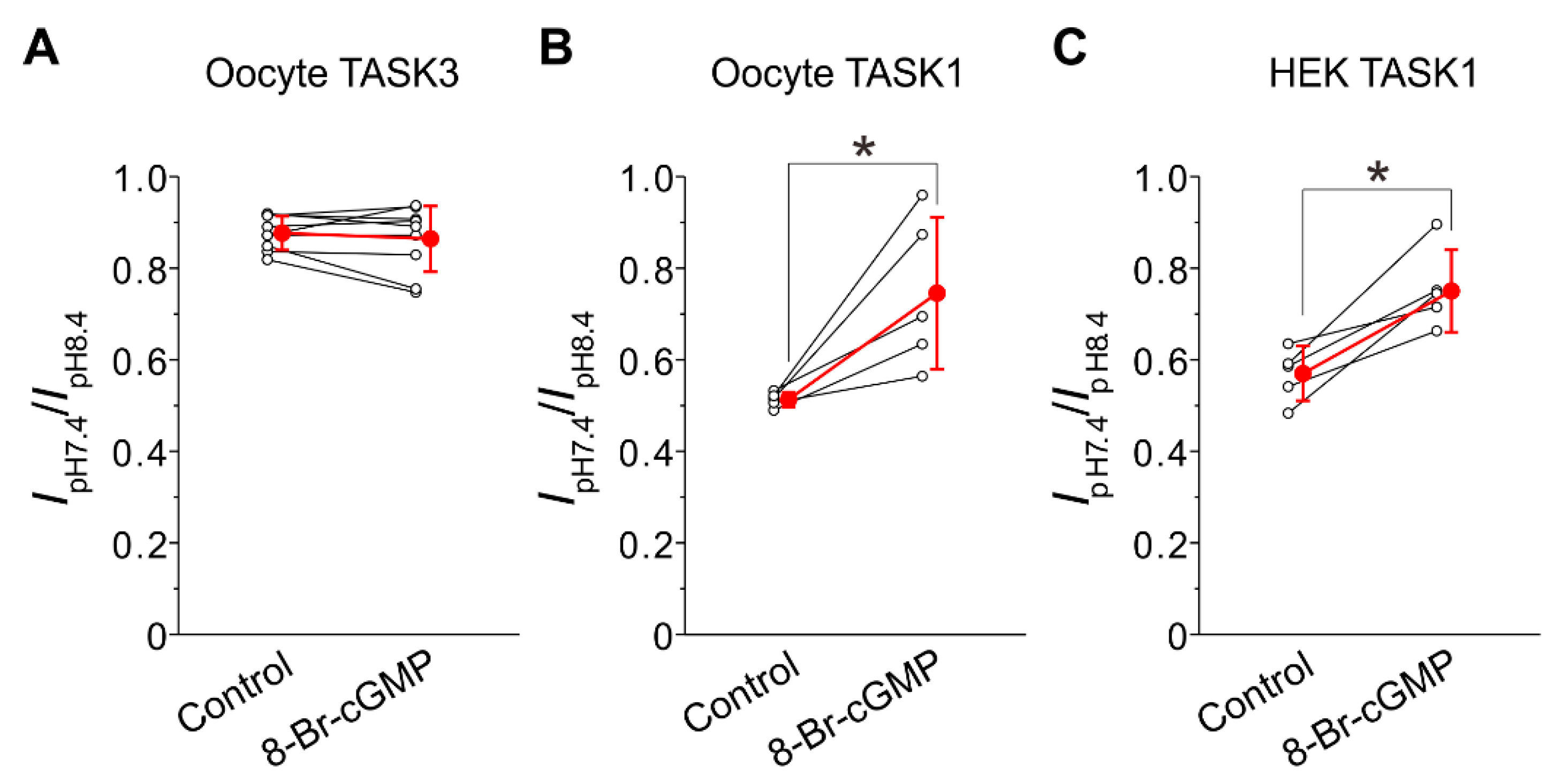
2.2. I-TASK3 Recorded from Xenopus Oocyte
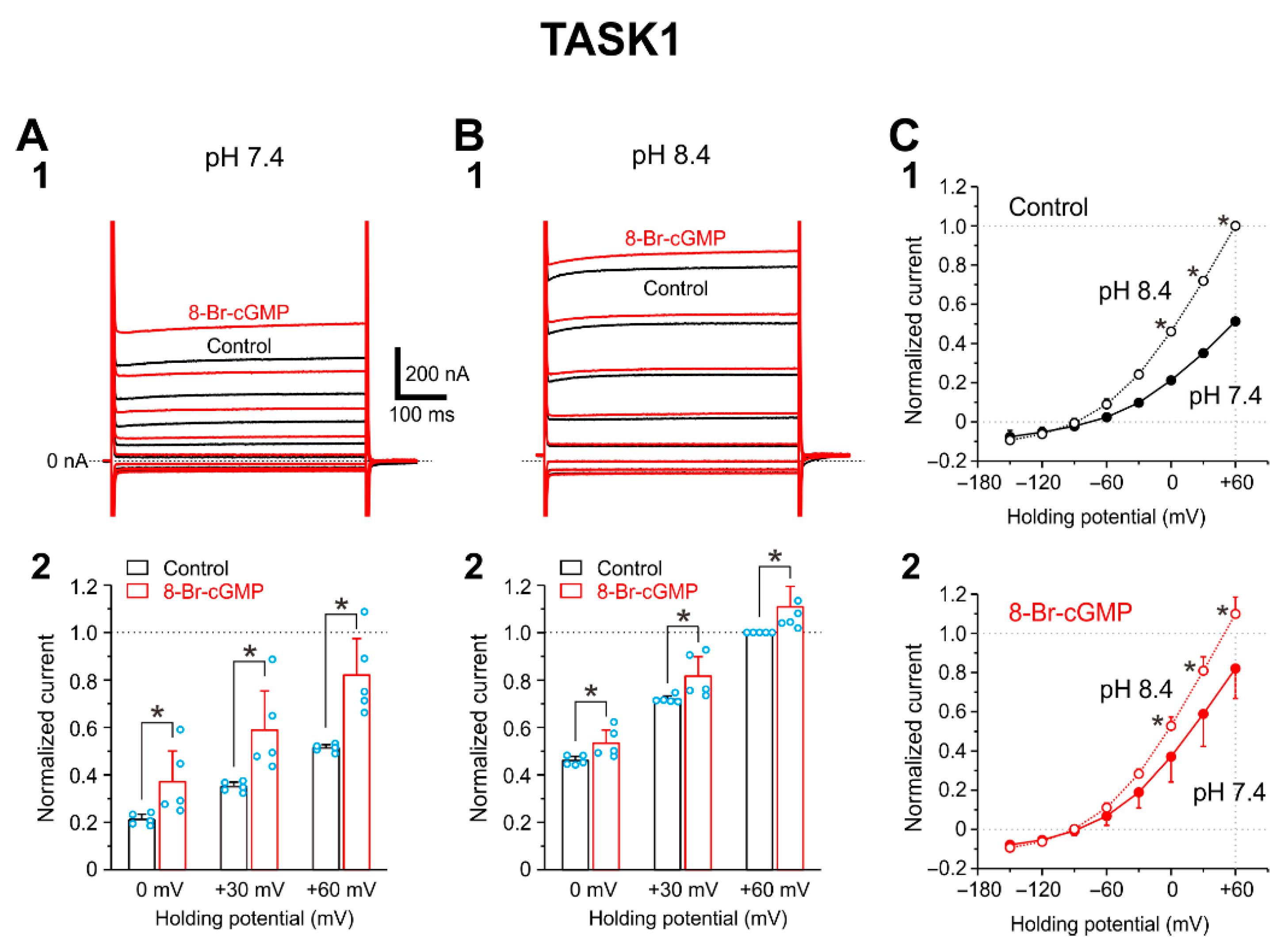
2.3. I-TASK1 Recorded from Xenopus Oocyte
2.4. Integration of Differential Effects of 8-Br-cGMP on TASK1 and TASK3 Channels
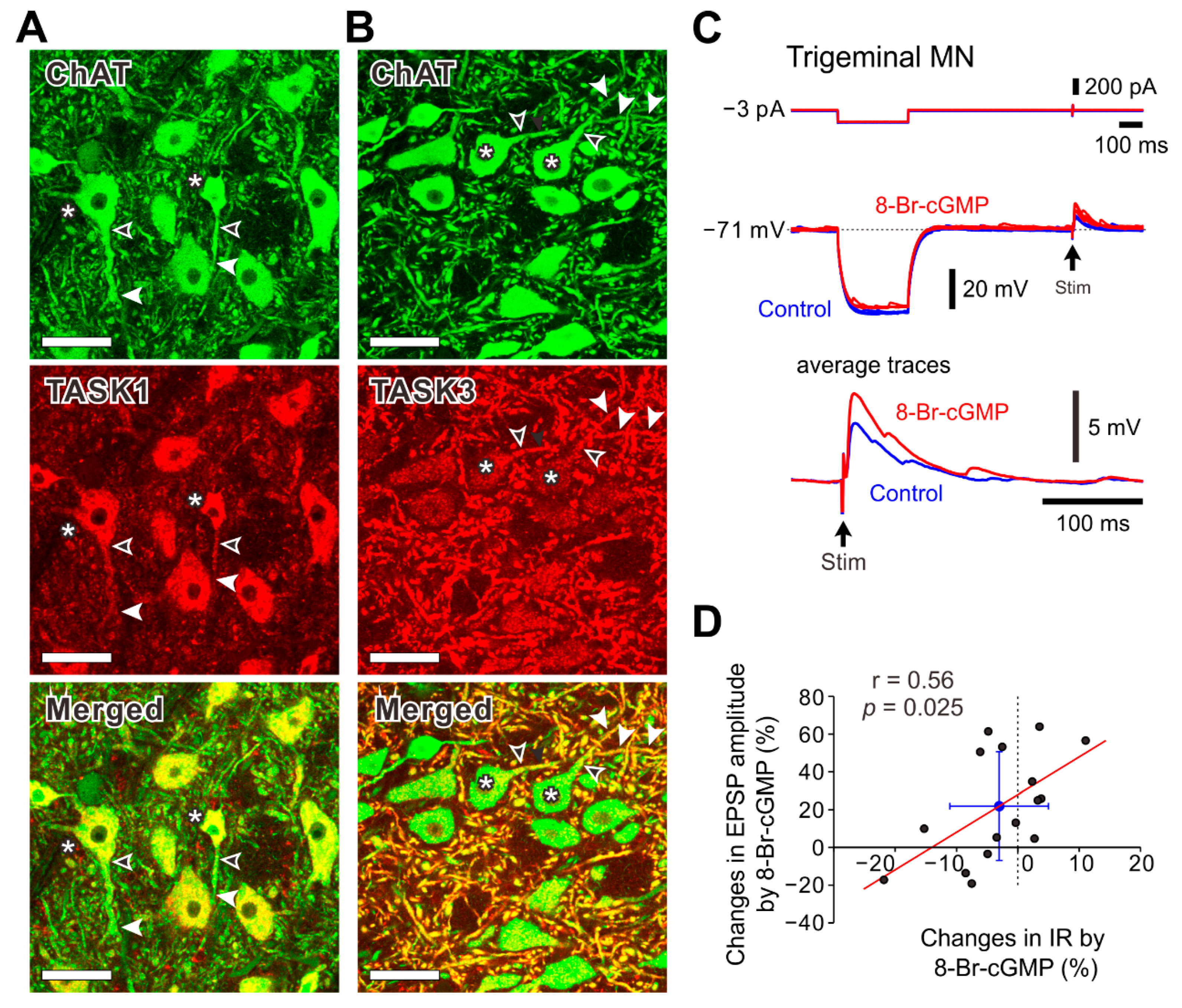
2.5. Subcellular Distributions of TASK1 and TASK3 Channels and Effects of 8-Br-cGMP on Excitatory Postsynaptic Potentials (EPSPs) in MNs in the Dorsolateral Trigeminal Motor Nucleus (dl-TMN)
3. Discussion
4. Materials and Methods
4.1. Whole-Cell Patch-Clamp Recordings from Cultured Mammalian Cells
4.2. Two-Electrode Voltage-Clamp Recordings from Xenopus Oocytes
4.3. Whole-Cell Recording from Motoneurons (MNs) in the Dorsolateral Trigeminal Nucleus (dl-TMN)
4.4. Fluorescence Immunohistochemistry
4.5. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aCSF | artificial cerebrospinal fluid |
| ChAT | choline acetyltransferase |
| CHO | Chinese hamster ovary |
| COS | CV-1 in origin with SV40 |
| Cy3 | cyanine 3 |
| dl-TMN | dorsolateral trigeminal motor nucleus |
| EPSP | excitatory postsynaptic potential |
| HEK | human embryonic kidney |
| I-TASK | TASK current |
| IR | input resistance |
| IR-DIC | infrared differential interference contrast |
| KCC2 | K+-Cl− cotransporter 2 |
| MN | motoneuron |
| NKCC1 | Na+, K+-2Cl− cotransporter |
| PKG | cGMP-dependent protein kinase |
| PLSD | protected least significant difference |
| PBS | phosphate-buffered saline |
| TASK | TWIK-related acid-sensitive K+ channel |
| TWIK | two-pore domain weak inwardly rectifying K+ channel |
| VRAC | volume regulated anion channel |
| XCD | Triton X-100, λ-carrageenan and donkey serum |
