Incretin Response to Mixed Meal Challenge in Active Cushing’s Disease and after Pasireotide Therapy
Abstract
1. Introduction
2. Material and Methods
3. Results
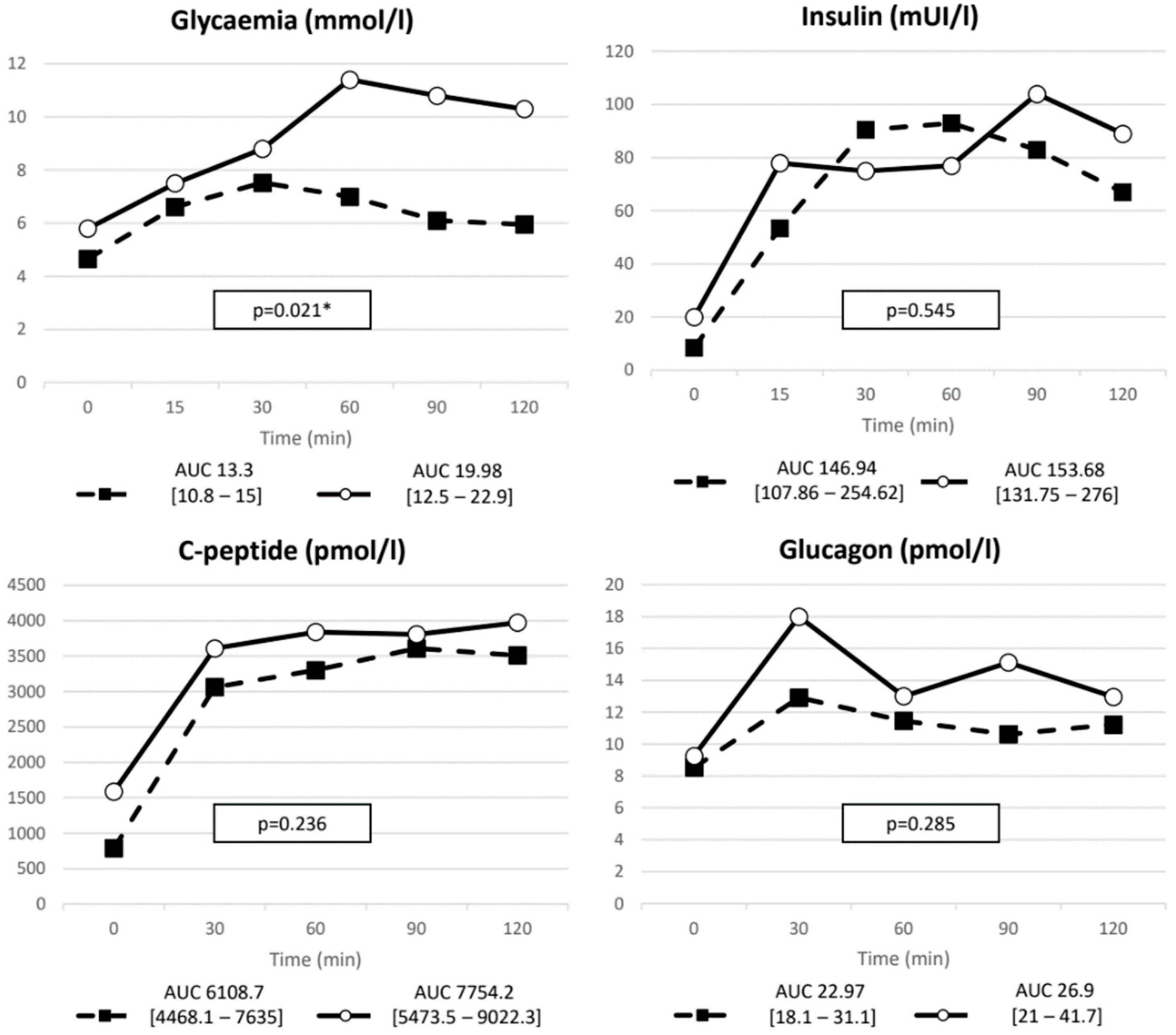
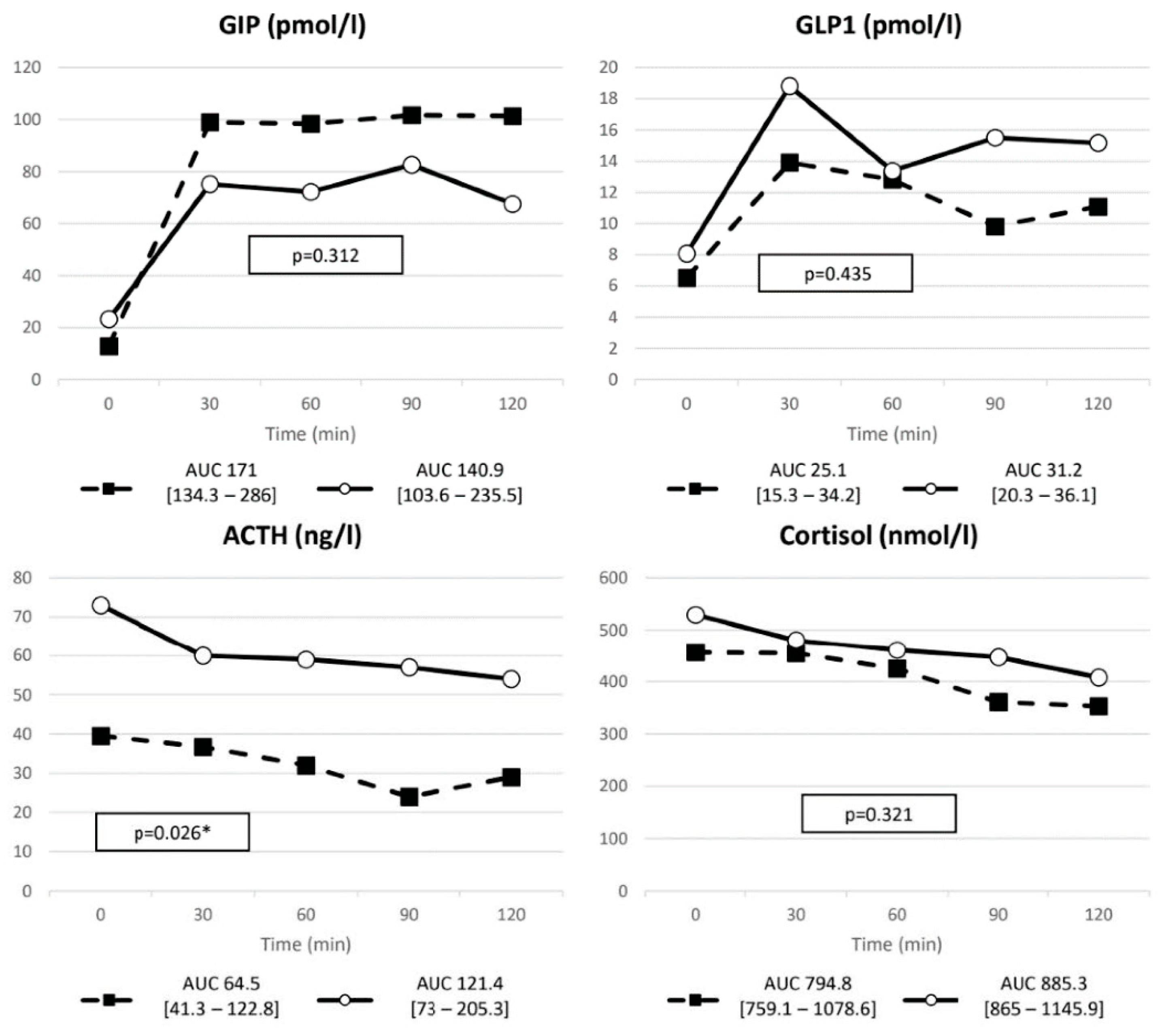
3.1. Baseline Metabolic Profile
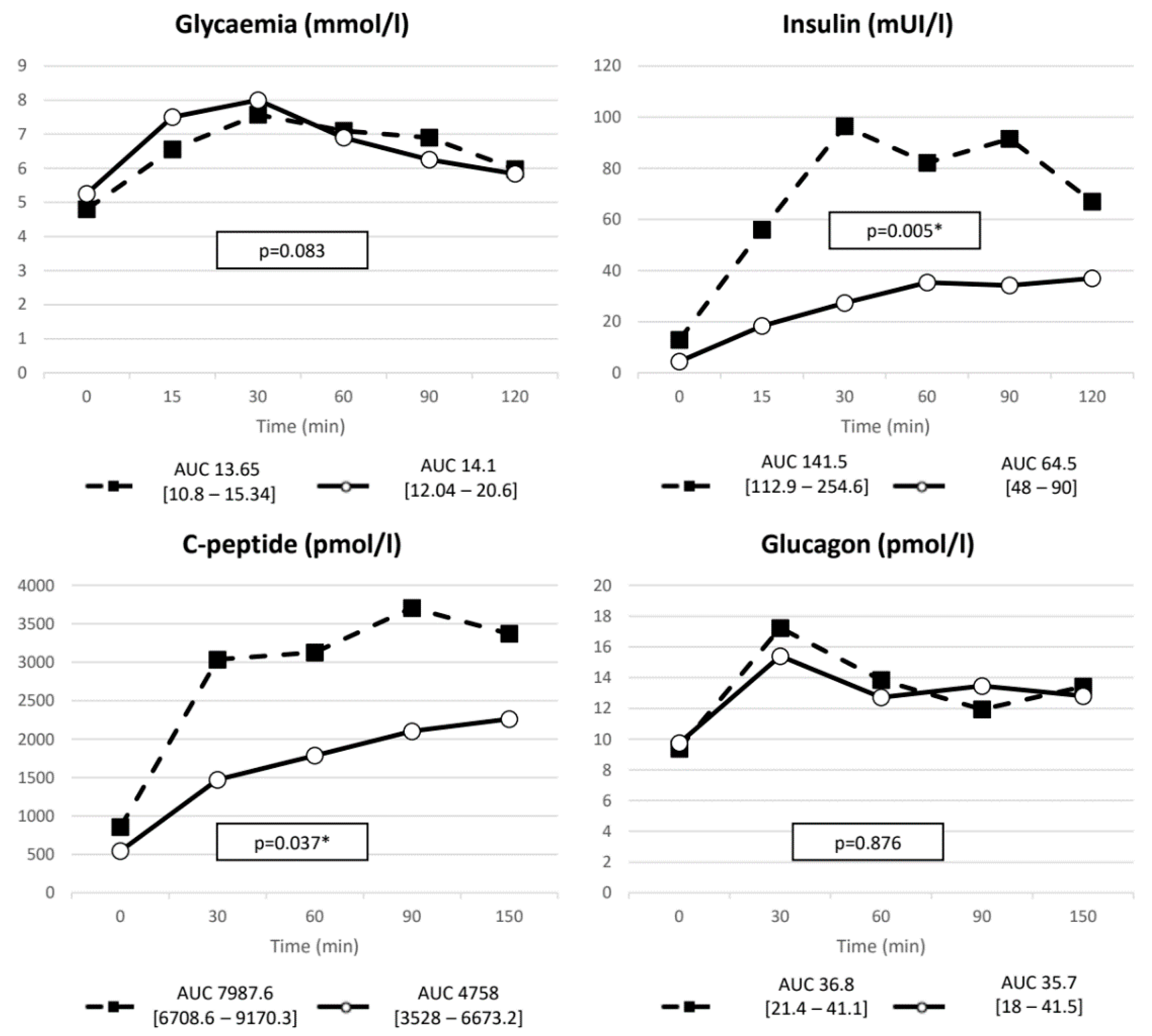
3.2. Results PAS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Barbot, M.; Ceccato, F.; Scaroni, C. Diabetes Mellitus Secondary to Cushing’s Disease. Front. Endocrinol. 2018, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Scaroni, C.; Zilio, M.; Foti, M.; Boscaro, M. Glucose Metabolism Abnormalities in Cushing Syndrome: From Molecular Basis to Clinical Management. Endocr. Rev. 2017, 38, 189–219. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Guarnotta, V.; Pivonello, R.; Amato, M.C.; Simeoli, C.; Ciresi, A.; Cozzolino, A.; Colao, A. Is diabetes in Cushing’s syndrome only a consequence of hypercortisolism? Eur. J. Endocrinol. 2014, 170, 311–319. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar] [CrossRef]
- Eriksen, M.; Jensen, D.H.; Tribler, S.; Holst, J.J.; Madsbad, S.; Krarup, T. Reduction of insulinotropic properties of GLP-1 and GIP after glucocorticoid-induced insulin resistance. Diabetologia 2015, 58, 920–928. [Google Scholar] [CrossRef]
- Jensen, D.H.; Aaboe, K.; Henriksen, J.E.; Vølund, A.; Holst, J.J.; Madsbad, S.; Krarup, T. Steroid-induced insulin resistance and impaired glucose tolerance are both associated with a progressive decline of incretin effect in first-degree relatives of patients with type 2 diabetes. Diabetologia 2012, 55, 1406–1416. [Google Scholar] [CrossRef]
