Decellularization of Human Pancreatic Fragments with Pronounced Signs of Structural Changes
Abstract
1. Introduction
2. Results
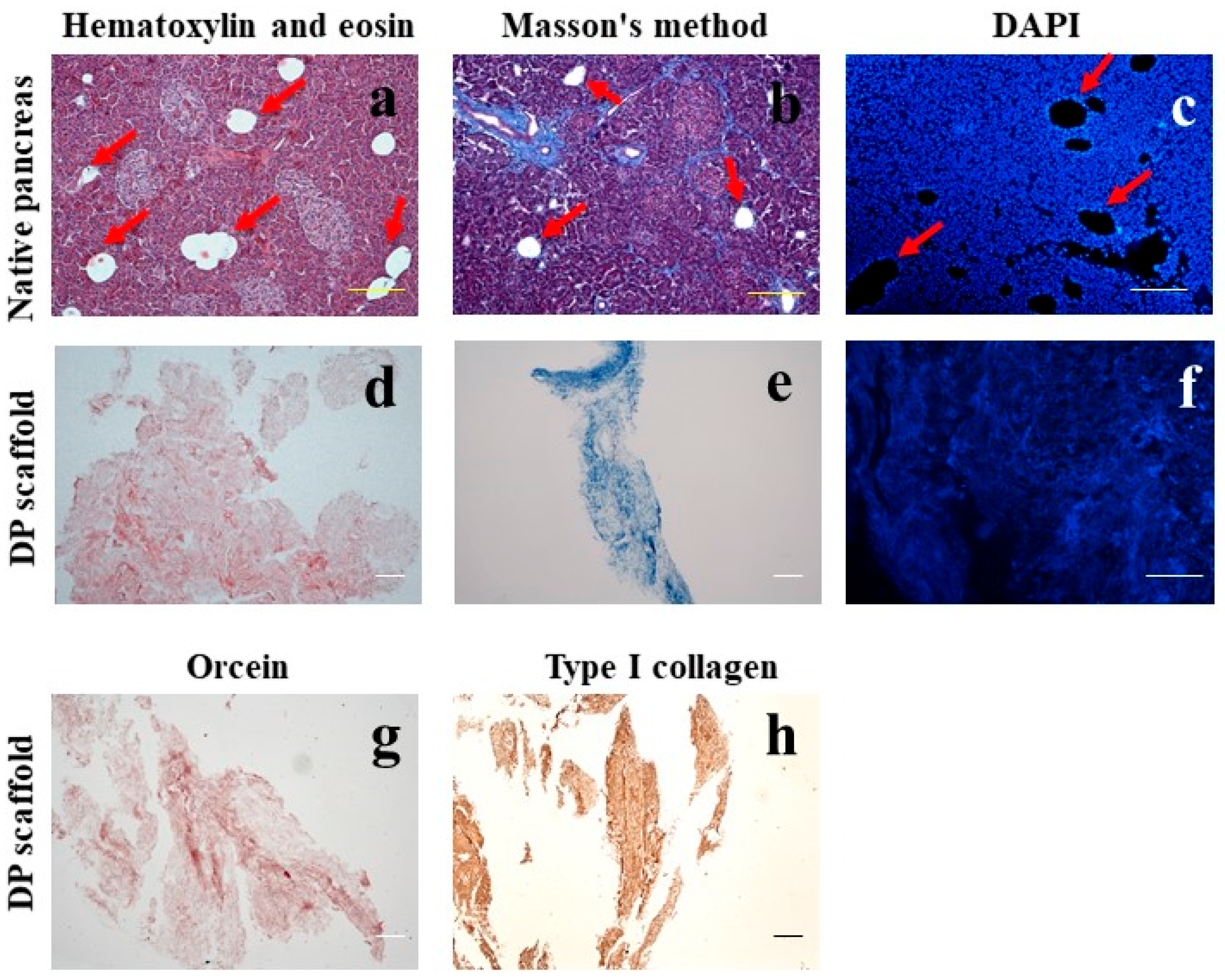
2.1. Morphological Analysis
2.1.1. Morphological Analysis of the Pancreas
2.1.2. Morphological Analysis of the DP Scaffold
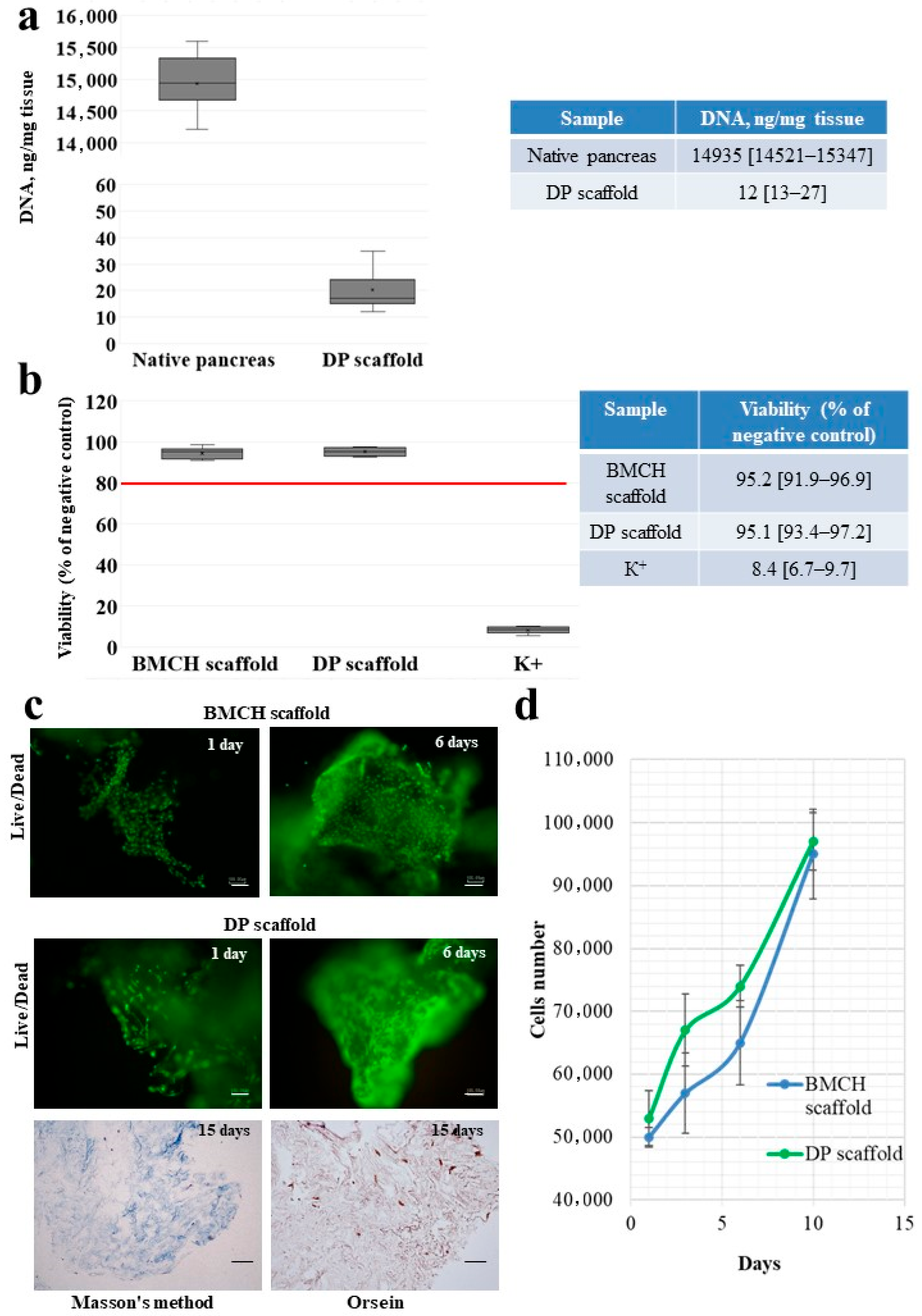
2.2. DNA Content in the DP Scaffold
2.3. Cytotoxicity of the DP Scaffold
2.4. Adhesion and Proliferation of MSC on the Scaffolds
2.5. Freshly Isolated Islets
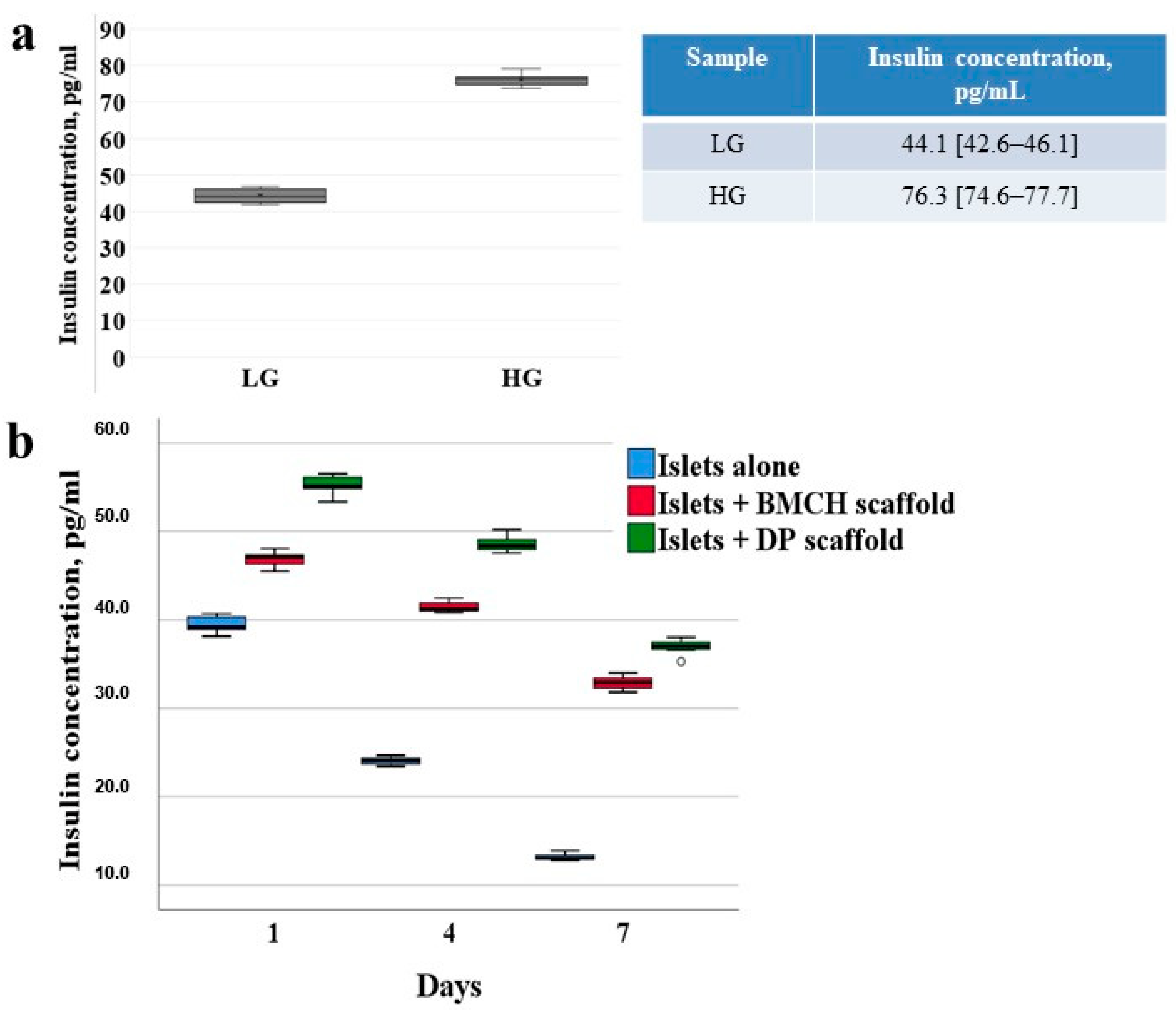
2.6. Viability of Cultured Islets
2.6.1. Islets Alone (Control Group)
2.6.2. Islets with Biopolymer Scaffold
2.6.3. Islets with Tissue-Specific Scaffold
2.7. Islet Insulin-Producing Function
3. Discussion
4. Materials and Methods
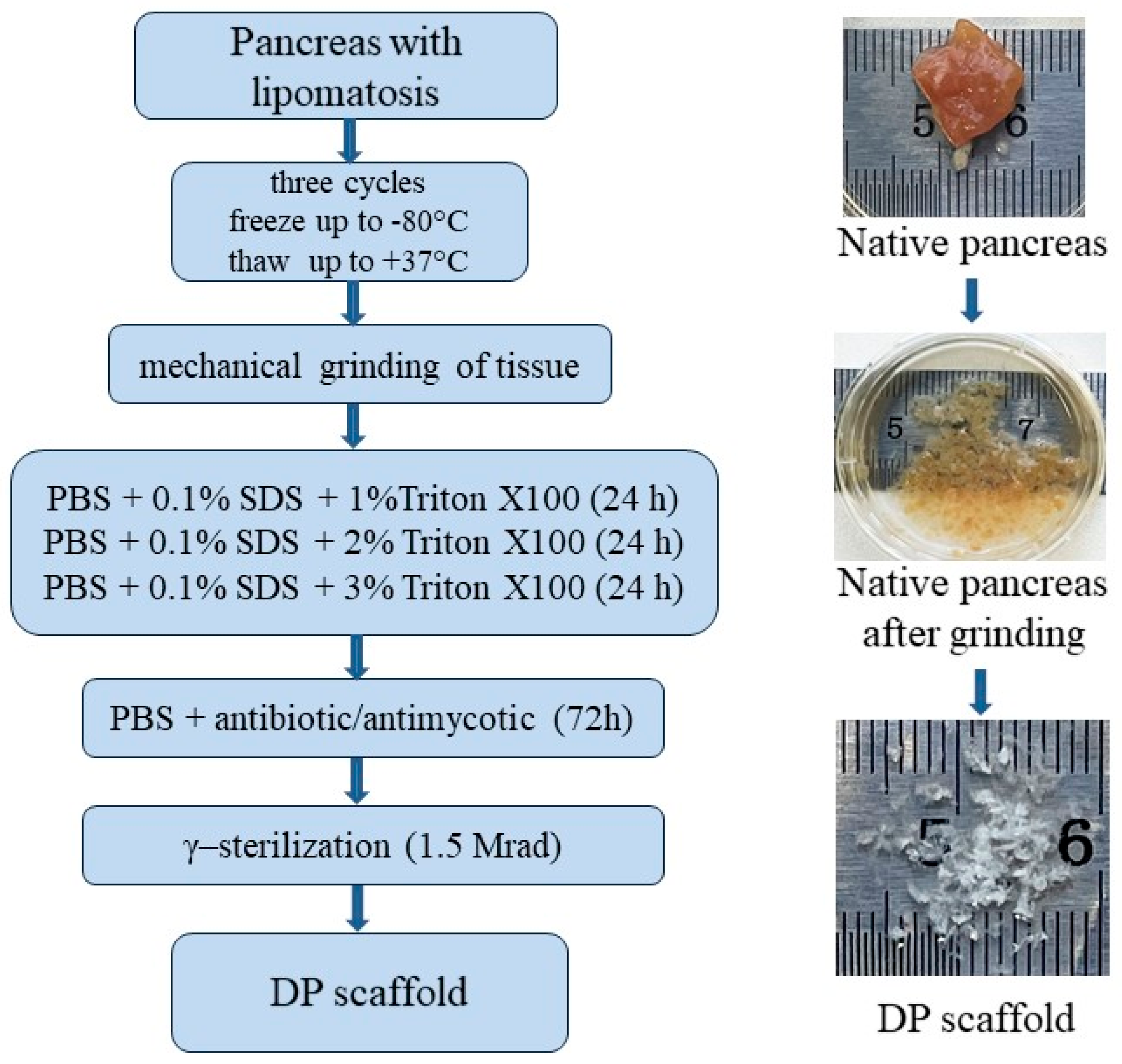
4.1. Pancreas Retrieval and Preparation
4.2. Pancreas Decellularization
4.3. Histology Staining
4.4. DNA Quantification
4.5. Biopolymer Microheterogeneous Collagen-Containing Hydrogel (The BMCH Scaffold)
4.6. Cytotoxicity
4.7. Cell Isolation, Adhesion and Proliferation
4.8. Islets Isolation and Dithizone Staining
4.9. Islet Culture
4.10. Cell Viability Assay
4.11. Enzyme Immunoassay (ELISA)
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eguchi, N.; Kimia Damyar, K.; Alexander, M.; Dafoe, D.; Lakey, J.R.T.; Ichii, H. Anti-oxidative therapy in islet cell transplantation. Antioxidants 2022, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Amer, L.D.; Mahoney, M.J.; Bryant, S.J. Tissue engineering approaches to cell-based type 1 diabetes therapy. Tissue Eng. 2014, 20, 455–467. [Google Scholar] [CrossRef]
