Magnesium and the Brain: A Focus on Neuroinflammation and Neurodegeneration
Abstract
1. Introduction
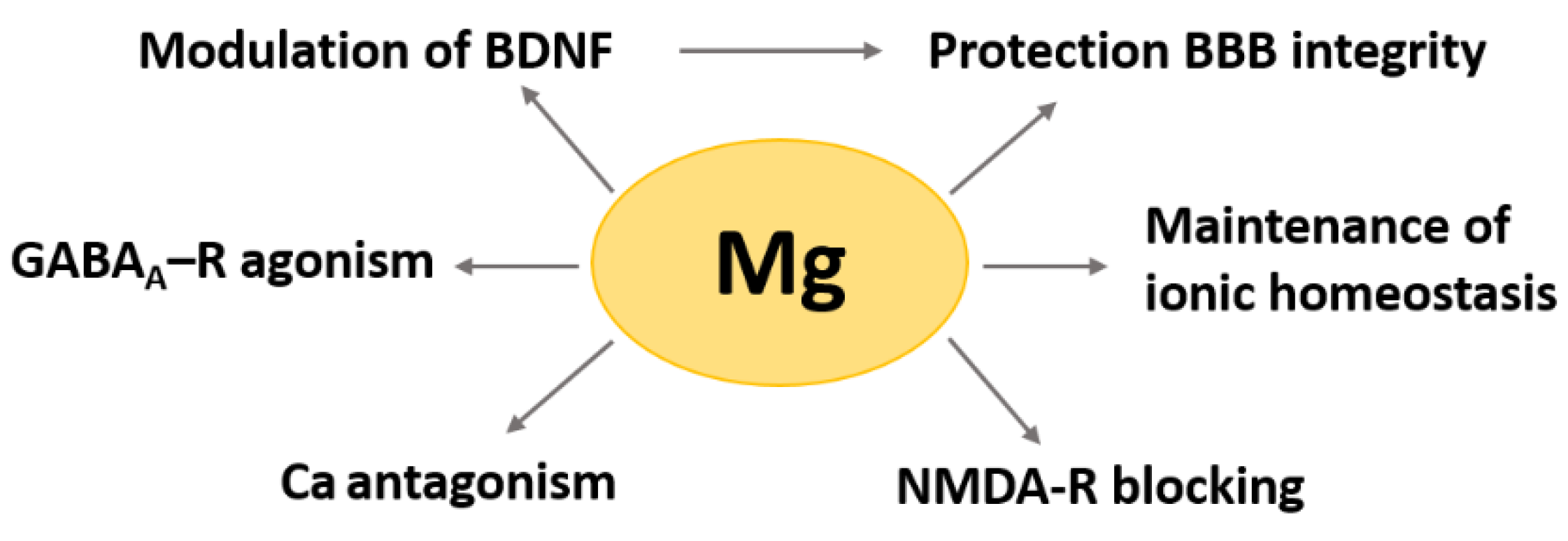
2. Magnesium in the Brain
3. Magnesium and Neuroinflammation
3.1. Magnesium and Inflammatory Mediators in the Brain
3.2. Magnesium and Other Contributors to Neuroinflammation
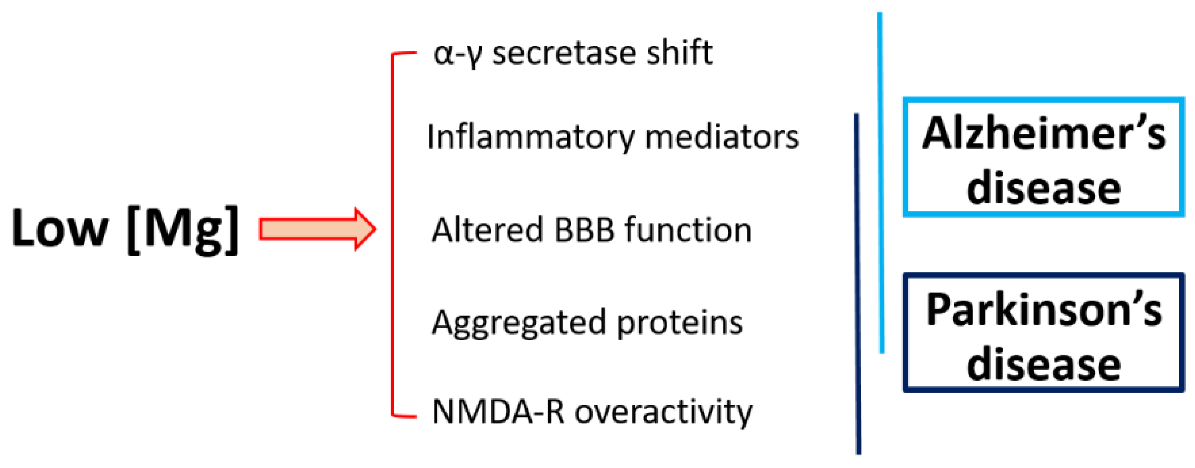
4. Magnesium and Neurodegeneration
4.1. Magnesium and Alzheimer’s Disease
4.2. Magnesium and Parkinson’s Disease
4.3. Magnesium and Multiple Sclerosis
5. Unanswered Questions in Mg, Neuroinflammation and Neurodegeneration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holm, N.G. The significance of Mg in prebiotic geochemistry. Geobiology 2012, 10, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.A.; Panonnummal, R. ’Magnesium’-the master cation-as a drug-possibilities and evidences. Biometals 2021, 34, 955–986. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Rosanoff, A.; Baniasadi, S.; Barbagallo, M.; Castiglioni, S.; Guerrero-Romero, F.; Iotti, S.; Mazur, A.; Micke, O.; Pourdowlat, G.; et al. The relevance of magnesium homeostasis in COVID-19. Eur. J. Nutr. 2022, 61, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- Poleszak, E. Benzodiazepine/GABA(A) receptors are involved in magnesium-induced anxiolytic-like behavior in mice. Pharmacol. Rep. 2008, 60, 483–489. [Google Scholar]
- Campo-Soria, C.; Chang, Y.; Weiss, D.S. Mechanism of action of benzodiazepines on GABAA receptors. Br. J. Pharmacol. 2006, 148, 984–990. [Google Scholar] [CrossRef]
- Yamanaka, R.; Shindo, Y.; Hotta, K.; Suzuki, K.; Oka, K. GABA-Induced Intracellular Mg(2+) Mobilization Integrates and Coordinates Cellular Information Processing for the Maturation of Neural Networks. Curr. Biol. 2018, 28, 3984–3991.e5. [Google Scholar] [CrossRef]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef]
- Romeo, V.; Cazzaniga, A.; Maier, J.A.M. Magnesium and the blood-brain barrier in vitro: Effects on permeability and magnesium transport. Magnes. Res. 2019, 32, 16–24. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Afsharfar, M.; Shahraki, M.; Shakiba, M.; Asbaghi, O.; Dashipour, A. The effects of magnesium supplementation on serum level of brain derived neurotrophic factor (BDNF) and depression status in patients with depression. Clin. Nutr. ESPEN 2021, 42, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Fedele, G.; Castiglioni, S.; Maier, J.A. The Presence of Blood-Brain Barrier Modulates the Response to Magnesium Salts in Human Brain Organoids. Int. J. Mol. Sci. 2022, 23, 5133. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, I.; Abumaria, N.; Wu, L.-J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.-G.; Zhuo, M.; et al. Enhancement of learning and memory by elevating brain magnesium. Neuron 2010, 65, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Funato, Y.; Meschi, E.; Jovanoski, K.D.; Miki, H.; Waddell, S. Magnesium efflux from Drosophila Kenyon cells is critical for normal and diet-enhanced long-term memory. eLife 2020, 9, e61339. [Google Scholar] [CrossRef]
- Yen, L.M.; Thwaites, C.L. Tetanus. Lancet Lond. Engl. 2019, 393, 1657–1668. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Tsuji, R.; Inoue, H.; Uehara, M.; Kida, S. Dietary magnesium deficiency induces the expression of neuroinflammation-related genes in mouse brain. Neuropsychopharmacol. Rep. 2021, 41, 230–236. [Google Scholar] [CrossRef]
