Abstract
Although peroxiredoxin 2 (PRDX2) plays a vital role in relieving oxidative stress, its physiological function in cartilage development remains almost unknown. In this study, we found that the expression of PRDX2 significantly increased in the chondrocytes compared with pre-chondrocytes. PRDX2 knockdown significantly decreased the expression of extracellular matrix (ECM) protein (Col2a and Aggrecan), which led to blocked cartilage formation. Moreover, PRDX2 knockdown also inhibited the expression of connective tissue growth factor (CTGF). CTGF is an important growth factor that regulates synthesis of ECM proteins. We explored the possible regulatory mechanism by which PRDX2 regulated the expression of CTGF. Our results demonstrated that PRDX2 knockdown downregulated the expression of CTGF by inhibiting Wnt5a/Yes-associated protein 1 (YAP1) pathway. In addition, PRDX2 knockdown promoted the expression of interleukin 6 (IL-6), indicating PRDX2 expression had an anti-inflammatory function during antler growth. Mechanistically, PRDX2 knockdown promoted cartilage matrix degradation by activating the IL-6-mediated Janus Kinase 2/Signal Transducer and Activator of Transcription 3 (JAK2/STAT3) signaling pathway. These results reveal that PRDX2 is a potential regulator that promotes cartilage extracellular matrix synthesis.
1. Introduction
Deer antler, a bony organ, grows in vitro, is easy to observe and can periodically regenerate. Antler cartilage is formed by endochondral ossification [1]. During this period, the tip of the antler tissue is stored through a transformation of perichondrium into mesenchymal cells, which differentiate to pre-chondrocytes and finally transform into chondrocytes (Figure 1) [2,3]. During early chondrogenesis, chondrocytes continue to divide and specifically express ECM proteins including type II collagen alpha (Col2a), cartilage oligomeric matrix protein (COMP) and Aggrecan, which promote cartilage ECM formation [4]. In the meantime, parts of chondrocytes step down from the cell cycle and continue to differentiate into hypertrophic chondrocytes. In hypertrophic chondrocytes (during late chondrogenesis), Runt-related transcription factor 2 (Runx2) is dominantly expressed and regulates type X collagen alpha (Col10a) expression [5]. Runx2 interacts with Osterix to induce the expression of matrix metalloproteinases 13 (MMP13), which results in calcification of matrices [6].
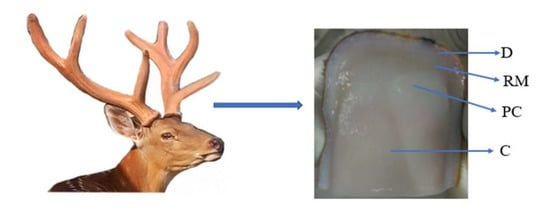
Figure 1.
Longitudinal section of antler tip tissue. D, dermis; RM, reserve mesenchymal; PC, pre-cartilage; C, cartilage.
The ECM, the living environment of chondrocytes, is a complex network composed of collagen, chondroitin sulfate, proteoglycans and various signaling molecules. It provides chondrocytes with a three-dimensional pore structure to facilitate their attachment, proliferation and secretion of the matrix [7,8]. Once the cartilage extracellular matrix is degraded, it can cause cartilage diseases such as osteoarthritis [9]. Therefore, exploring the mechanism of extracellular matrix formation has become an important means to treat cartilage damage repair.
PRDX2 is a member of the peroxiredoxin family which protects cells from oxidative stress by removing H2O2 and controlling reactive oxygen species (ROS) level [10]. Furthermore, PRDX2 is also involved in mediating other biological functions. It has been reported that PRDX2 as a potential inflammatory mediator regulates inflammatory response [11]. The high expression of PRDX2 is also related to the survival and proliferation of cancer cells, including gastric cancer, lung cancer and colon cancer [12,13]. A recent study has indicated that PRDX2 mediates atherosclerosis progression [14]. This also implies that PRDX2 has a wide range of biological activities in different types of cells. However, the biological function of PRDX2 for cartilage development remains unexplored.
Wnt signaling plays a significant role in various biological processes, such as angiogenesis, tumor growth, immune response and cartilage development [15,16,17,18]. The canonical Wnt pathway relies on the stabilization and nuclear translocation of β-catenin to regulate the expression of downstream genes. The activation of the canonical Wnt signal prevents the degradation of β-catenin by the APC complex, and β-catenin accumulates in the cytoplasm, thus realizing the transfer from the cytoplasm to the nucleus [19]. The non-canonical Wnt signaling pathway has multiple regulatory mechanisms, including the Wnt/mTOR, Wnt/JNK, Wnt/Ca+ and Wnt/YAP pathways, playing important roles in regulating cell growth, differentiation and apoptosis [20,21,22,23].
In recent years, it has been discovered that the Wnt/YAP1 signaling pathway can mediate various biological effects. YAP acts as a downstream effector of Wnt proteins to alter the Wnt signaling pathway, in which Wnt5a/b can induce the activation of YAP/TAZ signaling [24]. YAP1, a nuclear transcription factor and the key mediator of the Hippo signaling pathway, plays an important role in regulating cell proliferation, differentiation and survival [25,26]. Previous studies have shown that YAP1 can bind to TEADs in the nucleus and act as a transcriptional co-activator to regulate the expression of target genes, such as CYR61 and CTGF [27,28].
The CTGF/CCN2 (connective tissue growth factor) is the second member of the CCN family implicated in cell proliferation, differentiation and ECM production [29]. Many in vitro and in vivo studies have shown that CTGF as an osteogenesis-related protein plays an important role in regulating endochondral ossification [30]. CTGF promotes new bone formation by regulating extracellular matrix accumulation and intramembranous osteogenesis [31]. Loss of CTGF can cause severe chondrodysplasia [32]. In addition, IL-6 is a key inflammatory factor that regulates immune and inflammatory responses [33]. Previous studies have shown that inflammation is a common cause of cartilage destruction. The IL-6-mediated JAK2/STAT3 signaling pathway can induce chondrocyte apoptosis and MMP expression [34,35].