References
- Enyedi, P.; Czirjak, G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, I.; Zanzouri, M.; Honore, E.; Duprat, F.; Ehrengruber, M.U.; Lazdunski, M.; Patel, A.J. K+-dependent cerebellar granule neuron apoptosis. Role of task leak K+ channels. J. Biol. Chem. 2003, 278, 32068–32076. [Google Scholar] [CrossRef] [PubMed]
- Czirjak, G.; Enyedi, P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J. Biol. Chem. 2002, 277, 5426–5432. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.P.; Talley, E.M.; Manger, J.P.; Bayliss, D.A. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J. Neurosci. 2004, 24, 6693–6702. [Google Scholar] [CrossRef]
- Kang, D.; Han, J.; Talley, E.M.; Bayliss, D.A.; Kim, D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J. Physiol. 2004, 554, 64–77. [Google Scholar] [CrossRef]
- Okamoto, K.; Emura, N.; Sato, H.; Fukatsu, Y.; Saito, M.; Tanaka, C.; Morita, Y.; Nishimura, K.; Kuramoto, E.; Yin, D.X.; et al. The Possible Role of TASK Channels in Rank-Ordered Recruitment of Motoneurons in the Dorsolateral Part of the Trigeminal Motor Nucleus. eNeuro 2016, 3, e0138-16. [Google Scholar] [CrossRef]
- Toyoda, H.; Saito, M.; Okazawa, M.; Hirao, K.; Sato, H.; Abe, H.; Takada, K.; Funabiki, K.; Takada, M.; Kaneko, T.; et al. Protein kinase G dynamically modulates TASK1-mediated leak K+ currents in cholinergic neurons of the basal forebrain. J. Neurosci. 2010, 30, 5677–5689. [Google Scholar] [CrossRef]
- Talley, E.M.; Solorzano, G.; Lei, Q.; Kim, D.; Bayliss, D.A. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J. Neurosci. 2001, 21, 7491–7505. [Google Scholar] [CrossRef]