- Bosco, D.; Armanet, M.; Morel, P.; Niclauss, N.; Sgroi, A.; Muller, Y.D.; Giovannoni, L.; Parnaud, G.; Berney, T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 2010, 59, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-specific decellularization methods: Rationale and strategies to achieve regenerative compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Riopel, M.; Wang, K. Collagen matrix support of pancreatic islet survival and function. Front. Biosci. 2014, 19, 77–90. [Google Scholar] [CrossRef]
- Stendahl, J.C.; Kaufman, D.B.; Stupp, S.I. Extracellular matrix in pancreatic islets: Relevance to scaffold design and transplantation. Cell Transpl. 2009, 18, 1–12. [Google Scholar] [CrossRef]
- Lemos, N.E.; de Almeida Brondani, L.; Dieter, C.; Rheinheimer, J.; Bouças, A.P.; Leitão, C.B.; Crispim, D.; Bauer, A.C. Use of additives, scaffolds and extracellular matrix components for improvement of human pancreatic islet outcomes in vitro: A systematic review. Islets 2017, 9, 73–86. [Google Scholar] [CrossRef]
- Guruswamy Damodaran, R.; Vermette, P. Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture. J. Tissue Eng. Regen. Med. 2018, 12, 1230–1237. [Google Scholar] [CrossRef]
- Salvatori, M.; Katari, R.; Patel, T.; Peloso, A.; Mugweru, J.; Owusu, K.; Orlando, G. Extracellular matrix scaffold technology for bioartificial pancreas engineering: State of the art and future challenges. J. Diabetes Sci. Technol. 2014, 8, 159–169. [Google Scholar] [CrossRef]
