Libanoridin Isolated from Corydalis heterocarpa Inhibits Adipogenic Differentiation of Bone Marrow-Derived Mesenchymal Stromal Cells
Abstract
1. Introduction
2. Results
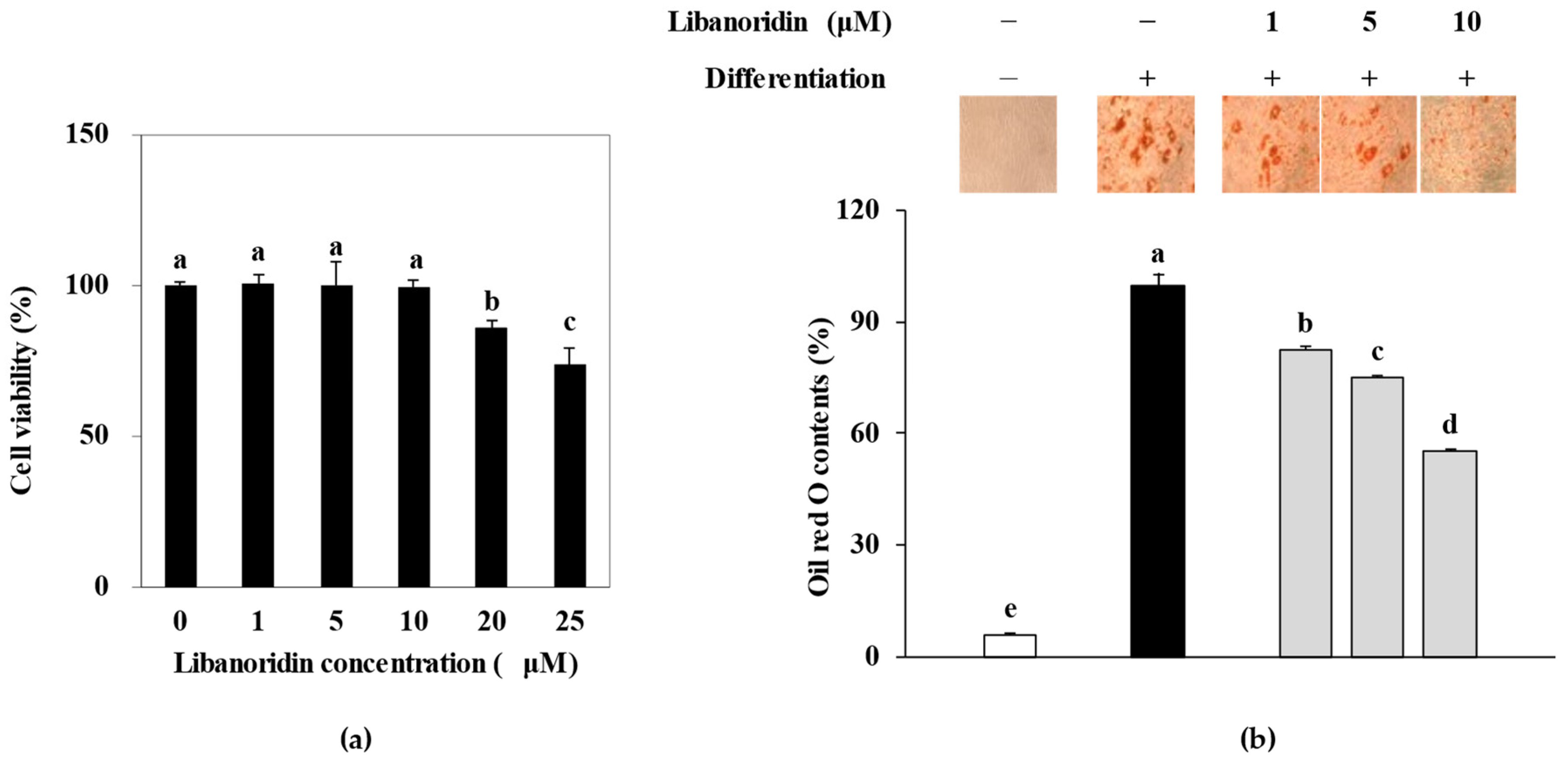
2.1. Inhibition of Lipid Accumulation
2.2. Supression of PPARγ Expression
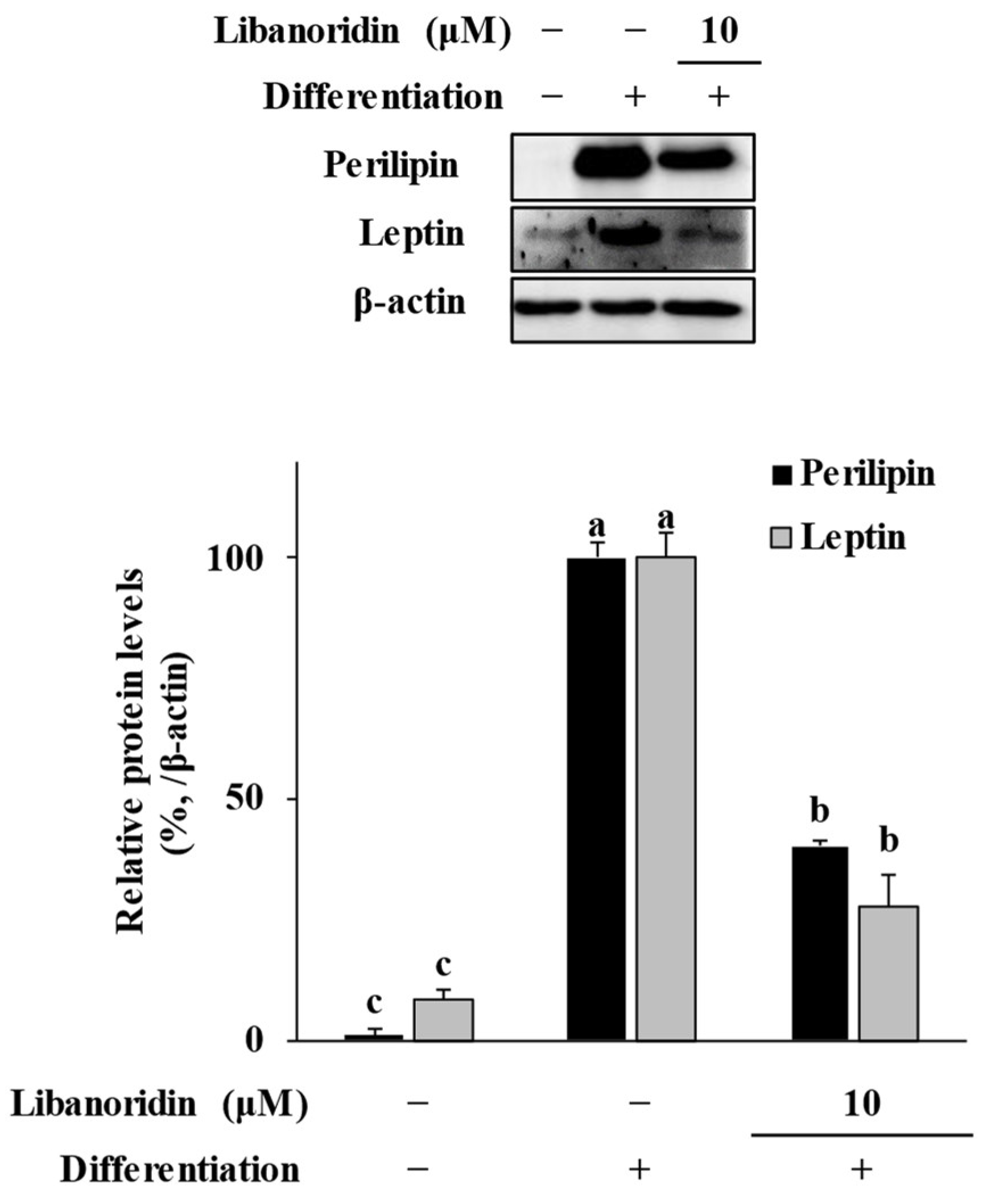
2.3. Suppression of Perilipin and Leptin Levels
2.4. Repression of MAPK Activation
3. Materials and Methods
3.1. Materials
3.2. Maintenance and Differentiation of hBM-MSCs
3.3. Assessment of Cytotoxicity
3.4. Oil Red O Staining of the Intracellular Lipid Deposits
3.5. Reverse Transcription Semi-Quantitative Polymerase Chain Reaction (RT-qPCR)
3.6. Immunoblotting
3.7. Immunofluorescence Staining
3.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardouin, P.; Rharass, T.; Lucas, S. Bone marrow adipose tissue: To be or not to be a typical adipose tissue? Front. Endocrinol. 2016, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Paccou, J.; Penel, G.; Chauveau, C.; Cortet, B.; Hardouin, P. Marrow adiposity and bone: Review of clinical implications. Bone 2019, 118, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gkastaris, K.; Goulis, D.G.; Potoupnis, M.; Anastasilakis, A.D.; Kapetanos, G. Obesity, osteoporosis and bone metabolism. J. Musculoskelet. Neuronal Interact. 2020, 20, 372. [Google Scholar] [PubMed]
- Suchacki, K.J.; Cawthorn, W.P.; Rosen, C.J. Bone marrow adipose tissue: Formation, function and regulation. Curr. Opin. Pharmacol. 2016, 28, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xu, A.; Leung, W.K. Obesity, bone loss, and periodontitis: The interlink. Biomolecules 2022, 12, 865. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Wang, C.; Meng, H.; Wang, X.; Zhao, C.; Peng, J.; Wang, Y. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis. Med. Sci. Monit. 2016, 22, 226. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Lu, L.; Yu, X. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. 2020, 52, 88–98. [Google Scholar] [CrossRef]
- Muruganandan, S.; Govindarajan, R.; Sinal, C.J. Bone marrow adipose tissue and skeletal health. Curr. Osteoporos. Rep. 2018, 16, 434–442. [Google Scholar] [CrossRef]
