Gradual Stress-Relaxation of Hydrogel Regulates Cell Spreading
Abstract
:1. Introduction
2. Results and Discussion
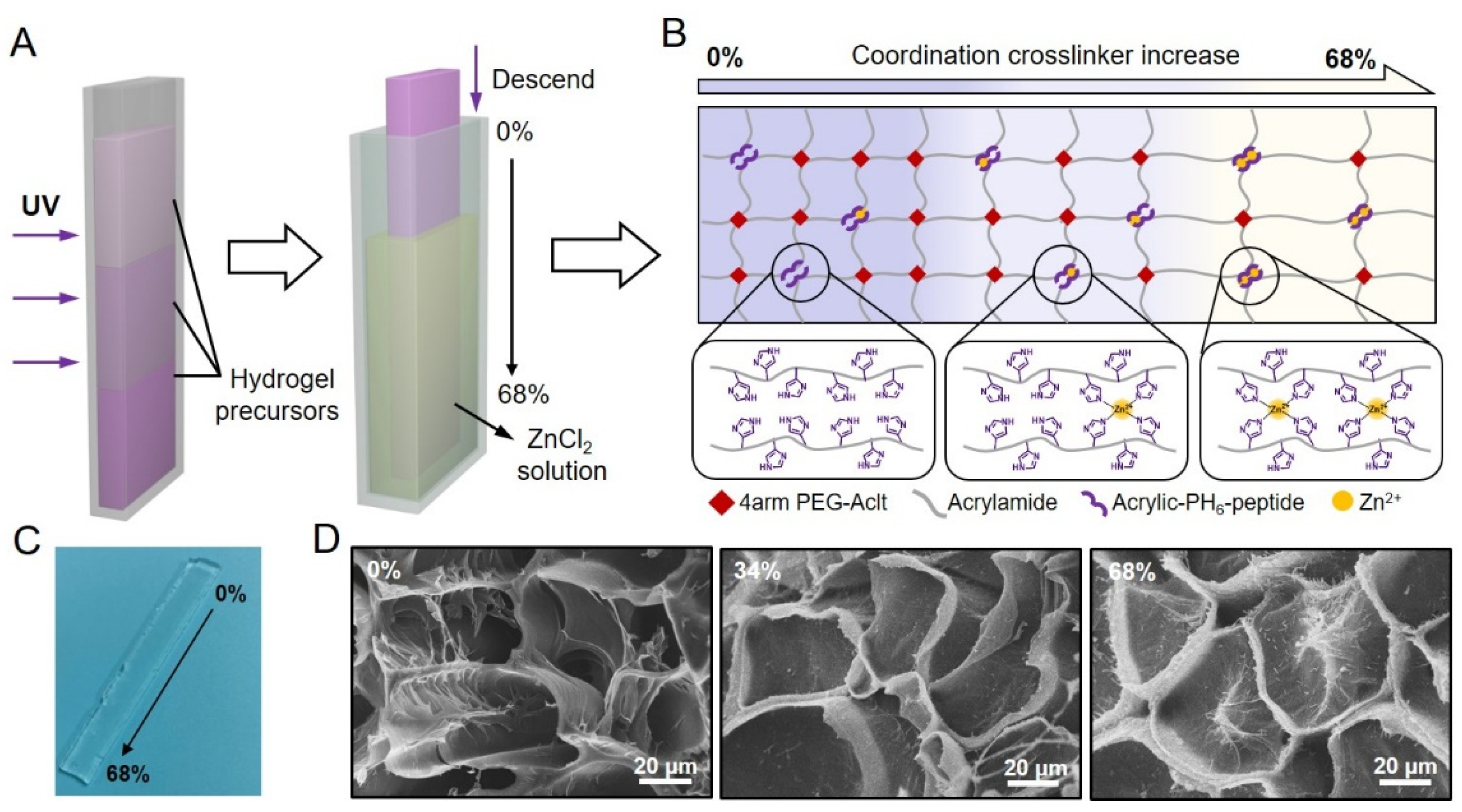
2.1. Designing Hydrogels with a Stress-Relaxation Gradient
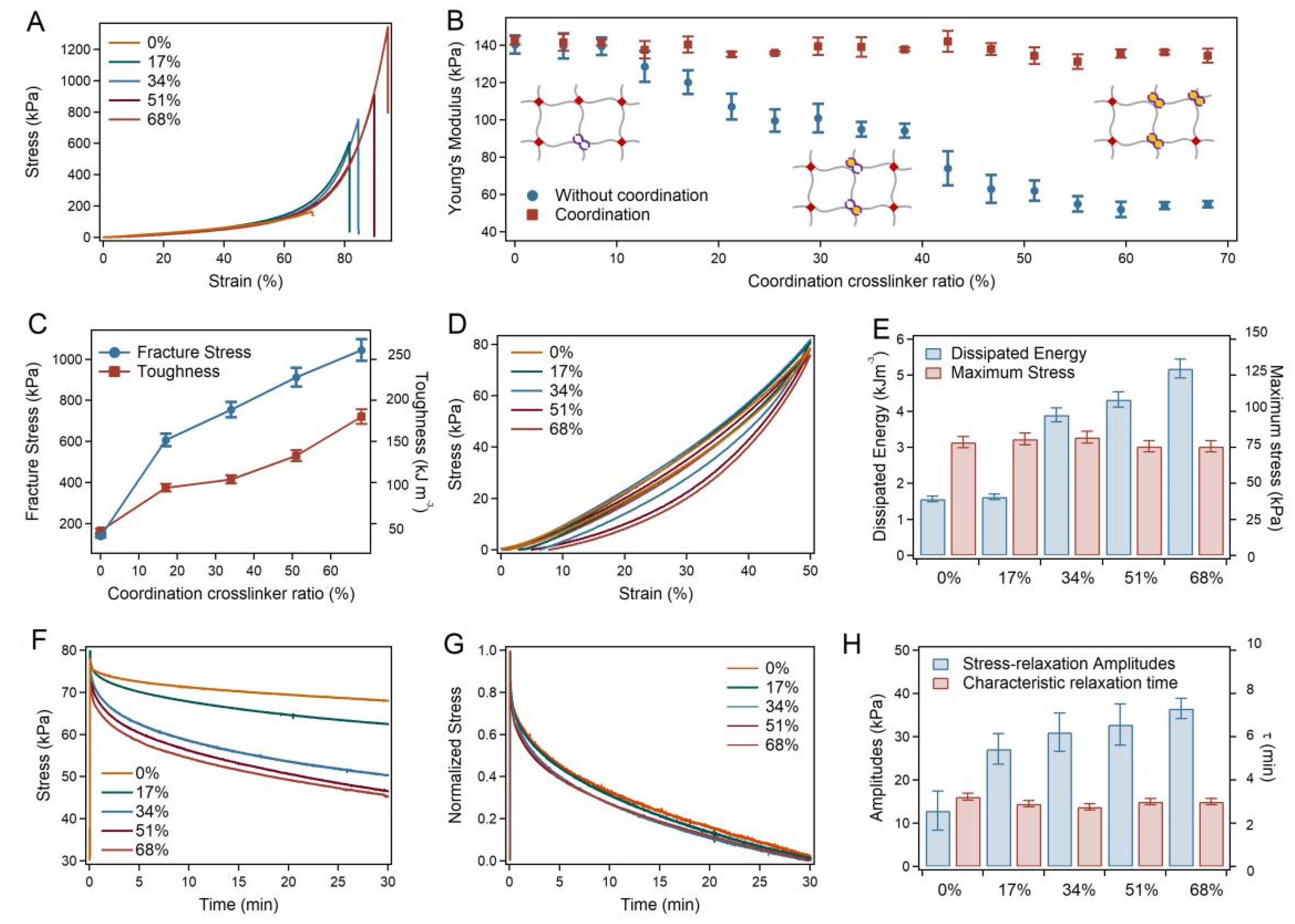
2.2. Mechanical Properties of the SRG Hydrogels
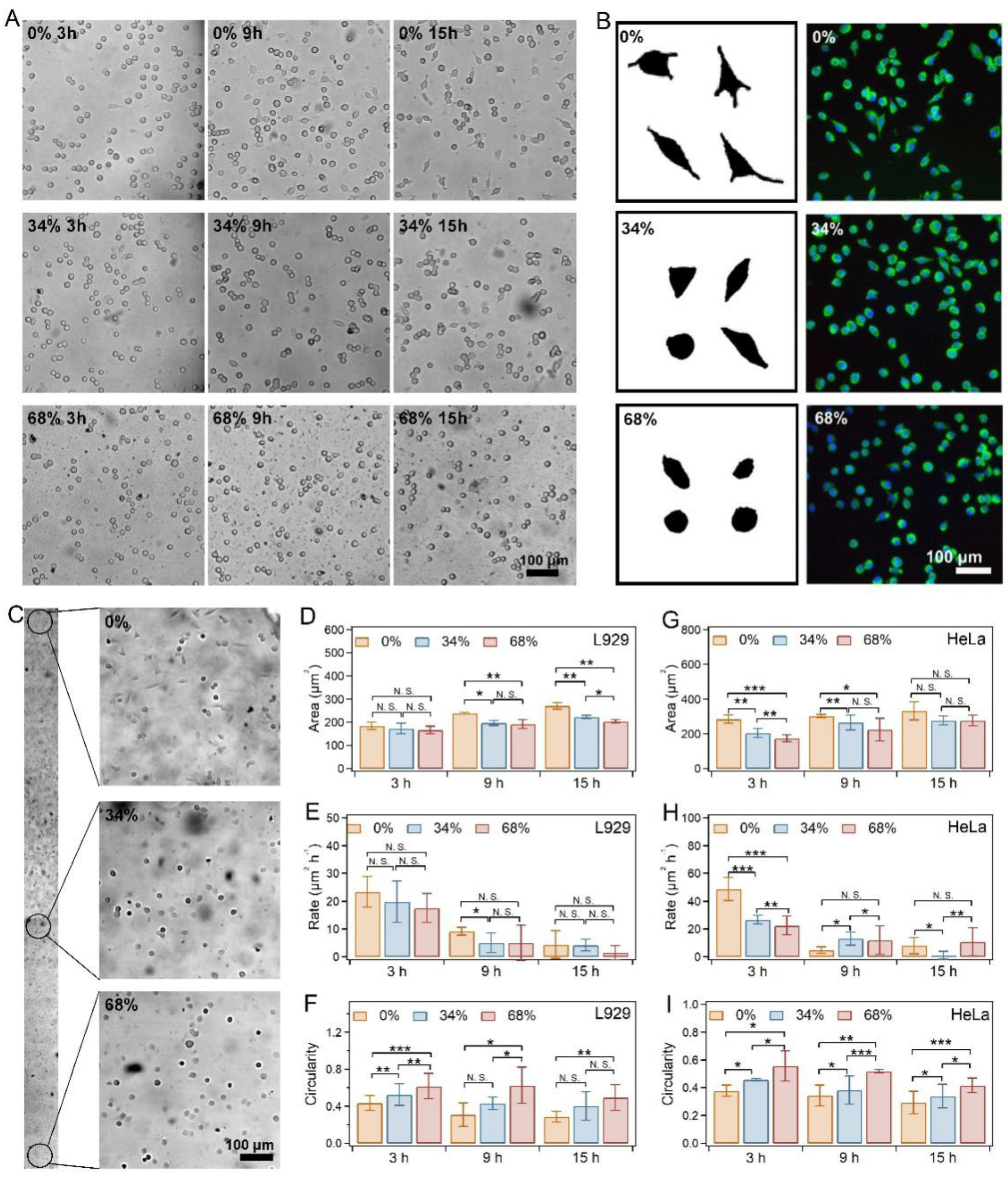
2.3. Cell Spreading on SRG Hydrogels
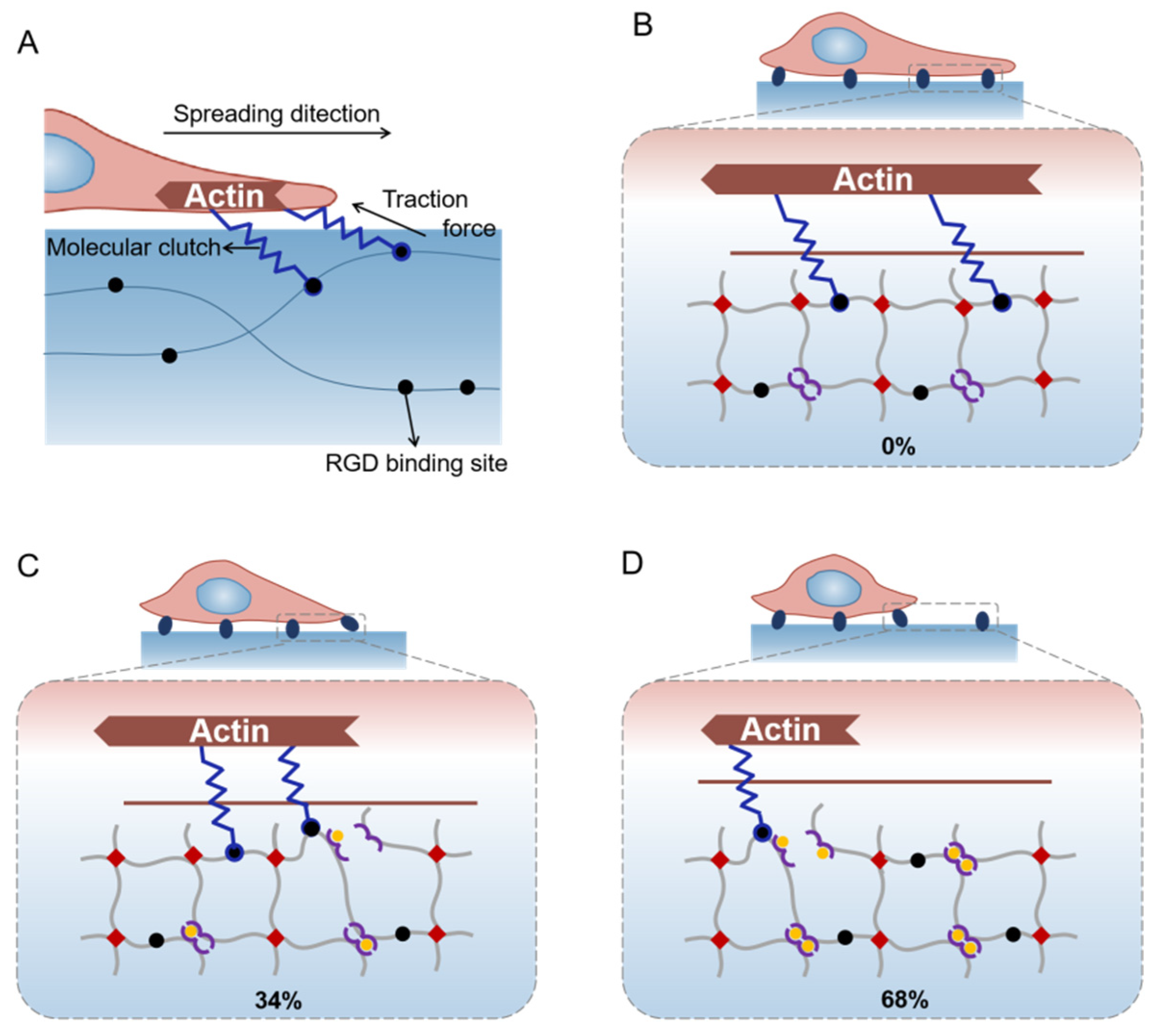
2.4. Hypothesis of Mechanisms for Cell Spreading Regulation with the Gradual Stress-Relaxation
3. Materials and Methods
3.1. Materials
3.2. Correlation between Young’s Modulus and Immersion Time
3.3. Preparation of the SRG Hydrogel
3.4. Scanning Electron Microscopy (SEM)
3.5. Mechanical Test
3.6. Swelling Ratios and Water Contents
3.7. Cell Culture
3.8. Immunostaining and Cell Morphology Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelham, R.J.; Wang, Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mierke, C.T.; Sauer, F.; Grosser, S.; Puder, S.; Fischer, T.; Käs, J.A. The two faces of enhanced stroma: Stroma acts as a tumor promoter and a steric obstacle. NMR Biomed. 2018, 31, e3831. [Google Scholar] [CrossRef] [PubMed]
- Brabek, J.; Mierke, C.; Rosel, D.; Vesely, P.; Fabry, B. The role of the tissue microenvironment in the regulation of cancer cell motility and invasion. Cell Commun. Signal. 2010, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Sapudom, J.; Rubner, S.; Martin, S.; Kurth, T.; Riedel, S.; Mierke, C.T.; Pompe, T. The phenotype of cancer cell invasion controlled by fibril diameter and pore size of 3D collagen networks. Biomaterials 2015, 52, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulrich, T.A.; de Juan Pardo, E.M.; Kumar, S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009, 69, 