Nanomaterials-Functionalized Hydrogels for the Treatment of Cutaneous Wounds
Abstract
1. Introduction
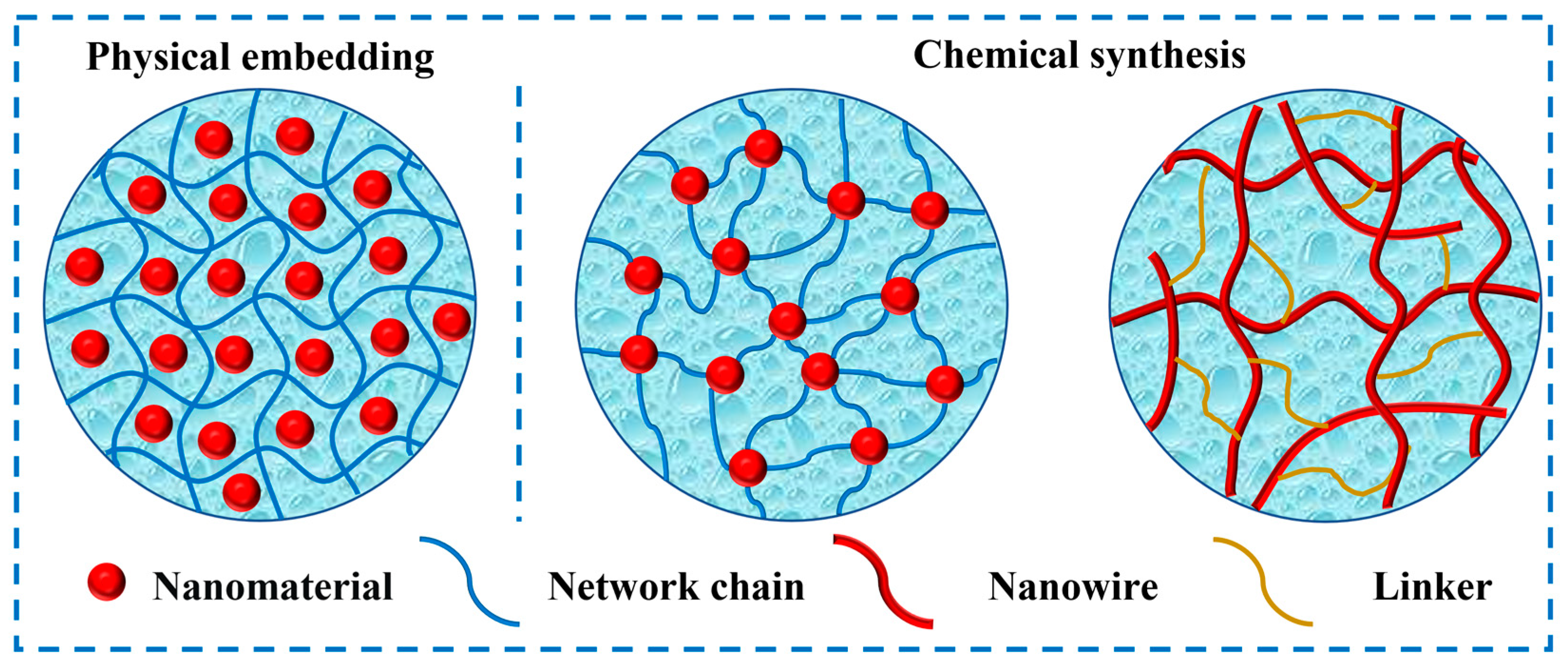
2. Fabrication Strategies
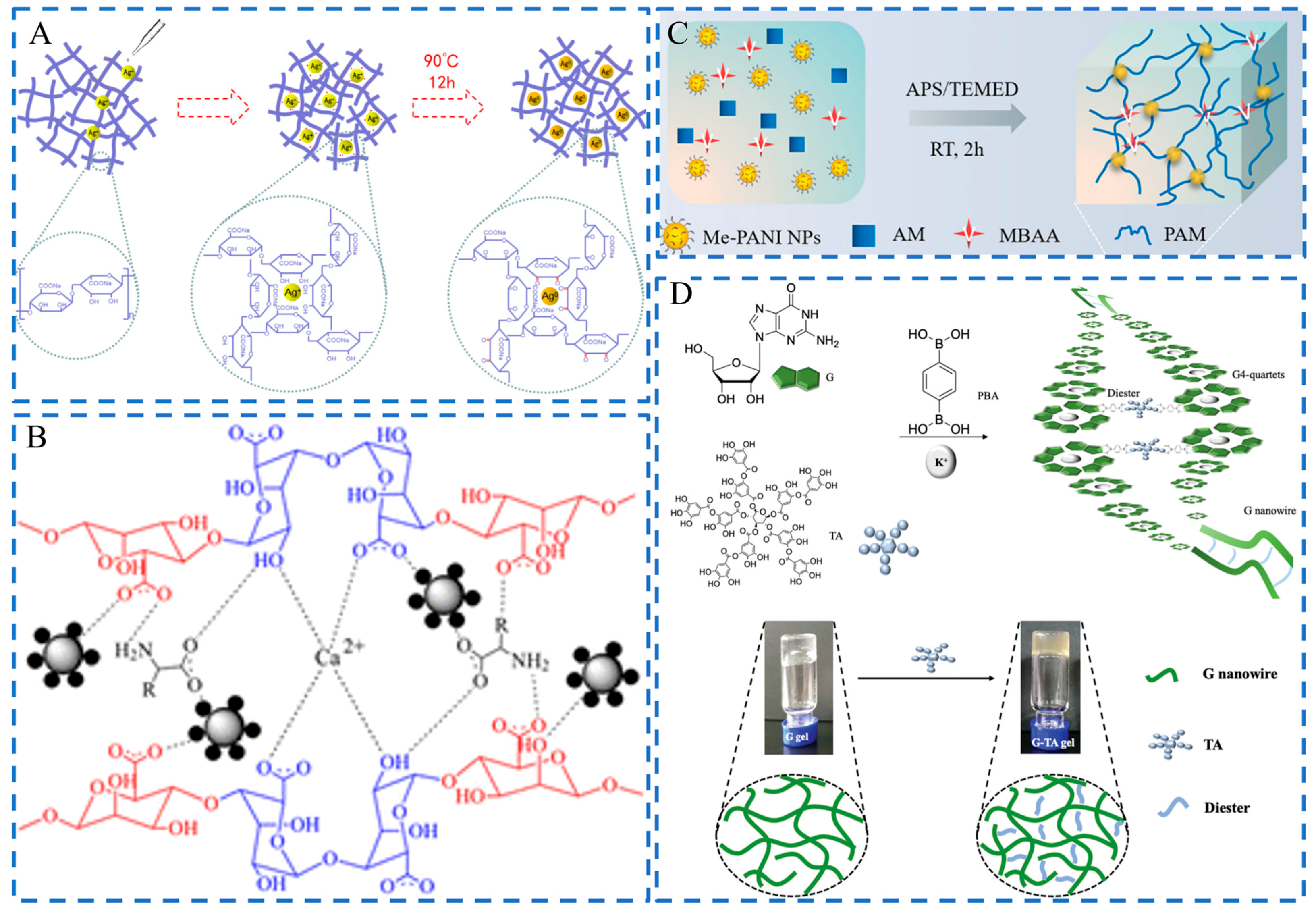
2.1. Physical Embedding
2.2. Chemical Synthesis
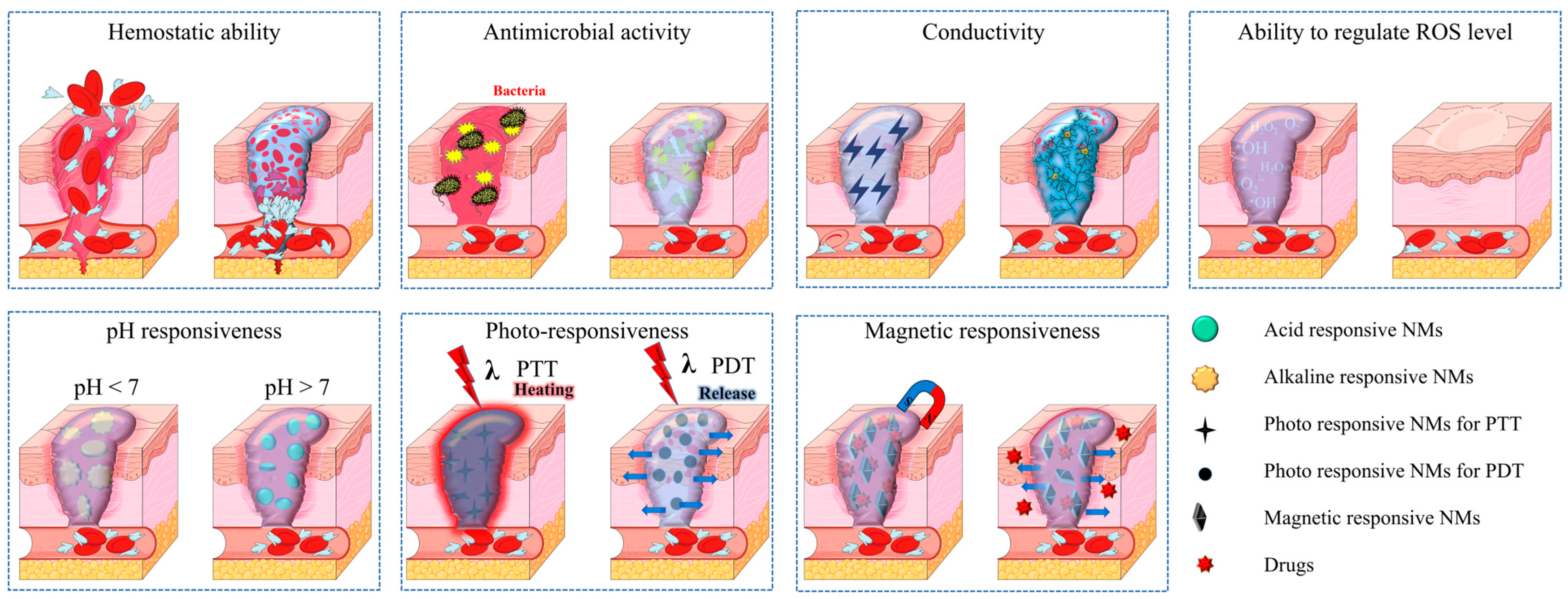
3. Functions
3.1. Hemostatic Ability
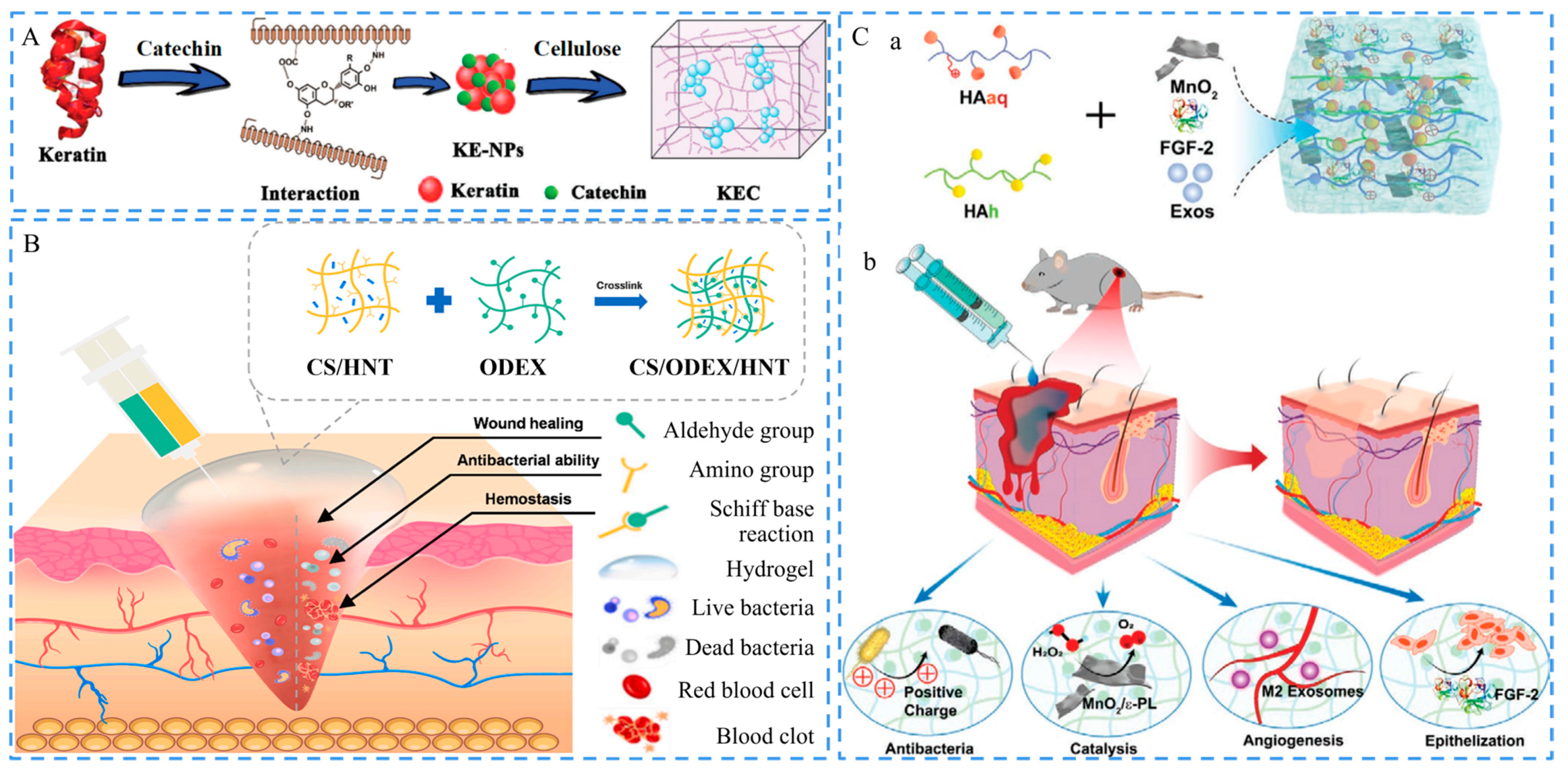
3.2. Antimicrobial Activity
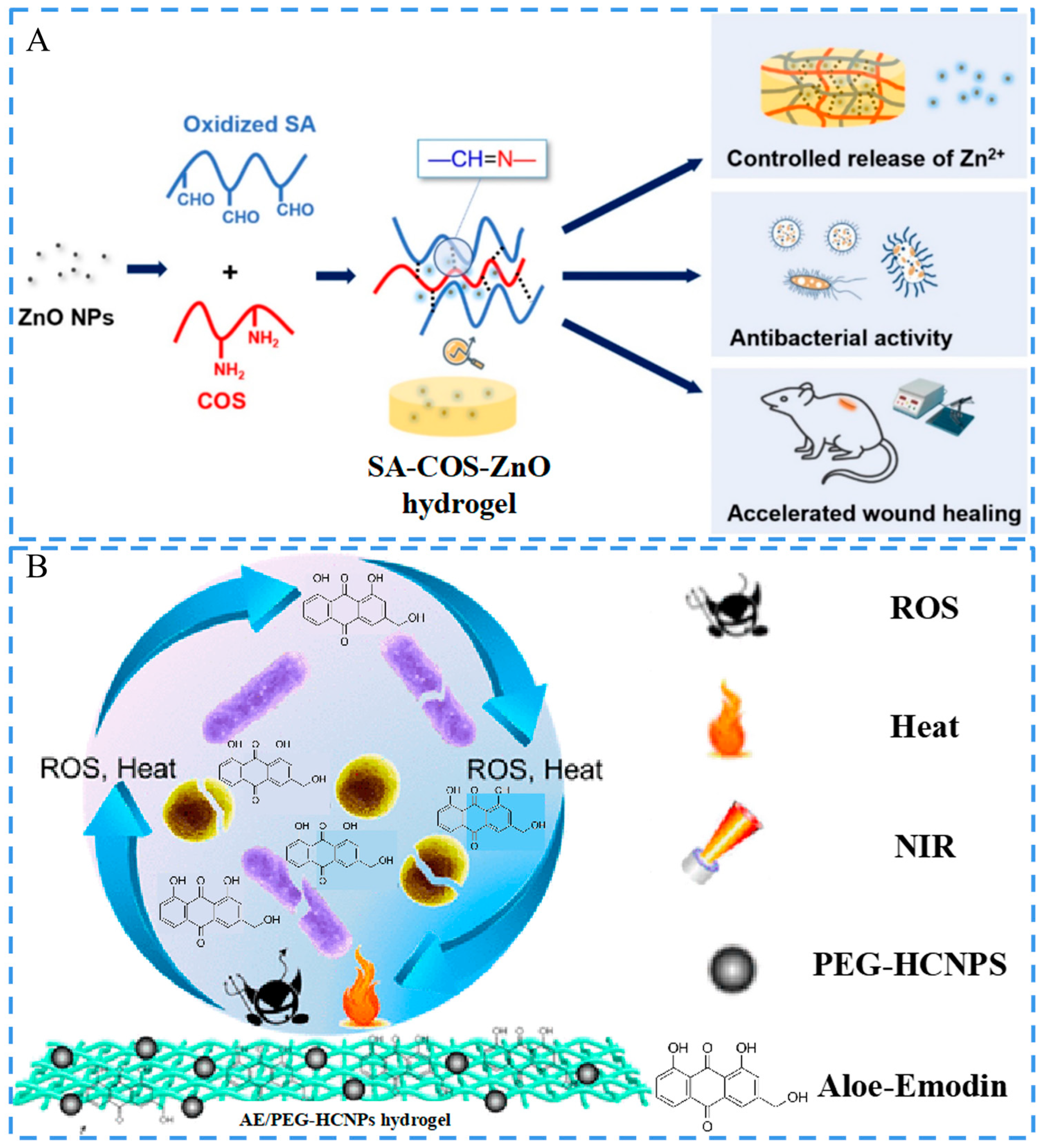
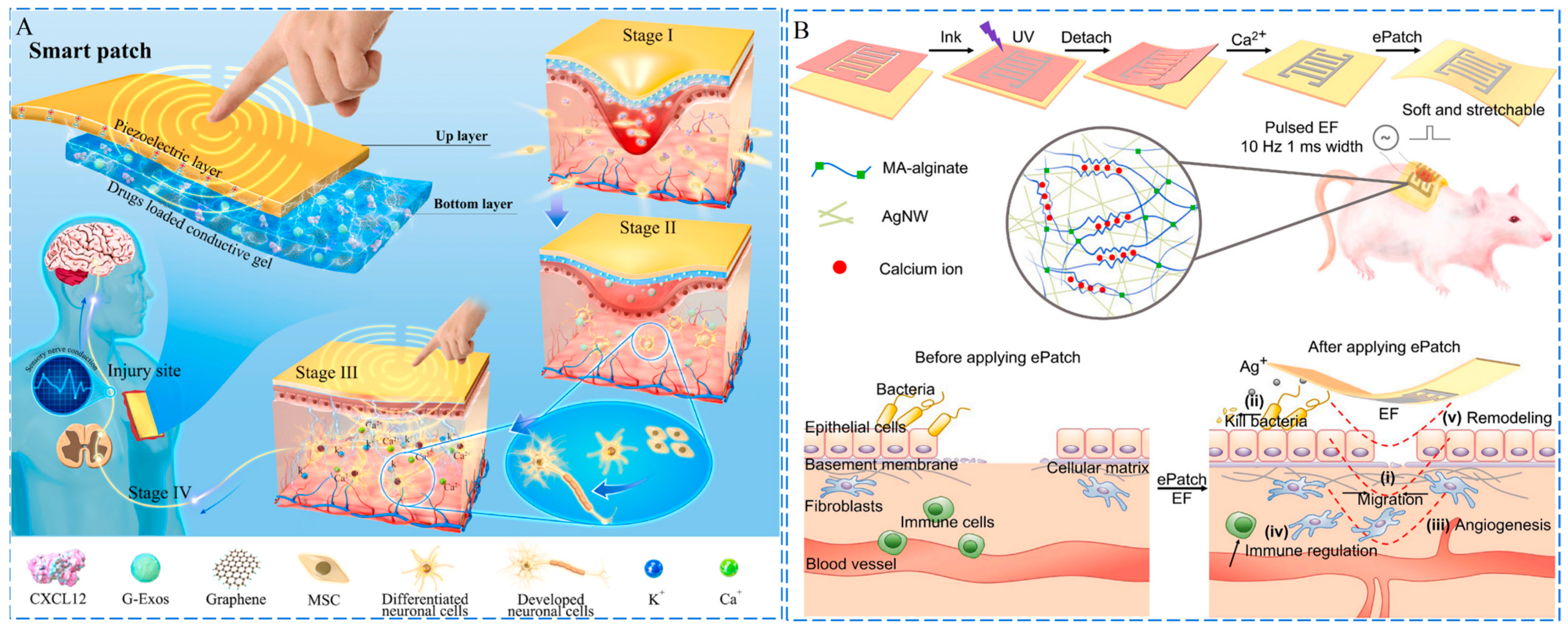
3.3. Conductivity
3.4. Regulation of ROS Level
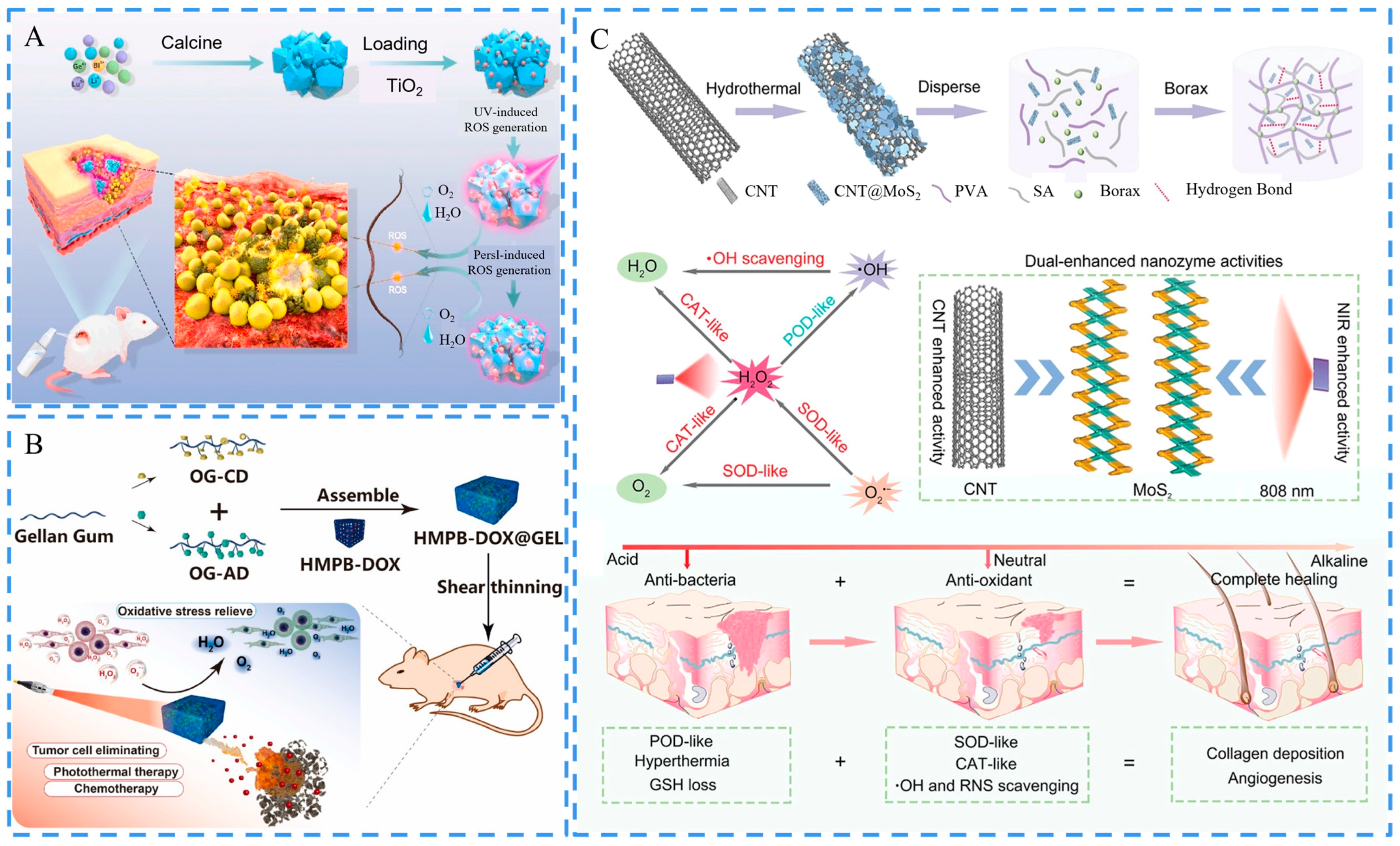
3.5. Stimulus Responsiveness
3.5.1. Photo-Responsiveness
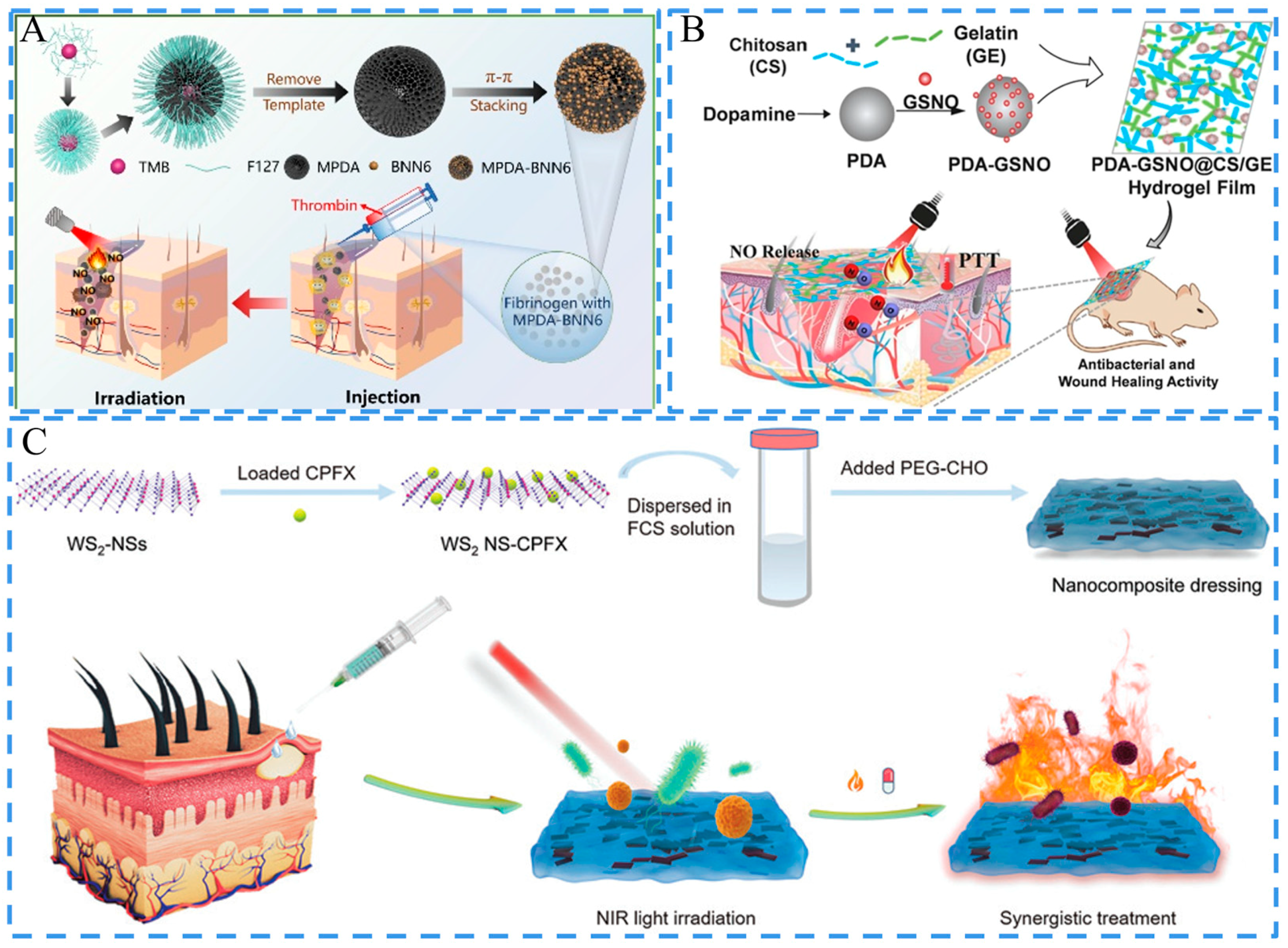
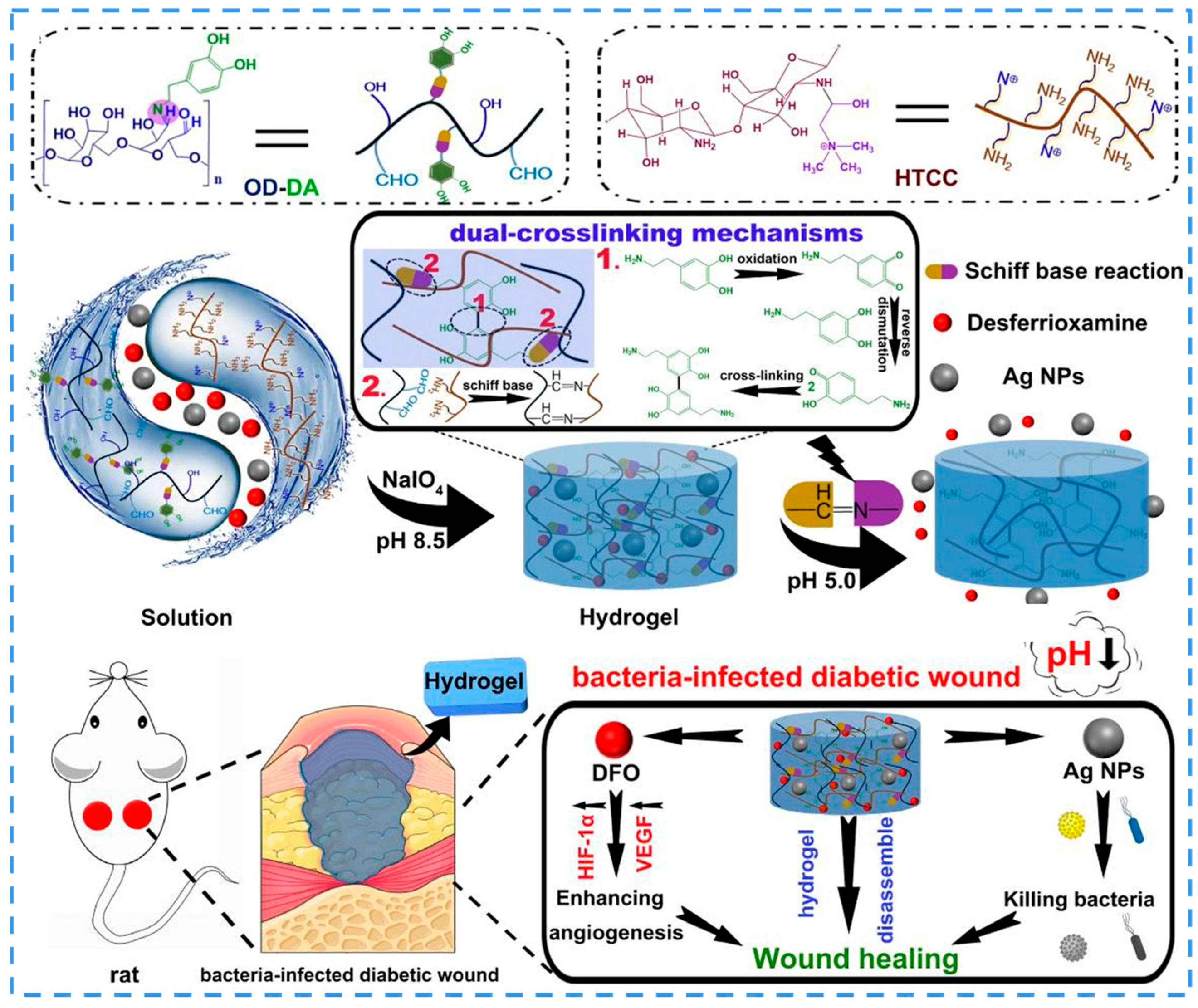
3.5.2. pH Responsiveness
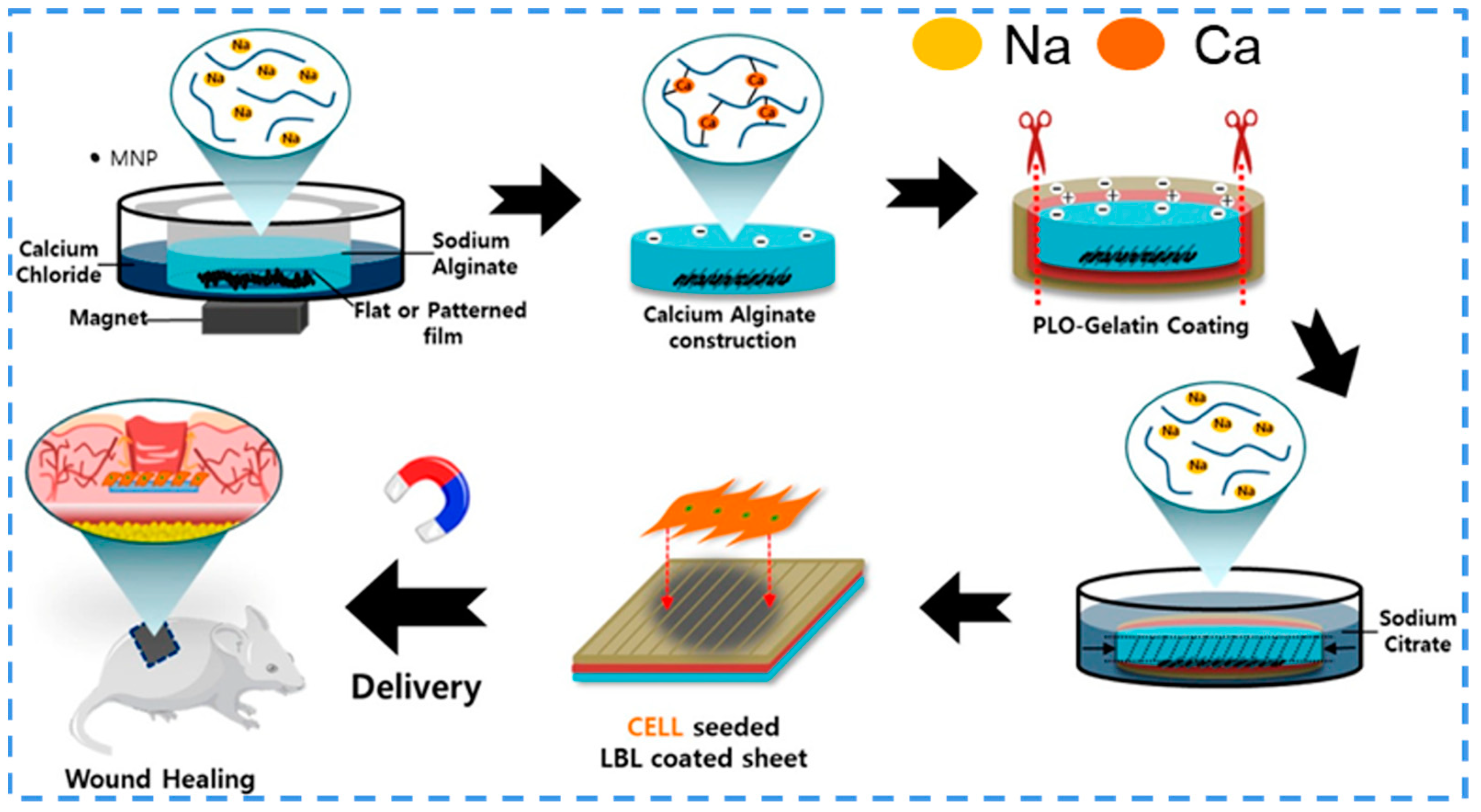
3.5.3. Magnetic Responsiveness
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.J.; Gao, G.R.; Xu, Z.Y.; Tang, D.N.; Chen, T. Recent Progress in Bionic Skin Based on Conductive Polymer Gels. Macromol. Rapid Commun. 2021, 42, 2100480. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Van, D.; Bogdanov, B.; Rooze, N.D.; Sch Ac Ht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar]
- Cubo, N.; Garcia, M.; Canizo, J.D.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2017, 9, 2843–2854. [Google Scholar] [CrossRef] [PubMed]
- Jarbrink, K.; Ni, G.; Sonnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 7. [Google Scholar] [CrossRef]
- Yannas, I.V.; Burke, J.F.; Orgill, D.P.; Skrabut, E.M. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 1982, 215, 174–176. [Google Scholar] [CrossRef]
- Ji, X.; Li, Z.; Hu, Y.; Xie, H.; Wu, W.; Song, F.; Liu, H.; Wang, J.; Jiang, M.; Lam, J.W.Y.; et al. Bioinspired Hydrogels with Muscle-Like Structure for AIEgen-Guided Selective Self-Healing. CCS Chem. 2021, 3, 1146–1156. [Google Scholar] [CrossRef]
- Liang, Y.P.; Zhao, X.; Hu, T.L.; Chen, B.J.; Yin, Z.H.; Ma, P.X.; Guo, B.L. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Ghobril, C.; Charoen, K.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. A Dendritic Thioester Hydrogel Based on Thiol-Thioester Exchange as a Dissolvable Sealant System for Wound Closure. Angew. Chem.-Int. Ed. 2013, 52, 14070–14074. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef]
- Wang, S.G.; Wang, F.; Shi, K.; Yuan, J.F.; Sun, W.L.; Yang, J.T.; Chen, Y.X.; Zhang, D.; Che, L.B. Osteichthyes skin-inspired tough and sticky composite hydrogels for dynamic adhesive dressings. Compos. Part B-Eng. 2022, 241, 110010. [Google Scholar] [CrossRef]
- Sun, G.M.; Zhang, X.J.; Shen, Y.I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, A.; Arani, A.R.; Moghanian, A.; Mozafari, M. Cerium-doped bioactive glass-loaded chitosan/polyethylene oxide nanofiber with elevated antibacterial properties as a potential wound dressing. Ceram. Int. 2021, 47, 9447–9461. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993–1003. [Google Scholar] [CrossRef]
- He, J.H.; Liang, Y.P.; Shi, M.T.; Guo, B.L. Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly(epsilon-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem. Eng. J. 2020, 385, 123464. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef]
- Feng, Y.F.; Li, X.F.; Zhang, Q.; Yan, S.Q.; Guo, Y.; Li, M.Z.; You, R.C. Mechanically robust and flexible silk protein/polysaccharide composite sponges for wound dressing. Carbohydr. Polym. 2019, 216, 17–24. [Google Scholar] [CrossRef]
- Li, D.W.; Tao, L.; Wu, T.; Wang, L.L.; Sun, B.B.; Ke, Q.F.; Mo, X.M.; Deng, B.Y. Mechanically-reinforced 3D scaffold constructed by silk nonwoven fabric and silk fibroin sponge. Colloids Surf. B-Biointerfaces 2020, 196, 111361. [Google Scholar] [CrossRef]
- Rameshbabu, A.P.; Bankoti, K.; Datta, S.; Subramani, E.; Apoorva, A.; Ghosh, P.; Maity, P.P.; Manchikanti, P.; Chaudhury, K.; Dhara, S. Silk Sponges Ornamented with a Placenta-Derived Extracellular Matrix Augment Full-Thickness Cutaneous Wound Healing by Stimulating Neovascularization and Cellular Migration. ACS Appl. Mater. Interfaces 2018, 10, 16977–16991. [Google Scholar] [CrossRef]
- Liu, Y.K.; Fan, J.C.; Lv, M.Q.; She, K.P.; Sun, J.L.; Lu, Q.Q.; Han, C.H.; Ding, S.T.; Zhao, S.; Wang, G.X.; et al. Photocrosslinking silver nanoparticles-aloe vera-silk fibroin composite hydrogel for treatment of full-thickness cutaneous wounds. Regen. Biomater. 2021, 8, rbab048. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Q.; Li, Z.L.; Huang, Y.; Yu, R.; Guo, B.L. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.L.; Zhang, Z.W.B.; Zhang, Y.; Wang, E.D.; Ma, B.; Xu, Q.; Ma, L.L.; Zhang, M.; Pei, G.; Chang, J. A novel “hot spring”-mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials 2021, 264, 120414. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef]
- Xuan, Q.Z.; Jiang, F.; Dong, H.; Zhang, W.X.; Zhang, F.Y.; Ma, T.H.; Zhuang, J.F.; Yu, J.L.; Wang, Y.B.; Shen, H.; et al. Bioinspired Intrinsic Versatile Hydrogel Fabricated by Amyloidal Toxin Simulant-Based Nanofibrous Assemblies for Accelerated Diabetic Wound Healing. Adv. Funct. Mater. 2021, 31, 2106705. [Google Scholar] [CrossRef]
- Qi, J.; Su, G.; Li, Z. Gel-Based Luminescent Conductive Materials and Their Applications in Biosensors and Bioelectronics. Materials 2021, 14, 6759. [Google Scholar] [CrossRef]
- Su, G.; Li, Z.; Dai, R. Recent Advances in Applied Fluorescent Polymeric Gels. ACS Appl. Polym. Mater. 2022, 4, 3131–3152. [Google Scholar] [CrossRef]
- Liu, D.P.; Yin, G.Q.; Le, X.X.; Chen, T. Supramolecular topological hydrogels: From material design to applications. Polym. Chem. 2022, 13, 1940–1952. [Google Scholar] [CrossRef]
- Li, Z.; Ji, X.; Xie, H.; Tang, B.Z. Aggregation-Induced Emission-Active Gels: Fabrications, Functions, and Applications. Adv. Mater. 2021, 33, e2100021. [Google Scholar] [CrossRef]
- Liu, H.; Wei, S.X.; Qiu, H.Y.; Si, M.Q.; Lin, G.Q.; Lei, Z.K.; Lu, W.; Zhou, L.; Chen, T. Supramolecular Hydrogel with Orthogonally Responsive R/G/B Fluorophores Enables Multi-Color Switchable Biomimetic Soft Skins. Adv. Funct. Mater. 2022, 32, 2108830. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Niazmand, R.; Dikshit, P.K.; Kim, B.S. Recent progress in polymeric non-invasive insulin delivery. Int. J. Biol. Macromol. 2022, 203, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.M.; Poorani, G.; Prabu, H.G.; Ravikumar, S.; Jeyakanthan, J. Biological synergy of greener gold nanoparticles by using Coleus aromaticus leaf extract. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 99, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zang, G.; Peng, Q.; Fan, J.; Liu, Y.; Zhang, G.; Zhao, Y.; Li, H.; Zhang, Y. In-situ growth of 3D rosette-like copper nanoparticles on carbon cloth for enhanced sensing of ammonia based on copper electrodissolution. Anal. Chim. Acta 2020, 1104, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhang, Y.; Yang, S.; Yuwen, T.; Liu, Y.; Fan, J.; Zang, G. Glucose determination behaviour of gold microspheres-electrodeposited carbon cloth flexible electrodes in neutral media. Anal. Chim. Acta 2021, 1159, 338442. [Google Scholar] [CrossRef] [PubMed]
