Involvement of Diamine Oxidase in Modification of Plasma Membrane Proton Pump Activity in Cucumis sativus L. Seedlings under Cadmium Stress
Abstract
1. Introduction
2. Results
2.1. Activities of DAO and PAO in Cucumber Roots
2.2. Activity of Plasma Membrane H+-ATPase in Roots of Cucumber Seedlings Treated with Cd and/or AG
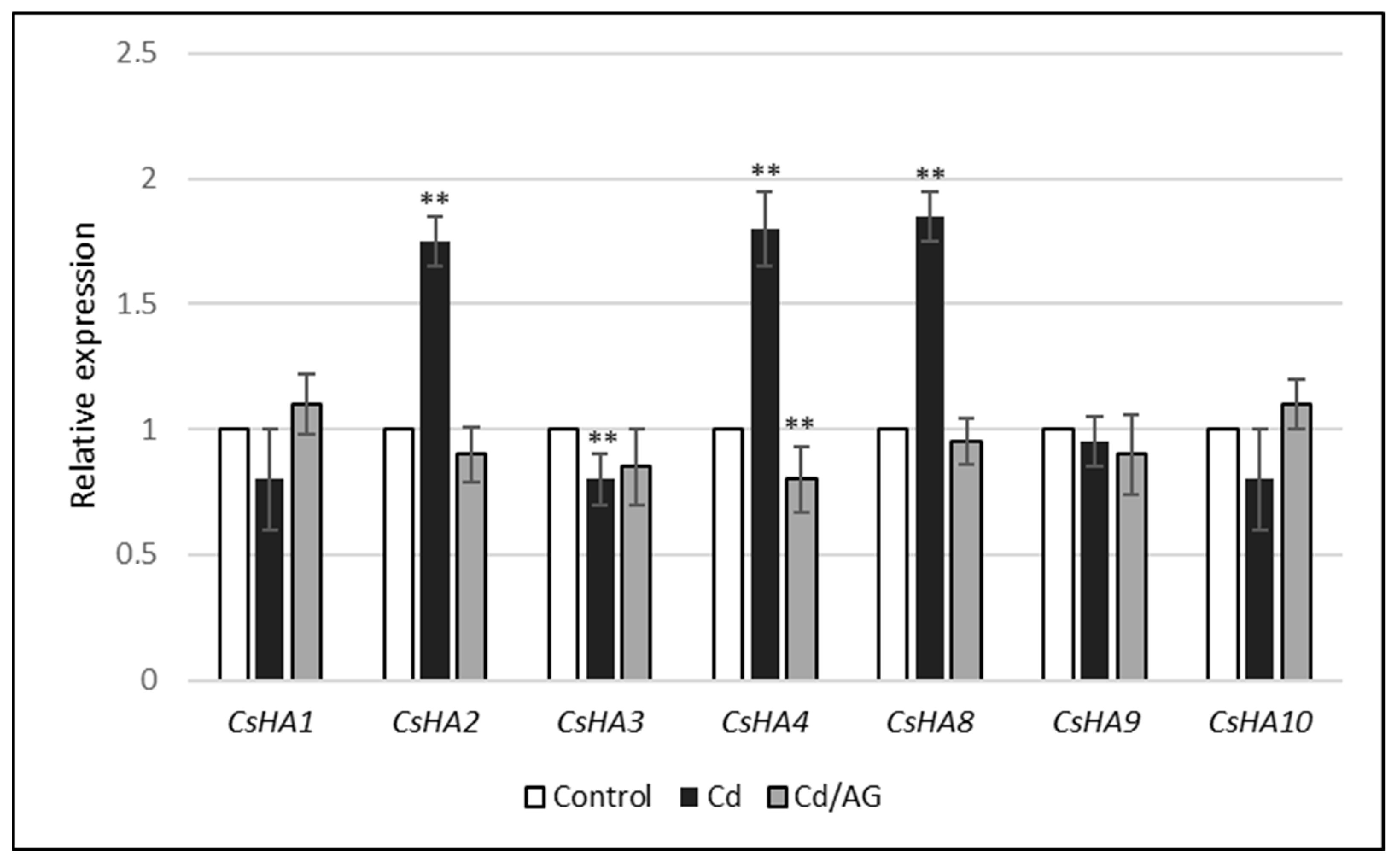
2.3. Effect of Cd and/or AG on the Expression Level of PM H+-ATPase Genes
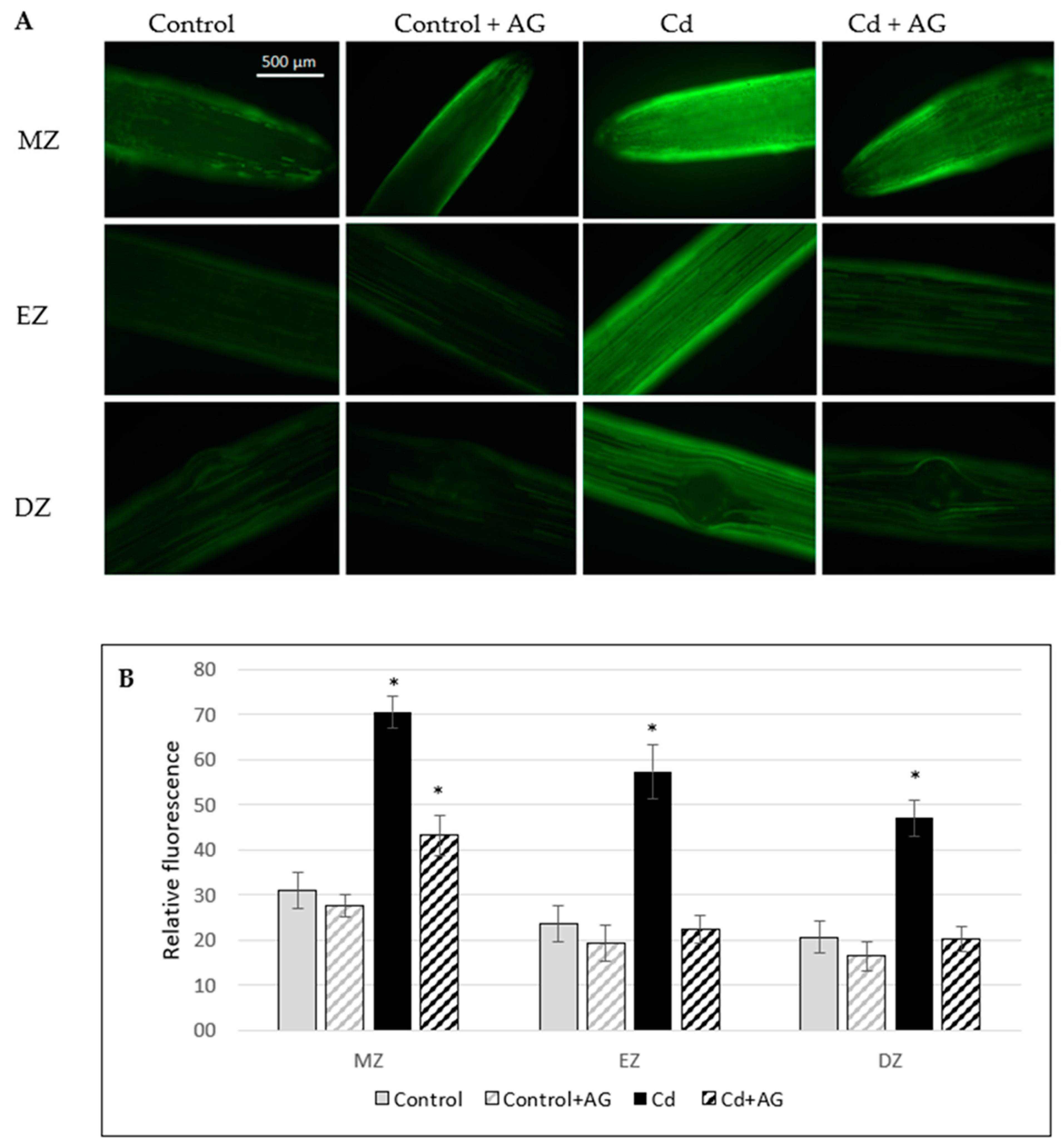
2.4. NO Level in Roots of Cucumber Seedlings Treated with Cd and/or AG
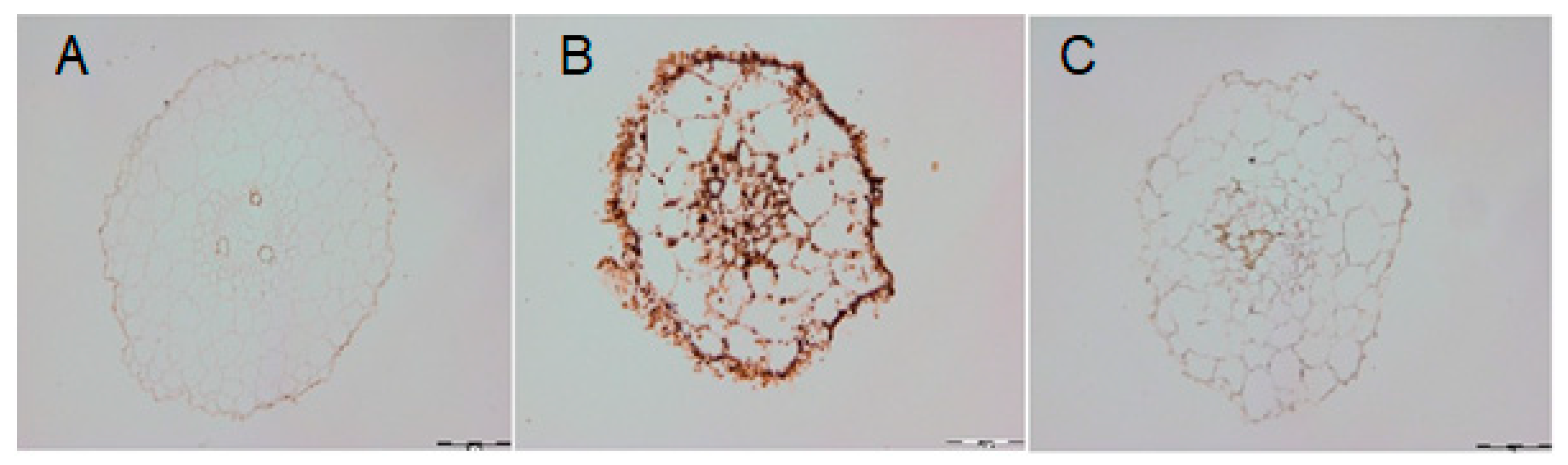
2.5. DAO-Induced H2O2 Level under Cadmium Stress
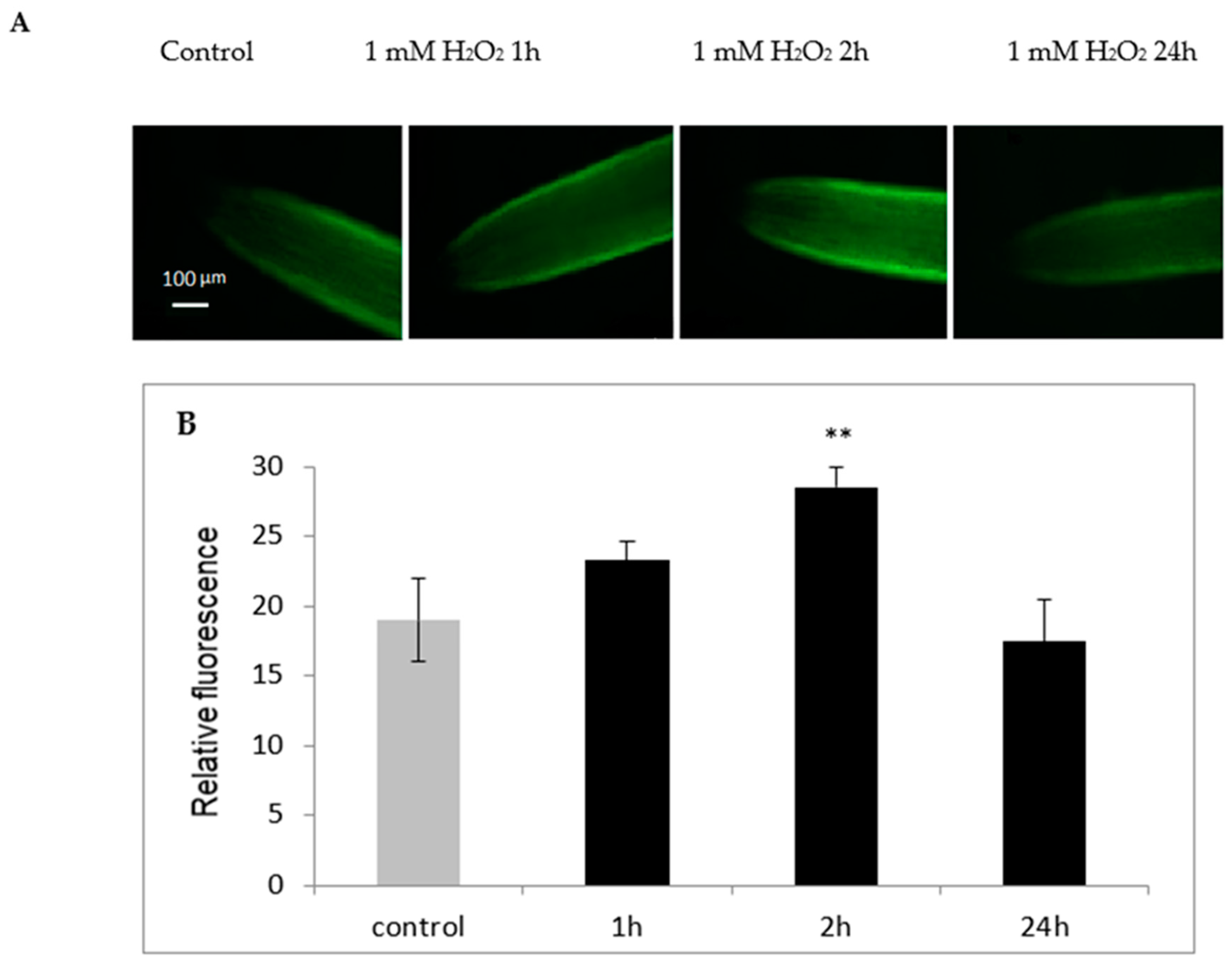
2.6. H2O2-Dependent NO Generation in the Roots of Cucumber Seedlings
3. Materials and Methods
3.1. Plant Material and Chemical Treatments
3.2. Assay of DAO and PAO
3.3. Plasma Membrane Isolation and PM H+-ATPase Activity Determination
3.4. Determination of Endogenous NO
3.5. Histochemical Detection of H2O2 in Cucumber Roots
3.6. Protein Determination
3.7. RNA Isolation and Analysis of Transcript Levels
3.8. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fancy, N.N.; Bahlmann, A.K.; Gary, J.; Loake, G.J. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2017, 40, 462–472. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Ma, X.; Guo, L.H.Y.; Ren, Z.; Kuang, Z.; Zhang, X.; Zhang, Z. Analysis of potential strategies for cadmium stress tolerance revealed by transcriptome analysis of upland cotton. Sci. Rep. 