Establishment of an Absolute Quantitative Method to Detect a Plasma tRNA-Derived Fragment and Its Application in the Non-Invasive Diagnosis of Gastric Cancer
Abstract
1. Introduction
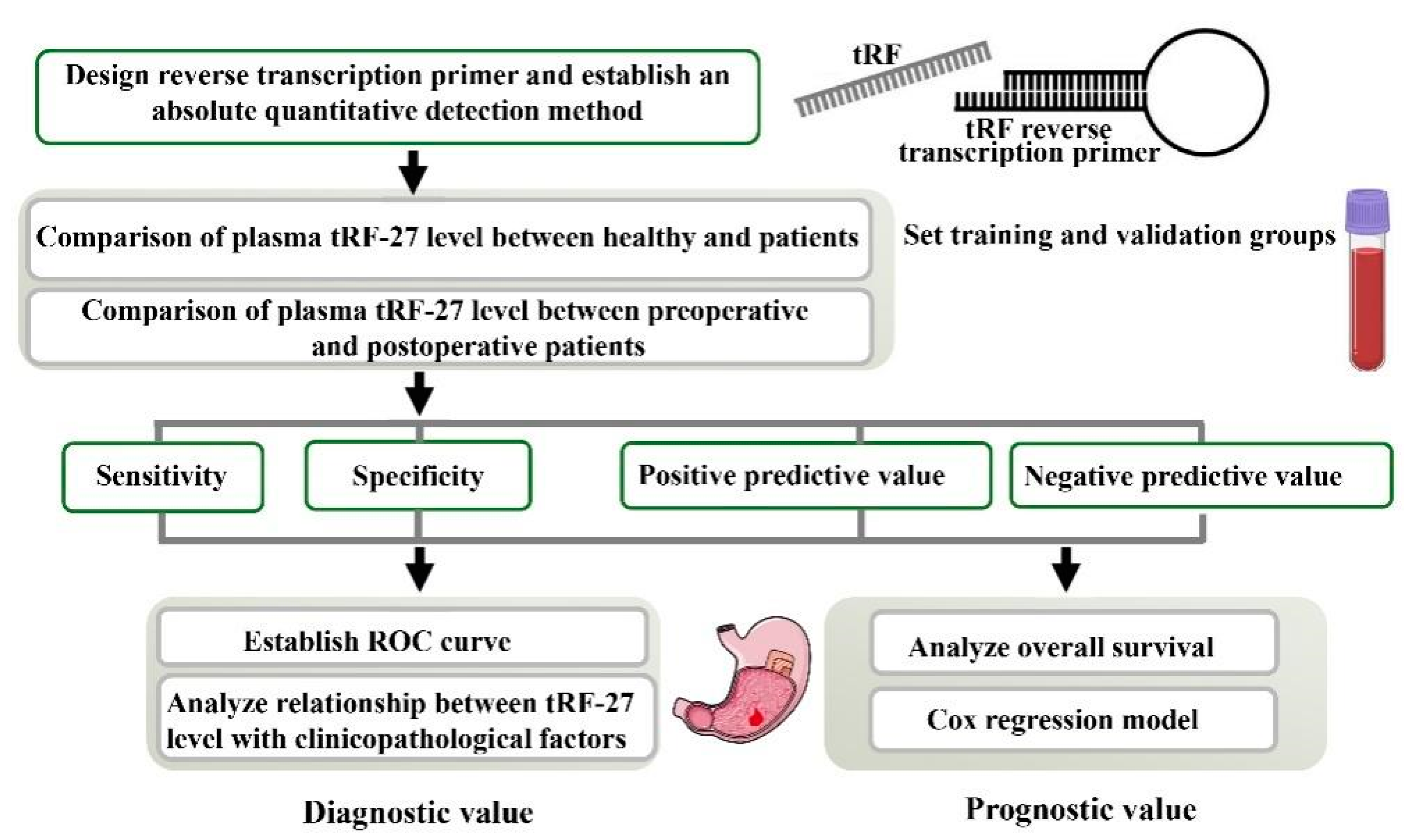
2. Results
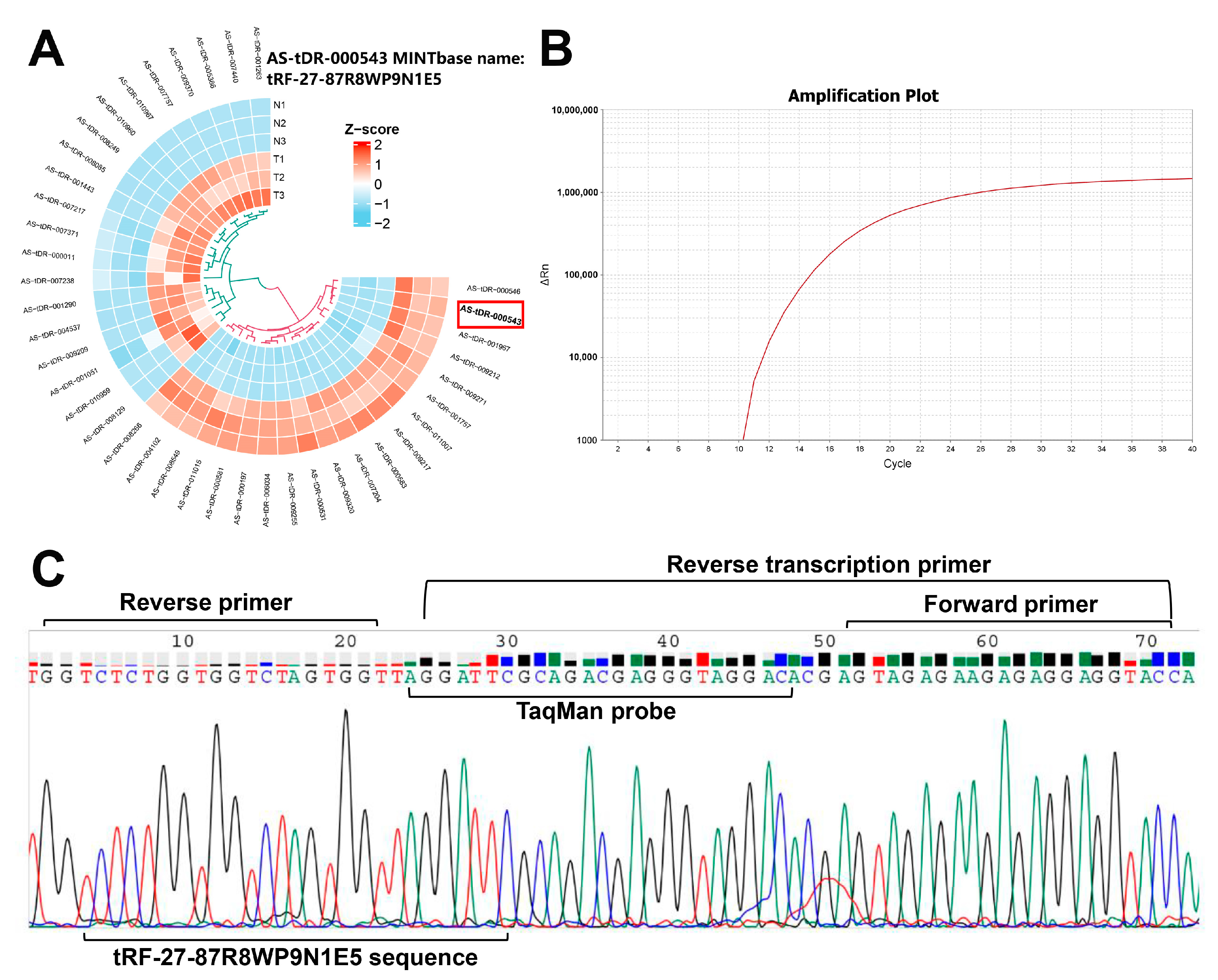
2.1. Successful Design of tRF-27-Specific Reverse Transcription Primer, TaqMan Probe, and Amplification Primers
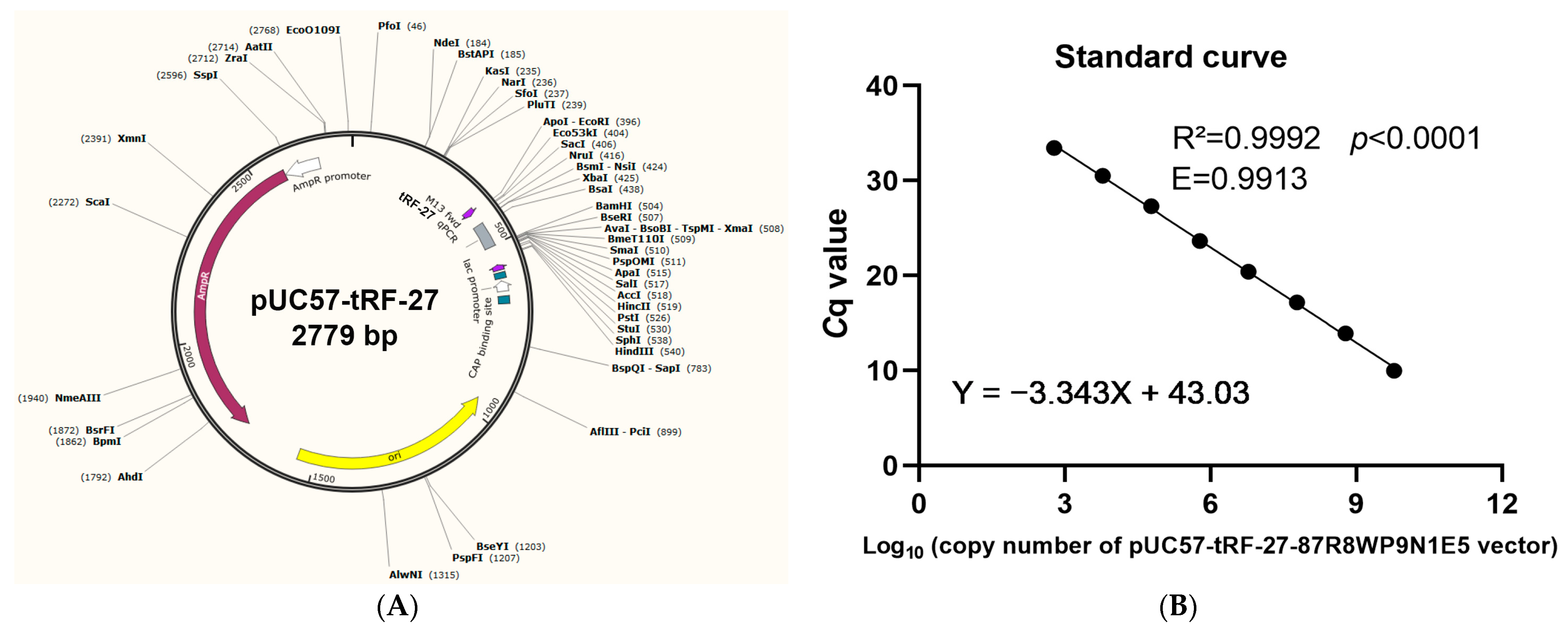
2.2. Establishment of an Absolute Quantitative Method to Detect tRF-27 in the Plasma
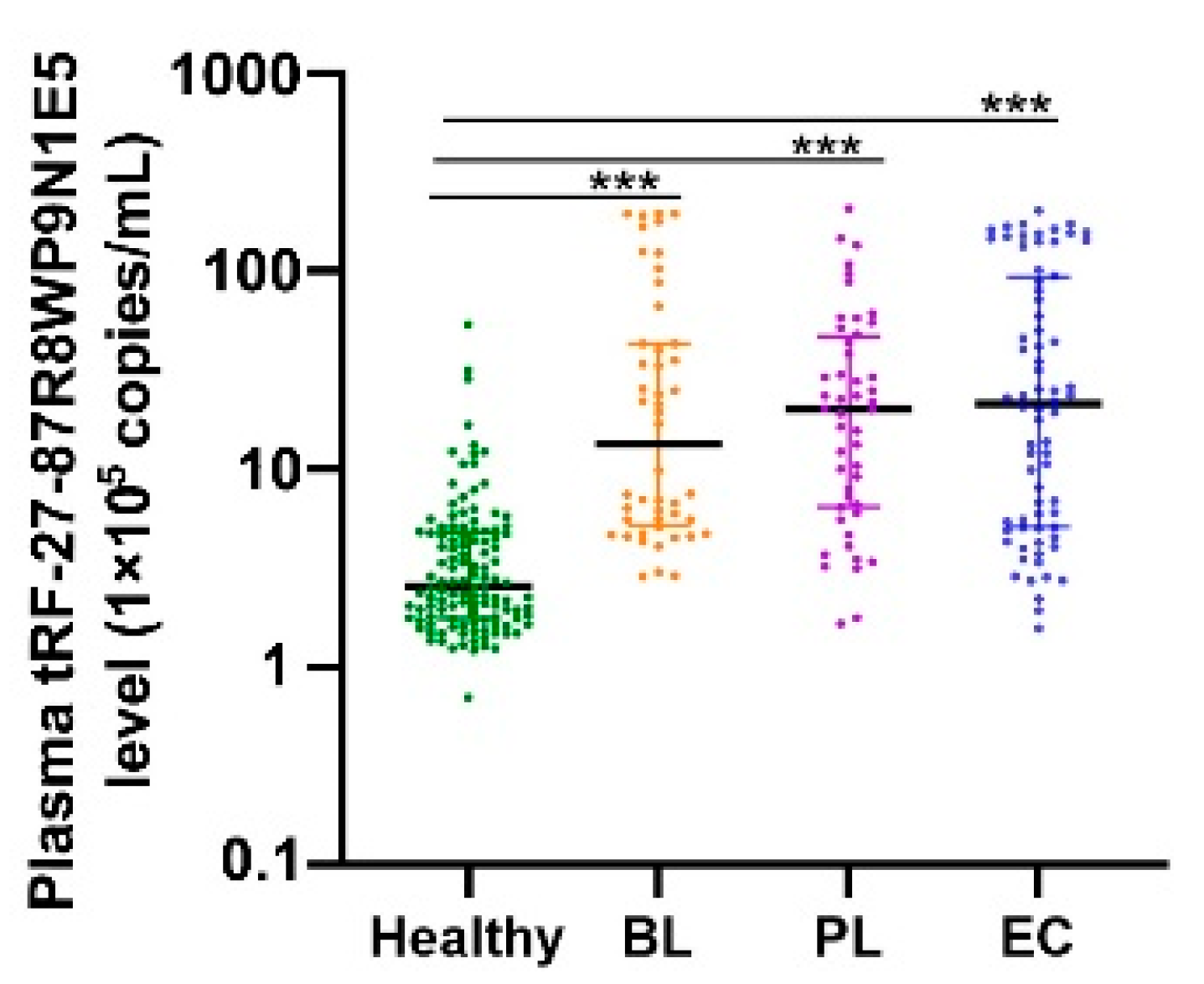
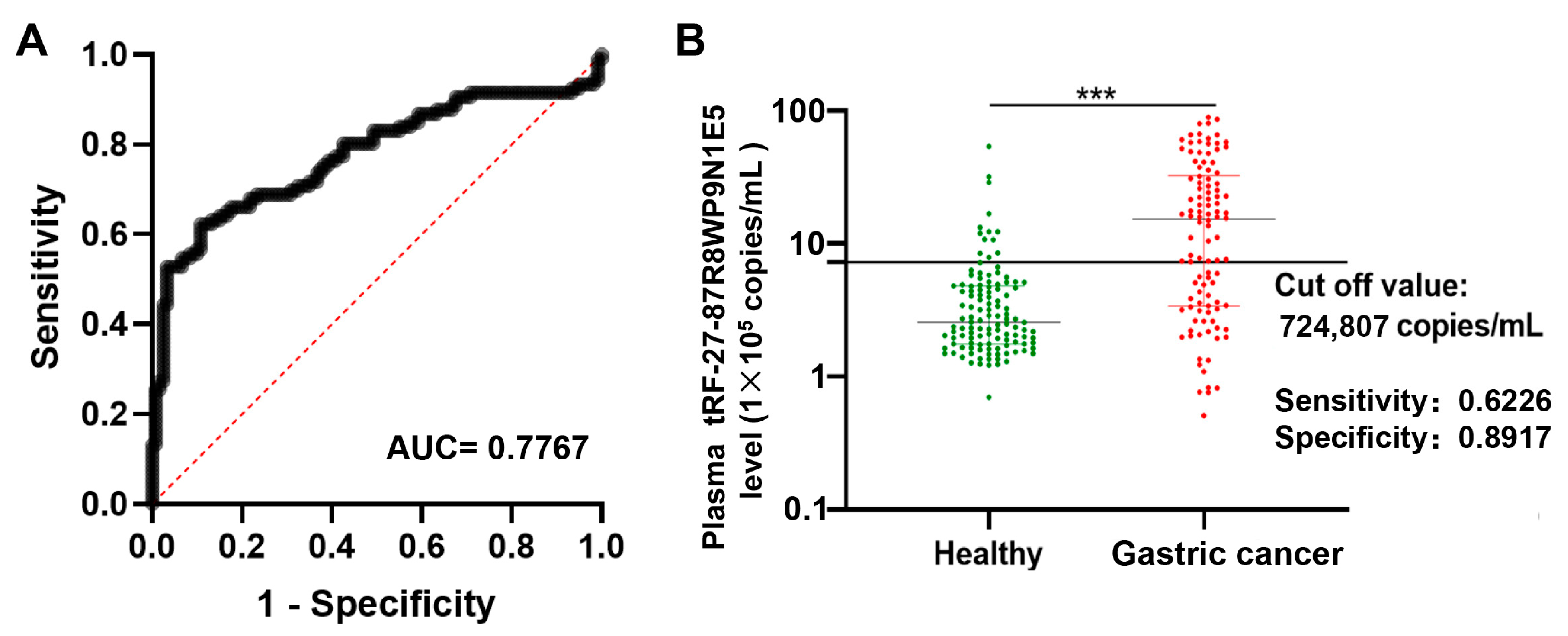
2.3. Plasma tRF-27 Levels Are Increased in Early Gastric Cancer Patients Compared to Those in Healthy Individuals
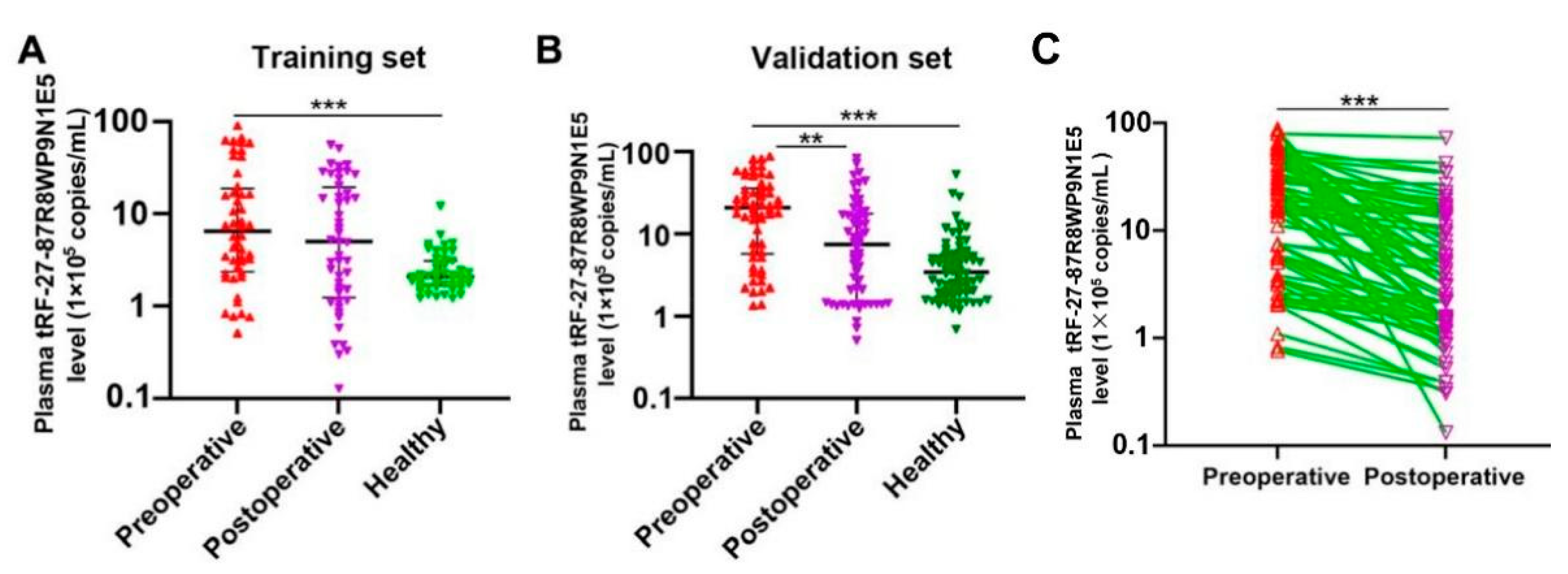
2.4. Plasma tRF-27 Levels Are Significantly Decreased in Postoperative Patients Compared to Preoperative Patients, and Tend to the Level of Healthy Individuals
2.5. tRF-27 Has Good Noninvasive Diagnostic Value in Gastric Cancer
2.6. Plasma tRF-27 Levels Are Closely Related to Tumor Size and Ki67 Expression in Preoperative Gastric Cancer Patients
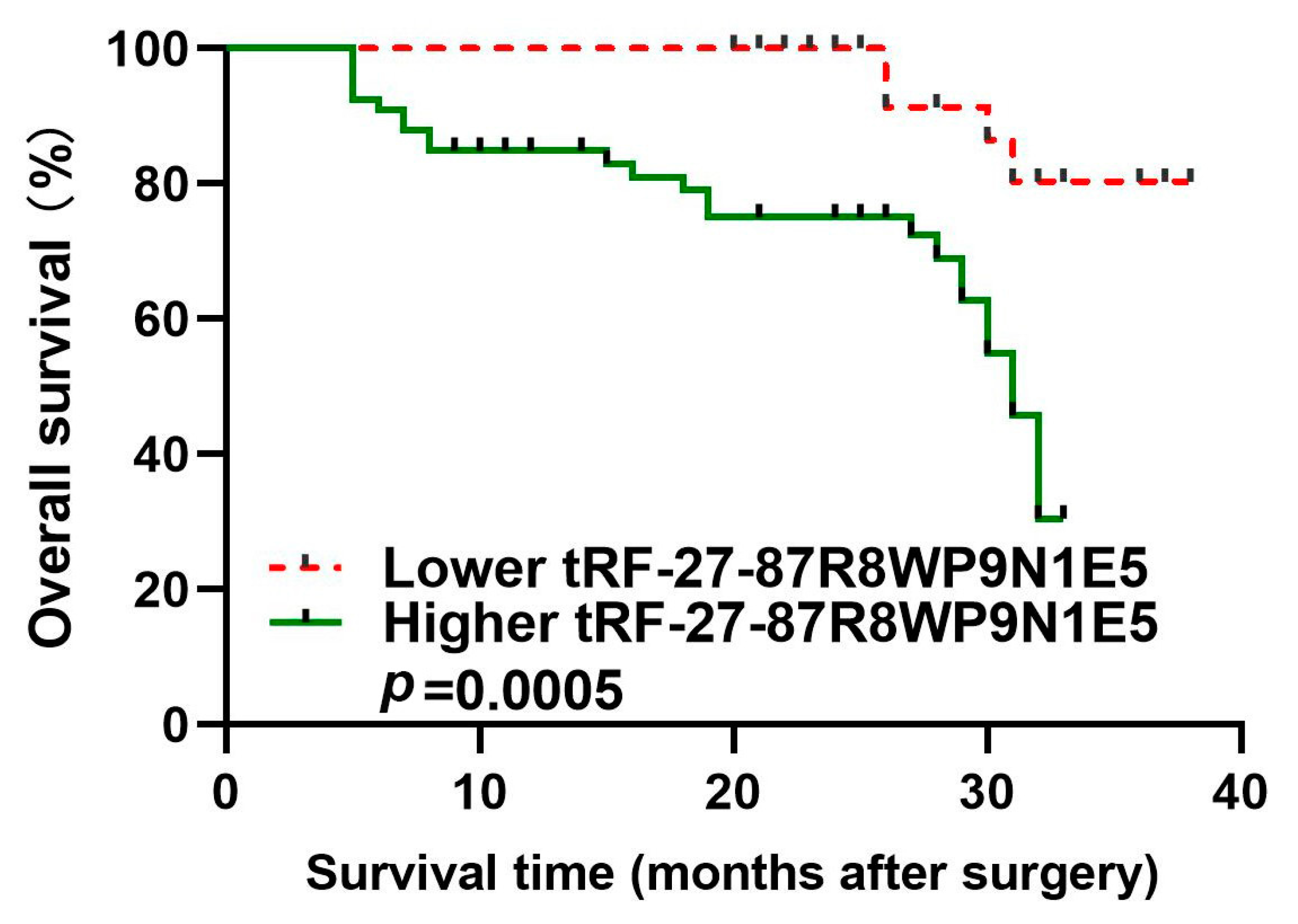
2.7. Prognostic Value of tRF-27 in Gastric Cancer Patients
2.8. Feasibility of tRF-27 as an Independent Predictor of Prognosis
3. Discussion
4. Materials and Methods
4.1. Design of a Specific Stem-Loop-Structure Reverse Transcription Primer of tRF-27
4.2. Design of a TaqMan Probe and Amplification Primers for qRT-PCR Detection
4.3. Establishment of an Absolute Quantitative Method to Measure Plasma tRF-27 Levels
4.4. Detection of tRF-27 Levels in Patients with Benign and Malignant Gastric Lesions
4.5. Investigation of Training and Validation Sets