- Zhang, J.; Mai, C.-L.; Xiong, Y.; Lin, Z.-J.; Jie, Y.-T.; Mai, J.-Z.; Liu, C.; Xie, M.-X.; Zhou, X.; Liu, X.-G. The Causal Role of Magnesium Deficiency in the Neuroinflammation, Pain Hypersensitivity and Memory/Emotional Deficits in Ovariectomized and Aged Female Mice. J. Inflamm. Res. 2021, 14, 6633–6656. [Google Scholar] [CrossRef]
- Gao, F.; Ding, B.; Zhou, L.; Gao, X.; Guo, H.; Xu, H. Magnesium sulfate provides neuroprotection in lipopolysaccharide-activated primary microglia by inhibiting NF-κB pathway. J. Surg. Res. 2013, 184, 944–950. [Google Scholar] [CrossRef]
- Johnson, M.B.; Young, A.D.; Marriott, I. The Therapeutic Potential of Targeting Substance P/NK-1R Interactions in Inflammatory CNS Disorders. Front. Cell. Neurosci. 2016, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell. Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef] [PubMed]
- Weglicki, W.B.; Chmielinska, J.J.; Tejero-Taldo, I.; Kramer, J.H.; Spurney, C.F.; Viswalingham, K.; Lu, B.; Mak, I.T. Neutral endopeptidase inhibition enhances substance P mediated inflammation due to hypomagnesemia. Magnes. Res. 2009, 22, 167S–173S. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qu, C.; Lu, X.; Zhang, S. Activation of microglia by histamine and substance P. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 34, 768–780. [Google Scholar] [CrossRef]
- Vink, R.; Gabrielian, L.; Thornton, E. The Role of Substance P in Secondary Pathophysiology after Traumatic Brain Injury. Front. Neurol. 2017, 8, 304. [Google Scholar] [CrossRef]
- Ghabriel, M.N.; Thomas, A.; Vink, R. Magnesium restores altered aquaporin-4 immunoreactivity following traumatic brain injury to a pre-injury state. Acta Neurochir. Suppl. 2006, 96, 402–406. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Jiang, S.; Fu, Y.; Avraham, S.; Avraham, H.K. The proinflammatory peptide substance P promotes blood-brain barrier breaching by breast cancer cells through changes in microvascular endothelial cell tight junctions. Int. J. Cancer 2014, 134, 1034–1044. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, D.-Y. Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide Biol. Chem. 2009, 20, 223–230. [Google Scholar] [CrossRef]
- Faraci, F.M. Protecting the brain with eNOS: Run for your life. Circ. Res. 2006, 99, 1029–1030. [Google Scholar] [CrossRef]
- An, L.; Shen, Y.; Chopp, M.; Zacharek, A.; Venkat, P.; Chen, Z.; Li, W.; Qian, Y.; Landschoot-Ward, J.; Chen, J. Deficiency of Endothelial Nitric Oxide Synthase (eNOS) Exacerbates Brain Damage and Cognitive Deficit in A Mouse Model of Vascular Dementia. Aging Dis. 2021, 12, 732–746. [Google Scholar] [CrossRef]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Maier, J.A.M. Endothelial cells and magnesium: Implications in atherosclerosis. Clin. Sci. 2012, 122, 397–407. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef]
- Garnier, Y.; Middelanis, J.; Jensen, A.; Berger, R. Neuroprotective effects of magnesium on metabolic disturbances in fetal hippocampal slices after oxygen-glucose deprivation: Mediation by nitric oxide system. J. Soc. Gynecol. Investig. 2002, 9, 86–92. [Google Scholar] [CrossRef]
- Sun, X.; Mei, Y.; Tong, E. Effect of magnesium on nitric oxide synthase of neurons in cortex during early period of cerebral ischemia. J. Tongji Med. Univ. 2000, 20, 13–15. [Google Scholar] [CrossRef]