4167–4174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuFort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Xue, B.; Tang, D.; Wu, X.; Xu, Z.; Gu, J.; Han, Y.; Zhu, Z.; Qin, M.; Zou, X.; Wang, W.; et al. Engineering hydrogels with homogeneous mechanical properties for controlling stem cell lineage specification. Proc. Natl. Acad. Sci. USA 2021, 118, e2110961118. [Google Scholar] [CrossRef]
- Nerger, B.A.; Nelson, C.M. Engineered extracellular matrices: Emerging strategies for decoupling structural and molecular signals that regulate epithelial branching morphogenesis. Curr. Opin. Biomed. Eng. 2020, 13, 103–112. [Google Scholar] [CrossRef]
- Mak, M. Impact of crosslink heterogeneity on extracellular matrix mechanics and remodeling. Comput. Struct. Biotechnol. J. 2020, 18, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Sauer, F.; Oswald, L.; de Schellenberger, A.A.; Tzschätzsch, H.; Schrank, F.; Fischer, T.; Braun, J.; Mierke, C.T.; Valiullin, R.; Sack, I.; et al. Collagen networks determine viscoelastic properties of connective tissues yet do not hinder diffusion of the aqueous solvent. Soft Matter 2019, 15, 3055–3064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunyer, R.; Trepat, X. Durotaxis. Curr. Biol. 2020, 30, R383–R387. [Google Scholar] [CrossRef] [PubMed]
- Shellard, A.; Mayor, R. Collective durotaxis along a self-generated stiffness gradient in vivo. Nature 2021, 600, 690–694. [Google Scholar] [CrossRef]
- Koser, D.E.; Thompson, A.J.; Foster, S.K.; Dwivedy, A.; Pillai, E.K.; Sheridan, G.K.; Svoboda, H.; Viana, M.; da F. Costa, L.; Guck, J.; et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016, 19, 1592–1598. [Google Scholar] [CrossRef] [Green Version]
- Hazur, J.; Endrizzi, N.; Schubert, D.W.; Boccaccini, A.R.; Fabry, B. Stress relaxation amplitude of hydrogels determines migration, proliferation, and morphology of cells in 3-D culture. Biomater. Sci. 2022, 10, 270–280. [Google Scholar] [CrossRef]