In this study, we analyzed the expression levels of PRDX family members in pre-chondrocytes and chondrocytes and found that as antler chondrocytes matured, PRDX2 expression increased significantly. It is unclear whether PRDX2 is involved in the process of cartilage development. Here, we aimed to investigate the specific biological effect of PRDX2 during the rapid growth of deer antler and explored the potential regulatory mechanism.
2. Results
2.1. Antler Chondrocytes Can Highly Express a Variety of Peroxiredoxins, and the Expression of PRDX2 Upregulates as the Chondrocytes Mature
Although the growth rate of an antler is extremely quick, the consumption of large amounts of oxygen does not cause oxidative stress. We analyzed the expression level of the PRDX family protein with qRT-PCR in antler chondrocytes. We found that PRDX1-6 were expressed in the antler chondrocytes, and the expression levels of PRDX1 and PRDX2 were high (Figure 2A). In a previous study, we successfully isolated and identified antler pre-chondrocytes and chondrocytes [36]. Here, we detected the expression levels of PRDXs between the pre-chondrocytes and the chondrocytes. Chondrocytes can highly express Col2a (chondrocytes marker molecule) compared to pre-chondrocytes (Figure 2B). We found that the expression of PRDX2 significantly increased as the chondrocytes matured (Figure 2C). We detected the location and expression of PRDX2 in the pre-cartilage layer and cartilage layer with immunohistochemistry staining. PRDX2 protein showed a strong signal in the cartilage layer of deer antler (Figure 2D).
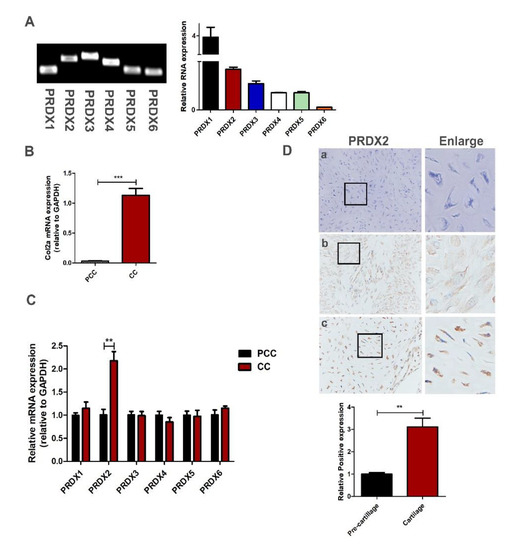
Figure 2.
Antler chondrocytes can highly express a variety of peroxiredoxins, and the expression of PRDX2 upregulates as the chondrocytes mature. (A) The expression level of PRDX family protein by qRT-PCR in antler chondrocytes. (B) The expression level Col2a (chondrocyte marker molecule) by qRT-PCR in antler pre-chondrocytes (PCC) and chondrocytes (CC). (C) The expression level of PRDX1-6 by qRT-PCR in PCC and CC. (D) Immunohistochemistry staining of PRDX2 in the antler pre-cartilage layer and cartilage layer. (a) Negative control, (b) pre-cartilage layer, (c) cartilage layer. Scale bar, 50 μm. The data include the means ± SD of three independent experiments. ** p < 0.01, *** p < 0.001.
2.2. PRDX2 Knockdown Inhibits the Synthesis of Cartilage Matrix Proteins
PRDX2 expression significantly increased in the chondrocytes compared with pre-chondrocytes, and we proposed that it may mediate early chondrogenesis. To determine whether PRDX2 mediates chondrogenesis, the interference fragment targeting the PRDX2 gene was transfected into proliferating chondrocytes of deer antler. The interference efficiency of PRDX2 was detected using Western blot analysis and qRT-PCR (Figure 3A,B). PRDX2 knockdown significantly inhibited the expression of cartilage matrix proteins (Col2a, Aggrecan and COMP) compared with the negative control (Figure 3C). In addition, the results from toluidine blue staining and Alcian blue staining showed PRDX2 knockdown reduced the synthesis of glycosaminoglycan, which inhibited the formation of the cartilage extracellular matrix (Figure 3D).
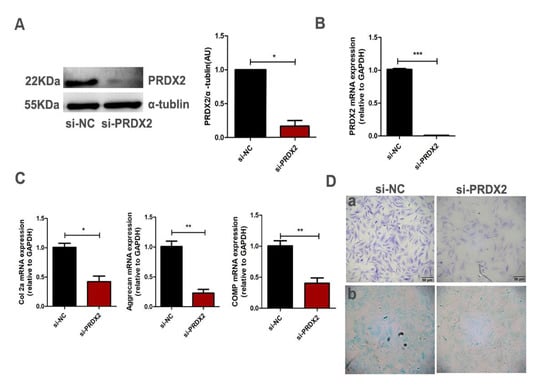
Figure 3.
PRDX2 knockdown inhibits the synthesis of cartilage matrix proteins. (A) The PRDX2 expression level was decreased after treatment with PRDX2 siRNA in the chondrocytes for 72 h by Western blot. (B) The PRDX2 expression level was decreased after treatment with PRDX2 siRNA for 72 h by q-PCR. (C) The Col2a, Aggrecan and COMP expression levels were decreased after treatment with PRDX2 siRNA for 72 h by qRT-PCR (D) toluidine blue staining (a) and Alcian blue staining (b) were performed after treatment with PRDX2 siRNA for 72 h. Scale bar, 50 μm. The data include the means ± SD of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001.
2.3. PRDX2 Knockdown Inhibits the Expression of CTGF
In vivo and in vitro studies have shown that CTGF plays a vital role in regulating the synthesis of the ECM and maintaining chondrocyte phenotype. We investigated whether PRDX2 regulated the synthesis of cartilage matrix proteins through the transcription of CTGF. We found that PRDX2 knockdown significantly inhibited the expression of CTGF (Figure 4A,B).