- Sankar, R.; Baskaran, A.; Shivashangari, K.S.; Ravikumar, V. Inhibition of pathogenic bacterial growth on excision wound by green synthesized copper oxide nanoparticles leads to accelerated wound healing activity in Wistar Albino rats. J. Mater. Sci.-Mater. Med. 2015, 26, 214. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.P.; Zhao, X.; Hu, T.L.; Han, Y.; Guo, B.L. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Zou, M.Z.; Liu, W.L.; Chen, H.S.; Bai, X.F.; Gao, F.; Ye, J.J.; Cheng, H.; Zhang, X.Z. Advances in nanomaterials for treatment of hypoxic tumor. Natl. Sci. Rev. 2021, 8, nwaa160. [Google Scholar] [CrossRef]
- Huang, S.; Hong, X.; Zhao, M.; Liu, N.; Liu, H.; Zhao, J.; Shao, L.; Xue, W.; Zhang, H.; Zhu, P.; et al. Nanocomposite hydrogels for biomedical applications. Bioeng. Transl. Med. 2022, 7, e10315. [Google Scholar] [CrossRef]
- Yah, C.S.; Simate, G.S. Nanoparticles as potential new generation broad spectrum antimicrobial agents. Daru 2015, 23, 43. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wu, W.; Lei, Y.; Gaucher, C.; Pei, S.; Zhang, J.; Xia, X. Edaravone-Loaded Alginate-Based Nanocomposite Hydrogel Accelerated Chronic Wound Healing in Diabetic Mice. Mar. Drugs 2019, 17, 285. [Google Scholar] [CrossRef] [PubMed]
- Linhart, A.N.; Wortman-Otto, K.M.; Deninger, I.; Dudek, A.L.; Lange, H.R.; Danhausen, D.M.; Graverson, C.F.; Beckmann, T.J.; Havens, M.A.; Keleher, J.J. Strategic Design of Antimicrobial Hydrogels Containing Biomimetic Additives for Enhanced Matrix Responsiveness and HDFa Wound Healing Rates. ACS Appl. Bio Mater. 2020, 3, 5750–5758. [Google Scholar] [CrossRef]
- Pang, Q.; Wu, K.H.; Jiang, Z.L.; Shi, Z.W.; Si, Z.Z.; Wang, Q.; Cao, Y.H.; Hou, R.X.; Zhu, Y.B. A Polyaniline Nanoparticles Crosslinked Hydrogel with Excellent Photothermal Antibacterial and Mechanical Properties for Wound Dressing. Macromol. Biosci. 2022, 22, 2100386. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Qi, J.; Zou, J.; Dan, H.; Zhao, H.; Chen, Q. A multifunctional supramolecular hydrogel for infected wound healing. Biomater. Sci. 2022, 10, 381–395. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, F.F.; Hua, Y.J.; Zhang, X.Z.; Ni, C.Y.; Pan, D.H.; Zhang, Y.Q.; Jiang, D.M.; Yang, L.; Lin, Q.N.; et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019, 10, 2060. [Google Scholar] [CrossRef]
- Clarke, D.L.; Quazi, M.A.; Reddy, K.; Thomson, S.R. Emergency operation for penetrating thoracic trauma in a metropolitan surgical service in South Africa. J. Thorac. Cardiovasc. Surg. 2011, 142, 563–568. [Google Scholar] [CrossRef]
- Dong, R.N.; Zhang, H.L.; Guo, B.L. Emerging hemostatic materials for non-compressible hemorrhage control. Natl. Sci. Rev. 2022, 9, nwac162. [Google Scholar] [CrossRef]
- Gaston, E.; Fraser, J.F.; Xu, Z.P.; Ta, H.T. Nano- and micro-materials in the treatment of internal bleeding and uncontrolled hemorrhage. Nanomedicine 2018, 14, 507–519. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Lu, W.; Ngai, T.; Le, X.X.; Zheng, J.; Zhao, N.; Huang, Y.J.; Wen, X.F.; Zhang, J.W.; Chen, T. Mussel-inspired multifunctional supramolecular hydrogels with self-healing, shape memory and adhesive properties. Polym. Chem. 2016, 7, 5343–5346. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, X.Y.; Ma, X.M.; Cui, X.X.; Yi, Z.; Li, X.D. Cellulose/keratin-catechin nanocomposite hydrogel for wound hemostasis. J. Mater. Chem. B 2018, 6, 6133–6141. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.; Li, X.; Lin, Z.; Chen, L.; Chen, H.; Jin, Y.; Zhang, T.; Xia, H.; Lu, Y.; et al. Ultrafast in-situ forming halloysite nanotube-doped chitosan/oxidized dextran hydrogels for hemostasis and wound repair. Carbohydr. Polym. 2021, 267, 118155. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef]
- Lin, J.; Wang, M.; Hu, H.; Yang, X.; Wen, B.; Wang, Z.; Jacobson, O.; Song, J.; Zhang, G.; Niu, G.; et al. Multimodal-Imaging-Guided Cancer Phototherapy by Versatile Biomimetic Theranostics with UV and gamma-Irradiation Protection. Adv. Mater. 2016, 28, 3273–3279. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.; Liang, Y.; Pan, G.; Guo, B. Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds. Chem. Eng. J. 2022, 427, 132039. [Google Scholar] [CrossRef]
- Lan, G.; Li, Q.; Lu, F.; Yu, K.; Lu, B.; Bao, R.; Dai, F. Improvement of platelet aggregation and rapid induction of hemostasis in chitosan dressing using silver nanoparticles. Cellulose 2019, 27, 385–400. [Google Scholar] [CrossRef]
- Jun, E.A.; Lim, K.M.; Kim, K.; Bae, O.N.; Noh, J.Y.; Chung, K.H.; Chung, J.H. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology 2011, 5, 157–167. [Google Scholar] [CrossRef]
- Hattori, H.; Ishihara, M. Feasibility of improving platelet-rich plasma therapy by using chitosan with high platelet activation ability. Exp. Ther. Med. 2017, 13, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, D.; Ji, N.; Lee, S.; Wang, G.; Zheng, Y.; Zhang, X.; Yang, L.; Qin, Z.; Yang, Y. Bioinspired Design of Sericin/Chitosan/Ag@MOF/GO Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing. Polymers 2021, 13, 2812. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Liu, P.; Yu, T.; Lin, C.; Yan, C.; Hu, Y.; Zhou, W.; Sun, Y.; Panayi, A.C.; et al. All-in-One: Multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing through Timed-Release of Exosome and Fibroblast Growth Factor. Small 2022, 18, e2104229. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.N.; Amirthalingam, S.; Mony, U.; Varma, P.K.; Jayakumar, R. Injectable chitosan-nano bioglass composite hemostatic hydrogel for effective bleeding control. Int. J. Biol. Macromol. 2019, 129, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; He, J.H.; Yang, Y.T.; Qiao, L.P.; Hu, J.; Zhang, J.; Guo, B.L. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater. 2022, 146, 119–130. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef]
- Rafique, A.; Mahmood Zia, K.; Zuber, M.; Tabasum, S.; Rehman, S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: A review. Int. J. Biol. Macromol. 2016, 87, 141–154. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, Y.; Zhou, Y.; Tan, C.; Liu, M. Gold@Halloysite nanotubes-chitin composite hydrogel with antibacterial and hemostatic activity for wound healing. Bioact. Mater. 2023, 20, 355–367. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: An eco-friendly sustainable synthesis method of wound healing patch. Int. J. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef]