- Fisher, S.A.; Tam, R.Y.; Shoichet, M.S. Tissue mimetics: Engineered hydrogel matrices provide biomimetic environments for cell growth. Tissue Eng. 2014, 20, 895–898. [Google Scholar] [CrossRef]
- Kumar, N.; Joisher, H.; Ganguly, A. Polymeric scaffolds for pancreatic tissue engineering: A review. Rev. Diabet. Stud. 2018, 14, 334–353. [Google Scholar] [CrossRef]
- Gazia, C.; Gaffley, M.; Asthana, A. Scaffolds for pancreatic tissue engineering. In Handbook of Tissue Engineering Scaffolds; Science Direct: Amsterdam, The Netherlands, 2019; Volume 2, pp. 765–786. [Google Scholar] [CrossRef]
- Coronel, M.; Stabler, C. Engineering a local microenvironment for pancreatic islet replacement. Curr. Opin. Biotechnol. 2013, 24, 900–908. [Google Scholar] [CrossRef]
- Napierala, H.; Hillebrandt, K.-H.; Haep, N.; Tang, P.; Tintemann, M.; Gassner, J.; Noesser, M.; Everwien, H.; Seiffert, N.; Kluge, M.; et al. Engineering an endocrine neo-pancreas by repopulation of a decellularized rat pancreas with islets of Langerhans. Sci. Rep. 2017, 2, 41777. [Google Scholar] [CrossRef]
- Llacua, L.A.; Faas, M.M.; de Vos, P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia 2018, 61, 1261–1272. [Google Scholar] [CrossRef]
- Sevastianov, V.I.; Basok, Y.B.; Kirsanova, L.A.; Grigoriev, A.M.; Kirillova, A.D.; Nemets, E.A.; Subbot, A.M.; Gautier, S.V. A comparison of the capacity of mesenchymal stromal cells for cartilage regeneration depending on collagen-based injectable biomimetic scaffold type. Life 2021, 11, 756. [Google Scholar] [CrossRef]