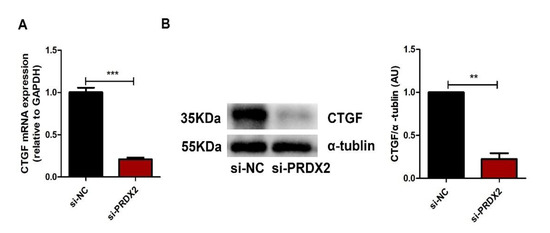
Figure 4.
PRDX2 knockdown inhibits the expression of CTGF. (A) The CTGF expression level was decreased after treatment with PRDX2 siRNA in the proliferative chondrocytes for 72 h by qPCR. (B) The CTGF protein level was detected after treatment with PRDX2 siRNA in the antler chondrocytes for 72 h by Western blot. The data include the means ± SD of three independent experiments. ** p < 0.01, *** p < 0.001.
2.4. PRDX2 Knockdown Inhibits the Expression of Wnt5a and Promotes Nuclear Translocation of β-Catenin
The Wnt/β-catenin pathway is a key regulator during chondrogenesis. To further explore how PRDX2 regulated the expression of CTGF, we evaluated whether PRDX2 regulated the expression of CTGF though activating the Wnt/β-catenin signaling pathway. We found that PRDX2 knockdown significantly decreased Wnt5a expression but increased β-catenin activity (Figure 5A). The immunofluorescence results showed that after PRDX2 knockdown, β-catenin had a strong signal in the nucleus (Figure 5B). In addition, PRDX2 knockdown significantly inhibited phosphorylation of GSK-3β and enhanced GSK-3β activation (Figure 5C,D). These results indicate that PRDX2 is involved in Wnt5a pathways (independent of β-catenin).
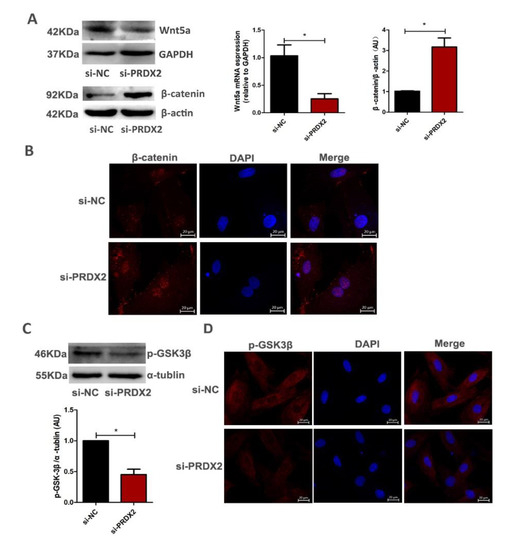
Figure 5.
PRDX2 knockdown inhibits the expression of Wnt5a and promotes nuclear translocation of β-catenin. (A) The Wnt5a and β-catenin expression levels were decreased after treatment with PRDX2 siRNA in the proliferative chondrocytes for 72 h by Western blot. (B) Control or PRDX2 siRNA was transfected for 72 h in proliferative chondrocytes. Localization of β-catenin was determined by immunofluorescence staining. The nuclei were stained with DAPI (blue), and the β-catenin was detected with CY3 (red). Scale bar, 20 μm. (C) The p-GSK-3β protein level was detected after treatment with PRDX2 siRNA in the antler chondrocytes for 72 h by Western blot. (D) Localization of p-GSK3β was determined by immunofluorescence staining. The nuclei were stained with DAPI (blue), and the p-GSK3β was detected with CY3 (red). Scale bar, 20 μm. The data include the means ± SD of three independent experiments. * p < 0.05.
2.5. PRDX2 Knockdown Reduces the Expression of CTGF by Inhibiting the Activity of YAP1
Previous studies have indicated that Wnt5a can induce YAP/TAZ activation to change the canonical Wnt signaling pathway [24,37]. Here, we performed Western blot analysis to examine the transcription activity of YAP1 after PRDX2 knockdown. Treatment with siPRDX2 significantly increased the expression of p-YAP1 and decreased the nuclear translocation of YAP1 (Figure 6A). The immunofluorescence results also showed that after PRDX2 knockdown, YAP1 had a weak signal in the nucleus compared with the negative control (Figure 6B). Next, the interference fragment targeting the YAP1 gene was transfected into chondrocytes. The protein level of YAP1 was significantly reduced compared with the negative control (Figure 6C,E). The expression of CTGF was significantly reduced after YAP1 knockdown by q-PCR (Figure 6D). These results indicate that PRDX2 regulated the expression of CTGF depending on the transcriptional activity of YAP1.
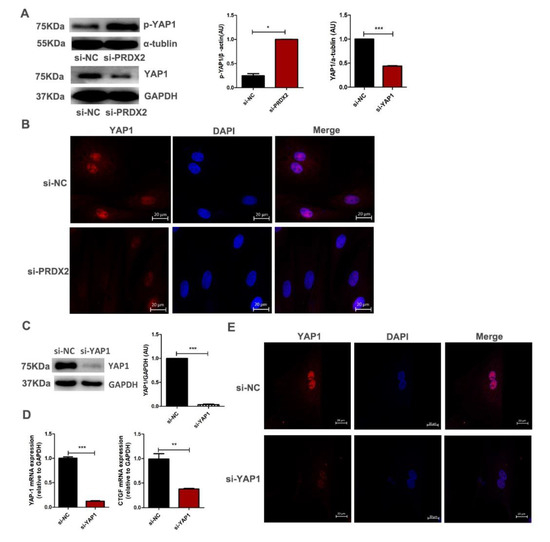
Figure 6.
PRDX2 knockdown reduces the expression of CTGF by inhibiting the activity of YAP1. (A) The YAP1 and p-YAP1 expression levels were decreased after treatment with PRDX2 siRNA in antler chondrocytes for 72 h by Western blot. (B) Control or PRDX2 siRNA was transfected for 72 h. Localization of YAP1 was determined by immunofluorescence staining. The nuclei were stained with DAPI (blue), and the YAP1 was detected with CY3 (red). Scale bar, 20 μm. (C) The protein level of YAP1 was decreased after treatment with YAP1 siRNA for 72 h by Western blot. (D) The CTGF expression level was decreased after treatment with YAP1 siRNA for 72 h by q-PCR. (E) The interference efficiency of YAP1 was examined by immunofluorescence staining. The YAP1 was detected with CY3 (red). Scale bar, 20 μm. The data include the means ± SD of three independent experiments, * p < 0.05, ** p < 0.01, *** p < 0.001.