- Khatib, N.; Ginsberg, Y.; Ben David, C.; Ross, M.G.; Vitner, D.; Zipori, Y.; Zamora, O.; Weiner, Z.; Beloosesky, R. Magnesium sulphate neuroprotection mechanism is placental mediated by inhibition of inflammation, apoptosis and oxidative stress. Placenta 2022, 127, 29–36. [Google Scholar] [CrossRef]
- Tachikawa, M.; Hosoya, K.; Terasaki, T. Pharmacological significance of prostaglandin E2 and D2 transport at the brain barriers. Adv. Pharmacol. 2014, 71, 337–360. [Google Scholar] [CrossRef]
- Figueiredo-Pereira, M.E.; Rockwell, P.; Schmidt-Glenewinkel, T.; Serrano, P. Neuroinflammation and J2 prostaglandins: Linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Front. Mol. Neurosci. 2014, 7, 104. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Mohammadi, H.; Shamshirian, A.; Eslami, S.; Shamshirian, D.; Ebrahimzadeh, M.A. Magnesium Sulfate Attenuates Lethality and Oxidative Damage Induced by Different Models of Hypoxia in Mice. Biomed Res. Int. 2020, 2020, 2624734. [Google Scholar] [CrossRef]
- Bagheri, G.; Rezaee, R.; Tsarouhas, K.; Docea, A.O.; Shahraki, J.; Shahriari, M.; Wilks, M.F.; Jahantigh, H.; Tabrizian, K.; Moghadam, A.A.; et al. Magnesium sulfate ameliorates carbon monoxide-induced cerebral injury in male rats. Mol. Med. Rep. 2019, 19, 1032–1039. [Google Scholar] [CrossRef]
- Sugimoto, J.; Romani, A.M.; Valentin-Torres, A.M.; Luciano, A.A.; Ramirez Kitchen, C.M.; Funderburg, N.; Mesiano, S.; Bernstein, H.B. Magnesium decreases inflammatory cytokine production: A novel innate immunomodulatory mechanism. J. Immunol. 2012, 188, 6338–6346. [Google Scholar] [CrossRef]
- Bourgognon, J.-M.; Cavanagh, J. The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv. 2020, 4, 2398212820979802. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Tai, Y.; Qiu, Y.; Bao, Z. Magnesium Lithospermate B Suppresses Lipopolysaccharide-Induced Neuroinflammation in BV2 Microglial Cells and Attenuates Neurodegeneration in Lipopolysaccharide-Injected Mice. J. Mol. Neurosci. 2018, 64, 80–92. [Google Scholar] [CrossRef]
- Marret, S.; Marpeau, L.; Zupan-Simunek, V.; Eurin, D.; Lévêque, C.; Hellot, M.-F.; Bénichou, J. Magnesium sulphate given before very-preterm birth to protect infant brain: The randomised controlled PREMAG trial*. BJOG 2007, 114, 310–318. [Google Scholar] [CrossRef]
- Le Dieu-Lugon, B.; Dupré, N.; Derambure, C.; Janin, F.; Gonzalez, B.J.; Marret, S.; Arabo, A.; Leroux, P. Effect of Neuroprotective Magnesium Sulfate Treatment on Brain Transcription Response to Hypoxia Ischemia in Neonate Mice. Int. J. Mol. Sci. 2021, 22, 4253. [Google Scholar] [CrossRef]
- Andretta, A.; Schieferdecker, M.E.M.; Petterle, R.R.; Dos Santos Paiva, E.; Boguszewski, C.L. Relations between serum magnesium and calcium levels and body composition and metabolic parameters in women with fibromyalgia. Adv. Rheumatol. Lond. Engl. 2020, 60, 18. [Google Scholar] [CrossRef]
- Pan, K.; Garaschuk, O. The role of intracellular calcium-store-mediated calcium signals in in vivo sensor and effector functions of microglia. J. Physiol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Lee, M.; Jantaratnotai, N.; McGeer, E.; McLarnon, J.G.; McGeer, P.L. Mg2+ ions reduce microglial and THP-1 cell neurotoxicity by inhibiting Ca2+ entry through purinergic channels. Brain Res. 2011, 1369, 21–35. [Google Scholar] [CrossRef]
- Huang, Z.; Gibb, A.J. Mg2+ block properties of triheteromeric GluN1-GluN2B-GluN2D NMDA receptors on neonatal rat substantia nigra pars compacta dopaminergic neurones. J. Physiol. 2014, 592, 2059–2078. [Google Scholar] [CrossRef]