- Abualhassan, N.; Sapozhnikov, L.; Pawlick, R.L.; Kahana, M.; Pepper, A.R.; Bruni, A.; Gala-Lopez, B.; Kin, T.; Mitrani, E.; Shapiro, A.M.J. Lung-derived microscaffolds facilitate diabetes reversal after mouse and human intraperitoneal islet transplantation. PLoS ONE 2016, 11, e0156053. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Tancos, Z.; Feher, L.Z.; Alfoldi, R.; Kobolak, J.; Dinnyes, A.; Puskas, L.G. Real architecture for 3D tissue (RAFT) culture system improves viability and maintains insulin and glucagon production of mouse pancreatic islet cells. Cytotechnology 2017, 69, 359–369. [Google Scholar] [CrossRef]
- Baranova, V.S.N.; Kirsanova, L.A.; Ponomareva, A.S.; Basok, Y.B.; Nemets, E.A.; Gautier, S.V. Comparative analysis of the influence of extracellular matrix biomimetics on the viability and insulin-producing function of isolated pancreatic islets. J. Gene Eng. Bio. Res. 2021, 3, 17–25. [Google Scholar] [CrossRef]
- Rana, D.; Zreigat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef]
- Mirmalek-Sani, S.-H.; Orlando, G.; McQuilling, J.; Pareta, R.; Mack, D.L.; Salvatori, M.; Farney, A.C.; Stratta, R.J.; Atala, A.; Opara, E.C.; et al. Porcine pancreas extracellular matrix as a platform endocrine pancreas bioengineering. Biomaterials 2013, 34, 5488–5495. [Google Scholar] [CrossRef]