- Kappe, C.; Fransson, L.; Wolbert, P.; Ortsäter, H. Glucocorticoids suppress GLP-1 secretion: Possible contribution to their diabetogenic effects. Clin. Sci. 2015, 129, 405–414. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- Brodovicz, K.G.; Girman, C.J.; Simonis-Bik, A.M.; Rijkelijkhuizen, J.M.; Zelis, M.; Bunck, M.C.; Mari, A.; Nijpels, G.; Eekhoff, E.; Dekker, J.M. Postprandial metabolic responses to mixed versus liquid meal tests in healthy men and men with type 2 diabetes. Diabetes Res. Clin. Pract. 2011, 94, 449–455. [Google Scholar] [CrossRef]
- Nieman, L.K. Recent Updates on the Diagnosis and Management of Cushing’s Syndrome. Endocrinol. Metab. 2018, 33, 139–146. [Google Scholar] [CrossRef]
- Sharma, S.T.; Nieman, L.K.; Feelders, R.A. Comorbidities in Cushing’s disease. Pituitary 2015, 18, 188–194. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef]
- Colao, A.; Petersenn, S.; Newell-Price, J.; Findling, J.W.; Boguszewski, C.; Maldonado, M.; Schoenherr, U.; Biol, D.; Mills, D.; Salgado, L.R.; et al. A 12-Month Phase 3 Study of Pasireotide in Cushing’s Disease. N. Engl. J. Med. 2012, 366, 914–924. [Google Scholar] [CrossRef]
- Henry, R.R.; Ciaraldi, T.P.; Armstrong, D.; Burke, P.; Ligueros-Saylan, M.; Mudaliar, S. Hyperglycemia Associated With Pasireotide: Results from a Mechanistic Study in Healthy Volunteers. J. Clin. Endocrinol. Metab. 2013, 98, 3446–3453. [Google Scholar] [CrossRef]
- Barbot, M.; Regazzo, D.; Mondin, A.; Zilio, M.; Lizzul, L.; Zaninotto, M.; Plebani, M.; Arnaldi, G.; Ceccato, F.; Scaroni, C. Is pasireotide-induced diabetes mellitus predictable? A pilot study on the effect of a single dose of pasireotide on glucose homeostasis. Pituitary 2020, 23, 534–542. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V. The Diagnosis of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef]
