Characterization of the Wave Phenomenon in Flash-Induced Fluorescence Relaxation and Its Application to Study Cyclic Electron Pathways in Microalgae
Abstract
:1. Introduction
1.1. Photosynthesis of Microalgae
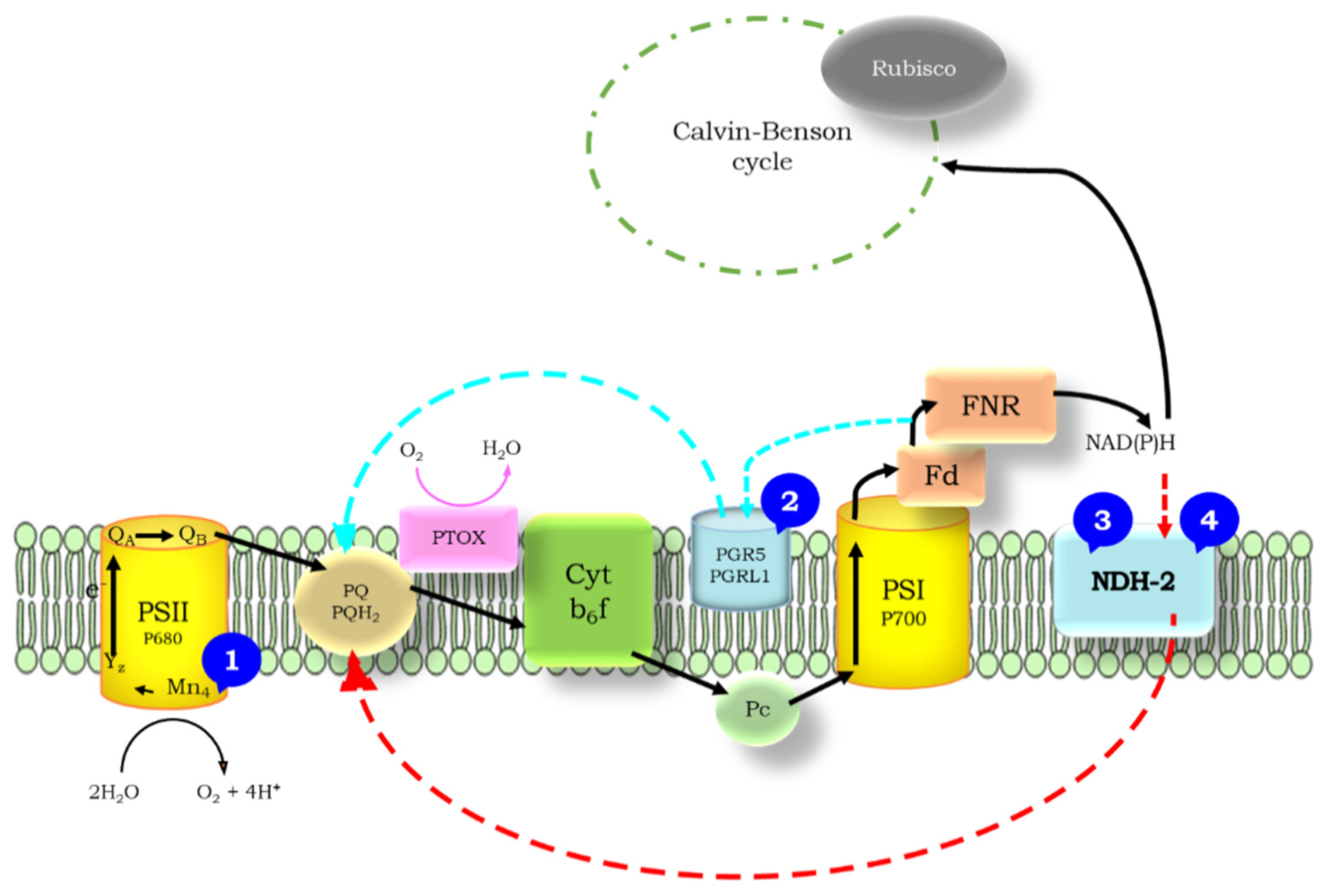
1.2. Cyclic Electron Flow
1.3. Flash-Induced Fluorescence Relaxation