4.6. Construction of a Receiver Operating Characteristic Curve to Evaluate the Diagnostic Value of tRF-27 in Gastric Cancer
4.7. Analysis of Clinicopathological Data and Construction of a Survival Curve
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Shen, Y.; Xie, Y.; Yu, X.; Zhang, S.; Wen, Q.; Ye, G.; Guo, J. Clinical Diagnostic Values of Transfer Rna-Derived Fragment Trf-19-3l7l73jd and Its Effects on The Growth of Gastric Cancer Cells. J. Cancer 2021, 12, 3230–3238. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Najafi, M.; Ang, H.L.; Moghadam, E.R.; Mahabady, M.K.; Zabolian, A.; Jafaripour, L.; Bejandi, A.K.; Hushmandi, K.; Saleki, H.; et al. PTEN, a Barrier for Proliferation and Metastasis of Gastric Cancer Cells: From Molecular Pathways to Targeting and Regulation. Biomedicines 2020, 8, 264. [Google Scholar] [CrossRef]
- Abadi, A.J.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Najafi, M.; Entezari, M.; Hushmandi, K.; Aref, A.R.; Khan, H.; Makvandi, P.; et al. The Role of Sox Family Transcription Factors in Gastric Cancer. Int. J. Biol. Macromol. 2021, 180, 608–624. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current Treatment and Recent Progress in Gastric Cancer. CA A Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Ruan, Y.; Li, Z.; Xie, Y.; Yan, Z.; Guo, J. Global Profile of Trna-Derived Small Rnas in Gastric Cancer Patient Plasma and Identification of tRF-33-P4R8YP9LON4VDP as a New Tumor Suppressor. Int. J. Med. Sci. 2021, 18, 1570–1579. [Google Scholar] [CrossRef]