- Fischer, B.; Rausch, U.; Wollny, P.; Westphal, H.; Seitz, J.; Aumüller, G. Immunohistochemical Localization of the Glucocorticoid Receptor in Pancreatic β-Cells of the Rat. Endocrinology 1990, 126, 2635–2641. [Google Scholar] [CrossRef]
- Škrha, J.; Hilgertová, J.; Jarolímková, M.; Kunešová, M.; Hill, M. Meal test for glucose-dependent insulinotropic peptide (GIP) in obese and type 2 diabetic patients. Physiol. Res. 2010, 59, 749–755. [Google Scholar] [CrossRef]
- Hansen, K.B.; Vilsbøll, T.; Bagger, J.I.; Holst, J.J.; Knop, F.K. Reduced Glucose Tolerance and Insulin Resistance Induced by Steroid Treatment, Relative Physical Inactivity, and High-Calorie Diet Impairs the Incretin Effect in Healthy Subjects. J. Clin. Endocrinol. Metab. 2010, 95, 3309–3317. [Google Scholar] [CrossRef]
- E Van Genugten, R.; Van Raalte, D.H.; Muskiet, M.; Heymans, M.; Pouwels, P.J.W.; Ouwens, M.; Mari, A.; Diamant, M. Does dipeptidyl peptidase-4 inhibition prevent the diabetogenic effects of glucocorticoids in men with the metabolic syndrome? A randomized controlled trial. Eur. J. Endocrinol. 2014, 170, 429–439. [Google Scholar] [CrossRef][Green Version]
- Færch, K.; Vaag, A.; Holst, J.J.; Glümer, C.; Pedersen, O.; Borch-Johnsen, K. Impaired fasting glycaemia vs impaired glucose tolerance: Similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia 2008, 51, 853–861. [Google Scholar] [CrossRef]
- Henkel, E.; Menschikowski, M.; Koehler, C.; Leonhardt, W.; Hanefeld, M. Impact of glucagon response on postprandial hyperglycemia in men with impaired glucose tolerance and type 2 diabetes mellitus. Metabolism 2005, 54, 1168–1173. [Google Scholar] [CrossRef]