- Zhou, X.; Hollern, D.; Liao, J.; Andrechek, E.; Wang, H. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell Death Dis. 2013, 4, e560. [Google Scholar] [CrossRef]
- Deshpande, L.S.; Lou, J.K.; Mian, A.; Blair, R.E.; Sombati, S.; Attkisson, E.; DeLorenzo, R.J. Time course and mechanism of hippocampal neuronal death in an in vitro model of status epilepticus: Role of NMDA receptor activation and NMDA dependent calcium entry. Eur. J. Pharmacol. 2008, 583, 73–83. [Google Scholar] [CrossRef]
- Clerc, P.; Young, C.A.; Bordt, E.A.; Grigore, A.M.; Fiskum, G.; Polster, B.M. Magnesium sulfate protects against the bioenergetic consequences of chronic glutamate receptor stimulation. PLoS ONE 2013, 8, e79982. [Google Scholar] [CrossRef]
- Hou, H.; Wang, L.; Fu, T.; Papasergi, M.; Yule, D.I.; Xia, H. Magnesium Acts as a Second Messenger in the Regulation of NMDA Receptor-Mediated CREB Signaling in Neurons. Mol. Neurobiol. 2020, 57, 2539–2550. [Google Scholar] [CrossRef]
- Pochwat, B.; Sowa-Kucma, M.; Kotarska, K.; Misztak, P.; Nowak, G.; Szewczyk, B. Antidepressant-like activity of magnesium in the olfactory bulbectomy model is associated with the AMPA/BDNF pathway. Psychopharmacology 2015, 232, 355–367. [Google Scholar] [CrossRef]
- Abiri, B.; Sarbakhsh, P.; Vafa, M. Randomized study of the effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammation, and SIRT1 in obese women with mild to moderate depressive symptoms. Nutr. Neurosci. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, L.H.; Yang, W.; Cui, R.J.; Xu, S.B. The Role of BDNF in the Neuroimmune Axis Regulation of Mood Disorders. Front. Neurol. 2019, 10, 515. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- León, J.; Acurio, J.; Bergman, L.; López, J.; Karin Wikström, A.; Torres-Vergara, P.; Troncoso, F.; Castro, F.O.; Vatish, M.; Escudero, C. Disruption of the Blood-Brain Barrier by Extracellular Vesicles from Preeclampsia Plasma and Hypoxic Placentae: Attenuation by Magnesium Sulfate. Hypertension 2021, 78, 1423–1433. [Google Scholar] [CrossRef]
- Imer, M.; Omay, B.; Uzunkol, A.; Erdem, T.; Sabanci, P.A.; Karasu, A.; Albayrak, S.B.; Sencer, A.; Hepgul, K.; Kaya, M. Effect of magnesium, MK-801 and combination of magnesium and MK-801 on blood-brain barrier permeability and brain edema after experimental traumatic diffuse brain injury. Neurol. Res. 2009, 31, 977–981. [Google Scholar] [CrossRef]
- Shadman, J.; Sadeghian, N.; Moradi, A.; Bohlooli, S.; Panahpour, H. Magnesium sulfate protects blood-brain barrier integrity and reduces brain edema after acute ischemic stroke in rats. Metab. Brain Dis. 2019, 34, 1221–1229. [Google Scholar] [CrossRef]
- Cenacchi, V.; Maier, J.A.; Perini, M.P. A potential protective role of magnesium in neuroCOVID. Magnes. Res. 2022, 35, 18–26. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Prim. 2021, 7, 33. [Google Scholar] [CrossRef]
- Nguyen, K.V. β-Amyloid precursor protein (APP) and the human diseases. AIMS Neurosci. 2019, 6, 273–281. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Nathan, C.; Calingasan, N.; Nezezon, J.; Ding, A.; Lucia, M.S.; La Perle, K.; Fuortes, M.; Lin, M.; Ehrt, S.; Kwon, N.S.; et al. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J. Exp. Med. 2005, 202, 1163–1169. [Google Scholar] [CrossRef]
- Andrási, E.; Igaz, S.; Molnár, Z.; Makó, S. Disturbances of magnesium concentrations in various brain areas in Alzheimer’s disease. Magnes. Res. 2000, 13, 189–196. [Google Scholar]