2.6. PRDX2 Knockdown Activates IL6-Induced JAK2/STAT3 Signaling Pathway in Chondrocytes
The JAK2/STAT3 pathway plays an important role in osteoarthritis (OA) pathogenesis. Activation of the JAK2/STAT3 pathway can accelerate the degradation of the cartilage matrix. Figure 7A shows that PRDX2 knockdown promotes the expression of IL-6. As expected, PRDX2 knockdown upregulated the phosphorylation levels of JAK2 and STAT3 (Figure 7B,C). These results indicate PRDX2 knockdown induced inflammatory response and inhibited the expression of cartilage matrix proteins through activating the IL6-induced JAK2/STAT3 signaling pathway.
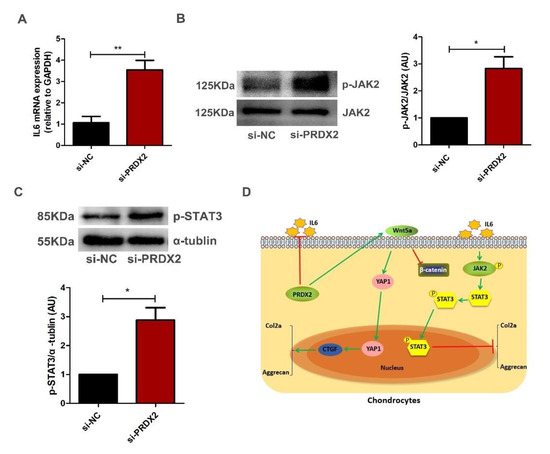
Figure 7.
PRDX2 knockdown activates IL6-induced JAK2/STAT3 signaling pathway in chondrocytes. (A) IL-6 expression levels were decreased after treatment with PRDX2 siRNA in the hypertrophic chondrocytes for 72 h by qRT-PCR. (B,C) The p-JAK2 and p-STAT3 protein levels were increased after treatment with PRDX2 siRNA for 72 h by Western blot. (D) PRDX2 knockdown inhibits extracellular matrix synthesis of chondrocytes by inhibiting Wnt5a/YAP1/CTGF and activating IL-6/JAK2/STAT3 pathways in deer antler. The data include the means ± SD of three independent experiments, * p < 0.05, ** p < 0.01.
3. Discussion
PRDX2 is a very powerful ROS-scavenging protein compared with other PRDX members involved in important physiological functions. In this work, we found that PRDX2 was expressed at different stages of chondrogenesis. PRDX2 expression was significantly increased in chondrocytes compared with pre-chondrocytes. PRDX2 knockdown inhibited the expression of Col2a, Aggrecan, COMP and reduced the synthesis of glycosaminoglycan, indicating that PRDX2 was involved in the cartilage ECM formation and maintained chondrocyte phenotype. Furthermore, PRDX2 knockdown markedly increased the expression of IL-6, indicating PRDX2 expression had an anti-inflammatory function during antler growth.
Previous studies have shown that CTGF is a significant matricellular protein that regulates cartilage growth [38,39,40]. Moreover, exogenous CTGF treatment increases the expression of cartilaginous matrix proteins, such as Col2a and Aggrecan, and promotes chondrocytes proliferation [41]. Here, we found that PRDX2 knockdown inhibited the expression of CTGF. PRDX2 involvement in regulating the cartilage matrix protein expression may depend on the activity of CTGF. We further explored the relationship between PRDX2 and CTGF as well as possible regulatory mechanisms. Previous studies have shown that YAP1 can bind to TEADs in the nucleus and act as a transcriptional co-activator to regulate the expression of target genes, such as CYR61 and CTGF [27,28]. We suspect that PRDX2 may affect the transcription of YAP1 in antler chondrocytes. Here, we observed that PRDX2 knockdown significantly promoted YAP1 phosphorylation and inactivation. In addition, relevant studies have shown that inhibition or downregulation of GSK-3β can promote nuclear aggregation of YAP1 [42]. PRDX2 knockdown upregulated the expression of GSK-3β (Figure 4C). These results suggested that PRDX2 regulated the expression of CTGF by activating the YAP1 signaling pathway. However, it is unclear how PRDX2 is associated with YAP1, and further study is required to explore this.
Non-canonical Wnt signaling mediates a variety of physiological functions, including embryonic development, cell differentiation and inhibition of canonical Wnt signaling [43,44]. Wnt5a has traditionally been considered a common ligand in non-canonical Wnt signaling. Wnt5a-driven signaling is able to inhibit canonical Wnt signaling by inhibiting the expression of Wnt target genes [24]. Ror2 receptor, as a major receptor of Wnt5a, plays a vital role in mediating non-canonical Wnt signaling. Wnt5a can inhibit the transcriptional activity of β-catenin by activating the phosphorylation of the Ror2 receptor [45,46]. We found that PRDX2 knockdown inhibited the Wnt5a expression and increased the β-catenin accumulation (Figure 4A). Obviously, Wnt5a inhibited the transcriptional activity of β-catenin. In addition, related research results show that Wnt5a can act as an activator of YAP/TAZ signaling, thereby altering the Wnt signaling pathway [24]. Our results indicated that PRDX2 activated the YAP/TAZ signal through Wnt5a in the chondrocytes of deer antler.