- Geisberger, G.; Gyenge, E.B.; Hinger, D.; Kach, A.; Maake, C.; Patzke, G.R. Chitosan-Thioglycolic Acid as a Versatile Antimicrobial Agent. Biomacromolecules 2013, 14, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, P.; Guo, B.L.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H.; Liu, H.; Jin, D.; Yin, M.; Lin, H.; Qu, X.; Liu, C. A reduced polydopamine nanoparticle-coupled sprayable PEG hydrogel adhesive with anti-infection activity for rapid wound sealing. Biomater. Sci. 2020, 8, 6946–6956. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, D.; He, X.; Ni, Y.; Che, L.; Wu, J.; Wu, B.; Wang, Y.; Wang, S.; Sha, D.; et al. Cationic peptide-based salt-responsive antibacterial hydrogel dressings for wound healing. Int. J. Biol. Macromol. 2021, 190, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhang, Y.; Chen, C.; Yu, F.; Tian, J.; Cai, H.; Jiang, X.; Zhang, L.; Zhang, W. An Antifouling and Antimicrobial Zwitterionic Nanocomposite Hydrogel Dressing for Enhanced Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 1621–1630. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef]
- Huang, S.; Chen, H.-J.; Deng, Y.-P.; You, X.-H.; Fang, Q.-h.; Lin, M. Preparation of novel stable microbicidal hydrogel films as potential wound dressing. Polym. Degrad. Stabil. 2020, 181, 109349. [Google Scholar] [CrossRef]
- Diab, S.E.; Tayea, N.A.; Elwakil, B.H.; Gad, A.A.M.; Ghareeb, D.A.; Olama, Z.A. Novel Amoxicillin-Loaded Sericin Biopolymeric Nanoparticles: Synthesis, Optimization, Antibacterial and Wound Healing Activities. Int. J. Mol. Sci. 2022, 23, 11654. [Google Scholar] [CrossRef]
- Song, K.; Hao, Y.; Liu, Y.; Cao, R.; Zhang, X.; He, S.; Wen, J.; Zheng, W.; Wang, L.; Zhang, Y. Preparation of pectin-chitosan hydrogels based on bioadhesive-design micelle to prompt bacterial infection wound healing. Carbohydr. Polym. 2023, 300, 120272. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Trevino, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, e1701503. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Ahmad, I.; Khalil, A.T.; Mukherjee, S.; Javed, R.; Ayaz, M.; Raza, A.; Shinwari, Z.K. Wound healing applications of biogenic colloidal silver and gold nanoparticles: Recent trends and future prospects. Appl. Microbiol. Biotechnol. 2018, 102, 4305–4318. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, J.; Wu, X.; Ren, Y.; Li, Z.; Ren, J. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef]
- Fan, Z.J.; Liu, B.; Wang, J.Q.; Zhang, S.Y.; Lin, Q.Q.; Gong, P.W.; Ma, L.M.; Yang, S.R. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Yang, Y.; Fan, A.; Chi, R.; Shi, J.; Zhang, X. Poly (vinyl alcohol) (PVA) hydrogel incorporated with Ag/TiO2 for rapid sterilization by photoinspired radical oxygen species and promotion of wound healing. Appl. Surf. Sci. 2019, 494, 708–720. [Google Scholar] [CrossRef]
- Shi, G.; Chen, W.; Zhang, Y.; Dai, X.; Zhang, X.; Wu, Z. An Antifouling Hydrogel Containing Silver Nanoparticles for Modulating the Therapeutic Immune Response in Chronic Wound Healing. Langmuir 2019, 35, 1837–1845. [Google Scholar] [CrossRef]
- Ghasemi, A.H.; Farazin, A.; Mohammadimehr, M.; Naeimi, H. Fabrication and characterization of biopolymers with antibacterial nanoparticles and Calendula officinalis flower extract as an active ingredient for modern hydrogel wound dressings. Mater. Today Commun. 2022, 31, 103513. [Google Scholar] [CrossRef]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A.; Pervaiz, E.; Janjua, H.A.; Hussain, Z. In-vitro and in-vivo study of superabsorbent PVA/Starch/g-C3N4/Ag@TiO2 NPs hydrogel membranes for wound dressing. Eur. Polym. J. 2020, 130, 109650. [Google Scholar] [CrossRef]
- Ragothaman, M.; Kannan Villalan, A.; Dhanasekaran, A.; Palanisamy, T. Bio-hybrid hydrogel comprising collagen-capped silver nanoparticles and melatonin for accelerated tissue regeneration in skin defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Li, G.H.; Ding, Y.; Sun, S.Q. Understanding the photothermal effect of gold nanostars and nanorods for biomedical applications. RSC Adv. 2014, 4, 30375–30383. [Google Scholar] [CrossRef]
- Majumder, S.; Ranjan Dahiya, U.; Yadav, S.; Sharma, P.; Ghosh, D.; Rao, G.K.; Rawat, V.; Kumar, G.; Kumar, A.; Srivastava, C.M. Zinc Oxide Nanoparticles Functionalized on Hydrogel Grafted Silk Fibroin Fabrics as Efficient Composite Dressing. Biomolecules 2020, 10, 710. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.N.; Han, W.W.; Jiang, T.Z.; Liu, F.; Zhao, X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- Tang, L.; Zhu, L.; Tang, F.; Yao, C.; Wang, J.; Li, L. Mild Synthesis of Copper Nanoparticles with Enhanced Oxidative Stability and Their Application in Antibacterial Films. Langmuir 2018, 34, 14570–14576. [Google Scholar] [CrossRef]
- Zhang, K.X.; Zhao, G.Y. An Effective Wound Healing Material Based on Gold Incorporation into a Heparin-Polyvinyl Alcohol Nanocomposite: Enhanced In Vitro and In Vivo Care of Perioperative Period. J. Clust. Sci. 2021, 33, 1655–1665. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Zare, E.N.; Salimi, F.; Goudarzi, I.; Tay, F.R.; Makvandi, P. Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef]
- Huang, T.; Yuan, B.; Jiang, W.; Ding, Y.; Jiang, L.; Ren, H.; Tang, J. Glucose oxidase and Fe3O4/TiO2/Ag3PO4 co-embedded biomimetic mineralization hydrogels as controllable ROS generators for accelerating diabetic wound healing. J. Mater. Chem. B 2021, 9, 6190–6200. [Google Scholar] [CrossRef]
- Deuel, H.; Neukom, H.; Weber, F. Reaction of boric acid with polysaccharides. Nature 1948, 161, 96–97. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Wang, Z.; Liu, Y.; Guo, J.; Zhu, Y.; Shao, J.; Li, J.; Wang, L.; Wang, K. Antibacterial, antioxidant and biocompatible nanosized quercetin-PVA xerogel films for wound dressing. Colloids Surf. B Biointerfaces 2022, 209, 112175. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Wu, Q.; Xu, Z.; Wang, Y.; Zhu, B.; Fan, L.; Gao, L. Aloe-Emodin/Carbon Nanoparticle Hybrid Gels with Light-Induced and Long-Term Antibacterial Activity. ACS Biomater. Sci. Eng. 2018, 4, 4391–4400. [Google Scholar] [CrossRef] [PubMed]
- Reczynska-Kolman, K.; Hartman, K.; Kwiecien, K.; Brzychczy-Wloch, M.; Pamula, E. Composites Based on Gellan Gum, Alginate and Nisin-Enriched Lipid Nanoparticles for the Treatment of Infected Wounds. Int. J. Mol. Sci. 2021, 23, 321. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Wang, Y.A.; Zhang, J.Y.; Hu, X.F.; Yang, Z.Y.; Guo, Y.; Wang, Y.B. In-situ doping of a conductive hydrogel with low protein absorption and bacterial adhesion for electrical stimulation of chronic wounds. Acta Biomater. 2019, 89, 217–226. [Google Scholar] [CrossRef]
- Hammer, B.E. Physical Properties of Tissues. Mosc. Univ. Math. Bull. 1991, 54, 128. [Google Scholar] [CrossRef]
- So, J.Y.; Lee, J.; Ahn, Y.; Kang, D.; Bae, W.G. The synergistic effect of biomimetic electrical stimulation and extracellular-matrix-mimetic nanopattern for upregulating cell activities. Biosens. Bioelectron. 2020, 167, 112470. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef]