- Cameron, A.R.; Frith, J.E.; Cooper-White, J.J. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 2011, 32, 5979–5993. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Darnell, M.; Klumpers, D.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Mooney, D.J. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015, 6, 6365. [Google Scholar] [CrossRef] [Green Version]
- Wisdom, K.M.; Adebowale, K.; Chang, J.; Lee, J.Y.; Nam, S.; Desai, R.; Rossen, N.S.; Rafat, M.; West, R.B.; Hodgson, R.; et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 2018, 9, 4144. [Google Scholar] [CrossRef] [Green Version]
- Sabeh, F.; Shimizu-Hirota, R.; Weiss, S.J. Protease-dependent versus -independent cancer cell invasion programs: Three-dimensional amoeboid movement revisited. J. Cell Biol. 2009, 185, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Wolf, K.; Lindert, M.T.; Krause, M.; Alexander, S.; Riet, J.T.; Willis, A.L.; Hoffman, R.L.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, T.; Swift, J.; Irianto, J.; Shin, J.-W.; Spinler, K.R.; Athirasala, A.; Diegmiller, R.; Dingal, P.C.D.P.; Ivanovska, I.L.; Discher, D.E. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol. 2014, 204, 669–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.; Gupta, V.K.; Lee, H.-P.; Lee, J.Y.; Wisdom, K.M.; Varma, S.; Flaum, E.M.; Davis, C.; West, R.B.; Chaudhuri, O. Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27(Kip1) signaling axis. Sci. Adv. 2019, 5, eaaw6171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.; Chaudhuri, O. Mitotic cells generate protrusive extracellular forces to divide in three-dimensional microenvironments. Nat. Phys. 2018, 14, 621–628. [Google Scholar] [CrossRef]