- Andrási, E.; Páli, N.; Molnár, Z.; Kösel, S. Brain aluminum, magnesium and phosphorus contents of control and Alzheimer-diseased patients. J. Alzheimers Dis. 2005, 7, 273–284. [Google Scholar] [CrossRef]
- Veronese, N.; Zurlo, A.; Solmi, M.; Luchini, C.; Trevisan, C.; Bano, G.; Manzato, E.; Sergi, G.; Rylander, R. Magnesium Status in Alzheimer’s Disease: A Systematic Review. Am. J. Alzheimers Dis. Other Demen. 2016, 31, 208–213. [Google Scholar] [CrossRef]
- Du, K.; Zheng, X.; Ma, Z.-T.; Lv, J.-Y.; Jiang, W.-J.; Liu, M.-Y. Association of Circulating Magnesium Levels in Patients with Alzheimer’s Disease From 1991 to 2021: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 13, 799824. [Google Scholar] [CrossRef]
- Cazzola, R.; Della Porta, M.; Manoni, M.; Iotti, S.; Pinotti, L.; Maier, J.A. Going to the roots of reduced magnesium dietary intake: A tradeoff between climate changes and sources. Heliyon 2020, 6, e05390. [Google Scholar] [CrossRef]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y. Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: The Hisayama Study. J. Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef]
- Liu, G.; Weinger, J.G.; Lu, Z.-L.; Xue, F.; Sadeghpour, S. Efficacy and Safety of MMFS-01, a Synapse Density Enhancer, for Treating Cognitive Impairment in Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2016, 49, 971–990. [Google Scholar] [CrossRef]
- Yu, X.; Guan, P.-P.; Guo, J.-W.; Wang, Y.; Cao, L.-L.; Xu, G.-B.; Konstantopoulos, K.; Wang, Z.-Y.; Wang, P. By suppressing the expression of anterior pharynx-defective-1α and -1β and inhibiting the aggregation of β-amyloid protein, magnesium ions inhibit the cognitive decline of amyloid precursor protein/presenilin 1 transgenic mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 5044–5058. [Google Scholar] [CrossRef]
- Wang, P.; Yu, X.; Guan, P.-P.; Guo, J.-W.; Wang, Y.; Zhang, Y.; Zhao, H.; Wang, Z.-Y. Magnesium ion influx reduces neuroinflammation in Aβ precursor protein/Presenilin 1 transgenic mice by suppressing the expression of interleukin-1β. Cell. Mol. Immunol. 2017, 14, 451–464. [Google Scholar] [CrossRef]
- Xu, Z.-P.; Li, L.; Bao, J.; Wang, Z.-H.; Zeng, J.; Liu, E.-J.; Li, X.-G.; Huang, R.-X.; Gao, D.; Li, M.-Z.; et al. Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model. PLoS ONE 2014, 9, e108645. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, H.I.; Cha, H.Y.; Yang, Y.J.; Kwon, S.H.; Yang, S.J. Diabetes and Alzheimer’s Disease: Mechanisms and Nutritional Aspects. Clin. Nutr. Res. 2018, 7, 229–240. [Google Scholar] [CrossRef]
- Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Femminella, G.D.; Livingston, N.R.; Raza, S.; van der Doef, T.; Frangou, E.; Love, S.; Busza, G.; Calsolaro, V.; Carver, S.; Holmes, C.; et al. Does insulin resistance influence neurodegeneration in non-diabetic Alzheimer’s subjects? Alzheimers Res. Ther. 2021, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.-G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Liu, Y.; Li, W.; Wang, Z.; Sun, Q.; Zhou, H.; Chen, X.; Yu, Y.; Wang, Y.; et al. TRPM7 Is Required for Normal Synapse Density, Learning, and Memory at Different Developmental Stages. Cell Rep. 2018, 23, 3480–3491. [Google Scholar] [CrossRef]
- Sun, Y.; Kamat, A.; Singh, B.B. Isoproterenol-Dependent Activation of TRPM7 Protects Against Neurotoxin-Induced Loss of Neuroblastoma Cells. Front. Physiol. 2020, 11, 305. [Google Scholar] [CrossRef]