CTGF has been identified a vital transcription factor that promotes synthesis of cartilage matrix proteins including Col2a and Aggrecan [41]. Degradation of the extracellular matrix has long been a hallmark of arthritic diseases, and inflammatory responses are the main cause of a series of structural damage [47]. A large number of inflammatory mediators including IL-6, TNF-α and IL-1β were detected in osteoarthritis [48]. In vitro and in vivo studies have shown that the JAK2/STAT3 pathway plays vital roles in osteoarthritis (OA) [35,49]. The activation of the JAK2/STAT3 pathway resulted in the production of matrix metalloproteinases (MMPs), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2, which would lead to cartilage destruction [34]. IL-6 is a well-known target protein upstream of the JAK2/STAT3 signaling pathway, and it can regulate articular cartilage degradation in OA [34,50]. PRDX2 knockdown inhibited the expression of cartilage matrix proteins by activating the IL6-induced JAK2/STAT3 signaling pathway in chondrocytes.
It is well known that cartilage disruption and loss of the extracellular matrix are the most distinctive features of OA [51]. Counteracting cartilage degradation by stimulating chondrocyte proliferation and extracellular matrix synthesis is a potential treatment for OA. In addition, the results of a study showed that PRDX2 expression showed lower levels in the cartilage tissue of OA patients [52]. In this study we found that PRDX2 knockdown downregulated the expression of cartilage matrix proteins. We reported that PRDX2 regulated CTGF expression through the Wnt5a/YAP1 pathway. Our data indicated that PRDX2 promoted extracellular matrix formation by inhibiting the IL-6/JAK2/STAT3 pathway. However, we only explored the effect of cartilage matrix protein expression by knocking down the expression of PRDX2. Whether overexpression of PRDX2 can promote the formation of the cartilage ECM is unclear. In addition, whether PRDX2 expression regulates chondrocyte differentiation remains to be further investigated.
4. Materials and Methods
4.1. Antler Issue Collection and Cell Culture
Deer antler samples (four antlers) were collected (Jinsanxin Farm, Wuhan, China). These cervus nippon are native to the northeastern part of China. The tips of antler tissues (about 5 cm) about 60 days after casting from healthy deer (two years old) were dissected into different layers (mesenchyme layer, pre-cartilage, and cartilage layer) separately as described previously [2]. Dissected tissues were cut up in DMEM/high glucose medium (Hyclone, GE Healthcare, Logan, UT, USA), centrifuged at 1000 rpm for 2 min and using 0.2% collagenase II (Sigma-Aldrich, Marlborough, MA, USA) digested for 30 min. The digested tissue layers were filtered through a cell strainer (pore size: 100 μm), and the cell suspension was centrifuged at 1300 rpm for 4 min. The cell pellets were resuspended in DMEM/high glucose medium containing 10% FBS (fetal bovine serum). Antler cells were cultured at 37 °C with 5% CO2. Finally, cell cryopreservation solution was added to freeze the cells in liquid nitrogen for long-term storage.
4.2. Immunohistochemistry Staining
For immunohistochemistry staining, antigen-treated, paraffin-embedded tissue was exposed to 3% hydrogen peroxide in the dark, and decolonization was performed with PBS 3 times for 5 min each. Blocking of the paraffin section was performed using PBS with goat serum for 40 min, and it was incubated with PRDX2 antibody (1:500) (Abcam, ab109367) at 4 °C overnight. Subsequently, the paraffin sections were washed with 1X-PBS 3 times and incubated with goat anti-rabbit IgG at 37 °C for 30 min following PBS washing. Before sealing paraffin sections with a neutral stain, they were counterstained using hematoxylin for 2 min and subjected to ddH2O washing and 1% hydrochloric acid alcohol differentiation for a few seconds, rinsed with ddH2O until ammonia water returned to blue and rinsed with ddH2O again.
4.3. RNA Interference
Antler chondrocytes were seeded in cell culture plates up to 60–70% confluence. The cells were transfected with 100 nM siRNA by Lipofectamine RNAiMAX Reagent in Opti-MEM medium (Life Technologies, Inc., Carlsbad, CA, USA) according to the instructions as compared to the control. After 48 h of transfection, cells were harvested for mRNA expression or protein expression. The siRNA sequence used in this study is as follows. PRDX2: 5′- AGGAAUAUUUCUCCAAACATT -3′, YAP1: 5′- GGUGACACUAUCAACCAAATT -3′.
4.4. Total RNA and Quantitative Real-Time PCR (qRT-PCR)
Post-transfection (48 h), total RNA from Antler cells was extracted using an RNA kit (Cat R6834-02, Omega Bio-Tek, Norcross, GA, USA) according to the kit instructions. Subsequently, the purity of RNA was determined for the absorbance at 260/280. The RNA with the absorbance value of 1.8–2.1 was used with the cDNA first-trans synthesis kit (CatKR118-02, TIANGEN Biotech, Beijing, China) for reverse transcription into cDNA. The expression of genes was analyzed using the method of 2−ΔΔCT. All the targeted primers were designed by Primer 5.0 software (Primer Biosoft, Palo Alto, CA, USA) (Table 1).

Table 1.
Primers used for RT-PCR in this study.
4.5. Western Blot Assay
Antler cells (10) were collected and lysed for 20 min using lysis buffer (RIPA lysis and 50 × Cocktail) (Servicebio, Wuhan, China). Protein concentration was examined using the BCA protein assay (Servicebio, Wuhan, China). The total protein (20 μg) was separated by SDS-polyacrylamide gel electrophoresis. Subsequently, proteins were transferred to polyvinylidene difluoride membranes and blocked with 5% skimmed milk or bovine serum albumin (BSA) powder with 0.1% Tween-20 in TBS for 2 h and incubated with primary antibody at 4 °C overnight. The following antibodies were used: anti-YAP1 (GTX129151, GeneTex, San Antonin, TX, USA, 1:1000), anti-p-YAP1 (S127) (ab76252, Abcam, Cambridge, UK, 1:2000), anti-GSK3 beta (phosphor S9) (ab75814, Abcam, 1:1000), anti-PRDX2 (ab109367, Abcam, 1:2000), anti-β-catenin (ab32572, Abcam, 1:2000), anti-Wnt5a (ab179824, Abcam, 1:2000), anti-Jak2 (3230, Cell Signaling Technology (CST) Biological reagents Company Ltd., Shanghai, China, 1:1000), anti-p-JAK2 (Try1007/1008) (3771, CST, 1:1000), anti-p-STAT3 (Tyr705) (9145, CST, 1:1000) and anti-Col2a (15943, Proteintech, Wuhan, China, 1:1000). After washing, membranes were incubated with secondary antibodies for 2 h. Lastly, the membranes were incubated with ECL chemiluminescence reagent and exposed to X-ray film for the observation of protein bands.