- Abrahami, D.; McDonald, E.G.; Schnitzer, M.E.; Barkun, A.N.; Suissa, S.; Azoulay, L. Proton Pump Inhibitors and Risk of Gastric Cancer: Population-Based Cohort Study. Gut 2022, 71, 16–24. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kim, Y.I.; Kook, M.C.; Park, B.; Joo, J. Family History of Gastric Cancer and Helicobacter pylori Treatment. New Engl. J. Med. 2020, 382, 427–436. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is Gastric Cancer Becoming a Rare Disease? A Global Assessment of Predicted Incidence Trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Hashimoto, I.; Oshima, T. Claudins and Gastric Cancer: An Overview. Cancers 2022, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Ruan, Y.; Sun, W.; Yu, R. Low Expression of hsa_circ_0001811 in Gastric Cancer and Its Role in Clinical Diagnosis. J. Clin. Lab. Anal. 2021, 35, e23642. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, J.; Oshima, Y.; Nanami, T.; Suzuki, T.; Yajima, S.; Shiratori, F.; Funahashi, K.; Shimada, H. Prognostic Impact of CEA/CA19-9 at the Time of Recurrence in Patients With Gastric Cancer. Surg. Today 2021, 51, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Z.Q.; Xu, Q.; Goyal, H.; Xu, H.G. Development and Validation of Novel Nomograms Using Serum Tumor Markers for the Prediction of Preoperative Histologic Grades in Gastroenteropancreatic Neuroendocrine Tumors. Front. Oncol. 2021, 11, 681149. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xie, Y.; Zhang, S.; Song, X.; Xiao, B.; Yan, Z. tRNA-Derived Fragments: Mechanisms Underlying Their Regulation of Gene Expression and Potential Applications as Therapeutic Targets in Cancers and Virus Infections. Theranostics 2021, 11, 461–469. [Google Scholar] [CrossRef]
- Xie, Y.; Yao, L.; Yu, X.; Ruan, Y.; Li, Z.; Guo, J. Action Mechanisms and Research Methods of tRNA-Derived Small RNAs. Signal Transduct. Target. Ther. 2020, 5, 109. [Google Scholar] [CrossRef]