- Choi, J.H.; Yarishkin, O.; Kim, E.; Bae, Y.; Kim, A.; Kim, S.C.; Ryoo, K.; Cho, C.H.; Hwang, E.M.; Park, J.Y. TWIK-1/TASK-3 heterodimeric channels contribute to the neurotensin-mediated excitation of hippocampal dentate gyrus granule cells. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef]
- Lloyd, E.E.; Marrelli, S.P.; Bryan, R.M., Jr. cGMP does not activate two-pore domain K+ channels in cerebrovascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1774–H1780. [Google Scholar] [CrossRef][Green Version]
- Toyoda, H.; Saito, M.; Sato, H.; Dempo, Y.; Ohashi, A.; Hirai, T.; Maeda, Y.; Kaneko, T.; Kang, Y. cGMP activates a pH-sensitive leak K+ current in the presumed cholinergic neuron of basal forebrain. J. Neurophysiol. 2008, 99, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Wenker, I.C.; Benoit, J.P.; Chen, X.; Liu, H.; Horner, R.L.; Mulkey, D.K. Nitric oxide activates hypoglossal motoneurons by cGMP-dependent inhibition of TASK channels and cGMP-independent activation of HCN channels. J. Neurophysiol. 2011, 107, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Abudara, V.; Alvarez, A.F.; Chase, M.H.; Morales, F.R. Nitric oxide as an anterograde neurotransmitter in the trigeminal motor pool. J. Neurophysiol. 2002, 88, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ordaz, B.; Vaca, L.; Franco, R.; Pasantes-Morales, H. Volume changes and whole cell membrane currents activated during gradual osmolarity decrease in C6 glioma cells: Contribution of two types of K+ channels. Am. J. Physiol. Cell Physiol. 2004, 286, C1399–C1409. [Google Scholar] [CrossRef][Green Version]
