The Structure and Function of Next-Generation Gingival Graft Substitutes—A Perspective on Multilayer Electrospun Constructs with Consideration of Vascularization
Abstract
1. Introduction
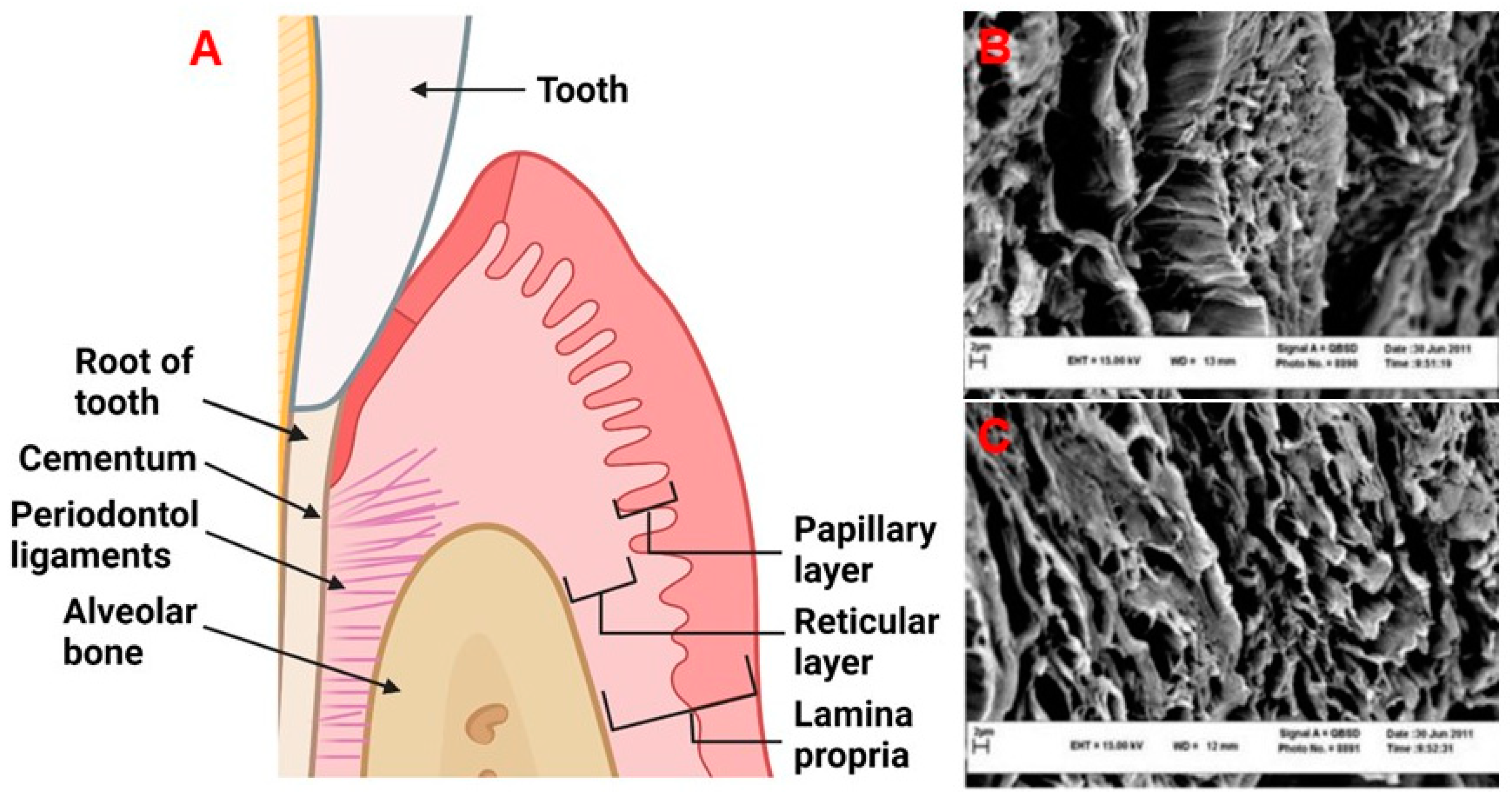
2. Physiology and Disease of the Periodontium and Gingival Tissues: Defining Structure Requirements
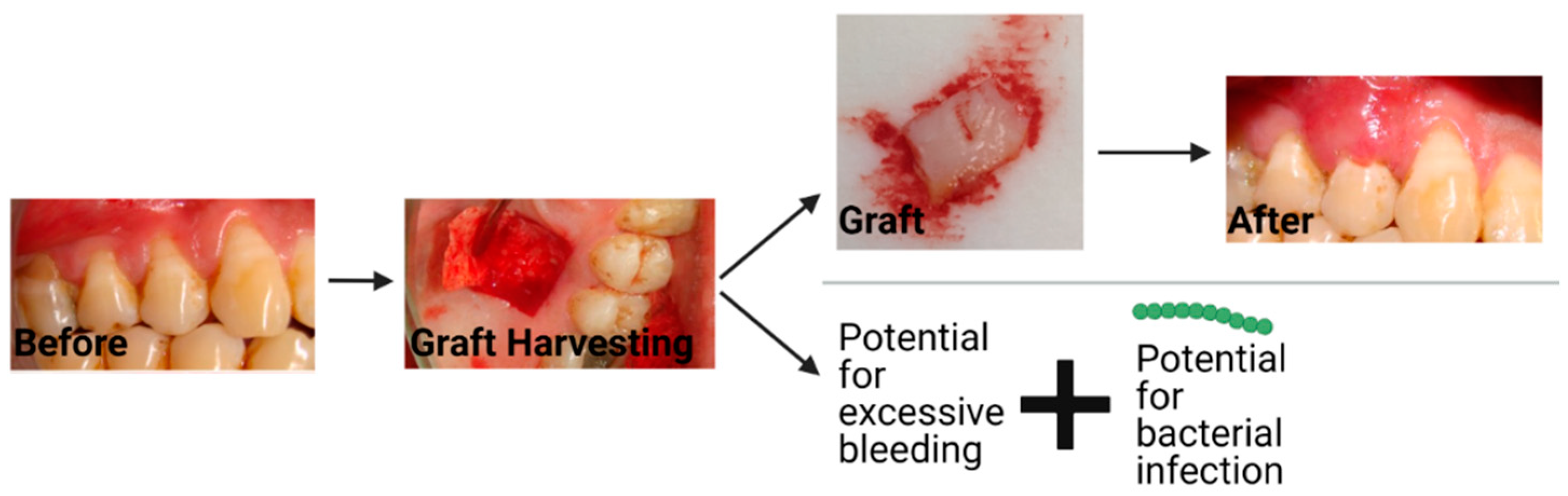
3. Current Material Options for Gingival Recession Treatment
4. Electrospinning Biomaterial Features for Gingival Tissue Engineering
5. Layered Structures in Electrospun Constructs
5.1. Horizontal Layers
5.2. Vertical Layers
6. Conventional Material Approaches
7. Current Direction in Material Development
Degradation of Scaffold Materials and Mechanical Properties
8. Cell Options for Use with Electrospun Scaffolds
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kassab, M.M.; Cohen, R.E. The etiology and prevalence of gingival recession. J. Am. Dent. Assoc. 2003, 134, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Thronton-Evans, G.O.; Genco, R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Merijohn, G.K. Management and prevention of gingival recession. Periodontol. 2000 2016, 71, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Colley, H.; Murdoch, C.; Hearnden, V.; Chai, W.L.; Brook, I.M.; Thornhill, M.H.; MacNeil, S. Tissue-engineered oral mucosa. J. Dent. Res. 2012, 91, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Mancini, L.; Fratini, A.; Tarallo, F.; Americo, L.M.; Marchetti, E. 3D analysis at implant sites after soft tissue augmentation with two types of collagen matrices: A pilot study. Plast. Aesthetic Res. 2021, 8, 26. [Google Scholar] [CrossRef]
- Kim, D.M.; Neiva, R. Periodontal Soft Tissue Non–Root Coverage Procedures: A Systematic Review From the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S56–S72. [Google Scholar] [CrossRef]
- Goudouri, O.M.; Kontonasaki, E.; Boccaccini, A.R. Layered Scaffolds for Periodontal Regeneration; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081009673. [Google Scholar]
- Benatti, B.B.; Silvério, K.G.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Physiological features of periodontal regeneration and approaches for periodontal tissue engineering utilizing periodontal ligament cells. J. Biosci. Bioeng. 2007, 103, 1–6. [Google Scholar] [CrossRef]
- Herford, A.S.; Akin, L.; Cicciu, M.; Maiorana, C.; Boyne, P.J. Use of a Porcine Collagen Matrix as an Alternative to Autogenous Tissue for Grafting Oral Soft Tissue Defects. J. Oral Maxillofac. Surg. 2010, 68, 1463–1470. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhu, M.; Wang, L.; Xiao, N.; Kong, D. Wet-spun poly (ε-caprolactone) microfiber scaffolds for oriented growth and infiltration of smooth muscle cells. Mater. Lett. 2014, 132, 59–62. [Google Scholar] [CrossRef]
- Smirani, R.; Rémy, M.; Devillard, R.; Naveau, A. Engineered Prevascularization for Oral Tissue Grafting: A Systematic Review. Tissue Eng. Part B Rev. 2020, 26, 383–398. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Brook, I.M.; Van Noort, R.; Scutt, A.M.; Thornhill, M.H. Tissue-engineered oral mucosa: A review of the scientific literature. J. Dent. Res. 2007, 86, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.E.; Listgarten, M.A. The gingival tissues: The architecture of periodontal protection. Periodontol. 2000 1997, 13, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Jati, A.S.; Furquim, L.Z.; Consolaro, A. Gingival recession: Its causes and types, and the importance of orthodontic treatment. Dental Press J. Orthod. 2016, 21, 18–29. [Google Scholar] [CrossRef]
- Mahdavishahri, N.; Matin, M.M.; Fereidoni, M.; Yarjanli, Z.; Rad, S.A.B.; Ahmadi, S.K. In vitro assay of human gingival scaffold in differentiation of rat’s bone marrow mesenchymal stem cells to keratinocystes. Iran. J. Basic Med. Sci. 2012, 15, 1185–1190. [Google Scholar] [CrossRef]
- Kaur, P.; Kakar, V. Collagen: Role in oral tissues: A review. Int. J. Sci. Res. 2014, 3, 273–276. [Google Scholar]
- Vindin, H.; Mithieux, S.M.; Weiss, A.S. Elastin architecture. Matrix Biol. 2019, 84, 4–16. [Google Scholar] [CrossRef]
- Schoen, F.J.; Mitchell, R. Tissues, the Extracellular Matrix, and Cell Biomaterial Interactions, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Oxford, UK, 2013. [Google Scholar]
- Hughes, C.C.W. Endothelial-stromal interactions in angiogenesis. Curr. Opin. Hematol. 2008, 15, 204–209. [Google Scholar] [CrossRef]