- Abumaria, N.; Li, W.; Clarkson, A.N. Role of the chanzyme TRPM7 in the nervous system in health and disease. Cell. Mol. Life Sci. 2019, 76, 3301–3310. [Google Scholar] [CrossRef]
- Sun, H.-S.; Jackson, M.F.; Martin, L.J.; Jansen, K.; Teves, L.; Cui, H.; Kiyonaka, S.; Mori, Y.; Jones, M.; Forder, J.P.; et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat. Neurosci. 2009, 12, 1300–1307. [Google Scholar] [CrossRef]
- Nadolni, W.; Zierler, S. The Channel-Kinase TRPM7 as Novel Regulator of Immune System Homeostasis. Cells 2018, 7, 109. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Chen, Y.; Wei, X.; Ma, G.-G.; Ding, J.; Lu, C.; Zhou, R.-P.; Hu, W. Immunomodulatory functions of TRPM7 and its implications in autoimmune diseases. Immunology 2022, 165, 3–21. [Google Scholar] [CrossRef]
- Romagnani, A.; Vettore, V.; Rezzonico-Jost, T.; Hampe, S.; Rottoli, E.; Nadolni, W.; Perotti, M.; Meier, M.A.; Hermanns, C.; Geiger, S.; et al. TRPM7 kinase activity is essential for T cell colonization and alloreactivity in the gut. Nat. Commun. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zhong, Y. Interleukin-17A: The Key Cytokine in Neurodegenerative Diseases. Front. Aging Neurosci. 2020, 12, 566922. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, V.; Anrather, J.; Orzi, F.; Iadecola, C. Th17 and Cognitive Impairment: Possible Mechanisms of Action. Front. Neuroanat. 2019, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Oberstein, T.J.; Taha, L.; Spitzer, P.; Hellstern, J.; Herrmann, M.; Kornhuber, J.; Maler, J.M. Imbalance of Circulating T(h)17 and Regulatory T Cells in Alzheimer’s Disease: A Case Control Study. Front. Immunol. 2018, 9, 1213. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, C.; Volpicelli, F.; Lippiello, P.; Buono, B.; Raucci, F.; Piccolo, M.; Iqbal, A.J.; Irace, C.; Miniaci, M.C.; Perrone Capano, C.; et al. Neutralization of IL-17 rescues amyloid-β-induced neuroinflammation and memory impairment. Br. J. Pharmacol. 2019, 176, 3544–3557. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet Lond. Engl. 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-Synuclein Aggregation in Parkinson’s Disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary intake of metals and risk of Parkinson’s disease: A case-control study in Japan. J. Neurol. Sci. 2011, 306, 98–102. [Google Scholar] [CrossRef]
- Tasset, I.; Sánchez-López, F.; Agüera, E.; Fernández-Bolaños, R.; Sánchez, F.M.; Cruz-Guerrero, A.; Gascón-Luna, F.; Túnez, I. NGF and nitrosative stress in patients with Huntington’s disease. J. Neurol. Sci. 2012, 315, 133–136. [Google Scholar] [CrossRef]
- Shen, Y.; Dai, L.; Tian, H.; Xu, R.; Li, F.; Li, Z.; Zhou, J.; Wang, L.; Dong, J.; Sun, L. Treatment of Magnesium-L-Threonate Elevates The Magnesium Level In The Cerebrospinal Fluid And Attenuates Motor Deficits And Dopamine Neuron Loss In A Mouse Model Of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2019, 15, 3143–3153. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, Y.; Fang, X.; Lu, Y.; Li, J.; Zhang, J.; Deng, X.; Li, S. The possible mechanism of Parkinson’s disease progressive damage and the preventive effect of GM1 in the rat model induced by 6-hydroxydopamine. Brain Res. 2014, 1592, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Alimonti, A.; Senofonte, O.; Pino, A.; Violante, N.; Petrucci, F.; Sancesario, G.; Forte, G. Metal changes in CSF and peripheral compartments of parkinsonian patients. J. Neurol. Sci. 2006, 248, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, M.; Wu, P.; Cornell, R. SLC41A1 and TRPM7 in magnesium homeostasis and genetic risk for Parkinson’s disease. J. Neurol. Neuromed. 2016, 1, 23–28. [Google Scholar]