- Lee, H.P.; Stowers, R.; Chaudhuri, O. Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nat. Commun. 2019, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Raeber, G.P.; Lutolf, M.P.; Hubbell, J.A. Molecularly engineered PEG hydrogels: A novel model system for proteolytically mediated cell migration. Biophys. J. 2005, 89, 1374–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Huebsch, N.; Mooney, D.J.; Suo, Z. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J. Appl. Phys. 2010, 107, 063509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, Y.; Qin, M.; Cheng, W.; Wang, W.; Cao, Y. Understanding and regulating cell-matrix interactions using hydrogels of designable mechanical properties. J. Bio. Nanotechnol. 2021, 17, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Gu, J.; Li, L.; Yu, W.; Yin, S.; Qin, M.; Jiang, Q.; Wang, W.; Cao, Y. Hydrogel tapes for fault-tolerant strong wet adhesion. Nat. Commun. 2021, 12, 7156. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Healy, K.E.; Rezania, A.; Stile, R.A. Designing biomaterials to direct biological responses. Ann. N. Y. Acad. Sci. 1999, 875, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Metters, A.T.; Anseth, K.S.; Bowman, C.N. Fundamental studies of biodegradable hydrogels as cartilage replacement materials. Biomed. Sci. Instrum. 1999, 35, 33–38. [Google Scholar] [PubMed]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Wu, D.; Lei, H.; Xie, X.; Zhou, L.; Zheng, P.; Cao, Y.; Zhang, Y. Self-sorting double network hydrogels with photo-definable biochemical cues as artificial synthetic extracellular matrix. Nano Res. 2022, 1–8. [Google Scholar] [CrossRef]