- Kishimoto, H.; Bae, Y.C.; Yoshida, A.; Moritani, M.; Takemura, M.; Nakagawa, S.; Nagase, Y.; Wada, T.; Sessle, B.J.; Shigenaga, Y. Central distribution of synaptic contacts of primary and secondary jaw muscle spindle afferents in the trigeminal motor nucleus of the cat. J. Comp. Neurol. 1998, 391, 50–63. [Google Scholar] [CrossRef]
- Yabuta, N.H.; Yasuda, K.; Nagase, Y.; Yoshida, A.; Fukunishi, Y.; Shigenaga, Y. Light microscopic observations of the contacts made between two spindle afferent types and alpha-motoneurons in the cat trigeminal motor nucleus. J. Comp. Neurol. 1996, 374, 436–450. [Google Scholar] [CrossRef]
- Zanzouri, M.; Lauritzen, I.; Duprat, F.; Mazzuca, M.; Lesage, F.; Lazdunski, M.; Patel, A. Membrane potential-regulated transcription of the resting K+ conductance TASK-3 via the calcineurin pathway. J. Biol. Chem. 2006, 281, 28910–28918. [Google Scholar] [CrossRef]
- Russell, J.M. Sodium-potassium-chloride cotransport. Physiol. Rev. 2000, 80, 211–276. [Google Scholar] [CrossRef]
- Orlov, S.N. Membrane Transporters in the Pathogenesis of Cardiovascular and Lung Disorder, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Deneka, D.; Sawicka, M.; Lam, A.K.M.; Paulino, C.; Dutzler, R. Structure of a volume-regulated anion channel of the LRRC8 family. Nature 2018, 558, 254–259. [Google Scholar] [CrossRef]
- Okada, Y.; Okada, T.; Sato-Numata, K.; Islam, M.R.; Ando-Akatsuka, Y.; Numata, T.; Kubo, M.; Shimizu, T.; Kurbannazarova, R.S.; Marunaka, Y.; et al. Cell Volume-Activated and Volume-Correlated Anion Channels in Mammalian Cells: Their Biophysical, Molecular, and Pharmacological Properties. Pharmacol. Rev. 2019, 71, 49–88. [Google Scholar] [CrossRef]