- Oyanagi, K.; Kawakami, E.; Kikuchi-Horie, K.; Ohara, K.; Ogata, K.; Takahama, S.; Wada, M.; Kihira, T.; Yasui, M. Magnesium deficiency over generations in rats with special references to the pathogenesis of the Parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Neuropathology 2006, 26, 115–128. [Google Scholar] [CrossRef]
- Kolisek, M.; Sponder, G.; Mastrototaro, L.; Smorodchenko, A.; Launay, P.; Vormann, J.; Schweigel-Röntgen, M. Substitution p.A350V in Na+/Mg2+ exchanger SLC41A1, potentially associated with Parkinson’s disease, is a gain-of-function mutation. PLoS ONE 2013, 8, e71096. [Google Scholar] [CrossRef]
- Hermosura, M.C.; Garruto, R.M. TRPM7 and TRPM2-Candidate susceptibility genes for Western Pacific ALS and PD? Biochim. Biophys. Acta 2007, 1772, 822–835. [Google Scholar] [CrossRef]
- Lin, L.; Yan, M.; Wu, B.; Lin, R.; Zheng, Z. Expression of magnesium transporter SLC41A1 in the striatum of 6-hydroxydopamine-induced parkinsonian rats. Brain Res. Bull. 2018, 142, 338–343. [Google Scholar] [CrossRef]
- Vaidya, B.; Sharma, S.S. Transient Receptor Potential Channels as an Emerging Target for the Treatment of Parkinson’s Disease: An Insight into Role of Pharmacological Interventions. Front. Cell Dev. Biol. 2020, 8, 584513. [Google Scholar] [CrossRef]
- Sun, Y.; Sukumaran, P.; Singh, B.B. Magnesium-Induced Cell Survival Is Dependent on TRPM7 Expression and Function. Mol. Neurobiol. 2020, 57, 528–538. [Google Scholar] [CrossRef]
- Dati, L.M.; Ulrich, H.; Real, C.C.; Feng, Z.P.; Sun, H.S.; Britto, L.R. Carvacrol promotes neuroprotection in the mouse hemiparkinsonian model. Neuroscience 2017, 356, 176–181. [Google Scholar] [CrossRef]
- Yang, C.P.; Zhang, Z.H.; Zhang, L.H.; Rui, H.C. Neuroprotective Role of MicroRNA-22 in a 6-Hydroxydopamine-Induced Cell Model of Parkinson’s Disease via Regulation of Its Target Gene TRPM7. J. Mol. Neurosci. 2016, 60, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huang, Y.; Bao, T.; Liu, C.; Liu, X.; Chen, X. The role of Th17 cells/IL-17A in AD, PD, ALS and the strategic therapy targeting on IL-17A. J. Neuroinflamm. 2022, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Rossier, P.; van Erven, S.; Wade, D.T. The effect of magnesium oral therapy on spasticity in a patient with multiple sclerosis. Eur. J. Neurol. 2000, 7, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Edwards, L.R. Magnesium Supplementation in Vitamin D Deficiency. Am. J. Ther. 2019, 26, e124–e132. [Google Scholar] [CrossRef]
- Cheung, M.M.; Dall, R.D.; Shewokis, P.A.; Altasan, A.; Volpe, S.L.; Amori, R.; Singh, H.; Sukumar, D. The effect of combined magnesium and vitamin D supplementation on vitamin D status, systemic inflammation, and blood pressure: A randomized double-blinded controlled trial. Nutrition 2022, 99–100, 111674. [Google Scholar] [CrossRef]
- Wasnik, S.; Sharma, I.; Baylink, D.J.; Tang, X. Vitamin D as a Potential Therapy for Multiple Sclerosis: Where Are We? Int. J. Mol. Sci. 2020, 21, 3102. [Google Scholar] [CrossRef]
- Karpińska, E.; Socha, K.; Soroczyńska, J.; Kochanowicz, J.; Jakoniuk, M.; Mariak, Z.; Borawska, M.H. Concentration of magnesium in the serum and the ability status of patients with relapsing-remitting multiple sclerosis. J. Elem. 2017, 22, 671–679. [Google Scholar] [CrossRef]