- Iwen, K.A.H.; Senyaman, O.; Schwartz, A.; Drenckhan, M.; Meier, B.; Hadaschik, D.; Klein, J. Melanocortin crosstalk with adipose functions: ACTH directly induces insulin resistance, promotes a pro-inflammatory adipokine profile and stimulates UCP-1 in adipocytes. J. Endocrinol. 2008, 196, 465–472. [Google Scholar] [CrossRef]
- Kumar, U.; Sasi, R.; Suresh, S.; Patel, A.; Thangaraju, M.; Metrakos, P.; Patel, S.C.; Patel, Y.C. Subtype-selective expression of the five somatostatin receptors (hSSTR1-5) in human pancreatic islet cells: A quantitative double-label immunohistochemical analysis. Diabetes 1999, 48, 77–85. [Google Scholar] [CrossRef]
- Guarnotta, V.; Pizzolanti, G.; Ciresi, A.; Giordano, C. Insulin sensitivity and secretion and adipokine profile in patients with Cushing’s disease treated with pasireotide. J. Endocrinol. Investig. 2018, 41, 1137–1147. [Google Scholar] [CrossRef]
- Campbell, S.A.; Johnson, J.; Light, P.E. Evidence for the existence and potential roles of intra-islet glucagon-like peptide-1. Islets 2021, 13, 32–50. [Google Scholar] [CrossRef]
- A Schmid, H.; Brueggen, J. Effects of somatostatin analogs on glucose homeostasis in rats. J. Endocrinol. 2012, 212, 49–60. [Google Scholar] [CrossRef]
- Samson, S.L.; Gu, F.; Feldt-Rasmussen, U.; Zhang, S.; Yu, Y.; Witek, P.; Kalra, P.; Pedroncelli, A.M.; Pultar, P.; Jabbour, N.; et al. Managing pasireotide-associated hyperglycemia: A randomized, open-label, Phase IV study. Pituitary 2021, 24, 887–903. [Google Scholar] [CrossRef]