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.G.; Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M.N.V. Iron-and manganese-assisted cadmium tolerance in Oryza sativa L.: Lowering of rhizotoxicity next to functional photosynthesis. Planta 2015, 241, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Shah, K. Evidences for suppression of cadmium induced oxidative stress in presence of sulphosalicylic acid in rice seedlings. Plant Growth Regul. 2015, 76, 99–110. [Google Scholar] [CrossRef]
- van Belleghem, F.; Cuypers, A.; Semane, B.; Smeets, K.; Vangronsveld, J.; d’Haen, J.; Valcke, R. Subcellular localization of cadmium in roots and leaves of Arabidopsis thaliana. New Phytol. 2007, 173, 495–508. [Google Scholar] [CrossRef]
- Ram, H.; Kaur, A.; Gandass, N.; Singh, S.; Desmukh, R.; Sonah, H.; Sharma, T.R. Molecular characterization and expression dynamics of MTP genes under various spatiotemporal stages and metal stress conditions in rice. PLoS ONE 2019, 14, e0217360. [Google Scholar] [CrossRef]
- Burzyński, M.; Kolano, E. In vivo and in vitro effects of copper and cadmium on the plasma membrane H+-ATPase from cucumber (Cucumis sativus L.) and maize (Zea mays L.) roots. Acta Physiol. Plant. 2003, 25, 39–45. [Google Scholar] [CrossRef]
- Kanczewska, J.; Marco, S.; Vandermeeren, C.; Maudoux, O.; Rigaud, J.L.; Boutry, M. Activation of the plant plasma membrane H+-ATPase by phosphorylation and binding of 14-3-3 proteins converts a dimmer into a hexamer. Proc. Natl. Acad. Sci. USA 2005, 102, 11675–11680. [Google Scholar] [CrossRef]