- Berkova, Z.; Zacharovova, K.; Patikova, A.; Leontovyc, I.; Hladikova, Z.; Cerveny, D.; Tihlarikova, E.; Nedela, V.; Girman, P.; Jirak, D.; et al. Decellularized pancreatic tail as matrix for pancreatic islet transplantation into the greater omentum in rats. J. Funct. Biomater. 2022, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.K.; Bertera, S.; Olsen, P.; Candiello, J.E.; Halfter, W.; Uechi, G.; Balasubramani, M.; Johnson, S.A.; Sicari, B.M.; Kollar, E.; et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013, 34, 6760–6772. [Google Scholar] [CrossRef] [PubMed]
- Klak, M.; Łojszczyk, I.; Berman, A.; Tymicki, G.; Adamiok-Ostrowska, A.; Sierakowski, M.; Olkowski, R.; Szczepankiewicz, A.; Kamiński, A.; Dobrzyń, A.; et al. Impact of porcine pancreas decellularization conditions on the quality of obtained dECM. Int. J. Mol. Sci. 2021, 22, 7005. [Google Scholar] [CrossRef] [PubMed]
- Sackett, S.D.; Tremmel, D.M.; Ma, F.; Feeney, A.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’Brien, C.; Li, L.; et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. 2018, 8, 10452. [Google Scholar] [CrossRef] [PubMed]
- Shirakigawa, N.; Ijima, H. Decellularized tissue engineering. In Advances in Biomaterials for Biomedical Applications; Springer Nature: Singapore, 2017; pp. 185–226. [Google Scholar] [CrossRef]
- Ponomareva, A.S.; Kirsanova, L.A.; Baranova, N.V.; Surguchenko, V.A.; Bubentsova, G.N.; Basok, Y.B.; Miloserdov, I.A.; Sevastianov, V.I. Decellularization of donor pancreatic fragment to obtain a tissue-specific matrix scaffold. Russ. J. Transpl. Artif. Organs 2020, 22, 123–133. [Google Scholar] [CrossRef]