- Colao, A.; De Block, C.; Gaztambide, M.S.; Kumar, S.; Seufert, J.; Casanueva, F.F. Managing hyperglycemia in patients with Cushing’s disease treated with pasireotide: Medical expert recommendations. Pituitary 2014, 17, 180–186. [Google Scholar] [CrossRef]

| DM− (n = 19) | DM+ (n = 7) | p | |
|---|---|---|---|
| Age (years) | 45 (35.5–55) | 54 (41.5–58) | 0.37 |
| BMI (kg/m2) | 24.97 (22.6–26.95) | 28.96 (26.6–38.6) | 0.012 |
| Waist (cm) | 91 (85.75–101) | 105.5 (104.25–131.5) | 0.006 |
| Hip (cm) | 99 (96.25–99.75) | 105 (102–119.5) | 0.14 |
| SBP (mmHg) | 130 (130–137.5) | 135 (130–140) | 0.47 |
| DBP (mmHg) | 90 (80–95) | 90 (90–97.5) | 0.13 |
| ACTH (ng/L) | 39.5 (30.5–62.75) | 73 (52–95.5) | 0.04 |
| F h 8 (nmol/L) | 508 (344.5–579.5) | 550.5 (395–628.75) | 0.48 |
| UFC/UNL | 3.24 (1.62–4.87) | 2.56 (1.48–3.5) | 0.49 |
| SL-cortisol h8 (nmol/L) | 12.6 (11.1–14.1) | 13.9 (10.2–22.8) | 1 |
| SL-cortisol h23 (nmol/L) | 11.9 (5.15–19.25) | 10.2 (7.4–27.55) | 0.533 |
| Glycemia (nmol/L) | 4.61 (4.5–4.95) | 5,8 (4.65–7.55) | 0.122 |
| HbA1c (mmol/mol) | 35.5 (31.25–36.75) | 42 (39–45.5) | 0.009 |
| Insulin (mIU/L) | 8.5 (6–12.5) | 20 (8–29) | 0.052 |
| C-peptide (pmol/L) | 778 (612.5–999.3) | 1589 (1092.6–2016.4) | 0.004 |
| GIP (pmol/L) | 12.83 (9.53–19.9) | 23.23 (13.7–79) | 0.078 |
| GLP-1 (pmol/L) | 6.53 (5.3–9.96) | 8.1 (5.8–14.9) | 0.193 |
| Glucagon (pmol/L) | 8.53 (3.86–12.75) | 9.25 (9.16–14.7) | 0.214 |
| Total cholesterol (mg/dL) | 188.16 (171–206.75) | 215.09 (199.7–257.1) | 0.108 |
| HDL(mg/dL) | 63 (55.5–69.5) | 65.34 (48–103.8) | 0.841 |
| LDL (mg/dL) | 104.2 (86.5–126) | 133.66 (109–160.75) | 0.424 |
| Triglyceride (mg/dL) | 74.4 (54.5–104.5) | 127.88 (96.9–165.8) | 0.072 |