- Duby, G.; Boutry, M. The plant plasma membrane proton pump ATPase: A highly regulated P-type ATPase with multiple physiological roles. Pflug. Arch.—Eur. J. Physiol. 2009, 457, 645–655. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, M.; Zheng, H.; Hayashi, Y.; Zhu, Y.; Kinoshita, T. Molecular basis of plasma membrane H+-ATPase function and potential application in the agricultural production. Plant Physiol. Biochem. 2021, 168, 10–16. [Google Scholar] [CrossRef]
- Janicka-Russak, M. Plant plasma membrane H+-ATPase in adaptation of plants to abiotic stresses. In Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives; Shanker, A., Venkateswarlu, B., Eds.; InTech: Rijeka, Croatia, 2011; pp. 197–218. [Google Scholar]
- Wdowikowska, A.; Klobus, G. The plasma membrane proton pump gene family in cucumber. Acta Physiol. Plant. 2016, 38, 135. [Google Scholar] [CrossRef]
- Janicka, M.; Reda, M.; Czyżewska, K.; Kabała, K. Involvement of signalling molecules NO, H2O2 and H2S in modification of plasma membrane proton pump in cucumber roots subjected to salt or low temperature stress. Funct. Plant Biol. 2018, 45, 428–439. [Google Scholar] [CrossRef]
- Ahn, S.; Im, Y.; Chung, G.; Seong, K.; Cho, B. Sensitivity of plasma membrane H+-ATPase of cucumber root system in response to low root temperature. Plant Cell Rep. 2000, 19, 831–835. [Google Scholar] [CrossRef]