- Salg, G.A.; Giese, N.A.; Schenk, M.; Hüttner, F.J.; Felix, K.; Probst, P.; Diener, M.K.; Hackert, T.; Kenngott, H.G. The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells. J. Tissue Eng. 2019, 10, 1–25. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Weber, L.M.; Hayda, K.N.; Anseth, K.S. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng. Part A 2008, 14, 1959–1968. [Google Scholar] [CrossRef]
- Lucas-Clerc, C.; Massart, C.; Campion, J.P.; Launois, B.; Nicol, M. Long-term culture of human pancreatic islets in an extracellular matrix: Morphological and metabolic effects. Mol. Cell. Endocrinol. 1993, 94, 9–20. [Google Scholar] [CrossRef]
- Wu, D.; Wan, J.; Huang, Y.; Guo, Y.; Xu, T.; Zhu, M.; Fan, X.; Zhu, S.; Ling, C.; Li, X.; et al. 3D culture of MIN-6 cells on decellularized pancreatic scaffold: In vitro and in vivo study. Biomed. Res. Int. 2015, 2015, 432645. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-engineered grafts from human decellularized extracellular matrices: A systematic review and future perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef]
- Paulo Zambon, J.; Atala, A.; Yoo, J.J. Methods to generate tissue-derived constructs for regenerative medicine applications. Methods 2020, 171, 3–10. [Google Scholar] [CrossRef]
- Starnecker, F.; König, F.; Hagl, C.; Thierfelder, N. Tissue-engineering acellular scaffolds-the significant influence of physical and procedural decellularization factors. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 153–162. [Google Scholar] [CrossRef]