- Pitaru, S.; Melcher, A.H. Orientation of gingival fibroblasts and newly-synthesized collagen fibers in vitro: Resemblance to transseptal and dento-gingival fibers. J. Periodontal Res. 1983, 18, 483–500. [Google Scholar] [CrossRef]
- Bullon, P.; Fioroni, M.; Goteri, G.; Rubini, C.; Battino, M. Immunohistochemical analysis of soft tissues in implants with healthy and peri-implantitis condition, and aggressive periodontitis. Clin. Oral Implant. Res. 2004, 15, 553–559. [Google Scholar] [CrossRef]
- Yoshida, S.; Noguchi, K.; Imura, K.; Miwa, Y.; Sunohara, M.; Sato, I. A morphological study of the blood vessels associated with periodontal probing depth in human gingival tissue. Okajimas Folia Anat. Jpn. 2011, 88, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Le, N.M.; Song, S.; Zhou, H.; Xu, J.; Li, Y.; Sung, C.E.; Sadr, A.; Chung, K.H.; Subhash, H.M.; Kilpatrick, L.; et al. A noninvasive imaging and measurement using optical coherence tomography angiography for the assessment of gingiva: An in vivo study. J. Biophotonics 2018, 11, e201800242. [Google Scholar] [CrossRef] [PubMed]
- Mikecs, B.; Vág, J.; Gerber, G.; Molnár, B.; Feigl, G.; Shahbazi, A. Revisiting the vascularity of the keratinized gingiva in the maxillary esthetic zone. BMC Oral Health 2021, 21, 160. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Molnár, E.; Molnár, B.; Lohinai, Z.; Tóth, Z.; Benyó, Z.; Hricisák, L.; Windisch, P.; Vág, J. Evaluation of Laser Speckle Contrast Imaging for the Assessment of Oral Mucosal Blood Flow following Periodontal Plastic Surgery: An Exploratory Study. Biomed Res. Int. 2017, 2017, 4042902. [Google Scholar] [CrossRef]
- Patel, M.; Nixon, P.J.; Chan, M.F.W. Gingival recession: Part 3. Surgical management using free grafts and guided tissue regeneration. Nat. Publ. Gr. 2011, 211, 353–358. [Google Scholar] [CrossRef][Green Version]
- Schmitt, C.M.; Tudor, C.; Kiener, K.; Wehrhan, F.; Schmitt, J.; Eitner, S.; Agaimy, A.; Schlegel, K.A. Vestibuloplasty: Porcine Collagen Matrix Versus Free Gingival Graft: A Clinical and Histologic Study. J. Periodontol. 2013, 84, 914–923. [Google Scholar] [CrossRef]
- Bassetti, R.G.; Stähli, A.; Bassetti, M.A.; Sculean, A. Soft tissue augmentation around osseointegrated and uncovered dental implants: A systematic review. Clin. Oral Investig. 2017, 21, 53–70. [Google Scholar] [CrossRef]
- Trivedi, S.R.; Bhavsar, N.V.; Dulani, K.; Trivedi, R. Clinical evaluation of subepithelial connective tissue graft and guided tissue regeneration for treatment of Miller’s class 1 gingival recession (comparative, split mouth, six months study). J. Clin. Exp. Dent. 2014, 6, 218–224. [Google Scholar] [CrossRef]
- Dragan, I.F.; Hotlzman, L.P.; Karimbux, N.Y.; Morin, R.A.; Bassir, S.H. Clinical Outcomes of Comparing Soft Tissue Alternatives to Free Gingival Graft: A Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2017, 17, 370–380. [Google Scholar] [CrossRef]
- Smith, P.C.; Martínez, C.; Martínez, J.; Mcculloch, C.A. Role of fibroblast populations in periodontal wound healing and tissue remodeling. Front. Physiol. 2019, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Sudbeck, B.D.; Pilcher, B.K.; Welgus, H.G.; Parks, W.C. Induction and repression of collagenase-1 by keratinocytes is controlled by distinct components of different extracellular matrix compartments. J. Biol. Chem. 1997, 272, 22103–22110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lim, J.; Liu, J.; Ponugoti, B.; Alsadun, S.; Tian, C.; Vafa, R.; Graves, D.T. FOXO1 expression in keratinocytes promotes connective tissue healing. Sci. Rep. 2017, 7, 42834. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.; Fontao, L.; Chaponnier, C.; Vasiliev, J.; Gabbiani, G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J. Cell Sci. 2001, 114, 3285–3296. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.; Broad, S.; Diekmann, D.; Evans, R.D.; Watt, F.M. β1 Integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol. Biol. Cell 2000, 11, 453–466. [Google Scholar] [CrossRef]
- Groeger, S.E.; Meyle, J. Epithelial barrier and oral bacterial infection. Periodontol. 2000 2015, 69, 46–67. [Google Scholar] [CrossRef]