- Janicka-Russak, M.; Kabała, K.; Burzyński, M.; Kłobus, G. Response of plasma membrane H+-ATPase to heavy metal stress in Cucumis sativus roots. J. Exp. Bot. 2008, 59, 3721–3728. [Google Scholar] [CrossRef]
- Janicka-Russak, M.; Kabała, K.; Burzyński, M. Different effect of cadmium and copper on H+-ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J. Exp. Bot. 2012, 63, 4133–4142. [Google Scholar] [CrossRef]
- Surowy, T.; Boyer, J. Low water potentials affect expression of genes encoding vegetative storage proteins and plasma membrane proton ATPase in soybean. Plant Mol. Biol. 1991, 16, 252–262. [Google Scholar] [CrossRef]
- Ouffatole, M.; Arango, M.; Boutry, M. Identification and expression of tree new Nicotianaplumbaginifolia genes which encode isoforms of a plasma-membrane H+-ATPase, and one of which is induced by mechanical stress. Planta 2000, 210, 715–722. [Google Scholar] [CrossRef]
- Fodor, E.; Szabo-Nagy, A.; Erdei, L. The effects of cadmium on the fluidity and H+-ATPase activity of plasma membrane from sunflower and wheat roots. J. Plant Physiol. 1995, 147, 87–92. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defense. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Tavladoraki, P.; Cona, A.; Federico, R.; Tempera, G.; Viceconte, N.; Saccoccio, S.; Battaglia, V.; Toninello, A.; Agostinelli, E. Polyamine catabolism: Target for antiproliferative therapies in animals and stress tolerance strategies in plants. Amino Acids 2012, 42, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.; Paschalidis, K.; Roubelakis-Angelakis, K. Plant polyamine catabolism. Plant Signals Behav. 