4.6. Immunofluorescence Assay
Cells were grown on circular glass coverslips in 24-well plates, fixed with 4% paraformaldehyde (15 min) and then permeabilized using TritonX-100 (3 min). After washing with PBS, cell coverslip blocking was performed using BSA 5% for 30 min, and then the coverslips were incubated with primary antibodies at 4 °C overnight. Cell coverslips were washed 3 times with PBST and incubated with FITC or CY3-conjugated secondary antibodies (Servicebio, Wuhan, China). Lastly, counterstaining of the nucleus was performed with DAPI (Servicebio, Wuhan, China).
4.7. Toluidine Blue and Alcian Blue Staining
The adherent cells were washed 3 times with phosphate buffered saline solution and fixed with 4% paraformaldehyde at room temperature for 15 min. Then, the cells were incubated at room temperature with the prepared toluidine blue solution (Servicebio, Wuhan, China) for 2 h and washed with distilled water 3 times. Prepared 0.3% Alcian blue solution (Servicebio, Wuhan, China) was incubated at room temperature for 2 h and then washed with distilled water. Finally, an upright microscope was used to collect pictures.
4.8. Statistical Analysis
Analysis of data was performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA, USA). Data were summarized as mean ±standard deviation (SD), and a significant difference between two groups was determined using the t test. p < 0.05 indicated a statistically significant difference.
5. Conclusions
Our results indicate that PRDX2 has a positive effect on chondrogenesis. PRDX2 expression can effectively promote the production of extracellular matrix proteins. Mechanistically, on the one hand, PRDX2 regulated the expression of CTGF though Wnt5a/YAP1 pathway. On the other hand, PRDX2 suppressed inflammation response by inhibiting IL-6/JAK2/STAT3 pathway in antler chondrocytes, which facilitated the formation of the cartilage extracellular matrix.
Author Contributions
X.S. designed and performed the experiments. L.Y. and J.X. provide technical assistance and supervised this study; X.G. analyzed data; J.P. and X.Z. provided reagents; X.S. helped with images and the initial draft, Z.R. edited and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China (Grant No.31972533) supported this work.
Institutional Review Board Statement
HZAUDE-2018-001.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request to the corresponding author.
Acknowledgments
The authors thank the critical lab of Agricultural Animal Genetics, Breeding, and Reproduction of Ministry of Education, College of Animal Science and Technology of Huazhong Agricultural University for providing the facilities in this study. We also thank the sika deer farm of Jinsanxin, Wuhan, Hubei, for delivering the sika deer antlers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Price, J.S.; Oyajobi, B.O.; Nalin, A.M.; Frazer, A.; Russell, R.G.; Sandell, L.J. Chondrogenesis in the regenerating antler tip in red deer: Expression of collagen types I, IIA, IIB, and X demonstrated by in situ nucleic acid hybridization and immunocytochemistry. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1996, 205, 332–347. [Google Scholar] [CrossRef]
- Li, C.; Clark, D.E.; Lord, E.A.; Stanton, J.A.; Suttie, J.M. Sampling technique to discriminate the different tissue layers of growing antler tips for gene discovery. Anat. Rec. 2002, 268, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; He, C.; Sun, X.; Wang, J.; Luo, C.; Liu, G.; Yang, L.; Xiong, J.; Huo, L. The Regulatory Mechanism of MLT/MT1 Signaling on the Growth of Antler Mesenchymal Cells. Molecules 2017, 22, 1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, C.; Yik, J.H.; Kishore, A.; Van Dinh, V.; Di Cesare, P.E.; Haudenschild, D.R. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: Interaction, regulation and role in chondrogenesis. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 37, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Andoh, M.; Ueno-Kudoh, H.; Sato, T.; Miyaki, S.; Asahara, H. Sox9 directly promotes Bapx1 gene expression to repress Runx2 in chondrocytes. Exp. Cell Res. 2009, 315, 2231–2240. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, R.; Wakabayashi, M.; Hata, K.; Matsubara, T.; Honma, S.; Wakisaka, S.; Kiyonari, H.; Shioi, G.; Yamaguchi, A.; Tsumaki, N.; et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J. Biol. Chem. 2012, 287, 33179–33190. [Google Scholar] [CrossRef] [Green Version]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhao, Y.H.; Yang, Q. Development of cartilage extracellular matrix in cartilage tissue engineering. Hua Xi Kou Qiang Yi Xue Za Zhi = Huaxi Kouqiang Yixue Zazhi = West China J. Stomatol. 2019, 37, 220–223. [Google Scholar]
- Kong, J.; Wang, J.; Gong, X.; Zheng, X.; Chen, T. Punicalagin Inhibits Tert-Butyl Hydroperoxide-Induced Apoptosis and Extracellular Matrix Degradation in Chondrocytes by Activating Autophagy and Ameliorates Murine Osteoarthritis. Drug Des. Dev. Ther. 2020, 14, 5521–5533. [Google Scholar] [CrossRef]
- Peskin, A.V.; Meotti, F.C.; de Souza, L.F.; Anderson, R.F.; Winterbourn, C.C.; Salvador, A. Intra-dimer cooperativity between the active site cysteines during the oxidation of peroxiredoxin 2. Free Radic. Biol. Med. 2020, 158, 115–125. [Google Scholar] [CrossRef]