- Di Maio, G.; Alessio, N.; Demirsoy, I.H.; Peluso, G.; Perrotta, S.; Monda, M.; Di Bernardo, G. Evaluation of browning agents on the white adipogenesis of bone marrow mesenchymal stromal cells: A contribution to fighting obesity. Cells 2021, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, D.; Xie, W.; Wen, L.; Chen, W.; Xu, J.; Ding, J.; Ren, D. PPAR-γ and Wnt regulate the differentiation of MSCs into adipocytes and osteoblasts respectively. Curr. Stem Cell Res. Ther. 2017, 13, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Wang, H.; Yi, X.; Zhang, H.; Chen, H.; Pan, Z. C/EBPα regulates the fate of bone marrow mesenchymal stem cells and steroid-induced avascular necrosis of the femoral head by targeting the PPARγ signalling pathway. Stem Cell Res. Ther. 2022, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L. Overview of treatment approaches to osteoporosis. Br. J. Pharmacol. 2021, 178, 1891–1906. [Google Scholar] [CrossRef]
- Fontalis, A.; Kenanidis, E.; Kotronias, R.A.; Papachristou, A.; Anagnostis, P.; Potoupnis, M.; Tsiridis, E. Current and emerging osteoporosis pharmacotherapy for women: State of the art therapies for preventing bone loss. Expert Opin. Pharmacother. 2019, 20, 1123–1134. [Google Scholar] [CrossRef]
- Takakura, N.; Matsuda, M.; Khan, M.; Hiura, F.; Aoki, K.; Hirohashi, Y.; Mori, K.; Yasuda, H.; Hirata, M.; Kitamura, C.; et al. A novel inhibitor of NF-κB-inducing kinase prevents bone loss by inhibiting osteoclastic bone resorption in ovariectomized mice. Bone 2020, 135, 115316. [Google Scholar] [CrossRef]
- Han, L.; Wang, B.; Wang, R.; Gong, S.; Chen, G.; Xu, W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res. Ther. 2019, 10, 377. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal stem cells: Cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef]
- Luo, J.; Yu, Z.; Tovar, J.; Nilsson, A.; Xu, B. Critical review on anti-obesity effects of phytochemicals through Wnt/β-catenin signaling pathway. Pharmacol. Res. 2022, 184, 106461. [Google Scholar] [CrossRef]
- Aguilera, Y.; Benítez, V. Phytochemicals: Dietary sources, innovative extraction, and health benefits. Foods 2021, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Murugan, D.D.; Balan, D.; Wong, P.F. Adipogenesis and therapeutic potentials of antiobesogenic phytochemicals: Insights from preclinical studies. Phytother. Res. 2021, 35, 5936–5960. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Kong, C.S.; Seo, Y.; Kim, M.M.; Kim, S.K. Anti-inflammatory effect of coumarins isolated from Corydalis heterocarpa in HT-29 human colon carcinoma cells. Food Chem. Toxicol. 2009, 47, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-A.; Lee, J.-I.; Kong, C.-S.; Yea, S.-S.; Seo, Y. Antiproliferative effect of extracts from Corydalis heterocarpa on human cancer cells. KSBB J. 2009, 24, 201–206. [Google Scholar]