- Huttenlocher, A.; Horwitz, A.R. Wound Healing with Electric Potential. N. Engl. J. Med. 2007, 356, 303–304. [Google Scholar] [CrossRef]
- Talikowska, M.; Fu, X.X.; Lisak, G. Application of conducting polymers to wound care and skin tissue engineering: A review. Biosens. Bioelectron. 2019, 135, 50–63. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hamblin, M.R.; Mozafari, M. Nanotechnology for angiogenesis: Opportunities and challenges. Chem. Soc. Rev. 2020, 49, 5008–5057. [Google Scholar] [CrossRef]
- Yu, R.; Li, Z.L.; Pan, G.Y.; Guo, B.L. Antibacterial conductive self-healable supramolecular hydrogel dressing for infected motional wound healing. Sci. China-Chem. 2022, 65, 2238–2251. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, P.; Wang, S.; Shi, J.W.; He, J.; Zhang, J.W.; Huang, Y.J.; Chen, T. Scalable fabrication of free-standing, stretchable CNT/TPE ultrathin composite films for skin adhesive epidermal electronics. J. Mater. Chem. C 2018, 6, 6666–6671. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Yue, O.; Hou, M.; Zhang, H.; Beyer, S.; Blocki, A.M.; Wang, Q.; Gong, G.; Liu, X.; et al. Skin-inspired gelatin-based flexible bio-electronic hydrogel for wound healing promotion and motion sensing. Biomaterials 2021, 276, 121026. [Google Scholar] [CrossRef]
- He, J.H.; Shi, M.T.; Liang, Y.P.; Guo, B.L. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Zhang, B.; He, J.; Shi, M.; Liang, Y.; Guo, B. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar] [CrossRef]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82–83, 160–167. [Google Scholar] [CrossRef]
- Scheib, J.; Höke, A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013, 9, 668–676. [Google Scholar] [CrossRef]
- Tan, M.H.; Xu, X.H.; Yuan, T.J.; Hou, X.; Wang, J.; Jiang, Z.H.; Peng, L.H. Self-powered smart patch promotes skin nerve regeneration and sensation restoration by delivering biological-electrical signals in program. Biomaterials 2022, 283, 121413. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, X.; Kim, H.J.; Zhang, S.; Zhou, X.; Chen, Y.; Ling, H.; Xue, Y.; Chen, Z.; Qu, M.; et al. Flexible patch with printable and antibacterial conductive hydrogel electrodes for accelerated wound healing. Biomaterials 2022, 285, 121479. [Google Scholar] [CrossRef]
- Deng, P.P.; Chen, F.X.; Zhang, H.D.; Chen, Y.; Zhou, J.P. Conductive, Self-Healing, Adhesive, and Antibacterial Hydrogels Based on Lignin/Cellulose for Rapid MRSA-Infected Wound Repairing. ACS Appl. Mater. Interfaces 2021, 13, 52333–52345. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, H.; Liu, Z.; Wang, S.; Liu, Z.; Chen, F.; Zhang, H.; Kong, J.; Zhou, F.; Zhang, Q. Conductive Antibacterial Hemostatic Multifunctional Scaffolds Based on Ti3C2Tx MXene Nanosheets for Promoting Multidrug-Resistant Bacteria-Infected Wound Healing. ACS Nano 2021, 15, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Xiao, C.; Guan, P.; Zou, Y.; Wen, H.; Liu, C.; Luo, Y.; Tan, G.; Wang, Q.; Li, Y.; et al. Extracellular Matrix-Based Conductive Interpenetrating Network Hydrogels with Enhanced Neurovascular Regeneration Properties for Diabetic Wounds Repair. Adv. Healthc. Mater. 2022, 11, e2101556. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Suo, H.; Xie, G.; Lyu, Q.; Mo, M.; Xie, Z.; Zhou, N.; Zhang, L.; Tao, J.; Zhu, J. Self-powered and photothermal electronic skin patches for accelerating wound healing. Nano Energy 2022, 93, 106906. [Google Scholar] [CrossRef]
- Yang, K.; Xu, H.; Cheng, L.; Sun, C.Y.; Wang, J.; Liu, Z. In Vitro and In Vivo Near-Infrared Photothermal Therapy of Cancer Using Polypyrrole Organic Nanoparticles. Adv. Mater. 2012, 24, 5586–5592. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Xu, X.J.; Yang, W.; Chen, J.X.; Fang, X.S. Materials and Designs for Wearable Photodetectors. Adv. Mater. 2019, 31, e1808138. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shen, L.; Lu, Y.; Hu, C.; Liang, Z.; Long, L.; Ning, N.; Chen, J.; Guo, Y.; Yang, Z.; et al. Intrinsic Antibacterial and Conductive Hydrogels Based on the Distinct Bactericidal Effect of Polyaniline for Infected Chronic Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 52308–52320. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Guo, J.S.; Sun, W.; Kim, J.P.; Lu, X.L.; Li, Q.Y.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.G.; Qian, G.Y.; et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018, 72, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Snitkin, E.S.; Yockey, L.J.; Bermudez, D.M.; Liechty, K.W.; Segre, J.A.; Sequencing, N.C. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc. Natl. Acad. Sci. USA 2010, 107, 14799–14804. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Tian, X.G.; Kim, J.P.; Xie, D.H.; Ao, X.; Shan, D.Y.; Lin, Q.L.; Hudock, M.R.; Bai, X.C.; Yang, J. Citrate-based materials fuel human stem cells by metabonegenic regulation. Proc. Natl. Acad. Sci. USA 2018, 115, E11741–E11750. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Yuan, L.; Sheng, X.; Clark, L.H.; Lu, Z.; Bae-Jump, V.L. Glutaminase inhibitor compound 968 inhibits cell proliferation and sensitizes paclitaxel in ovarian cancer. Am. J. Transl. Res. 2016, 8, 4265–4277. [Google Scholar] [PubMed]
- Yang, Y.; Liang, Y.; Chen, J.; Duan, X.; Guo, B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact. Mater. 2022, 8, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Yin, H.; Chen, X.; Chen, T.H.; Liu, H.M.; Rao, S.S.; Tan, Y.J.; Qian, Y.X.; Liu, Y.W.; Hu, X.K.; et al. Angstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci. Adv. 2020, 6, eaba0942. [Google Scholar] [CrossRef]
- Zhu, Y.-Q.; Huang, W.-Q.; Chen, G.; Xia, L.; You, Y.-Z.; Yu, Y. Sticking-bacteria gel enhancing anti-multidrug-resistant microbial therapy under ultrasound. Nano Res. 2022, 15, 9105–9113. [Google Scholar] [CrossRef]
- Liu, L.; Shi, J.; Sun, X.; Zhang, Y.; Qin, J.; Peng, S.; Xu, J.; Song, L.; Zhang, Y. Thermo-responsive hydrogel-supported antibacterial material with persistent photocatalytic activity for continuous sterilization and wound healing. Compos. Part B Eng. 2022, 229, 109459. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Wang, J.; Zhang, Y.; Dong, M.; Bu, T.; Li, L.; Liu, Y.; Wang, L. Multifunctional Injectable Hydrogel Dressings for Effectively Accelerating Wound Healing: Enhancing Biomineralization Strategy. Adv. Funct. Mater. 2021, 31, 2100093. [Google Scholar] [CrossRef]
- Zhang, X.J.; Lin, S.J.; Liu, S.W.; Tan, X.L.; Dai, Y.; Xia, F. Advances in organometallic/organic nanozymes and their applications. Coord. Chem. Rev. 2021, 429, 213652. [Google Scholar] [CrossRef]