- Jian, Y.; Wu, B.; Yang, X.; Peng, Y.; Zhang, D.; Yang, Y.; Qiu, H.; Lu, H.; Zhang, J.; Chen, T. Stimuli-responsive hydrogel sponge for ultrafast responsive actuator. Supramol. Mater. 2022, 1, 100002. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, J.; Gu, J.; Li, W.; Cao, Y. Light controlled biomaterials for regulating cell migration and differentiation. Smart Mater. Med. 2022, 3, 209–216. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2015, 14, 102. [Google Scholar] [CrossRef] [Green Version]
- Lehn, J.-M. Supramolecular materials: Dynamic, responsive, adaptive. Supramol. Mater. 2022, 1, 100007. [Google Scholar] [CrossRef]
- Grindy, S.C.; Learsch, R.; Mozhdehi, D.; Cheng, J.; Barrett, D.G.; Guan, Z.; Messersmith, P.B.; Holten-Andersen, N. Control of hierarchical polymer mechanics with bioinspired metal-coordination dynamics. Nat. Mater. 2015, 14, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, M.; Cao, Y.; Wang, W. Designing the mechanical properties of peptide-based supramolecular hydrogels for biomedical applications. Sci. China-Phys. Mech. Astron. 2014, 57, 849–858. [Google Scholar] [CrossRef]
- Treloar, L.R.G. The Physics of Rubber Elasticity; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Aaron, B.B.; Gosline, J.M. Elastin as a random-network elastomer: A mechanical and optical analysis of single elastin fibers. Biopolymers 1981, 20, 1247–1260. [Google Scholar] [CrossRef]
- Elvin, C.M.; Carr, A.G.; Huson, M.G.; Maxwell, J.M.; Pearson, R.D.; Vuocolo, T.; Liyou, N.E.; Wong, D.C.C.; Merritt, D.J.; Dixon, N.E. Synthesis and properties of crosslinked recombinant pro-resilin. Nature 2005, 437, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Zenga, X.; Nyquist, Y.; Zhanga, Q.; Butt, H.-J.; Wu, S. Light-induced assembly of colloidal nanoparticles based on photo-controlled metal–ligand coordination. Supramol. Mater. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Ayoob, A.M.; Appel, E.A.; Borenstein, J.T.; Langer, R.; Anderson, D.G. Mixed reversible covalent crosslink kinetics enable precise, hierarchical mechanical tuning of hydrogel networks. Adv. Mater. 2017, 29, 1605947. [Google Scholar] [CrossRef]