- Narayan Biswal, B.; Narayan Das, S.; Kumar Das, B.; Rath, R. Alteration of cellular metabolism in cancer cells and its therapeutic. J. Oral Maxillofac. Pathol. 2017, 21, 244–251. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Koyama, T.; Kojima, T.; Kato, H.; Ono, Y.; Saito, C. Keratinocytes of tissue-engineered human oral mucosa promote re-epithelialization after intraoral grafting in athymic mice. J. Oral Maxillofac. Surg. 2012, 70, 1199–1214. [Google Scholar] [CrossRef]
- Izumi, K.; Feinberg, S.E.; Terashi, H.; Marcelo, C.L. Evaluation of transplanted tissue-engineered oral mucosa equivalents in severe combined immunodeficient mice. Tissue Eng. 2003, 9, 163–174. [Google Scholar] [CrossRef]
- McGuire, M.K.; Scheyer, E.T. Randomized, Controlled Clinical Trial to Evaluate a Xenogeneic Collagen Matrix as an Alternative to Free Gingival Grafting for Oral Soft Tissue Augmentation. J. Periodontol. 2014, 85, 1333–1341. [Google Scholar] [CrossRef]
- Vallecillo, C.; Toledano-Osorio, M.; Vallecillo-Rivas, M.; Toledano, M.; Osorio, R. In Vitro biodegradation pattern of collagen matrices for soft tissue augmentation. Polymers 2021, 13, 2633. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue response to a porous collagen matrix used for soft tissue augmentation. Materials 2019, 12, 3721. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.L.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.-C.; Laurell, L.; Geivelis, M.; Lingen, M.W.; Maddalozzo, D.; Increased, A.; Gingiva, A. Acellular Dermal Matrix Allografts to Achieve Increased Attached Gingiva. Part 1. A Clinical Study. J. Periodontol. 2000, 71, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Romeu, D.; De Resende, B.; Luiz, S.; Greghi, A.; Siqueira, A.F.; Augusto, C.; Benfatti, M.; Damante, C.A.; Schutzer, M.; Zangrando, R.; et al. Acellular dermal matrix allograft versus free gingival graft: A histological evaluation and split-mouth randomized clinical trial. Clin. Oral Investig. 2019, 23, 539–550. [Google Scholar] [CrossRef]
- Baker, B.M.; Handorf, A.M.; Ionescu, L.C.; Li, W.J.; Mauck, R.L. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert Rev. Med. Devices 2009, 6, 515–532. [Google Scholar] [CrossRef]
- Liao, S.; Li, B.; Ma, Z.; Wei, H.; Chan, C.; Ramakrishna, S. Biomimetic electrospun nanofibers for tissue regeneration. Biomed. Mater. 2006, 1, R45–R53. [Google Scholar] [CrossRef]
- Fomby, P.; Cherlin, A.J.; Hadjizadeh, A.; Doillon, C.J.; Sueblinvong, V.; Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; et al. A review of key challenges of electropsun scaffolds for tissue-engineering applications. Ann. Am. Thorac. Soc. 2010, 12, 181–204. [Google Scholar] [CrossRef]
- Ortega, Í.; Ryan, A.J.; Deshpande, P.; MacNeil, S.; Claeyssens, F. Combined microfabrication and electrospinning to produce 3-D architectures for corneal repair. Acta Biomater. 2013, 9, 5511–5520. [Google Scholar] [CrossRef]
- Zou, B.; Liu, Y.; Luo, X.; Chen, F.; Guo, X.; Li, X. Electrospun fibrous scaffolds with continuous gradations in mineral contents and biological cues for manipulating cellular behaviors. Acta Biomater. 2012, 8, 1576–1585. [Google Scholar] [CrossRef]
- Reid, J.A.; Mcdonald, A.; Id, A.C. Modulating electrospun polycaprolactone scaffold morphology and composition to alter endothelial cell proliferation and angiogenic gene response. PLoS ONE 2020, 15, e0240332. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Shukla, K.P.; Moctezuma, M.; Liping, T. Cellular and molecular responses of smooth muscle cells to surface nanotopography. J. Nanosci. Nanotechnol. 2007, 7, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef]
- Yang, M.; Guo, Z.; Li, T.; Li, J.; Chen, L.; Wang, J.; Wu, J.; Wu, Z. Synergetic effect of chemical and topological signals of gingival regeneration scaffold on the behavior of human gingival fibroblasts. J. Biomed. Mater. Res. Part A 2019, 107, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shin, H.J.; Cho, I.H.; Kang, Y.M.; Kim, I.A.; Park, K.D.; Shin, J.W. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials 2005, 26, 1261–1270. [Google Scholar] [CrossRef]
- Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun fibrous mats with high porosity as potential scaffolds for skin tissue engineering. Biomacromolecules 2008, 9, 1795–1801. [Google Scholar] [CrossRef]
- Türker, E.; Yildiz, Ü.H.; Arslan Yildiz, A. Biomimetic hybrid scaffold consisting of co-electrospun collagen and PLLCL for 3D cell culture. Int. J. Biol. Macromol. 2019, 139, 1054–1062. [Google Scholar] [CrossRef]