- Zhu, L.; Ge, J.; Li, T.; Shen, Y.; Guo, J. tRNA-Derived Fragments and tRNA Halves: The New Players in Cancers. Cancer Lett. 2019, 452, 31–37. [Google Scholar] [CrossRef]
- Di Fazio, A.; Schlackow, M.; Pong, S.K.; Alagia, A.; Gullerova, M. Dicer Dependent tRNA Derived Small RNAs Promote nascent RNA Silencing. Nucleic Acids Res. 2022, 50, 1734–1752. [Google Scholar] [CrossRef]
- Mo, D.; He, F.; Zheng, J.; Chen, H.; Tang, L.; Yan, F. tRNA-Derived Fragment tRF-17-79MP9PP Attenuates Cell Invasion and Migration via THBS1/TGF-β1/Smad3 Axis in Breast Cancer. Front. Oncol. 2021, 11, 656078. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Q.; Zhou, Y. Screening and Comprehensive Analysis of Cancer-Associated tRNA-Derived Fragments. Front. Genet. 2021, 12, 747931. [Google Scholar] [CrossRef]
- Zhu, L.; Li, T.; Shen, Y.; Yu, X.; Xiao, B.; Guo, J. Using tRNA Halves as Novel Biomarkers for the Diagnosis of Gastric Cancer. Cancer Biomark. Sect. A Dis. Markers 2019, 25, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Cui, W.; Xie, Q.; Peng, W.; Zhang, H.; Gao, Y.; Zhang, C.; Duan, C. Plasma tRNA-Derived Small RNAs Signature as a Predictive and Prognostic Biomarker in Lung Adenocarcinoma. Cancer Cell Int. 2022, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, C.; Fang, L.; Yu, W. Serum Transfer RNA-Derived Fragment tRF-31-79MP9P9NH57SD Acts as a Novel Diagnostic Biomarker for Non-Small Cell Lung Cancer. J. Clin. Lab. Anal. 2022, 36, e24492. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, Z.; Cai, H.; Peng, Y.; Yang, L.; Wang, Z. Identifying Differentially Expressed tRNA-Derived Small Fragments as a Biomarker for the Progression and Metastasis of Colorectal Cancer. Dis. Markers 2022, 2022, 2646173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Z.; Yu, X.; Ruan, Y.; Shen, Y.; Shao, Y.; Zhang, X.; Ye, G.; Guo, J. The tRNA-Derived Fragment 5026a Inhibits the Proliferation of Gastric Cancer Cells by Regulating the PTEN/PI3K/AKT Signaling Pathway. Stem Cell Res. Ther. 2021, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Wang, Y.; Xie, Y.; Yu, X.; Zhang, S.; Ge, J.; Li, Z.; Ye, G.; Guo, J. Extracellular Vesicles-Associated tRNA-Derived Fragments (tRFs): Biogenesis, Biological Functions, and Their Role as Potential Biomarkers in Human Diseases. J. Mol. Med. 2022, 100, 679–695. [Google Scholar] [CrossRef]
- Loher, P.; Telonis, A.G.; Rigoutsos, I. MINTmap: Fast and Exhaustive Profiling of Nuclear and Mitochondrial tRNA Fragments from Short RNA-seq Data. Sci. Rep. 2017, 7, 41184. [Google Scholar] [CrossRef]
- Loher, P.; Telonis, A.G.; Rigoutsos, I. Accurate Profiling and Quantification of tRNA Fragments from RNA-Seq Data: A Vade Mecum for MINTmap. Methods Mol. Biol. 2018, 1680, 237–255. [Google Scholar] [CrossRef]
- Karousi, P.; Adamopoulos, P.G.; Papageorgiou, S.G.; Pappa, V.; Scorilas, A.; Kontos, C.K. A Novel, Mitochondrial, Internal tRNA-Derived RNA Fragment Possesses Clinical Utility as a Molecular Prognostic Biomarker in Chronic Lymphocytic Leukemia. Clin. Biochem. 2020, 85, 20–26. [Google Scholar] [CrossRef]
- Huang, L.T.; Cui, M.; Silva, M.; Okuda, K.; Shimada, Y.; Wang, J.H.; Wang, Y.B. Expression Profiles of tRNA-Derived Fragments and Their Potential Roles in Lung Adenocarcinoma. Ann. Transl. Med. 2022, 10, 196. [Google Scholar] [CrossRef]