- Dooling, L.J.; Tirrell, D.A. Engineering the dynamic properties of protein networks through sequence variation. ACS Cent. Sci. 2016, 2, 812–819. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Dong, C.; Jiang, H.; Bin, X.; Gao, X.; Qin, M.; Wang, W.; Bin, C.; Cao, Y. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat. Commun. 2018, 9, 620. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Gu, J.; Jiang, Y.; Zhou, Y.; Zhu, Z.; Ma, T.; Cheng, Y.; Ji, Z.; Jiao, Y.; Xue, B.; et al. Regulating the homogeneity of thiol-maleimide Michael-type addition-based hydrogels using amino biomolecules. Gels 2021, 7, 206. [Google Scholar] [CrossRef]
- Morgan, W.T. The histidine-rich glycoprotein of serum has a domain rich in histidine, proline, and glycine that binds heme and metals. Biochemistry 1985, 24, 1496–1501. [Google Scholar] [CrossRef]
- Sun, W.; Xue, B.; Fan, Q.; Tao, R.; Wang, C.; Wang, X.; Li, Y.; Qin, M.; Wang, W.; Chen, B.; et al. Molecular engineering of metal-coordination interactions for strong, tough and fast-recovery hydrogels. Sci. Adv. 2020, 6, eaaz9531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Xue, B.; Zhu, Z.; Shen, Z.; Qin, M.; Wang, W.; Cao, Y. Strong and injectable hydrogels based on multivalent metal ion-peptide cross-linking. Chem. Res. Chin. Univ. 2020, 36, 962–969. [Google Scholar] [CrossRef]
- Zeng, L.; Song, M.; Gu, J.; Xu, Z.; Xue, B.; Li, Y.; Cao, Y. A highly stretchable, tough, fast self-healing hydrogel based on peptide-metal ion coordination. Biomimetics 2019, 4, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, J.A.; Oldenhuis, N.J.; Novitsky, A.L.; Samson, E.M.; Thrift, W.J.; Ragan, R.; Guan, Z. Large continuous mechanical gradient formation via metal-ligand interactions. Angew. Chem. Int. Ed. 2017, 56, 15575–15579. [Google Scholar] [CrossRef] [PubMed]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Stuart, M.A.C.