2008, 3, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Altabell, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef]
- Sujkowska-Rybkowska, M.; Borucki, W. Localization of hydrogen peroxide accumulation and diamine oxidase activity in pea root nodules under aluminum stress. Micron 2014, 57, 13–22. [Google Scholar] [CrossRef]
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveria, V.; Handro, W.; Floh, E.I.S.; Schere, G.F.E. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef]
- Groβ, F.; Rudolf, E.E.; Thiele, B.; Durner, J.; Astier, J. Copper amine oxidase 8 regulates arginine-dependent nitric oxide production in Arab. Thaliana. J. Exp. Bot. 2017, 68, 2149–2162. [Google Scholar]
- Gill, S.S.; Hasanuzzaman, M.; Nahar, K.; Macovei, A.; Tuteja, N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2013, 63, 254–261. [Google Scholar] [CrossRef]
- Holmsted, B.; Larsson, L.; Tham, R. Further studies on spectrometric method for the determination of amine oxidase activity. Biochim. Biophys. Acta 1961, 48, 182–186. [Google Scholar] [CrossRef]
- Federico, R.; Angelini, R. Distribution of polyamines and their related catabolic enzymes in etiolated and light-grown Leguminosae seedlings. Planta 1988, 173, 317–321. [Google Scholar] [CrossRef]