- Li, J.; Jin, L.; Gao, Y.; Gao, P.; Ma, L.; Zhu, B.; Yin, X.; Sui, S.; Chen, S.; Jiang, Z.; et al. Low Expression of tRF-Pro-CGG Predicts Poor Prognosis in Pancreatic Ductal Adenocarcinoma. J. Clin. Lab. Anal. 2021, 35, e23742. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Moradi, N.; Ohadian Moghadam, S.; Heidarzadeh, S. Application of Next-Generation Sequencing in the Diagnosis of Gastric Cancer. Scand. J. Gastroenterol. 2022, 57, 842–855. [Google Scholar] [CrossRef]
- Tao, X.; Shao, Y.; Yan, J.; Yang, L.; Ye, Q.; Wang, Q.; Lu, R.; Guo, J. Biological Roles and Potential Clinical Values of Circular RNAs in Gastrointestinal Malignancies. Cancer Biol. Med. 2021, 18, 437–457. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, Z.; Ma, D.; Guo, J.; Sun, W. Hsa_circ_0003195 as a Biomarker for Diagnosis and Prognosis of Gastric Cancer. Int. J. Clin. Oncol. 2022, 27, 354–361. [Google Scholar] [CrossRef]
- Shao, Y.; Qi, C.; Yan, J.; Lu, R.; Ye, G.; Guo, J. Biological and Clinical Implications of hsa_circ_0086720 in Gastric Cancer and Its Clinical Application. J. Clin. Lab. Anal. 2022, 36, e24369. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Chen, X.; Li, G. Prognostic Factors in the Treatment of Gastric Mucosal Atypical Hyperplasia by Endoscopic Submucosal Dissection. BMC Surg. 2022, 22, 382. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n (%) | High (%) | Low (%) | p-Value |
|---|---|---|---|---|
| All cases | 106 (100) | 66 (62.3) | 40 (37.7) | |
| Gender | 0.987 | |||
| Male | 69 (65.1) | 43 (65.2) | 26 (65.0) | |
| Female | 37 (34.9) | 23 (34.8) | 14 (35.0) | |
| Age (y) | 0.758 | |||
| <60 | 31 (29.2) | 20 (30.3) | 11 (27.5) | |
| ≥60 | 75 (70.8) | 46 (69.7) | 29 (72.5) | |
| Tumor size (cm) | 0.026 | |||
| <5 | 71 (67.0) | 39 (59.1) | 32 (80.0) | |
| ≥5 | 35 (33.0) | 27 (40.9) | 8 (20.0) | |