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dave, P.C.; Dingal, P.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.W.; et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef] [Green Version]
- Mandal, K.; Gong, Z.; Rylander, A.; Shenoy, V.B.; Janmey, P.A. Opposite responses of normal hepatocytes and hepatocellular carcinoma cells to substrate viscoelasticity. Biomater. Sci. 2020, 8, 1316–1328. [Google Scholar] [CrossRef]
- Charrier, E.E.; Pogoda, K.; Li, R.; Chan, Y.P.; Janmey, P.A. A novel method to make viscoelastic polyacrylamide gels for cell culture and traction force microscopy. APL Bioeng. 2020, 4, 036104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Sun, W.; Chen, H.; Wang, J.; Xue, B.; Cao, Y. Gradual Stress-Relaxation of Hydrogel Regulates Cell Spreading. Int. J. Mol. Sci. 2022, 23, 5170. https://doi.org/10.3390/ijms23095170
Yu W, Sun W, Chen H, Wang J, Xue B, Cao Y. Gradual Stress-Relaxation of Hydrogel Regulates Cell Spreading. International Journal of Molecular Sciences. 2022; 23(9):5170. https://doi.org/10.3390/ijms23095170
Chicago/Turabian StyleYu, Wenting, Wenxu Sun, Huiyan Chen, Juan Wang, Bin Xue, and Yi Cao. 2022. "Gradual Stress-Relaxation of Hydrogel Regulates Cell Spreading" International Journal of Molecular Sciences 23, no. 9: 5170. https://doi.org/10.3390/ijms23095170
APA StyleYu, W., Sun, W., Chen, H., Wang, J., Xue, B., & Cao, Y. (2022). Gradual Stress-Relaxation of Hydrogel Regulates Cell Spreading. International Journal of Molecular Sciences, 23(9), 5170. https://doi.org/10.3390/ijms23095170