- Kim, Y.A.; Lee, J.I.; Kong, C.S.; Choe, J.C.; Oh, K.S.; Seo, Y. Antioxidant activity of dihydrofurocoumarins from Corydalis heterocarpa. Biotechnol. Bioprocess Eng. 2014, 19, 771–779. [Google Scholar] [CrossRef]
- Oh, J.H.; Karadeniz, F.; Seo, Y.; Kong, C.-S. Hyunganol II exerts antiadipogenic properties via MAPK-mediated suppression of PPARγ expression in human bone marrow-derived mesenchymal stromal cells. Evid. Based Complement. Alternat. Med. 2022, 2022, 4252917. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kong, C.S.; Park, H.H.; Lee, E.; Jang, M.S.; Nam, K.H.; Seo, Y. Anti-Inflammatory activity of heterocarpin from the salt marsh plant Corydalis heterocarpa in LPS-induced RAW 264.7 macrophage cells. Molecules 2015, 20, 14474–14486. [Google Scholar] [CrossRef]
- Ye, C.F.; Pan, Y.M.; Zhou, H. Regulation of vitamin D receptor and Genistein on bone metabolism in mouse osteoblasts and the molecular mechanism of osteoporosis. J. Biol. Regul. Homeost. Agents 2018, 32, 497–505. [Google Scholar]
- Heim, M.; Frank, O.; Kampmann, G.; Sochocky, N.; Pennimpede, T.; Fuchs, P.; Hunziker, W.; Weber, P.; Martin, I.; Bendik, I. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 2004, 145, 848–859. [Google Scholar] [CrossRef]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Li, Z.; Hardij, J.; Bagchi, D.P.; Scheller, E.L.; MacDougald, O.A. Development, regulation, metabolism and function of bone marrow adipose tissues. Bone 2018, 110, 134–140. [Google Scholar] [CrossRef]
- Palhinha, L.; Liechocki, S.; Hottz, E.D.; da Silva Pereira, J.A.; de Almeida, C.J.; Moraes-Vieira, P.M.M.; Bozza, P.T.; Maya-Monteiro, C.M. Leptin induces proadipogenic and proinflammatory signaling in adipocytes. Front. Endocrinol. 2019, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ding, J.; Chen, A.; Song, Y.; Xu, C.; Tian, F.; Zhao, J. Aerobic exercise improves adipogenesis in diet-induced obese mice via the lncSRA/p38/JNK/PPARγ pathway. Nutr. Res. 2022, 105, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Lee, H.S.; Han, H.K.; Choi, C.I. Saikosaponin A and D inhibit adipogenesis via the AMPK and MAPK signaling pathways in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2021, 22, 11409. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Seo, Y.; Jang, M.S.; Kong, C.S. Effect and comparison of luteolin and its derivative sodium luteolin- 4′-sulfonate on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells through AMPK-mediated PPARγ signaling. Evid. Based Complement. Alternat. Med. 2020, 2020, 8894910. [Google Scholar] [CrossRef] [PubMed]
- Machinal-Quélin, F.; Dieudonné, M.N.; Leneveu, M.C.; Pecquery, R.; Giudicelli, Y. Proadipogenic effect of leptin on rat preadipocytes in vitro: Activation of MAPK and STAT3 signaling pathways. Am. J. Physiol. Cell Physiol. 2002, 282, 853–863. [Google Scholar] [CrossRef]
- Reusch, J.E.B.; Colton, L.A.; Klemm, D.J. CREB activation induces adipogenesis in 3T3-L1 cells. Mol. Cell. Biol. 2000, 20, 1008–1020. [Google Scholar] [CrossRef]
- Cong, Q.; Jia, H.; Biswas, S.; Li, P.; Qiu, S.; Deng, Q.; Guo, X.; Ma, G.; Ling Chau, J.F.; Wang, Y.; et al. P38α MAPK regulates lineage commitment and OPG synthesis of bone marrow stromal cells to prevent bone loss under physiological and pathological conditions. Stem Cell Rep. 2016, 6, 566–578. [Google Scholar] [CrossRef]
- Liu, G.X.; Zhu, J.C.; Chen, X.Y.; Zhu, A.Z.; Liu, C.C.; Lai, Q.; Chen, S.T. Inhibition of adipogenic differentiation of bone marrow mesenchymal stem cells by erythropoietin via activating ERK and P38 MAPK. Genet. Mol. Res. 2015, 14, 6968–6977. [Google Scholar] [CrossRef]
- Deng, W.; Chen, H.; Su, H.; Wu, X.; Xie, Z.; Wu, Y.; Shen, H. IL6 receptor facilitates adipogenesis differentiation of human mesenchymal stem cells through activating p38 pathway. Int. J. Stem Cells 2020, 13, 1158829. [Google Scholar] [CrossRef]
- Ji, J.; Zhu, J.; Hu, X.; Wang, T.; Zhang, X.; Hou, A.J.; Wang, H. (2S)-7,4′-dihydroxy-8-prenylflavan stimulates adipogenesis and glucose uptake through p38MAPK pathway in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2015, 460, 578–582. [Google Scholar] [CrossRef]