- Tong, Z.; Guo, Q.; Xu, G.; Gao, Y.; Yang, H.; Ding, Y.; Wang, W.; Mao, Z. Supramolecular hydrogel-loaded Prussian blue nanoparticles with photothermal and ROS scavenging ability for tumor postoperative treatments. Compos. Part B Eng. 2022, 237, 109872. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Adaptive Hydrogels Based on Nanozyme with Dual-Enhanced Triple Enzyme-Like Activities for Wound Disinfection and Mimicking Antioxidant Defense System. Adv. Healthc Mater. 2022, 11, e2101849. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Le, T.M.D.; Lee, S.M.; Kim, H.J.; Ko, Y.J.; Jeong, J.H.; Thambi, T.; Lee, D.S.; Son, S.U. Microporous Organic Nanoparticles Anchoring CeO2 Materials: Reduced Toxicity and Efficient Reactive Oxygen Species-Scavenging for Regenerative Wound Healing. ChemNanoMat 2020, 6, 1104–1110. [Google Scholar] [CrossRef]
- Sener, G.; Hilton, S.A.; Osmond, M.J.; Zgheib, C.; Newsom, J.P.; Dewberry, L.; Singh, S.; Sakthivel, T.S.; Seal, S.; Liechty, K.W.; et al. Injectable, self-healable zwitterionic cryogels with sustained microRNA-cerium oxide nanoparticle release promote accelerated wound healing. Acta Biomater. 2020, 101, 262–272. [Google Scholar] [CrossRef]
- Ou, Q.; Zhang, S.; Fu, C.; Yu, L.; Xin, P.; Gu, Z.; Cao, Z.; Wu, J.; Wang, Y. More natural more better: Triple natural anti-oxidant puerarin/ferulic acid/polydopamine incorporated hydrogel for wound healing. J. Nanobiotechnol. 2021, 19, 237. [Google Scholar] [CrossRef]
- Tang, P.; Han, L.; Li, P.; Jia, Z.; Wang, K.; Zhang, H.; Tan, H.; Guo, T.; Lu, X. Mussel-Inspired Electroactive and Antioxidative Scaffolds with Incorporation of Polydopamine-Reduced Graphene Oxide for Enhancing Skin Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 7703–7714. [Google Scholar] [CrossRef]
- Ramanan, V.V.; Katz, J.S.; Guvendiren, M.; Cohen, E.R.; Marklein, R.A.; Burdick, J.A. Photocleavable side groups to spatially alter hydrogel properties and cellular interactions. J. Mater. Chem. 2010, 20, 8920–8926. [Google Scholar] [CrossRef]
- Ruskowitz, E.R.; DeForest, C.A. Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nat. Rev. Mater. 2018, 3, 17087. [Google Scholar] [CrossRef]
- Dong, H.; Yang, K.; Zhang, Y.; Li, Q.; Xiu, W.; Ding, M.; Shan, J.; Mou, Y. Photocatalytic Cu2WS4 Nanocrystals for Efficient Bacterial Killing and Biofilm Disruption. Int. J. Nanomed. 2022, 17, 2735–2750. [Google Scholar] [CrossRef]
- Wang, Y.F.; Meng, H.M.; Song, G.S.; Li, Z.H.; Zhang, X.B. Conjugated-Polymer-Based Nanomaterials for Photothermal Therapy. ACS Appl. Polym. Mater. 2020, 2, 4258–4272. [Google Scholar] [CrossRef]
- Rajendran, M. Quinones as photosensitizer for photodynamic therapy: ROS generation, mechanism and detection methods. Photodiagnosis Photodyn. Ther. 2016, 13, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Sun, I.C.; Hwang, H.S.; Yoon, H.Y.; Kim, K. Light-triggered photodynamic nanomedicines for overcoming localized therapeutic efficacy in cancer treatment. Adv. Drug Deliv. Rev. 2022, 186, 114344. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; ChawPattnayak, B.; Dash, P.; Nayak, B.; Mohapatra, S. Papaya-Derived Carbon-Dot-Loaded Fluorescent Hydrogel for NIR-Stimulated Photochemotherapy and Antibacterial Activity. ACS Appl. Polym. Mater. 2021, 4, 369–380. [Google Scholar] [CrossRef]
- Belali, S.; Karimi, A.R.; Hadizadeh, M. Cell-specific and pH-sensitive nanostructure hydrogel based on chitosan as a photosensitizer carrier for selective photodynamic therapy. Int. J. Biol. Macromol. 2018, 110, 437–448. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, N.; Sahi, S.V. Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics 2021, 13, 1174. [Google Scholar] [CrossRef]
- Li, Y.Y.; Jiang, C.H.; Zhang, D.W.; Wang, Y.; Ren, X.Y.; Ai, K.L.; Chen, X.S.; Lu, L.H. Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor. Acta Biomater. 2017, 47, 124–134. [Google Scholar] [CrossRef]
- Jin, A.T.; Wang, Y.T.; Lin, K.L.; Jiang, L. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Liu, K.Z.; Wang, K.F.; Fang, L.M.; Weng, L.T.; Zhang, H.P.; Tang, Y.H.; Ren, F.Z.; Zhao, C.C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef]
- Lv, X.; Xu, Y.; Ruan, X.; Yang, D.; Shao, J.; Hu, Y.; Wang, W.; Cai, Y.; Tu, Y.; Dong, X. An injectable and biodegradable hydrogel incorporated with photoregulated NO generators to heal MRSA-infected wounds. Acta Biomater. 2022, 146, 107–118. [Google Scholar] [CrossRef]
- Wang, W.; Sheng, H.; Cao, D.; Zhang, F.; Zhang, W.; Yan, F.; Ding, D.; Cheng, N. S-nitrosoglutathione functionalized polydopamine nanoparticles incorporated into chitosan/gelatin hydrogel films with NIR-controlled photothermal/NO-releasing therapy for enhanced wound healing. Int. J. Biol. Macromol. 2022, 200, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, P.; Tang, P.; Wang, X.; Zhou, T.; Wang, K.; Ren, F.; Guo, T.; Lu, X. Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion. Nanoscale 2019, 11, 15846–15861. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, W.; Liu, H.; Li, X.; Qin, X.; Wang, X.; Yu, J.; Li, B.; Li, F.; Huang, L.; et al. Conductive hydrogel dressings based on cascade reactions with photothermal effect for monitoring and treatment of diabetic wounds. Compos. Part B Eng. 2022, 242, 110098. [Google Scholar] [CrossRef]
- Das, B.; Dadhich, P.; Pal, P.; Dhara, S. Single step synthesized sulfur and nitrogen doped carbon nanodots from whey protein: Nanoprobes for longterm cell tracking crossing the barrier of photo-toxicity. RSC Adv. 2016, 6, 60794–60805. [Google Scholar] [CrossRef]
- Xiang, Y.; Mao, C.; Liu, X.; Cui, Z.; Jing, D.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; et al. Rapid and Superior Bacteria Killing of Carbon Quantum Dots/ZnO Decorated Injectable Folic Acid-Conjugated PDA Hydrogel through Dual-Light Triggered ROS and Membrane Permeability. Small 2019, 15, e1900322. [Google Scholar] [CrossRef]
- Nayak, S.; Prasad, S.R.; Mandal, D.; Das, P. Hybrid DNA-Carbon Dot-Poly(vinylpyrrolidone) Hydrogel with Self-Healing and Shape Memory Properties for Simultaneous Trackable Drug Delivery and Visible-Light-Induced Antimicrobial Photodynamic Inactivation. ACS Appl. Bio Mater. 2020, 3, 7865–7875. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.C.; Younis, M.R.; Zhou, Y.; Wang, C.; Xia, X.H. In Situ Fabrication of Ultrasmall Gold Nanoparticles/2D MOFs Hybrid as Nanozyme for Antibacterial Therapy. Small 2020, 16, e2000553. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, X.; Bu, T.; Wang, Q.; Zhang, H.; Jia, P.; Li, L.; Wang, L. Construction of a photothermal hydrogel platform with two-dimensional PEG@zirconium-ferrocene MOF nanozymes for rapid tissue repair of bacteria-infected wounds. Acta Biomater. 2021, 135, 342–355. [Google Scholar] [CrossRef]