- Okada, Y.; Sabirov, R.Z.; Merzlyak, P.G.; Numata, T.; Sato-Numata, K. Properties, Structures, and Physiological Roles of Three Types of Anion Channels Molecularly Identified in the 2010’s. Front. Physiol. 2021, 12, 805148. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Okada, Y.; Nilius, B. Biophysics and Physiology of the Volume-Regulated Anion Channel (VRAC)/Volume-Sensitive Outwardly Rectifying Anion Channel (VSOR). Pflugers. Arch. 2016, 468, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Fahlke, C. Ion permeation and selectivity in ClC-type chloride channels. Am. J. Physiol. Renal Physiol. 2001, 280, F748–F757. [Google Scholar] [CrossRef]
- Katz, Y.; Menon, V.; Nicholson, D.A.; Geinisman, Y.; Kath, W.L.; Spruston, N. Synapse distribution suggests a two-stage model of dendritic integration in CA1 pyramidal neurons. Neuron 2009, 63, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Lesage, F.; Barhanin, J. Molecular physiology of pH-sensitive background K(2P) channels. Physiology 2011, 26, 424–437. [Google Scholar] [CrossRef]
- Morton, M.J.; O’Connell, A.D.; Sivaprasadarao, A.; Hunter, M. Determinants of pH sensing in the two-pore domain K+ channels TASK-1 and -2. Pflugers Arch. 2003, 445, 577–583. [Google Scholar] [CrossRef]
- Kim, Y.; Bang, H.; Kim, D. TASK-3, a new member of the tandem pore K+ channel family. J. Biol. Chem. 2000, 275, 9340–9347. [Google Scholar] [CrossRef]
- Shigenaga, Y.; Mitsuhiro, Y.; Yoshida, A.; Cao, C.Q.; Tsuru, H. Morphology of single mesencephalic trigeminal neurons innervating masseter muscle of the cat. Brain Res. 1988, 445, 392–399. [Google Scholar] [CrossRef]