- Aouadi, M.; Jager, J.; Laurent, K.; Gonzalez, T.; Cormont, M.; Binétruy, B.; Le Marchand-Brustel, Y.; Tanti, J.F.; Bost, F. p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Lett. 2007, 581, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, J.Y.; Qi, Y.; Park, S.; Lee, H.L.; Yamabe, N.; Kim, H.; Jang, D.S.; Kang, K.S. Phytochemicals from the flowers of Prunus persica (L.) Batsch: Anti-adipogenic effect of mandelamide on 3T3-L1 preadipocytes. Bioorg. Med. Chem. Lett. 2021, 49, 128326. [Google Scholar] [CrossRef]
- San, H.T.; Khine, H.E.E.; Sritularak, B.; Prompetchara, E.; Chaotham, C.; Che, C.-T.; Likhitwitayawuid, K. Pinostrobin: An adipogenic suppressor from Fingerroot (Boesenbergia rotunda) and its possible mechanisms. Foods 2022, 11, 3024. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, S.; Yamaguchi, T.; Takahashi, S.I.; Hirose, F.; Osumi, T. Negative regulation of adipogenesis from human mesenchymal stem cells by Jun N-terminal kinase. Biochem. Biophys. Res. Commun. 2005, 326, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Huang, Z.; Yin, X.; Zhang, J.; Gong, L.; Chen, J.; Rong, K.; Xu, J.; Lu, L.; Cui, L. Role of c-Jun N-terminal kinase in the osteogenic and adipogenic differentiation of human adipose-derived mesenchymal stem cells. Exp. Cell Res. 2015, 339, 112–121. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadeniz, F.; Oh, J.H.; Jang, M.S.; Seo, Y.; Kong, C.-S. Libanoridin Isolated from Corydalis heterocarpa Inhibits Adipogenic Differentiation of Bone Marrow-Derived Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2023, 24, 254. https://doi.org/10.3390/ijms24010254
Karadeniz F, Oh JH, Jang MS, Seo Y, Kong C-S. Libanoridin Isolated from Corydalis heterocarpa Inhibits Adipogenic Differentiation of Bone Marrow-Derived Mesenchymal Stromal Cells. International Journal of Molecular Sciences. 2023; 24(1):254. https://doi.org/10.3390/ijms24010254
Chicago/Turabian StyleKaradeniz, Fatih, Jung Hwan Oh, Mi Soon Jang, Youngwan Seo, and Chang-Suk Kong. 2023. "Libanoridin Isolated from Corydalis heterocarpa Inhibits Adipogenic Differentiation of Bone Marrow-Derived Mesenchymal Stromal Cells" International Journal of Molecular Sciences 24, no. 1: 254. https://doi.org/10.3390/ijms24010254
APA StyleKaradeniz, F., Oh, J. H., Jang, M. S., Seo, Y., & Kong, C.-S. (2023). Libanoridin Isolated from Corydalis heterocarpa Inhibits Adipogenic Differentiation of Bone Marrow-Derived Mesenchymal Stromal Cells. International Journal of Molecular Sciences, 24(1), 254. https://doi.org/10.3390/ijms24010254