- Qiu, Y.C.; Leung, S.F.; Wei, Z.H.; Lin, Q.F.; Zheng, X.L.; Zhang, Y.G.; Fan, Z.Y.; Yang, S.H. Enhanced Charge Collection for Splitting of Water Enabled by an Engineered Three-Dimensional Nanospike Array. J. Phys. Chem. C 2014, 118, 22465–22472. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mater. Chem B 2019, 7, 2534–2548. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qiao, Y.; Zhang, H.; Zhao, J.; Li, W.; Xie, T.; Zhong, D.; Wei, Q.; Hua, S.; Yu, Y.; et al. Gold-silver nanoshells promote wound healing from drug-resistant bacteria infection and enable monitoring via surface-enhanced Raman scattering imaging. Biomaterials 2020, 234, 119763. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. J. Mater. Chem B 2020, 8, 3908–3917. [Google Scholar] [CrossRef] [PubMed]
- McArdle, C.; Lagan, K.M.; McDowell, D.A. The pH of wound fluid in diabetic foot ulcers—the way forward in detecting clinical infection? Curr. Diabetes Rev. 2014, 10, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Yu, J.; Wang, C.F.; Chen, S.; Li, Q.; Guo, K.; Qing, R.; Wang, G.; Ren, J. Micro-Gel Ensembles for Accelerated Healing of Chronic Wound via pH Regulation. Adv. Sci. 2022, 9, e2201254. [Google Scholar] [CrossRef]
- Lu, Y.M.; Wu, Y.; Liang, J.; Libera, M.R.; Sukhishvili, S.A. Self-defensive antibacterial layer-by-layer hydrogel coatings with pH-triggered hydrophobicity. Biomaterials 2015, 45, 64–71. [Google Scholar] [CrossRef]
- George, L.; Bavya, M.C.; Rohan, K.V.; Srivastava, R. A therapeutic polyelectrolyte-vitamin C nanoparticulate system in polyvinyl alcohol-alginate hydrogel: An approach to treat skin and soft tissue infections caused by Staphylococcus aureus. Colloids Surf. B Biointerfaces 2017, 160, 315–324. [Google Scholar] [CrossRef]
- Liu, K.; Dai, L.; Li, C. A lignocellulose-based nanocomposite hydrogel with pH-sensitive and potent antibacterial activity for wound healing. Int. J. Biol. Macromol. 2021, 191, 1249–1254. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Cuestas, M.L.; Perez, C.J.; Campo Dall Orto, V.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.J.; Long, L.Y.; Kong, Q.S.; Luo, R.F.; Wang, Y.B. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Munoz-Gonzalez, P.U.; Rooney, P.; Mohd Isa, I.L.; Pandit, A.; Delgado, J.; Flores-Moreno, M.; Castellano, L.E.; Mendoza-Novelo, B. Development and characterization of an immunomodulatory and injectable system composed of collagen modified with trifunctional oligourethanes and silica. Biomater. Sci. 2019, 7, 4547–4557. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, J.; Huang, W.; Yan, B.; Peng, Q.; Liu, J.; Chen, L.; Zeng, H. Injectable and Self-Healing Nanocomposite Hydrogels with Ultrasensitive pH-Responsiveness and Tunable Mechanical Properties: Implications for Controlled Drug Delivery. Biomacromolecules 2020, 21, 2409–2420. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dou, G.; Li, Z.; Wang, X.; Jin, R.; Liu, Y.; Kuang, H.; Huang, X.; Yang, X.; Yang, X.; et al. Hybrid Biomaterial Initiates Refractory Wound Healing via Inducing Transiently Heightened Inflammatory Responses. Adv. Sci. 2022, 9, e2105650. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.R.; Cabuil, V.; Lalot, T.; Thouvenot, R. Magnetic nanoparticles trapped in pH 7 hydrogels as a tool to characterize the properties of the polymeric network. Adv. Mater. 2000, 12, 417–420. [Google Scholar] [CrossRef]
- Shi, L.; Zeng, Y.; Zhao, Y.; Yang, B.; Ossipov, D.; Tai, C.W.; Dai, J.; Xu, C. Biocompatible Injectable Magnetic Hydrogel Formed by Dynamic Coordination Network. ACS Appl. Mater. Interfaces 2019, 11, 46233–46240. [Google Scholar] [CrossRef]
- Kozissnik, B.; Bohorquez, A.C.; Dobson, J.; Rinaldi, C. Magnetic fluid hyperthermia: Advances, challenges, and opportunity. Int. J. Hyperthermia 2013, 29, 706–714. [Google Scholar] [CrossRef]
- Patwa, R.; Soundararajan, N.; Mulchandani, N.; Bhasney, S.M.; Shah, M.; Kumar, S.; Kumar, A.; Katiyar, V. Silk nano-discs: A natural material for cancer therapy. Biopolymers 2018, 109, e23231. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhai, X.Y.; Fan, M.; Tan, H.P.; Chen, Y. Thermal-reversible and self-healing hydrogel containing magnetic microspheres derived from natural polysaccharides for drug delivery. Eur. Polym. J. 2021, 157, 110644. [Google Scholar] [CrossRef]
- Forouzandehdel, S.; Forouzandehdel, S.; Rezghi Rami, M. Synthesis of a novel magnetic starch-alginic acid-based biomaterial for drug delivery. Carbohydr. Res. 2020, 487, 107889. [Google Scholar] [CrossRef]
- Nezami, S.; Sadeghi, M.; Mohajerani, H. A novel pH-sensitive and magnetic starch-based nanocomposite hydrogel as a controlled drug delivery system for wound healing. Polym. Degrad. Stabil. 2020, 179, 109255. [Google Scholar] [CrossRef]
- Noh, M.; Choi, Y.H.; An, Y.H.; Tahk, D.; Cho, S.; Yoon, J.W.; Jeon, N.L.; Park, T.H.; Kim, J.; Hwang, N.S. Magnetic Nanoparticle-Embedded Hydrogel Sheet with a Groove Pattern for Wound Healing Application. ACS Biomater. Sci. Eng. 2019, 5, 3909–3921. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Dong, W.; Zhao, S.; Du, T.; Wang, Y.; Yao, J.; Liu, Z.; Sun, D.; Zhang, M. An injectable adhesive antibacterial hydrogel wound dressing for infected skin wounds. Biomater. Adv. 2022, 134, 112584. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Bai, Q.; Wu, W.; Sun, N.; Cui, N.; Lu, T. Gelatin-based adhesive hydrogel with self-healing, hemostasis, and electrical conductivity. Int. J. Biol. Macromol. 2021, 183, 2142–2151. [Google Scholar] [CrossRef]
- Zu, Y.; Wang, Y.; Yao, H.; Yan, L.; Yin, W.; Gu, Z. A Copper Peroxide Fenton Nanoagent-Hydrogel as an In Situ pH-Responsive Wound Dressing for Effectively Trapping and Eliminating Bacteria. ACS Appl. Bio Mater. 2022, 5, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.H.; Lin, C.J.; Liu, C.C.; Yeh, J.Z.; Fan, G.Y.; Tsai, H.D.; Chung, C.F.; Hsu, S.D. Hemostasis and Anti-Inflammatory Abilities of AuNPs-Coated Chitosan Dressing for Burn Wounds. J. Pers. Med. 2022, 12, 1089. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef] [PubMed]
- Muthiah Pillai, N.S.; Eswar, K.; Amirthalingam, S.; Mony, U.; Kerala Varma, P.; Jayakumar, R. Injectable Nano Whitlockite Incorporated Chitosan Hydrogel for Effective Hemostasis. ACS Appl. Bio Mater. 2019, 2, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Preethi, G.U.; Unnikrishnan, B.S.; Sreekutty, J.; Archana, M.G.; Anupama, M.S.; Shiji, R.; Raveendran Pillai, K.; Joseph, M.M.; Syama, H.P.; Sreelekha, T.T. Semi-interpenetrating nanosilver doped polysaccharide hydrogel scaffolds for cutaneous wound healing. Int. J. Biol. Macromol. 2020, 142, 712–723. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Song, S.; Yang, K.; Liu, H.; Yang, Z.; Wang, J.; Yang, B.; Lin, Q. Skin-Inspired Antibacterial Conductive Hydrogels for Epidermal Sensors and Diabetic Foot Wound Dressings. Adv. Funct. Mater. 2019, 29, 1901474. [Google Scholar] [CrossRef]