- Goyal, R.; Vega, M.E.; Pastino, A.K.; Singh, S.; Guvendiren, M.; Kohn, J.; Murthy, N.S.; Schwarzbauer, J.E. Development of hybrid scaffolds with natural extracellular matrix deposited within synthetic polymeric fibers. J. Biomed. Mater. Res. Part A 2017, 105, 2162–2170. [Google Scholar] [CrossRef]
- Wright, L.D.; Andric, T.; Freeman, J.W. Utilizing NaCl to increase the porosity of electrospun materials. Mater. Sci. Eng. C 2011, 31, 30–36. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Green, E.M.; Mansfield, J.C.; Bell, J.S.; Winlove, C.P. The structure and micromechanics of elastic tissue. Interface Focus 2014, 4, 20130058. [Google Scholar] [CrossRef] [PubMed]
- Han, D.G.; Ahn, C.B.; Lee, J.H.W.; Hwang, Y.; Kim, J.H.; Park, K.Y.; Lee, J.H.W.; Son, K.H. Optimization of electrospun poly(caprolactone) fiber diameter for vascular scaffolds to maximize smooth muscle cell infiltration and phenotype modulation. Polymers 2019, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Yang, S.; Yu, S.; Banerjee, A.; Myung, N.V.; Nam, J. Modulation of piezoelectric properties in electrospun PLLA nanofibers for application-specific self-powered stem cell culture platforms. Nano Energy 2021, 89, 106444. [Google Scholar] [CrossRef]
- Goonoo, N. Modulating Immunological Responses of Electrospun Fibers for Tissue Engineering. Adv. Biosyst. 2017, 1, 1700093. [Google Scholar] [CrossRef] [PubMed]
- Ooya, K.; Yooya, Y. Scanning electron microscopy of the epithelium-connective tissue interface in human gingiva. J. Periodontal Res. 1981, 16, 135–139. [Google Scholar] [CrossRef]
- Winning, T.A.; Townsend, G.C. Oral Mucosal Embryology and Histology. Clin. Dermatol. 2000, 18, 499–511. [Google Scholar] [CrossRef]
- McCullen, S.D.; Miller, P.R.; Gittard, S.D.; Gorga, R.E.; Pourdeyhimi, B.; Narayan, R.J.; Loboa, E.G. In situ collagen polymerization of layered cell-seeded electrospun scaffolds for bone tissue engineering applications. Tissue Eng. Part C Methods 2010, 16, 1095–1105. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Yang, Y.; Trivedi, H.M.; Masters, J.G.; Grenier, D. A Dual Zinc plus Arginine formulation attenuates the pathogenic properties of Porphyromonas gingivalis and protects gingival keratinocyte barrier function in an in vitro model. J. Oral Microbiol. 2020, 12, 1798044. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Tait, A.; Glynne-Jones, P.; Hill, A.R.; Smart, D.E.; Blume, C.; Hammarstrom, B.; Fisher, A.L.; Grossel, M.C.; Swindle, E.J.; Hill, M.; et al. Engineering multi-layered tissue constructs using acoustic levitation. Sci. Rep. 2019, 9, 9789. [Google Scholar] [CrossRef] [PubMed]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 3481–3498. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G.; Tarone, G. Positional Control of Cell Fate Through Joint Integrin/Receptor Protein Kinase Signaling. Annu. Rev. Cell Dev. Biol. 2003, 19, 173–206. [Google Scholar] [CrossRef] [PubMed]
- Mostefaoui, Y.; Claveau, I.; Rouabhia, M. In vitro analyses of tissue structure and interleukin-1β expression and production by human oral mucosa in response to Candida albicans infections. Cytokine 2004, 25, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Pitaru, S.; Melcher, A.H. Organization of an oriented fiber system in vitro by human gingival fibroblasts attached to dental tissue: Relationship between cells and mineralized and demineralized tissue. J. Periodontal Res. 1987, 22, 6–13. [Google Scholar] [CrossRef]
- Aukhil, I.; Fernyhough, W.S. Orientation of gingival fibroblasts in simulated periodontol spaces in vitro: SEM observations. J. Periodontol. 1986, 57, 405–412. [Google Scholar] [CrossRef]
- Nakajima, K.; Abe, T.; Tanaka, M.; Hara, Y. Periodontal tissue engineering by transplantation of multilayered sheets of phenotypically modified gingival fibroblasts. J. Periodontal Res. 2008, 43, 681–688. [Google Scholar] [CrossRef]
- Clark, R.A.F.; McCoy, G.A.; Folkvord, J.M.; McPherson, J.M. TGF-β1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: A fibronectin matrix-dependent event. J. Cell. Physiol. 1997, 170, 69–80. [Google Scholar] [CrossRef]
- Karande, T.S.; Ong, J.L.; Agrawal, M.C. Diffusion in musculoskeletal tissue engineering scaffolds: Design issues related to porosity, permeability, architecture, and nutrient mixing. Ann. Biomed. Eng. 2004, 32, 1728–1743. [Google Scholar] [CrossRef]