- Checconi, P.; Salzano, S.; Bowler, L.; Mullen, L.; Mengozzi, M.; Hanschmann, E.M.; Lillig, C.H.; Sgarbanti, R.; Panella, S.; Nencioni, L.; et al. Redox proteomics of the inflammatory secretome identifies a common set of redoxins and other glutathionylated proteins released in inflammation, influenza virus infection and oxidative stress. PLoS ONE 2015, 10, e0127086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; He, J.; Tang, M.; Sun, H. Prdx2 Upregulation Promotes the Growth and Survival of Gastric Cancer Cells. Pathol. Oncol. Res. POR 2020, 26, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Z.; Zhu, S.; Lu, H.; Peng, D.; Soutto, M.; Naz, H.; Peek, R., Jr.; Xu, H.; Zaika, A.; et al. PRDX2 protects against oxidative stress induced by H. pylori and promotes resistance to cisplatin in gastric cancer. Redox Biol. 2020, 28, 101319. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Wang, W.; Liu, L.; Zhang, Q.; Zhang, J.; Wang, B.; Wang, S.; Hou, L.; Gao, C.; et al. PRDX2 Protects Against Atherosclerosis by Regulating the Phenotype and Function of the Vascular Smooth Muscle Cell. Front. Cardiovasc. Med. 2021, 8, 624796. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef]
- Vallée, A.; Guillevin, R.; Vallée, J.N. Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/β-catenin pathway in gliomas. Rev. Neurosci. 2018, 29, 71–91. [Google Scholar] [CrossRef]
- Hou, L.; Shi, H.; Wang, M.; Liu, J.; Liu, G. MicroRNA-497-5p attenuates IL-1β-induced cartilage matrix degradation in chondrocytes via Wnt/β-catenin signal pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 3108–3118. [Google Scholar]
- Li, Z.; Wang, Y.; Xiang, S.; Zheng, Z.; Bian, Y.; Feng, B.; Weng, X. Chondrocytes-derived exosomal miR-8485 regulated the Wnt/β-catenin pathways to promote chondrogenic differentiation of BMSCs. Biochem. Biophys. Res. Commun. 2020, 523, 506–513. [Google Scholar] [CrossRef]
- Wang, W.; Smits, R.; Hao, H.; He, C. Wnt/β-Catenin Signaling in Liver Cancers. Cancers 2019, 11, 926. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Dong, X.; Liu, Y.; Ni, B.; Sai, N.; You, L.; Sun, M.; Yao, Y.; Qu, C.; Yin, X.; et al. Itraconazole exerts anti-liver cancer potential through the Wnt, PI3K/AKT/mTOR, and ROS pathways. Biomed. Pharmacother. 2020, 131, 110661. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, C.; Li, Y.; Wang, Y.; Gao, L.; Lu, K.; Chang, G.; Li, Y.; Qin, S.; Zhang, D. Vangl2 is essential for myocardial remodeling activated by Wnt/JNK signaling. Exp. Cell Res. 2018, 365, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Korn, C.; Wojtarowicz, J.; Mogler, C.; Augustin, I.; Boutros, M.; Niehrs, C.; Augustin, H.G. Endothelial RSPO3 Controls Vascular Stability and Pruning through Non-canonical WNT/Ca2+/NFAT Signaling. Dev. Cell 2016, 36, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yang, J.; Bao, M.; Zeng, K.; Fu, S.; Wang, C.; Ye, L. Wnt signaling in bone metastasis: Mechanisms and therapeutic opportunities. Life Sci. 2018, 208, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Kim, Y.C.; Yu, B.; Moroishi, T.; Mo, J.S.; Plouffe, S.W.; Meng, Z.; Lin, K.C.; Yu, F.X.; Alexander, C.M.; et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell 2015, 162, 780–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Lei, D.; Huang, S.; Yang, Y.; Jiang, C.; Shi, H.; Chen, W.; Zhao, Q.; You, Z.; Ye, X. A novel biodegradable external stent regulates vein graft remodeling via the Hippo-YAP and mTOR signaling pathways. Biomaterials 2020, 258, 120254. [Google Scholar] [CrossRef]
- Melhuish, T.A.; Kowalczyk, I.; Manukyan, A.; Zhang, Y.; Shah, A.; Abounader, R.; Wotton, D. Myt1 and Myt1l transcription factors limit proliferation in GBM cells by repressing YAP1 expression. Biochim. Et Biophys. Acta. Gene Regul. Mech. 2018, 1861, 983–995. [Google Scholar] [CrossRef]
- Chai, J.; Xu, S.; Guo, F. TEAD1 mediates the oncogenic activities of Hippo-YAP1 signaling in osteosarcoma. Biochem. Biophys. Res. Commun. 2017, 488, 297–302. [Google Scholar] [CrossRef]
- Moon, S.; Lee, S.; Caesar, J.A.; Pruchenko, S.; Chaqour, B.J.S.E.J. A CTGF-YAP Regulatory Pathway is Essential for Angiogenesis and Barriergenesis in the Retina. iScience 2020, 23, 101184. [Google Scholar] [CrossRef]
- Woods, A.; Pala, D.; Kennedy, L.; McLean, S.; Rockel, J.S.; Wang, G.; Leask, A.; Beier, F. Rac1 signaling regulates CTGF/CCN2 gene expression via TGFbeta/Smad signaling in chondrocytes. Osteoarthr. Cartil. 2009, 17, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Kanaan, R.A.; Aldwaik, M.; Al-Hanbali, O.A. The role of connective tissue growth factor in skeletal growth and development. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2006, 12, Ra277–Ra281. [Google Scholar]
- Jiang, W.; Takeshita, N.; Maeda, T.; Sogi, C.; Oyanagi, T.; Kimura, S.; Yoshida, M.; Sasaki, K.; Ito, A.; Takano-Yamamoto, T. Connective tissue growth factor promotes chemotaxis of preosteoblasts through integrin α5 and Ras during tensile force-induced intramembranous osteogenesis. Sci. Rep. 2021, 11, 2368. [Google Scholar] [CrossRef] [PubMed]