- Yang, E.; Hou, W.; Liu, K.; Yang, H.; Wei, W.; Kang, H.; Dai, H. A multifunctional chitosan hydrogel dressing for liver hemostasis and infected wound healing. Carbohydr. Polym. 2022, 291, 119631. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Reddy, K.K.; Pyarasani, R.D.; Akbari-Fakhrabadi, A.; Amalraj, J. Chitosan-pluronic based Cu nanocomposite hydrogels for prototype antimicrobial applications. Int. J. Biol. Macromol. 2020, 143, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Venkataprasanna, K.S.; Prakash, J.; Vignesh, S.; Bharath, G.; Venkatesan, M.; Banat, F.; Sahabudeen, S.; Ramachandran, S.; Devanand Venkatasubbu, G. Fabrication of Chitosan/PVA/GO/CuO patch for potential wound healing application. Int. J. Biol. Macromol. 2020, 143, 744–762. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Su, C.F.; Huang, C.C.; Jan, J.S. Biomimetic hydrogels based on L-Dopa conjugated gelatin as pH-responsive drug carriers and antimicrobial agents. Colloids Surf. B Biointerfaces 2020, 196, 111316. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Bano, S.; Poojary, S.S.; Priyadarshi, R.; Choudhary, A.; Kumar, D.; Negi, Y.S. Comparative analysis of TiO2 and Ag nanoparticles on xylan/chitosan conjugate matrix for wound healing application. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 376–385. [Google Scholar] [CrossRef]
- Cheng, H.; Shi, Z.; Yue, K.; Huang, X.; Xu, Y.; Gao, C.; Yao, Z.; Zhang, Y.S.; Wang, J. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021, 124, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zu, D.; Kong, L.; Chen, S.; Yao, J.; Lin, J.; Lu, L.; Wu, B.; Fang, B. Construction of antibacterial nano-silver embedded bioactive hydrogel to repair infectious skin defects. Biomater. Res. 2022, 26, 36. [Google Scholar] [CrossRef]
- Xu, M.; Li, Q.; Fang, Z.; Jin, M.; Zeng, Q.; Huang, G.; Jia, Y.G.; Wang, L.; Chen, Y. Conductive and antimicrobial macroporous nanocomposite hydrogels generated from air-in-water Pickering emulsions for neural stem cell differentiation and skin wound healing. Biomater. Sci. 2020, 8, 6957–6968. [Google Scholar] [CrossRef]
- Jia, Z.; Lv, X.; Hou, Y.; Wang, K.; Ren, F.; Xu, D.; Wang, Q.; Fan, K.; Xie, C.; Lu, X. Mussel-inspired nanozyme catalyzed conductive and self-setting hydrogel for adhesive and antibacterial bioelectronics. Bioact. Mater. 2021, 6, 2676–2687. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, C.; Zhao, M.; Liu, N.; Chen, Z.; Liu, J.; Li, G.; Deng, Y.; Sai, X.; Huang, H.; et al. Histatin-1 loaded multifunctional, adhesive and conductive biomolecular hydrogel to treat diabetic wound. Int. J. Biol. Macromol. 2022, 209, 1020–1031. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, S.; Cheng, F.; He, X.; Liu, Z.; Wang, W.; Zhou, L.; Zhang, Q. Bioactive anti-inflammatory, antibacterial, conductive multifunctional scaffold based on MXene@CeO2 nanocomposites for infection-impaired skin multimodal therapy. Chem. Eng. J. 2021, 424, 130148. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, M.; Zhang, Z.; Ren, G.; Liu, Y.; Wu, S.; Shen, J. Facile synthesis of ZnO QDs@GO-CS hydrogel for synergetic antibacterial applications and enhanced wound healing. Chem. Eng. J. 2019, 378, 122043. [Google Scholar] [CrossRef]
- Wang, M.; Xia, A.; Wu, S.; Shen, J. Facile Synthesis of the Cu, N-CDs@GO-CS Hydrogel with Enhanced Antibacterial Activity for Effective Treatment of Wound Infection. Langmuir 2021, 37, 7928–7935. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, X.; Yang, K.; Li, Q.; Wan, R.; Hu, G.; Ye, J.; Zhang, Y.; He, J.; Gu, H.; et al. Peroxidase- and UV-triggered oxidase mimetic activities of the UiO-66-NH2/chitosan composite membrane for antibacterial properties. Biomater. Sci. 2021, 9, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Y.; Zhang, Y.W.; Liu, X.Z.; Ma, X.F.; Qin, X.T.; Jia, S.R.; Zhong, C. Aggregation-induced emission-active amino acid/berberine hydrogels with enhanced photodynamic antibacterial and anti-biofilm activity. Chem. Eng. J. 2021, 413, 127542. [Google Scholar] [CrossRef]
- Huang, S.; Xu, S.; Hu, Y.; Zhao, X.; Chang, L.; Chen, Z.; Mei, X. Preparation of NIR-responsive, ROS-generating and antibacterial black phosphorus quantum dots for promoting the MRSA-infected wound healing in diabetic rats. Acta Biomater. 2022, 137, 199–217. [Google Scholar] [CrossRef]
- Lu, H.; Liu, J.; Yu, M.; Li, P.; Huang, R.; Wu, W.; Hu, Z.; Xiao, Y.; Jiang, F.; Xing, X. Photothermal-enhanced antibacterial and antioxidant hydrogel dressings based on catechol-modified chitosan-derived carbonized polymer dots for effective treatment of wound infections. Biomater. Sci. 2022, 10, 2692–2705. [Google Scholar] [CrossRef]
- Yu, R.; Li, M.; Li, Z.L.; Pan, G.Y.; Liang, Y.Q.; Guo, B.L. Supramolecular Thermo-Contracting Adhesive Hydrogel with Self-Removability Simultaneously Enhancing Noninvasive Wound Closure and MRSA-Infected Wound Healing. Adv. Healthc. Mater. 2022, 11, 2102749. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Ji, X.; Gong, J.; Hu, Y.; Wu, W.; Wang, X.; Peng, H.Q.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Bioinspired Simultaneous Changes in Fluorescence Color, Brightness, and Shape of Hydrogels Enabled by AIEgens. Adv. Mater. 2020, 32, e1906493. [Google Scholar] [CrossRef]
- Hoare, T.; Santamaria, J.; Goya, G.F.; Irusta, S.; Lin, D.; Lau, S.; Padera, R.; Langer, R.; Kohane, D.S. A Magnetically Triggered Composite Membrane for On-Demand Drug Delivery. Nano Lett. 2009, 9, 3651–3657. [Google Scholar] [CrossRef]
- Caldorera-Moore, M.E.; Liechty, W.B.; Peppas, N.A. Responsive Theranostic Systems: Integration of Diagnostic Imaging Agents and Responsive Controlled Release Drug Delivery Carriers. Acc. Chem. Res. 2011, 44, 1061–1070. [Google Scholar] [CrossRef]
- Wu, B.Y.; Le, X.X.; Jian, Y.K.; Lu, W.; Yang, Z.Y.; Zheng, Z.K.; Theato, P.; Zhang, J.W.; Zhang, A.; Chen, T. pH and Thermo Dual-Responsive Fluorescent Hydrogel Actuator. Macromol. Rapid Commun. 2019, 40, e1800648. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Bergert, M.; Lembo, S.; Sharma, S.; Russo, L.; Milovanovic, D.; Gretarsson, K.H.; Bormel, M.; Neveu, P.A.; Hackett, J.A.; Petsalaki, E.; et al. Cell Surface Mechanics Gate Embryonic Stem Cell Differentiation. Cell Stem Cell 2021, 28, 209–216.e4. [Google Scholar] [CrossRef] [PubMed]
- Bongiorno, T.; Kazlow, J.; Mezencev, R.; Griffiths, S.; Olivares-Navarrete, R.; McDonald, J.F.; Schwartz, Z.; Boyan, B.D.; McDevitt, T.C.; Sulchek, T. Mechanical stiffness as an improved single-cell indicator of osteoblastic human mesenchymal stem cell differentiation. J. Biomech. 2014, 47, 2197–2204. [Google Scholar] [CrossRef]
- Lima, J.; Goncalves, A.I.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. The effect of magnetic stimulation on the osteogenic and chondrogenic differentiation of human stem cells derived from the adipose tissue (hASCs). J. Magn. Magn. Mater. 2015, 393, 526–536. [Google Scholar] [CrossRef]