| VAI | 1.03 (0.67–1.25) | 1.31 (0.85–1.89) | 0.612 |
| HOMA-B | 1.7 (1.1–2.34) | 2.45 (1–3.47) | 0.545 |
| HOMA-IR | 1.81 (1.3–2.45) | 3.73 (2.1–8.6) | 0.027 |
| HOMA-S (%) | 55.35 (40.8–77.2) | 26.79 (11.8–47.6) | 0.027 |
| FIRI | 1.63 (1.17–2.2) | 3.36 (1.9–7.7) | 0.027 |
| QUICKI | 0.35 (0.33–0.37) | 0.315 (0.28–0.34) | 0.017 |
| Insulin:glucagon ratio | 13.63 (9.14–22.82) | 10.14 (3.58–26.85) | 0.33 |
| DM− (n = 19) | DM+ (n = 7) | p | ||
|---|---|---|---|---|
| Glycemia | ∆ (nmol/L) | 3.27 (1.9–4.1) | 3.2 (3–5.7) | 0.485 |
| nAUC | 3.63 (1.99–5.19) | 4.75 (2.875–9.2) | 0.115 | |
| Insulin | ∆ (mIU/L) | 107.5 (77.75–163.4) | 108 (18–167) | 0.586 |
| nAUC | 129.3 (9.36–224.76) | 109.75 (20.5–236) | 0.809 | |
| C-peptide | ∆ (pmol/L) | 3145.4 (2165.4–3486.4) | 2781 (872.4–4006.2) | 0.931 |
| nAUC | 4429.2 (3004.7–5619.5) | 3757.9 (888.6–5512.7) | 0.285 | |
| GIP | ∆ (pmol/L) | 116.5 (84–149.8) | 60.6 (35.7–100.85) | 0.019 |
| nAUC | 148.71 (106.5–221) | 89.97 (55.36–134.35) | 0.035 | |
| GLP-1 | ∆ (pmol/L) | 8.43 (4.9–13.6) | 8.63 (3.64–13.6) | 0.623 |
| nAUC | 9.49 (3.57–19.5) | 7.19 (2.57–19.1) | 0.583 | |
| Glucagon | ∆ (pmol/L) | 6.84 (2.7–11.2) | 8.13 (3.22–25.64) | 0.544 |
| nAUC | 6.1 (1.14–10.36) | 7.16 (2.66–23.34) | 0.402 | |
| Baseline | 2 Months | p | |
|---|---|---|---|
| BMI (kg/m2) | 24.97 (21.85–28.95) | 25.01 (23–33.3) | 0.203 |
| Waist circumference (cm) | 91 (80.5–109.5) | 87 (82–104) | 0.344 |
| SBP (mmHg) | 130 (125–135) | 130 (120–136.25) | 0.729 |
| DBP (mmHg) | 87.5 (77.5–100) | 82.5 (80–91.25) | 0.493 |
| ACTH (ng/L) | 41.9 (31–75) | 33.75 (21–73.7) | 0.139 |
| S-cortisol h 8 (nmol/L) | 510 (447.8–665.8) | 514.5 (370.3–566) | 0.445 |