| Differentiation | 0.671 | |||
| Poor | 77 (72.6) | 47 (71.2) | 30 (75.0) | |
| Moderate–Well | 29 (27.4) | 19 (28.8) | 10 (25.0) | |
| TNM stage | 0.402 | |||
| 0 and I | 35 (33.0) | 21 (31.8) | 14 (35.0) | |
| II | 22 (20.8) | 17 (25.8) | 5 (12.5) | |
| II | 41 (38.7) | 22 (33.3) | 19 (47.5) | |
| IV | 8 (7.5) | 6 (9.1) | 2 (5.0) | |
| T stage | 0.191 | |||
| Tis and T1 | 29 (27.4) | 17 (25.8) | 12 (30.0) | |
| T2 and T3 | 20 (18.9) | 16 (24.2) | 4 (10.0) | |
| T4 | 57 (53.7) | 33 (50.0) | 24 (60.0) | |
| Lymphatic metastasis | 0.691 | |||
| N0 and N1 | 61 (57.5) | 37 (56.1) | 24 (60.0) | |
| N2 and N3 | 45 (42.5) | 29 (43.9) | 16 (40.0) | |
| Distal metastasis | 0.439 | |||
| M0 | 98 (92.4) | 60 (90.9) | 38 (95.0) | |
| M1 | 8 (7.6) | 6 (9.1) | 2 (5.0) | |
| CEA | 0.845 | |||
| Negative | 91 (85.8) | 57 (86.4) | 34 (85.0) | |
| Positive | 15 (14.2) | 9 (13.6) | 6 (15.0) | |
| CA19-9 | 0.339 | |||
| Negative | 91 (85.8) | 55 (83.3) | 36 (90.0) | |
| Positive | 15 (14.2) | 11 (16.7) | 4 (10.0) | |
| Ki 67 (%) | 0.005 | |||
| <50 | 38 (35.8) | 17 (25.8) | 21 (52.5) | |
| ≥50 | 68 (64.2) | 49 (74.2) | 19 (47.5) |
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| Univariate analysis | |||
| Gender (Male vs. Female) | 0.542 | 0.216–1.362 | 0.193 |
| Age (<60 y vs. ≥60 y) | 1.120 | 0.483–2.598 | 0.791 |
| Tumor size (<5 cm vs. ≥5 cm) | 0.508 | 0.230–1.122 | 0.094 |
| Differentiation (Poor vs. Moderate–Well) | 0.677 | 0.269–1.700 | 0.406 |
| TNM stage (0 and I vs. II vs. III vs. IV) | 1.442 | 0.969–2.146 | 0.071 |
| Invasion depth (Tis and T1 vs. T2 and T3 vs. T4) | 1.587 | 0.956–2.637 | 0.074 |
| Lymphatic metastasis (N0 and N1 vs. N2 and N3) | 0.455 | 0.204–1.013 | 0.054 |