- Maredziak, M.; Smieszek, A.; Tomaszewski, K.A.; Lewandowski, D.; Marycz, K. The effect of low static magnetic field on osteogenic and adipogenic differentiation potential of human adipose stromal/stem cells. J. Magn. Magn. Mater. 2016, 398, 235–245. [Google Scholar] [CrossRef]
| Function | Nanomaterial | Hydrogel Matrix | Mechanism | Ref |
|---|---|---|---|---|
| Hemostatic Ability | polydopamine decorated Ag NPs (PDA@Ag NPs) | Oxidized Alg and catechol-modified gelatin | Good adhesion reduced blood loss and gelatin promoted platelet aggregation. | [192] |
| GO | dopamine grafted gelatin (GelDA) | The catechol group of dopamine (DA) attached to wound and stopped bleeding. | [193] | |
| transferrin conjugated CuO2 NPs (CP@Tf NPs) | copolymer of N-isopropylacrylamide (NIPAM), acrylamide (Aam), N-[3-(dimethylamino)propyl]-methacrylamide (DMPA), and methylene-N,N-bis(acrylamide) | Abundant amino acid groups of the hydrogel attracted negatively charged red blood cells to gather and form blood clotting, and Cu2+ promoted coagulation. | [194] | |
| Au NPs | CS | Au NPs stimulated the intrinsic coagulation pathway. | [195] | |
| multiwalled carbon nanotubes (MWCNTs) | copolymer of glycidyl methacrylate functionalized quaternized-CS (QCSG) and PF127 | MWCNTs trigger platelets activation and Ca2+ from extracellular activated the release of platelet membrane microparticles. | [196] | |
| nano whitlockite (nWH) | CS | Various coagulation factors involved in the coagulation cascade were activated by the Ca2+, Mg2+, and PO43− released from nWH and amine groups of CS. | [197] | |
| Antimicrobial activities | Ag NPs | galacto-xyloglucan and PAM | The released Ag+ affected the replication and/or inactivation of the microbial flora. | [198] |
| PDA@Ag NPs | PANI and PVA | Ag NPs released Ag+ and bind to bacteria to destroy them. | [199] | |
| Zn doped nWH (Zn-nWH) | copolymer of methacrylate anhydride quaternized CS (QCSMA) and methacrylate anhydride DA (DAMA) | The Zn2+ released from Zn-nWH synerging with QCSMA achieved a high antibacterial effect. | [200] | |
| Cu NPs | CS/ PF127 | Depolarization of the cell membrane through interaction between the cell membrane and Cu NPs weakened the cell outer membrane, and Cu2+ penetrated the cell and mediated the ROS to block the bacterial cell metabolism. | [201] | |
| GO/CuO nanocomposite | CS and PVA | NPs accumulate around bacteria, causing bacterial oxidative stress, DNA damage, and lactate dehydrogenase (LDH) release. | [202] | |
| Ag NPs | copolymer of L-DA, PEG, and gelatin (GPLD) | Star-shaped topology cationic GPLD with and/or certain functional group on the side chain showed antimicrobial activity. | [203] | |
| TiO2 NPs or Ag NPs | xylan and CS | Incorporation of TiO2 NPs or Ag NPs in the gel matrix provided synergistic effects in killing bacterial. | [204] | |
| CeO NPs | DA-modified GelMA | CeO NPs cleaned extracellular ROS and prevented intracellular ROS production. | [205] | |
| Ag NPs | silk fibroin (SF) | Ag NPs destroyed the bacterial structure and inhibited the inflammatory response. | [21] | |
| Ag NPs | porcine dermal decellularized extracellular matrix | Ag NPs destroyed the structure of bacteria. | [206] | |
| reduced graphene oxide (rGO) | DA modified HA (DA-HA) | High temperature (above 50℃) could kill bacteria through destroying some enzymes and proteins. | [8] | |
| Conductivity | CNTs | GelMA | The conductive hydrogel could promote the NE-C4 stem cells proliferation and differentiation. | [207] |
| CNTs | DA-gelatin/CS/PDA | The conductive hydrogel could adjust electrical signals and promote wound healing via improving blood flow, enhancing migration, and reducing edema. | [37] | |
| CNTs | N-carboxyethyl CS/benzaldehyde-terminated PF127 | The CNT-based conductive hydrogel showed photothermal ability to shorten the healing process of infected wound. | [114] | |
| GO-graft-cyclodextrin | quaternized CS-graft-cyclodextrin/quaternized CS-graft-adamantane | The hydrogel could regulate cell adhesion, proliferation, and migration with/without ES. | [115] | |
| TA-chelated Ag NPs | PAAc | The conductive hydrogels facilitated the earliest stage of myotube formation. | [208] | |
| Ppy NPs | GelMA/CS-catechol | The conductive hydrogel could regulate cellular behavior and benefit better integration and growth with tissues. | [209] | |
| Ti3C2Tx MXene@CeO2 nanocomposites | Polyethyleneimine grafted-PF127/oxidized SA | The conductive hydrogel is beneficial to the proliferation and migration of fibroblasts under the ES. | [210] | |
| Photo-responsiveness | ZnO QDs@GO | CS | ROS, and the Zn2+ released from ZnO QDs under acid environment killed the bacteria. | [211] |
| Cu, N-doped carbon dots (Cu, N-CDs)@GO NSs | CS | The hydrogel could absorb 808 nm light and convert the energy into thermal energy due to the photothermal effects of Cu, N-CDs, and GO NSs. | [212] | |
| UiO-66-NH2 MOF NPs | CS | The MOF could produce active oxygen (·OH) by pre-UV-irradiation or in the presence of a trace amount of H2O2 to kill the bacteria. | [213] | |
| berberine chloride NPs | N-(9-fluorenylmethoxycarbonyl)-L-phenylalanine | The hydrogel exhibited AIE behavior and 1O2 generation under white light, and kill bacteria via a PDT mechanism, and in turn penetrate and eradicate biofilms. | [214] | |
| black phosphorus QDs (BPQDs) | PVA/Alg | BPQDs were photo responsive, ROS-generating, and antibacterial, which could promote the MRSA-infected wound healing. | [215] | |
| catechol-modified CS-derived carbonized polymer dots (CPDs) | PVA | The PVA@CPDs hydrogel could reach the desired temperature quickly under irradiation, thus efficiently killing the bacteria and preventing overheating of normal tissues. | [216] | |
| PANI NPs | PAM | The hydrogel could convert light energy into heat upon NIR irradiation and be used as a photothermal antibacterial material. | [45] | |
| Ppy NTs | quaternized CS-graft-β-cyclodextrin, adenine | The PTT enhanced wound healing by promoting collagen deposition. | [217] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Su, G.; Zhang, R.; Dai, R.; Li, Z. Nanomaterials-Functionalized Hydrogels for the Treatment of Cutaneous Wounds. Int. J. Mol. Sci. 2023, 24, 336. https://doi.org/10.3390/ijms24010336
Liu Y, Su G, Zhang R, Dai R, Li Z. Nanomaterials-Functionalized Hydrogels for the Treatment of Cutaneous Wounds. International Journal of Molecular Sciences. 2023; 24(1):336. https://doi.org/10.3390/ijms24010336
Chicago/Turabian StyleLiu, Yangkun, Gongmeiyue Su, Ruoyao Zhang, Rongji Dai, and Zhao Li. 2023. "Nanomaterials-Functionalized Hydrogels for the Treatment of Cutaneous Wounds" International Journal of Molecular Sciences 24, no. 1: 336. https://doi.org/10.3390/ijms24010336
APA StyleLiu, Y., Su, G., Zhang, R., Dai, R., & Li, Z. (2023). Nanomaterials-Functionalized Hydrogels for the Treatment of Cutaneous Wounds. International Journal of Molecular Sciences, 24(1), 336. https://doi.org/10.3390/ijms24010336