| UFC/UNL | 3.26 (1.73–3.88) | 0.93 (0.4–1.98) | 0.005 |
| SL-cortisol h23 (nmol/L) | 8.3 (3.7–15.3) | 4.23 (3.8–5.9) | 0.091 |
| Glycemia (nmol/L) | 4.8 (4.5–5.1) | 5.26 (5–7.6) | 0.009 |
| HbA1c (mmol/mol) | 35 (29.5–36.5) | 41 (36.75–63.25) | 0.012 |
| Insulin (mIU/L) | 12.9 (7.5–17.35) | 4.45 (2–9) | 0.008 |
| C-peptide (pmol/L) | 857.5 (687–1068.6) | 544.65 (278.1–753.2) | 0.037 |
| GIP (pmol/L) | 14.38 (9.44–25.2) | 11.96 (6.8–19) | 0.285 |
| GLP-1 (pmol/L) | 8.17 (6.5–12.5) | 6.47 (4–13.15) | 0.646 |
| Glucagon (pmol/L) | 9.39 (4.36–15.5) | 9.75 (5.4–13.5) | 0.646 |
| Total cholesterol (mmol/L) | 5.1 (4–5.9) | 5.14 (4.28–5.85) | 0.499 |
| HDL (mmol/L) | 1.71 (1.64–2) | 1.6 (1.38–1.76) | 0.400 |
| LDL (mmol/L) | 2.89 (2.5–4.2) | 3.24 (2.33–4.38) | 0.735 |
| Triglycerides (mmol/L) | 0.67 (0.5–1.14) | 0.87 (0.51–2.15) | 0.779 |
| VAI | 1.06 (0.6–2.2) | 0.85 (0.52–2.15) | 0.249 |
| HOMA-B | 1.89 (1.15–2.69) | 0.44 (0.2–0.86) | 0.005 |
| HOMA-IR | 2.66 (1.5–3.9) | 1.1 (0.6–2) | 0.007 |
| HOMA-S (%) | 37.7 (26.1–66.7) | 94.9 (49.8–168) | 0.009 |
| FIRI | 2.4 (1.37–3.47) | 0.99 (0.54–1.83) | 0.007 |
| QUICKI | 0.33 (0.31–0.36) | 0.73 (0.6–0.89) | 0.005 |
| Insulin:glucagon ratio | 10.3 (7.89–22.2) | 5.23 (3.53–7.06) | 0.009 |
| Baseline | 2 Months | p | ||
|---|---|---|---|---|
| Glycemia | ∆ (nmol/L) | 3.1 (2.2–3.83) | 3.15 (2.4–4.25) | 0.475 |
| nAUC | 3.63 (1.81–5.19) | 3.44 (2.2–5.34) | 0.799 | |
| Insulin | ∆ (mIU/L) | 109 (80.75–150.75) | 40.5 (26–75.75) | 0.005 |
| nAUC | 112.46 (97.9–224.8) | 53.88 (34.6–82.75) | 0.009 | |
| C-peptide | ∆ (pmol/L) | 3195 (2606.5–3395.4) | 1930.3 (1519.7–2677.3) | 0.017 |
| nAUC | 5763.1 (4186.7–7005) | 3082.1 (2536.8–4597.3) | 0.022 | |