| Distal metastasis (M0 vs. M1) | 0.557 | 0.166–1.864 | 0.342 |
| CEA (Negative vs. Positive) | 1.037 | 0.354–3.039 | 0.947 |
| CA19-9 (Negative vs. Positive) | 0.430 | 0.168–1.103 | 0.079 |
| Ki 67 (<50% vs. ≥50%) | 0.491 | 0.196–1.230 | 0.129 |
| Expression of tRF-27 in plasma (High vs. Low) | 0.175 | 0.058–0.525 | 0.002 |
| Multivariate analysis | |||
| Expression of tRF-27 in plasma (High vs. Low) | 0.144 | 0.042–0.491 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Song, X.; Xie, Y.; Zhang, S.; Guo, J. Establishment of an Absolute Quantitative Method to Detect a Plasma tRNA-Derived Fragment and Its Application in the Non-Invasive Diagnosis of Gastric Cancer. Int. J. Mol. Sci. 2023, 24, 322. https://doi.org/10.3390/ijms24010322
Yu X, Song X, Xie Y, Zhang S, Guo J. Establishment of an Absolute Quantitative Method to Detect a Plasma tRNA-Derived Fragment and Its Application in the Non-Invasive Diagnosis of Gastric Cancer. International Journal of Molecular Sciences. 2023; 24(1):322. https://doi.org/10.3390/ijms24010322
Chicago/Turabian StyleYu, Xiuchong, Xuemei Song, Yaoyao Xie, Shuangshuang Zhang, and Junming Guo. 2023. "Establishment of an Absolute Quantitative Method to Detect a Plasma tRNA-Derived Fragment and Its Application in the Non-Invasive Diagnosis of Gastric Cancer" International Journal of Molecular Sciences 24, no. 1: 322. https://doi.org/10.3390/ijms24010322
APA StyleYu, X., Song, X., Xie, Y., Zhang, S., & Guo, J. (2023). Establishment of an Absolute Quantitative Method to Detect a Plasma tRNA-Derived Fragment and Its Application in the Non-Invasive Diagnosis of Gastric Cancer. International Journal of Molecular Sciences, 24(1), 322. https://doi.org/10.3390/ijms24010322