- ISO 10993-5: 1999; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO Standards: Geneva, Switzerland, 2009.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevastianov, V.I.; Ponomareva, A.S.; Baranova, N.V.; Kirsanova, L.A.; Basok, Y.B.; Nemets, E.A.; Kruglov, D.N.; Miloserdov, I.A.; Gautier, S.V. Decellularization of Human Pancreatic Fragments with Pronounced Signs of Structural Changes. Int. J. Mol. Sci. 2023, 24, 119. https://doi.org/10.3390/ijms24010119
Sevastianov VI, Ponomareva AS, Baranova NV, Kirsanova LA, Basok YB, Nemets EA, Kruglov DN, Miloserdov IA, Gautier SV. Decellularization of Human Pancreatic Fragments with Pronounced Signs of Structural Changes. International Journal of Molecular Sciences. 2023; 24(1):119. https://doi.org/10.3390/ijms24010119
Chicago/Turabian StyleSevastianov, Victor I., Anna S. Ponomareva, Natalia V. Baranova, Lyudmila A. Kirsanova, Yulia B. Basok, Evgeniy A. Nemets, Dmitry N. Kruglov, Igor A. Miloserdov, and Sergey V. Gautier. 2023. "Decellularization of Human Pancreatic Fragments with Pronounced Signs of Structural Changes" International Journal of Molecular Sciences 24, no. 1: 119. https://doi.org/10.3390/ijms24010119
APA StyleSevastianov, V. I., Ponomareva, A. S., Baranova, N. V., Kirsanova, L. A., Basok, Y. B., Nemets, E. A., Kruglov, D. N., Miloserdov, I. A., & Gautier, S. V. (2023). Decellularization of Human Pancreatic Fragments with Pronounced Signs of Structural Changes. International Journal of Molecular Sciences, 24(1), 119. https://doi.org/10.3390/ijms24010119