| GIP | ∆ (pmol/L) | 126.1 (91–228.2) | 113.16 (77.5–223.1) | 0.241 |
| nAUC | 187.7 (175.9–457.8) | 205.7 (141.1–320) | 0.203 | |
| GLP-1 | ∆ (pmol/L) | 9.29 (4.7–14.1) | 10.2 (4.6–12.4) | 0.646 |
| nAUC | 8.38 (4.7–26.2) | 15.55 (6.52–18.37) | 0.799 | |
| Glucagon | ∆ (pmol/L) | 7.91 (1–12.6) | 7.1 (4.1–12) | 0.799 |
| nAUC | 10.69 (−3–17.14) | 8.76 (4.8–14.2) | 0.386 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbot, M.; Mondin, A.; Regazzo, D.; Guarnotta, V.; Basso, D.; Giordano, C.; Scaroni, C.; Ceccato, F. Incretin Response to Mixed Meal Challenge in Active Cushing’s Disease and after Pasireotide Therapy. Int. J. Mol. Sci. 2022, 23, 5217. https://doi.org/10.3390/ijms23095217
Barbot M, Mondin A, Regazzo D, Guarnotta V, Basso D, Giordano C, Scaroni C, Ceccato F. Incretin Response to Mixed Meal Challenge in Active Cushing’s Disease and after Pasireotide Therapy. International Journal of Molecular Sciences. 2022; 23(9):5217. https://doi.org/10.3390/ijms23095217
Chicago/Turabian StyleBarbot, Mattia, Alessandro Mondin, Daniela Regazzo, Valentina Guarnotta, Daniela Basso, Carla Giordano, Carla Scaroni, and Filippo Ceccato. 2022. "Incretin Response to Mixed Meal Challenge in Active Cushing’s Disease and after Pasireotide Therapy" International Journal of Molecular Sciences 23, no. 9: 5217. https://doi.org/10.3390/ijms23095217
APA StyleBarbot, M., Mondin, A., Regazzo, D., Guarnotta, V., Basso, D., Giordano, C., Scaroni, C., & Ceccato, F. (2022). Incretin Response to Mixed Meal Challenge in Active Cushing’s Disease and after Pasireotide Therapy. International Journal of Molecular Sciences, 23(9), 5217. https://doi.org/10.3390/ijms23095217