- Hong, S.; Jung, B.Y.; Hwang, C. Multilayered Engineered Tissue Sheets for Vascularized Tissue Regeneration. Tissue Eng. Regen. Med. 2017, 14, 371–381. [Google Scholar] [CrossRef]
- Amirsadeghi, A.; Jafari, A.; Eggermont, L.J.; Hashemi, S.S.; Bencherif, S.A.; Khorram, M. Vascularization strategies for skin tissue engineering. Biomater. Sci. 2020, 8, 4052–4073. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Brook, I.M.; Van Noort, R.; Scutt, A. Development, optimization and characterization of a full-thickness tissue engineered human oral mucosal model for biological assessment of dental biomaterials. J. Mater. Sci. Med. 2008, 19, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.W.C.; Rose, E.E.; Santerre, J.P. Perfused culture of gingival fibroblasts in a degradable/polar/hydrophobic/ionic polyurethane (D-PHI) scaffold leads to enhanced proliferation and metabolic activity. Acta Biomater. 2013, 9, 6867–6875. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Benner, M.; Fienitz, T.; Happe, A.; Kreppel, M.; Nickenig, H.J.; Zöller, J.E. Biodegradation pattern and tissue integration of native and cross-linked porcine collagen soft tissue augmentation matrices—An experimental study in the rat. Head Face Med. 2014, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef]
- Strange, A.P. Quantitative Nanohistology: Collagen Disorders of Connective and Mineralised Tissues; University College London Eastman Dental Institute: London, UK, 2019. [Google Scholar]
- Choi, J.J.E.; Zwirner, J.; Ramani, R.S.; Ma, S.; Hussaini, H.M.; Waddell, J.N. Mechanical properties of human oral mucosa tissues are site dependent: A combined biomechanical, histological and ultrastructural approach. Clin. Exp. Dent. Res. 2020, 6, 602–611. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Zhang, S.; Sculean, A.; Staehli, A.; Bosshardt, D.D. Tissue integration and degradation of a porous collagen-based scaffold used for soft tissue augmentation. Materials 2020, 13, 2420. [Google Scholar] [CrossRef]
- Friberg, B.; Jemt, T. Soft Tissue Augmentation in Connection to Dental Implant Treatment Using a Synthetic, Porous Material—A Case Series with a 6-Month Follow-Up. Clin. Implant Dent. Relat. Res. 2012, 14, 872–881. [Google Scholar] [CrossRef]
- Gisselfält, K.; Edberg, B.; Flodin, P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules 2002, 3, 951–958. [Google Scholar] [CrossRef]
- Wolff, J.; Farré-Guasch, E.; Sándor, G.K.; Gibbs, S.; Jager, D.J.; Forouzanfar, T. Soft tissue augmentation techniques and materials used in the oral cavity: An overview. Implant Dent. 2016, 25, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Danesh-meyer, M.J.; Wikesjö, U.M.E.; Wikesjo, U.M.E. Gingival recession defects and guided tissue regeneration: A review. J. Periodontal Res. 2001, 36, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Buurma, B.; Gu, K.; Transplantation, R.R.; Buurma, B.; Gu, K.; Rutherford, R.B. Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. Eur. J. Oral Sci. 1999, 107, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.; Bullock, A.J.; Blackwood, K.A.; Chapple, C.R.; Macneil, S. Developing biodegradable scaffolds for tissue engineering of the urethra. BJU Int. 2010, 107, 296–302. [Google Scholar] [CrossRef]
- Blackwood, K.A.; McKean, R.; Canton, I.; Freeman, C.O.; Franklin, K.L.; Cole, D.; Brook, I.; Farthing, P.; Rimmer, S.; Haycock, J.W.; et al. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials 2008, 29, 3091–3104. [Google Scholar] [CrossRef]
- Hakki, S.S.; Korkusuz, P.; Purali, N.; Bozkurt, B.; Kus, M.; Duran, I. Attachment, proliferation and collagen type I mRNA expression of human gingival fibroblasts on different biodegradable membranes. Connect. Tissue Res. 2013, 54, 260–266. [Google Scholar] [CrossRef]
- Kriegebaum, U.; Mildenberger, M.; Mueller-Richter, U.D.A.; Klammert, U.; Kuebler, A.C.; Reuther, T. Tissue engineering of human oral mucosa on different scaffolds: In vitro experiments as a basis for clinical applications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, S190–S198. [Google Scholar] [CrossRef]
- Chahal, S.; Kumar, A.; Hussian, F.S.J. Development of biomimetic electrospun polymeric biomaterials for bone tissue engineering. A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 1308–1355. [Google Scholar] [CrossRef]