- Kłobus, G. The role of plasma membrane-bound activities in nitrate transport into sealed plasma membrane vesicles from Cucumis sativus L. roots. In Structure and Function of Roots; Developments in Plant and Soil Science; Baluška, F., Čiamporová, M., Gašparíková, O., Barlow, P.W., Eds.; Kulwer Academic Publisher: Dordrecht, The Netherlands, 1995; pp. 133–140. [Google Scholar]
- Gallahger, S.R.; Leonard, R.T. Effect of vanadate, molybdate and azide on membrane associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982, 70, 1335–1340. [Google Scholar] [CrossRef]
- Kłobus, G.; Buczek, J. The role of plasma membrane oxidoreductase activity in proton transport. J. Plant Physiol. 1995, 146, 103–107. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.Z.; Wei, Z.; Collinge, Y. Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principles of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lv, J.; Qi, J.; Shi, Q.; Shen, D.; Zhang, S.; Shao, G.; Li, H.; Sun, Z.; Weng, Y.; Shang, Y.; et al. Genetic diversity and population structure of cucumber (Cucumis sativus L.). PLoS ONE 2012, 7, e46919. [Google Scholar] [CrossRef]
- Serrano, R. Structure and function of plasma membrane ATPase. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 61–94. [Google Scholar] [CrossRef]
- Janicka-Russak, M.; Kabała, K.; Wdowikowska, A.; Kłobus, G. Response of plasma membrane H+-ATPase to low temperature in cucumber roots. J. Plant Res. 2012, 125, 291–300. [Google Scholar] [CrossRef]