- Hall-Glenn, F.; Aivazi, A.; Akopyan, L.; Ong, J.R.; Baxter, R.R.; Benya, P.D.; Goldschmeding, R.; van Nieuwenhoven, F.A.; Hunziker, E.B.; Lyons, K.M. CCN2/CTGF is required for matrix organization and to protect growth plate chondrocytes from cellular stress. J. Cell Commun. Signal. 2013, 7, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Tao, P.; Zhou, Q.; Li, J.; Yu, Z.; Wang, X.; Li, J.; Li, C.; Yan, M.; Zhu, Z.; et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 2017, 8, 20741–20750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Ding, Z.; Liu, F.; Shan, W.; Cheng, C.; Xu, J.; He, W.; Huang, W.; Ma, J.; Yin, Z. Dopamine delays articular cartilage degradation in osteoarthritis by negative regulation of the NF-κB and JAK2/STAT3 signaling pathways. Biomed. Pharmacother. 2019, 119, 109419. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; He, J.; Sun, Y.; Dong, X.; Yao, J.; Gu, H.; Liu, L. Leptin Induced TLR4 Expression via the JAK2-STAT3 Pathway in Obesity-Related Osteoarthritis. Oxidative Med. Cell. Longev. 2021, 2021, 7385160. [Google Scholar] [CrossRef]
- Sun, X.; Gu, X.; Li, K.; Li, M.; Peng, J.; Zhang, X.; Yang, L.; Xiong, J. Melatonin Promotes Antler Growth by Accelerating MT1-Mediated Mesenchymal Cell Differentiation and Inhibiting VEGF-Induced Degeneration of Chondrocytes. Int. J. Mol. Sci. 2022, 23, 759. [Google Scholar] [CrossRef]
- Tu, B.; Yao, J.; Ferri-Borgogno, S.; Zhao, J.; Chen, S.; Wang, Q.; Yan, L.; Zhou, X.; Zhu, C.; Bang, S.; et al. YAP1 oncogene is a context-specific driver for pancreatic ductal adenocarcinoma. JCI Insight 2019, 4, e130811. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Hattori, T.; Ono, M.; Uehara, J.; Kubota, S.; Kuboki, T.; Takigawa, M. CCN family 2/connective tissue growth factor (CCN2/CTGF) stimulates proliferation and differentiation of auricular chondrocytes. Osteoarthr. Cartil. 2008, 16, 787–795. [Google Scholar] [CrossRef] [Green Version]
- Yosimichi, G.; Kubota, S.; Nishida, T.; Kondo, S.; Yanagita, T.; Nakao, K.; Takano-Yamamoto, T.; Takigawa, M. Roles of PKC, PI3K and JNK in multiple transduction of CCN2/CTGF signals in chondrocytes. Bone 2006, 38, 853–863. [Google Scholar] [CrossRef]
- Hori, A.; Nishida, T.; Takashiba, S.; Kubota, S.; Takigawa, M. Regulatory mechanism of CCN2 production by serotonin (5-HT) via 5-HT2A and 5-HT2B receptors in chondrocytes. PLoS ONE 2017, 12, e0188014. [Google Scholar] [CrossRef]
- Xing, X.; Li, Z.; Yu, Z.; Cheng, G.; Li, D.; Li, Z. Effects of connective tissue growth factor (CTGF/CCN2) on condylar chondrocyte proliferation, migration, maturation, differentiation and signalling pathway. Biochem. Biophys. Res. Commun. 2018, 495, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Thongon, N.; Castiglioni, I.; Zucal, C.; Latorre, E.; Provenzani, A.J.O. The GSK3β inhibitor BIS I reverts YAP-dependent EMT signature in PDAC cell lines by decreasing SMADs expression level. Oncotarget 2016, 7, 26551–26566. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.E.; Punnia-Moorthy, G.; Henry, C.E.; Llamosas, E.; Nixdorf, S.; Olivier, J.; Caduff, R.; Ward, R.L.; Heinzelmann-Schwarz, V. The non-canonical Wnt ligand, Wnt5a, is upregulated and associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Gynecol. Oncol. 2014, 134, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int. J. Oncol. 2017, 51, 1357–1369. [Google Scholar] [CrossRef] [Green Version]
- Flores-Hernández, E.; Velázquez, D.M.; Castañeda-Patlán, M.C.; Fuentes-García, G.; Fonseca-Camarillo, G.; Yamamoto-Furusho, J.K.; Romero-Avila, M.T.; García-Sáinz, J.A.; Robles-Flores, M. Canonical and non-canonical Wnt signaling are simultaneously activated by Wnts in colon cancer cells. Cell. Signal. 2020, 72, 109636. [Google Scholar] [CrossRef]
- Curto, J.; Del Valle-Pérez, B.; Villarroel, A.; Fuertes, G.; Vinyoles, M.; Peña, R.; García de Herreros, A.; Duñach, M. CK1ε and p120-catenin control Ror2 function in noncanonical Wnt signaling. Mol. Oncol. 2018, 12, 611–629. [Google Scholar] [CrossRef] [Green Version]
- Bin, S.; Xin, L.; Lin, Z.; Jinhua, Z.; Rui, G.; Xiang, Z. Targeting miR-10a-5p/IL-6R axis for reducing IL-6-induced cartilage cell ferroptosis. Exp. Mol. Pathol. 2021, 118, 104570. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Sun, X.; Zhou, L.; Zhao, J. Long non-coding RNA DANCR regulates proliferation and apoptosis of chondrocytes in osteoarthritis via miR-216a-5p-JAK2-STAT3 axis. Biosci. Rep. 2018, 38, BSR20181228. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.A.; Kim, J.Y.; Yang, S.H.; Han, S.S.; Joo, K.W.; Kim, Y.S.; Kim, D.K. The role of local IL6/JAK2/STAT3 signaling in high glucose-induced podocyte hypertrophy. Kidney Res. Clin. Pract. 2016, 35, 212–218. [Google Scholar] [CrossRef]
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagh, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.H.; Fu, Q.W.; Li, L.X.; Zhou, R.; Liu, N.; Peng, J.H.; Chen, Y. Prx II reduces oxidative stress and cell senescence in chondrocytes by activating the p16-CDK4/6-pRb-E2F signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3448–3458. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).