- Zuhr, O.; Bäumer, D.; Hürzeler, M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: Critical elements in design and execution. J. Clin. Periodontol. 2014, 41, S123–S142. [Google Scholar] [CrossRef]
- Hanumantharao, S.N.; Rao, S. Multi-Functional Electrospun Nanofibers from Polymer Blends for Scaffold Tissue Egnineering. Fibers 2019, 7, 66. [Google Scholar] [CrossRef]
- Aytac, Z.; Dubey, N.; Daghrery, A.; Ferreira, J.A.; de Souza Araújo, I.J.; Castilho, M.; Malda, J.; Bottino, M.C. Innovations in craniofacial bone and periodontal tissue engineering–from electrospinning to converged biofabrication. Int. Mater. Rev. 2021, 67, 347–384. [Google Scholar] [CrossRef]
- Piccirillo, G.; Ditaranto, M.V.; Feuerer, N.F.S.; Carvajal Berrio, D.A.; Brauchle, E.M.; Pepe, A.; Bochicchio, B.; Schenke-Layland, K.; Hinderer, S. Non-invasive characterization of hybrid gelatin:poly-l-lactide electrospun scaffolds using second harmonic generation and multiphoton imaging. J. Mater. Chem. B 2018, 6, 6399–6412. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, M.A.; Rabiee, S.M.; Jahanshahi, M.; Nasiri, F. Electrospun Poly-ε-Caprolactone (PCL)/Dicalcium Phosphate Dihydrate (DCPD) Composite Scaffold for Tissue Engineering Application. Mol. Biotechnol. 2019, 61, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Álvarez-Pérez, M.A.; Genesca, J.; Gómez, K.K.; Covelo, A. Evaluation of the biocompatibility of a PVA/SA scaffold with a human gingival fibroblast (HGF) by using electrochemical impedance spectroscopy. Bioelectrochemistry 2020, 131, 107386. [Google Scholar] [CrossRef]
- Black, J.P.; Sefton, M.V. Complement activation by PVA as measured by ELIFA (enzyme-linked immunoflow assay) for SC5b-9. Biomaterials 2000, 21, 2287–2294. [Google Scholar] [CrossRef]
- Cazander, G.; Jukema, G.N.; Nibbering, P.H. Complement activation and inhibition in wound healing. Clin. Dev. Immunol. 2012, 2012, 534291. [Google Scholar] [CrossRef]
- Guo, Z.; Genlong, J.; Huang, Z.; Li, H.; Ge, Y.; Wu, Z.; Yu, P.; Li, Z. Synergetic effect of growth factor and topography on fibroblast proliferation. Biomed. Phys. Eng. Express 2020, 6, 065036. [Google Scholar] [CrossRef]
- Baranowska-Korczyc, A.; Warowicka, A.; Jasiurkowska-Delaporte, M.; Grześkowiak, B.; Jarek, M.; Maciejewska, B.M.; Jurga-Stopa, J.; Jurga, S. Antimicrobial electrospun poly(ε-caprolactone) scaffolds for gingival fibroblast growth. RSC Adv. 2016, 6, 19647–19656. [Google Scholar] [CrossRef]
- Wright, M.E.; Parrag, I.C.; Yang, M.; Santerre, J.P. Electrospun polyurethane nanofiber scaffolds with ciprofloxacin oligomer versus free ciprofloxacin: Effect on drug release and cell attachment. J. Control. Release 2017, 250, 107–115. [Google Scholar] [CrossRef]
- Vidal-gutiérrez, X.; Prado-prone, G.; Rodil, S.E.; Velasquillo, C. Bismuth subsalicylate incorporated in polycaprolactone-gelatin membranes by electrospinning to prevent bacterial colonization Bismuth subsalicylate incorporated in polycaprolactone-gelatin membranes by electrospinning to prevent bacterial colonization. Biomed. Mater 2021, 16, 045036. [Google Scholar] [CrossRef]
- Wright, M.E.E.; Wong, A.T.; Levitt, D.; Parrag, I.C.; Yang, M.; Santerre, P.J. Influence of ciprofloxacin-based additives on the hydrolysis of nanofiber polyurethane membranes. J. Biomed. Mater. Res. Part A 2018, 106, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Han, Y.; Zhang, F.; Ge, Z.; Liu, X.; Lu, Q. Buccal mucosa repair with electrospun silk fibroin matrix in a rat model. Int. J. Artif. Organs 2015, 38, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Xin, T.; Xiao, W.; Zhu, H.; Zhang, Q.; Liu, L.; Cheng, R.; Wang, Z. Vascularized silk electrospun fiber for promoting oral mucosa regeneration. NPG Asia Mater. 2020, 12, 39. [Google Scholar] [CrossRef]
- Canciani, E.; Gagliano, N.; Paino, F. Polyblend Nano fibers to Regenerate Gingival Tissue: A Preliminary In Vitro Study. Front. Mater. 2021, 8, 184. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Joshi, M.K.; Lee, J.; Maharjan, B.; Ko, S.W.; Park, C.H.; Kim, C.S. Heterogeneous electrospun polycaprolactone/polyethylene glycol membranes with improved wettability, biocompatibility, and mineralization. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 105–113. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Mathes, S.H.; Wohlwend, L.; Uebersax, L.; Von Mentlen, R.; Thoma, D.S.; Jung, R.E.; GöRlach, C.; Graf-Hausner, U.; Von Mentlen, R.; Thoma, D.S.; et al. A bioreactor test system to mimic the biological and mechanical environment of oral soft tissues and to evaluate substitutes for connective tissue grafts. Biotechnol. Bioeng. 2010, 107, 1029–1039. [Google Scholar] [CrossRef]