- Jakubowska, D.; Janicka, M. The role of brassinosteroids in the regulation of the plasma membrane H+-ATPase and NADPH oxidase under cadmium stress. Plant Sci. 2017, 264, 37–47. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, D.; Clarke, A.; Hancock, J.T. Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol. 2002, 128, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Tsuichihara, N.; Etoh, T.; Iwai, S. Reactive oxygen species and nitric oxide are involved in ABA inhibition of stomatal opening. Plant Cell Environ. 2007, 30, 1320–1325. [Google Scholar] [CrossRef]
- Qiao, W.; Li, C.; Fan, L.M. Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ. Exp. Bot. 2014, 100, 84–93. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Yang, Y.; Wu, H.; Wang, D.; Liu, J. Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant Cell Environ. 2007, 30, 775–785. [Google Scholar] [CrossRef]
- Wimalasekera, R.; Villa, C.; Begum, T.; Schere, G.F.E. Copper Amine Oxidase1 (CuAO1) of Arabidopsis thaliana contributes to abscisic acid- and polyamine-induced nitric oxide biosynthesis and abscisic acid signal transduction. Mol. Plant 2011, 4, 663–678. [Google Scholar] [CrossRef] [PubMed]
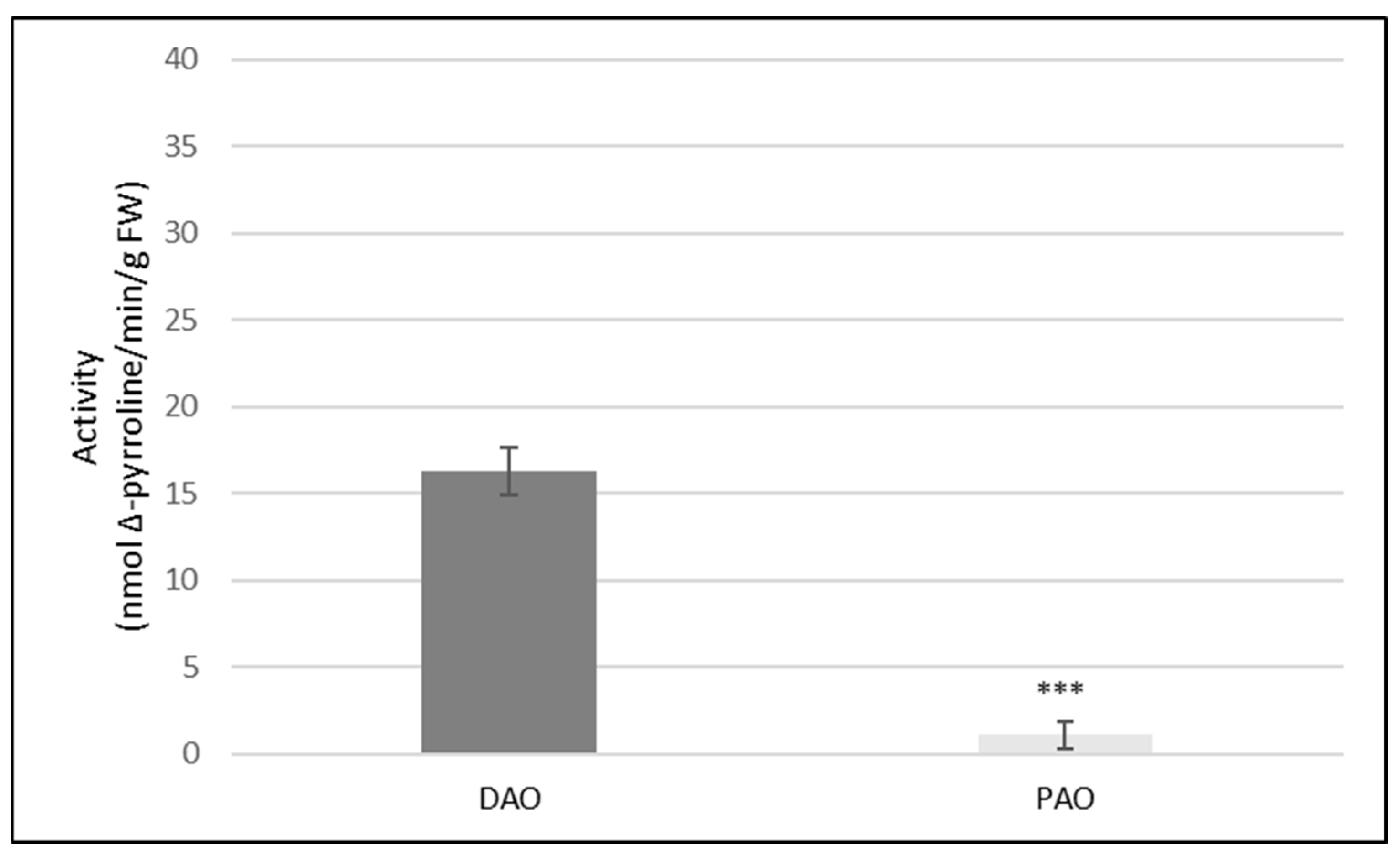
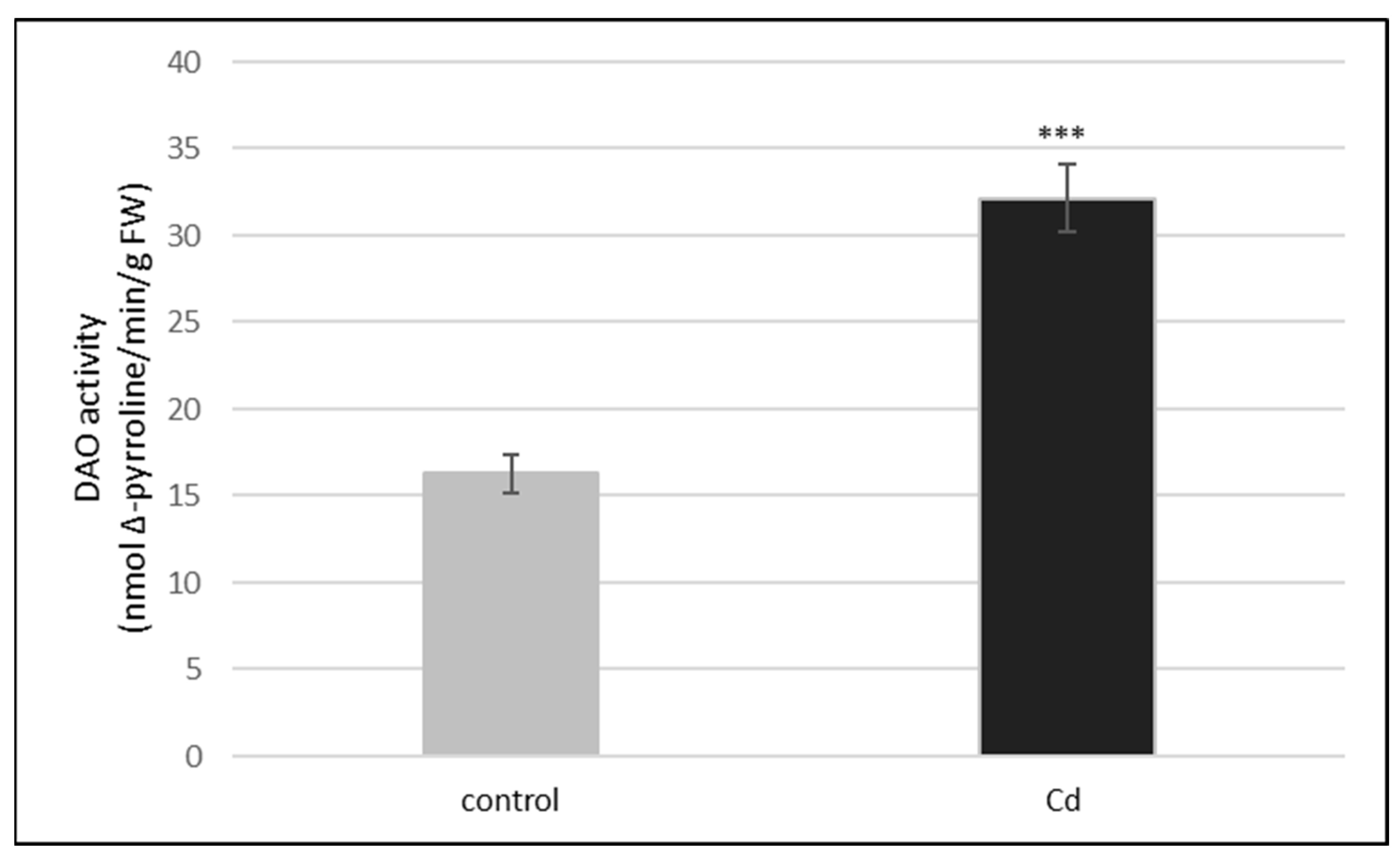
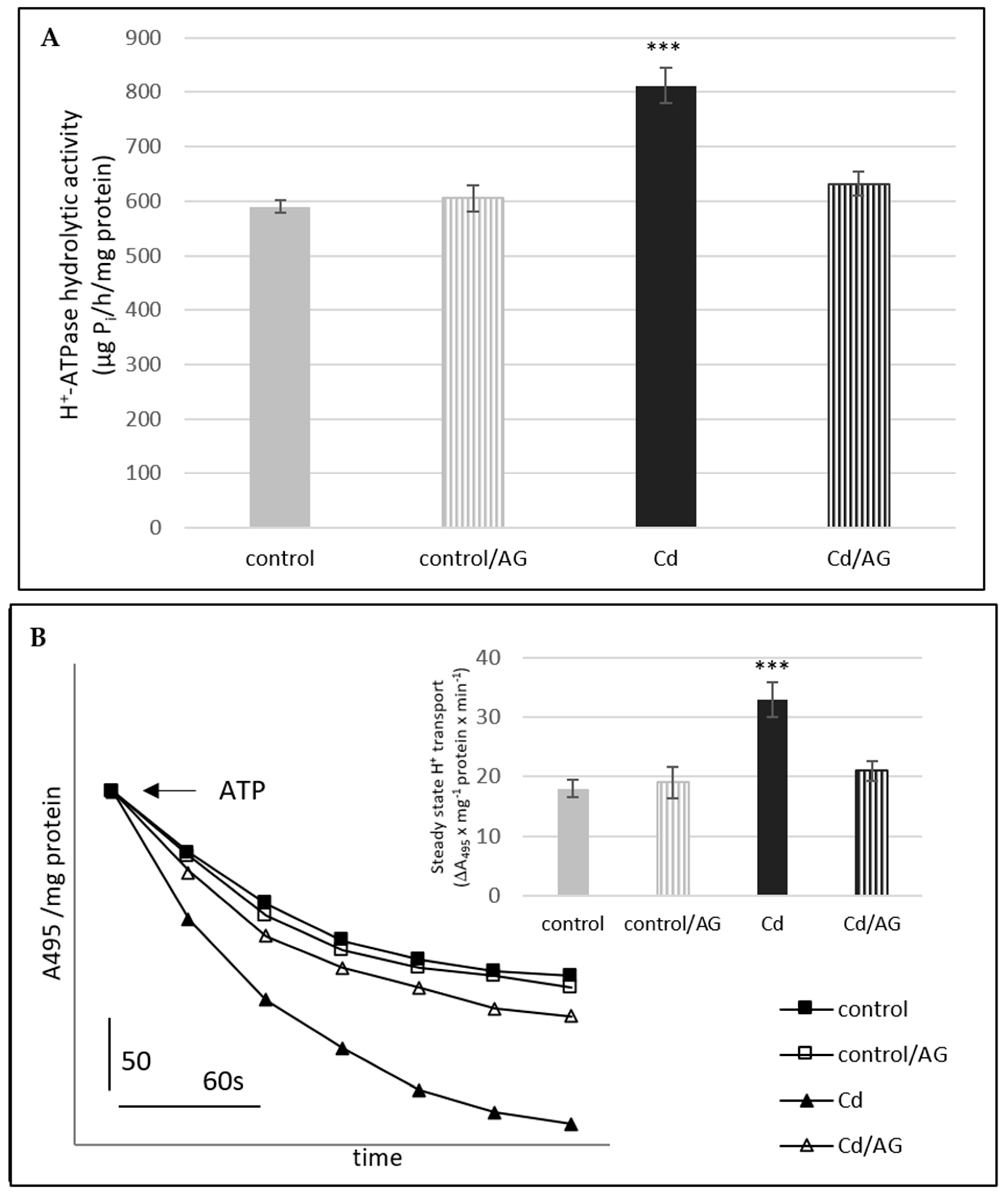
| DAO Activity (pmol Δ-Pyrroline/min/g FW) | ||
|---|---|---|
| −AG | +AG | |
| Control | 16.3 (±1.1) | 2.7 (±0.7) |
| Cd | 32.1 (±1.9) | 3.3 (±1.2) |
| Gene | Transcript Level (Fluorescence Units) |
|---|---|