- Henneman, E. The size principle and its relation to transmission failure in Ia projections to spinal motoneurons. Ann. N. Y. Acad. Sci. 1991, 627, 165–168. [Google Scholar] [CrossRef]
- Inanobe, A.; Fujita, S.; Makino, Y.; Matsushita, K.; Ishii, M.; Chachin, M.; Kurachi, Y. Interaction between the RGS domain of RGS4 with G protein alpha subunits mediates the voltage-dependent relaxation of the G protein-gated potassium channel. J. Physiol. 2001, 535, 133–143. [Google Scholar] [CrossRef]
- Dascal, N. The use of Xenopus oocytes for the study of ion channels. CRC Crit. Rev. Biochem. 1987, 22, 317–387. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, M.; Tanaka, C.; Toyoda, H.; Kang, Y. Subcellular Localization of Homomeric TASK3 Channels and Its Presumed Functional Significances in Trigeminal Motoneurons. Int. J. Mol. Sci. 2023, 24, 344. https://doi.org/10.3390/ijms24010344
Saito M, Tanaka C, Toyoda H, Kang Y. Subcellular Localization of Homomeric TASK3 Channels and Its Presumed Functional Significances in Trigeminal Motoneurons. International Journal of Molecular Sciences. 2023; 24(1):344. https://doi.org/10.3390/ijms24010344
Chicago/Turabian StyleSaito, Mitsuru, Chie Tanaka, Hiroki Toyoda, and Youngnam Kang. 2023. "Subcellular Localization of Homomeric TASK3 Channels and Its Presumed Functional Significances in Trigeminal Motoneurons" International Journal of Molecular Sciences 24, no. 1: 344. https://doi.org/10.3390/ijms24010344
APA StyleSaito, M., Tanaka, C., Toyoda, H., & Kang, Y. (2023). Subcellular Localization of Homomeric TASK3 Channels and Its Presumed Functional Significances in Trigeminal Motoneurons. International Journal of Molecular Sciences, 24(1), 344. https://doi.org/10.3390/ijms24010344