- Berton, F.; Porrelli, D.; Di Lenarda, R.; Turco, G. A Critical Review on the production of electrospun nanofibres for guided bone regeneration in oral surgery. Nanomaterials 2020, 10, 16. [Google Scholar] [CrossRef]
- Billiar, K.; Murray, J.; Laude, D.; Abraham, G.; Bachrach, N. Effects of carbodiimide crosslinking conditions on the physical properties of laminated intestinal submucosa. J. Biomed. Mater. Res. 2001, 56, 101–108. [Google Scholar] [CrossRef]
- da Silva Pereira, S.L.; Sallum, A.W.; Casati, M.Z.; Caffesse, R.G.; Weng, D.; Nociti, F.H.; Sallum, E.A. Comparison of Bioabsorbable and Non-Resorbable Membranes in the Treatment of Dehiscence-Type Defects. A Histomorphometric Study in Dogs. J. Periodontol. 2000, 71, 1306–1314. [Google Scholar] [CrossRef]
- Koibayash, K.; Suzuki, T.; Nomoto, Y.; Tada, Y.; Miyake, M.; Hazama, A.; Wada, I.; Nakamura, T.; Omori, K. A tissue-engineered trachea derived from a framed collagen scaffold, gingival fibroblasts and adipose-derived stem cells. Biomaterials 2010, 31, 4855–4863. [Google Scholar] [CrossRef] [PubMed]
- Costea, D.E.; Loro, L.L.; Dimba, E.A.O.; Vintermyr, O.K.; Johannessen, A.C. Crucial Effects of Fibroblasts and Keratinocyte Growth Factor on Morphogenesis of Reconstituted Human Oral Epithelium. J. Investig. Dermatol. 2003, 121, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, X.; Yan, Y.; Zhang, R. Liver tissue responses to gelatin and gelatin/chitosan gels. J. Biomed. Mater. Res. Part A 2008, 87, 62–68. [Google Scholar] [CrossRef]
- Leszczynski, R.; Stodolak, E.; Wieczorek, J.; Orlowska-Heitzman, J.; Gumula, T.; Blazewicz, S. In vivo biocompatibility assessment of (PTFE-PVDF-PP) terpolymer-based membrane with potential application for glaucoma treatment. J. Mater. Sci. Mater. Med. 2010, 21, 2843–2851. [Google Scholar] [CrossRef]
- Keun Kwon, I.; Kidoaki, S.; Matsuda, T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: Structural characteristics, mechanical properties and cell adhesion potential. Biomaterials 2005, 26, 3929–3939. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Qi, N. Controllable strucutre, properties, and degradation of the electrospun PLGA/PLA-blended nanofibrous scaffolds. J. Appl. Polym. Sci. 2012, 125, E468–E476. [Google Scholar] [CrossRef]
- Matsui, R.; Cvitkovitch, D. Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol. 2010, 5, 403–417. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef]
- Kim, H.W.; Yu, H.S.; Lee, H.H. Nanofibrous matrices of poly(lactic acid) and gelatin polymeric blends for the improvement of cellular responses. J. Biomed. Mater. Res. Part A 2008, 87, 25–32. [Google Scholar] [CrossRef]
- Balguid, A.; Mol, A.; Van Marion, M.H.; Bank, R.A.; Bouten, C.V.C.; Baaijens, F.P.T. Tailoring fiber diameter in electrospun poly(ε-Caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng. Part A 2009, 15, 437–444. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, C.; Ma, X.; Hu, L.; Chen, L.; Wang, C. In vitro and in vivo degradation behavior of aqueous-derived electrospun silk fibroin scaffolds. Polym. Degrad. Stab. 2010, 95, 1679–1685. [Google Scholar] [CrossRef]
- Sheikholeslam, M.; Wright, M.E.E.; Cheng, N.; Oh, H.H.; Wang, Y.; Datu, A.K.; Santerre, J.P.; Amini-nik, S.; Jeschke, M.G. Electrospun polyurethane−gelatin composite: A new tissue-engineered scaffold for application in skin regeneration and repair of complex wounds. ACS Biomater. Sci. Eng. 2020, 6, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Kim, N.R.; Park, S.; Park, J.B. Use of Artelon® Cosmetic in soft tissue augmentation in dentistry. Clin. Cosmet. Investig. Dent. 2011, 3, 33–37. [Google Scholar] [CrossRef]
- Wright, M.E.E.; Yu, J.K.; Jain, D.; Maeda, A.; Yeh, S.A.; DaCosta, R.S.; Lin, C.P.; Santerre, P.J. Engineering functional microvessels in synthetic polyurethane random-pore scaffolds by harnessing perfusion flow. Biomaterials 2020, 256, 120183. [Google Scholar] [CrossRef]