| CsHA1 | 0.01 |
| CsHA2 | 14.23 |
| CsHA3 | 13.25 |
| CsHA4 | 0.83 |
| CsHA8 | 7.33 |
| CsHA9 | 2.17 |
| CsHA10 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janicka, M.; Reda, M.; Napieraj, N.; Michalak, A.; Jakubowska, D.; Kabała, K. Involvement of Diamine Oxidase in Modification of Plasma Membrane Proton Pump Activity in Cucumis sativus L. Seedlings under Cadmium Stress. Int. J. Mol. Sci. 2023, 24, 262. https://doi.org/10.3390/ijms24010262
Janicka M, Reda M, Napieraj N, Michalak A, Jakubowska D, Kabała K. Involvement of Diamine Oxidase in Modification of Plasma Membrane Proton Pump Activity in Cucumis sativus L. Seedlings under Cadmium Stress. International Journal of Molecular Sciences. 2023; 24(1):262. https://doi.org/10.3390/ijms24010262
Chicago/Turabian StyleJanicka, Małgorzata, Małgorzata Reda, Natalia Napieraj, Adrianna Michalak, Dagmara Jakubowska, and Katarzyna Kabała. 2023. "Involvement of Diamine Oxidase in Modification of Plasma Membrane Proton Pump Activity in Cucumis sativus L. Seedlings under Cadmium Stress" International Journal of Molecular Sciences 24, no. 1: 262. https://doi.org/10.3390/ijms24010262
APA StyleJanicka, M., Reda, M., Napieraj, N., Michalak, A., Jakubowska, D., & Kabała, K. (2023). Involvement of Diamine Oxidase in Modification of Plasma Membrane Proton Pump Activity in Cucumis sativus L. Seedlings under Cadmium Stress. International Journal of Molecular Sciences, 24(1), 262. https://doi.org/10.3390/ijms24010262