- de Oliveira, M.; Gianeti, T.M.R.; da Rocha, F.C.G.; Lisboa-Filho, P.N.; Piacenti-Silva, M. A preliminary study of the concentration of metallic elements in the blood of patients with multiple sclerosis as measured by ICP-MS. Sci. Rep. 2020, 10, 13112. [Google Scholar] [CrossRef]
- Nirooei, E.; Kashani, S.M.A.; Owrangi, S.; Malekpour, F.; Niknam, M.; Moazzen, F.; Nowrouzi-Sohrabi, P.; Farzinmehr, S.; Akbari, H. Blood Trace Element Status in Multiple Sclerosis: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2022, 200, 13–26. [Google Scholar] [CrossRef]
- Kamermans, A.; Planting, K.E.; Jalink, K.; van Horssen, J.; de Vries, H.E. Reactive astrocytes in multiple sclerosis impair neuronal outgrowth through TRPM7-mediated chondroitin sulfate proteoglycan production. Glia 2019, 67, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Rowe, W.J. Correcting magnesium deficiencies may prolong life. Clin. Interv. Aging 2012, 7, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Killilea, D.W.; Maier, J.A.M. A connection between magnesium deficiency and aging: New insights from cellular studies. Magnes. Res. 2008, 21, 77–82. [Google Scholar] [PubMed]
- Nelke, C.; Schroeter, C.B.; Pawlitzki, M.; Meuth, S.G.; Ruck, T. Cellular senescence in neuroinflammatory disease: New therapies for old cells? Trends Mol. Med. 2022, 28, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yañez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet. Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef]
- Chmielinska, J.J.; Tejero-Taldo, M.I.; Mak, I.T.; Weglicki, W.B. Intestinal and cardiac inflammatory response shows enhanced endotoxin receptor (CD14) expression in magnesium deficiency. Mol. Cell. Biochem. 2005, 278, 53–57. [Google Scholar] [CrossRef]
- Zimowska, W.; Girardeau, J.P.; Kuryszko, J.; Bayle, D.; Rayssiguier, Y.; Mazur, A. Morphological and immune response alterations in the intestinal mucosa of the mouse after short periods on a low-magnesium diet. Br. J. Nutr. 2002, 88, 515–522. [Google Scholar] [CrossRef]
- Pyndt Jørgensen, B.; Winther, G.; Kihl, P.; Nielsen, D.S.; Wegener, G.; Hansen, A.K.; Sørensen, D.B. Dietary magnesium deficiency affects gut microbiota and anxiety-like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015, 27, 307–311. [Google Scholar] [CrossRef]
- Winther, G.; Pyndt Jørgensen, B.M.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sørensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, J.A.M.; Locatelli, L.; Fedele, G.; Cazzaniga, A.; Mazur, A. Magnesium and the Brain: A Focus on Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 223. https://doi.org/10.3390/ijms24010223
Maier JAM, Locatelli L, Fedele G, Cazzaniga A, Mazur A. Magnesium and the Brain: A Focus on Neuroinflammation and Neurodegeneration. International Journal of Molecular Sciences. 2023; 24(1):223. https://doi.org/10.3390/ijms24010223
Chicago/Turabian StyleMaier, Jeanette A. M., Laura Locatelli, Giorgia Fedele, Alessandra Cazzaniga, and André Mazur. 2023. "Magnesium and the Brain: A Focus on Neuroinflammation and Neurodegeneration" International Journal of Molecular Sciences 24, no. 1: 223. https://doi.org/10.3390/ijms24010223
APA StyleMaier, J. A. M., Locatelli, L., Fedele, G., Cazzaniga, A., & Mazur, A. (2023). Magnesium and the Brain: A Focus on Neuroinflammation and Neurodegeneration. International Journal of Molecular Sciences, 24(1), 223. https://doi.org/10.3390/ijms24010223