- Hussaini, S.H.; Dean, D.M.; Kelly, M.; Cuttica, D.J. Safety Profile of Artelon Use for Soft Tissue Reconstruction in Foot and Ankle Surgery. Foot Ankle Orthop. 2020, 5, 2473011420S0026. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H.; Sienkiewicz, M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater. Sci. Eng. C 2015, 46, 166–176. [Google Scholar] [CrossRef]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjo, U.M.E. Biology and principles of periodontal wound healing/regeneration. Periodontol. 2000 2006, 41, 30–47. [Google Scholar] [CrossRef]
- Battiston, K.G.; Cheung, J.W.C.; Jain, D.; Santerre, J.P. Biomaterials in co-culture systems: Towards optimizing tissue integration and cell signaling within scaffolds. Biomaterials 2014, 35, 4465–4476. [Google Scholar] [CrossRef]
- Heller, M.; Frerick-Ochs, E.V.; Bauer, H.K.; Schiegnitz, E.; Flesch, D.; Brieger, J.; Stein, R.; Al-Nawas, B.; Brochhausen, C.; Thüroff, J.W.; et al. Tissue engineered pre-vascularized buccal mucosa equivalents utilizing a primary triculture of epithelial cells, endothelial cells and fibroblasts. Biomaterials 2016, 77, 207–215. [Google Scholar] [CrossRef]
- Antonyshyn, J.A.; Mazzoli, V.; McFadden, M.J.; Gramolini, A.O.; Hofer, S.O.P.; Simmons, C.A.; Santerre, J.P. Mitigating the non-specific uptake of immunomagnetic microparticles enables the extraction of endothelium from human fat. Commun. Biol. 2021, 4, 1205. [Google Scholar] [CrossRef]
- Antonyshyn, J.A.; McFadden, M.J.; Gramolini, A.O.; Hofer, S.O.P.; Santerre, J.P. Limited endothelial plasticity of mesenchymal stem cells revealed by quantitative phenotypic comparisons to representative endothelial cell controls. Stem Cells Transl. Med. 2019, 8, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Nemcek, S.; Bloom, D.D.; Goodrich, D.A.; Kim, J.; Mosher, D.F.; Hematti, P. Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol 2017, 136, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, K.; Kato, R.; Okada, M.; Sugimura, T.; Latif, M.A.; Hori, Y.; Narita, Y.; Ueda, M.; Honda, H.; Kagami, H. Gingival and dermal fibroblasts: Their similarities and differences revealed from gene expression. J. Biosci. Bioeng. 2011, 111, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Izadyari Aghmiuni, A.; Heidari Keshel, S.; Sefat, F.; Akbarzadeh Khiyavi, A.; Izadyari, A.; Heidari, S.; Sefat, F.; Akbarzadeh, A. Quince seed mucilage-based scaffold as a smart biological substrate to mimic mechanobiological behavior of skin and promote fibroblasts proliferation and h-ASCs differentiation into keratinocytes. Int. J. Biol. Macromol. 2020, 142, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Seol, Y.J.; Gruber, R.; Giannobile, W.V. Pre-clinical models for oral and periodontal reconstructive therapies. J. Dent. Res. 2009, 88, 1065–1076. [Google Scholar] [CrossRef] [PubMed]


| Tissue Area | Type of Vessel | Diameter (µm) | Average Depth (µm) |
|---|---|---|---|
| Free gingiva | Capillary loops | ≤30 | 50–200 |
| Connective vessels | 50–100 | 200–700 | |
| Large blood vessels | 200–400 | ≥500 | |
| Attached gingiva | Capillary loops | ≤15 | 50–200 |
| Connective vessels | - | - | |
| Large blood vessels | 200–500 | ≥600 | |
| Alveolar mucosa | Capillary loops | ≤15 | 50–200 |
| Connective vessels | 200–600 | 200–700 | |
| Large blood vessels | ≥600 | ≥700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webb, B.C.W.; Glogauer, M.; Santerre, J.P. The Structure and Function of Next-Generation Gingival Graft Substitutes—A Perspective on Multilayer Electrospun Constructs with Consideration of Vascularization. Int. J. Mol. Sci. 2022, 23, 5256. https://doi.org/10.3390/ijms23095256
Webb BCW, Glogauer M, Santerre JP. The Structure and Function of Next-Generation Gingival Graft Substitutes—A Perspective on Multilayer Electrospun Constructs with Consideration of Vascularization. International Journal of Molecular Sciences. 2022; 23(9):5256. https://doi.org/10.3390/ijms23095256
Chicago/Turabian StyleWebb, Brian C. W., Michael Glogauer, and J. Paul Santerre. 2022. "The Structure and Function of Next-Generation Gingival Graft Substitutes—A Perspective on Multilayer Electrospun Constructs with Consideration of Vascularization" International Journal of Molecular Sciences 23, no. 9: 5256. https://doi.org/10.3390/ijms23095256
APA StyleWebb, B. C. W., Glogauer, M., & Santerre, J. P. (2022). The Structure and Function of Next-Generation Gingival Graft Substitutes—A Perspective on Multilayer Electrospun Constructs with Consideration of Vascularization. International Journal of Molecular Sciences, 23(9), 5256. https://doi.org/10.3390/ijms23095256