1.4. The Wave Phenomenon of the Fluorescence Relaxation
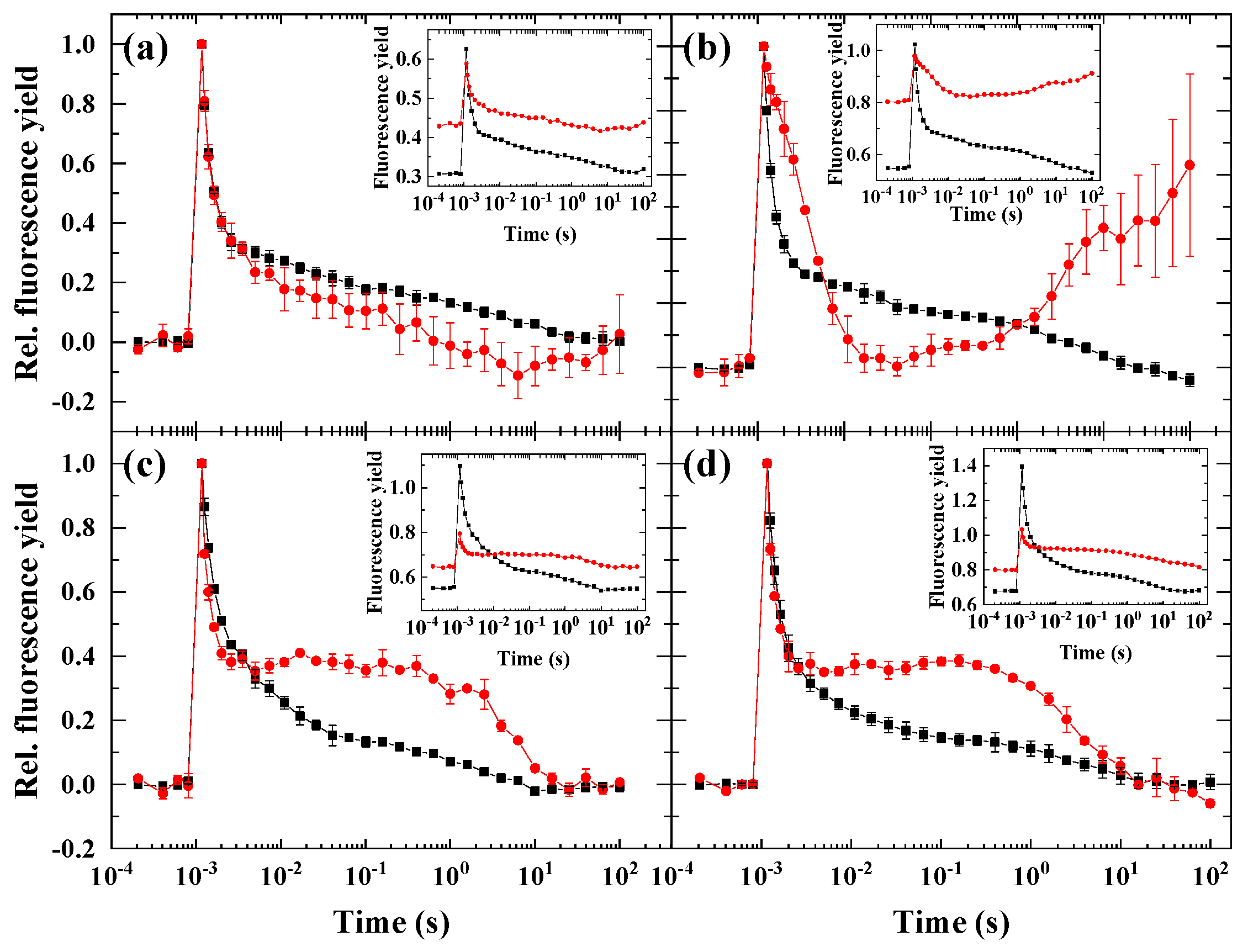
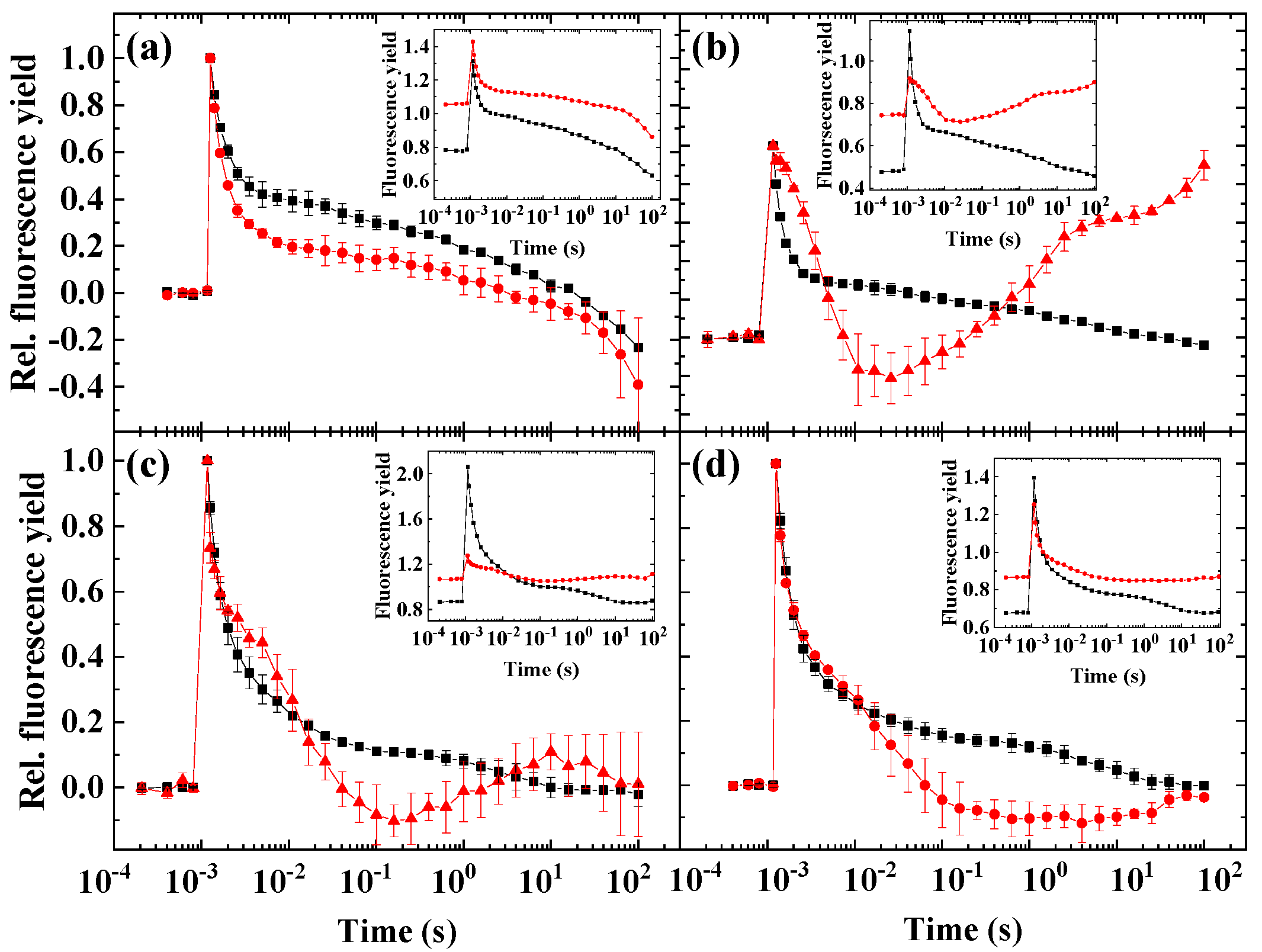
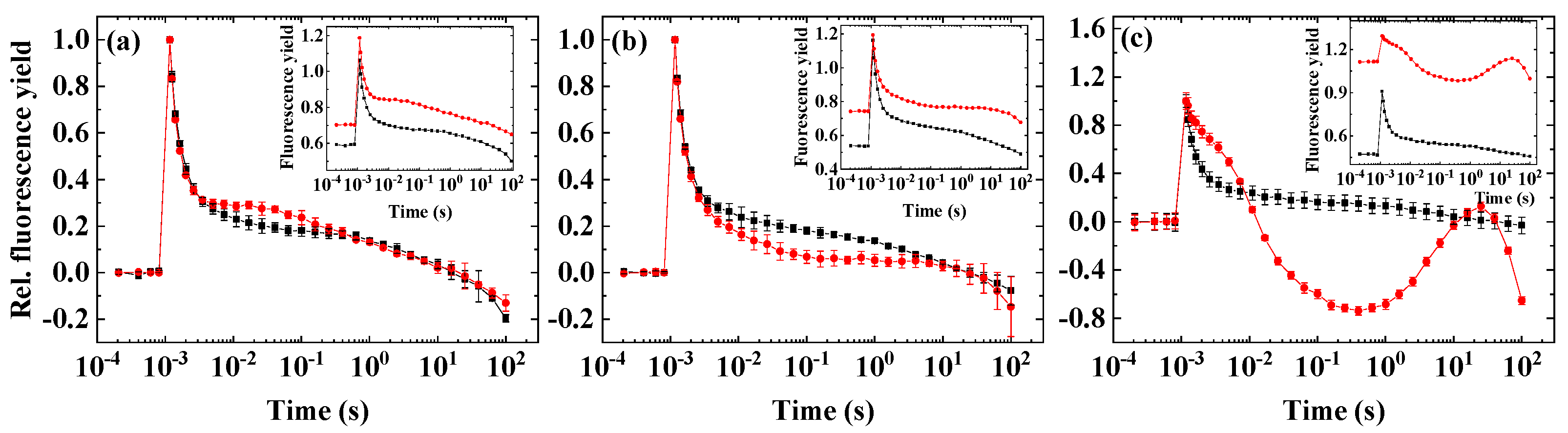
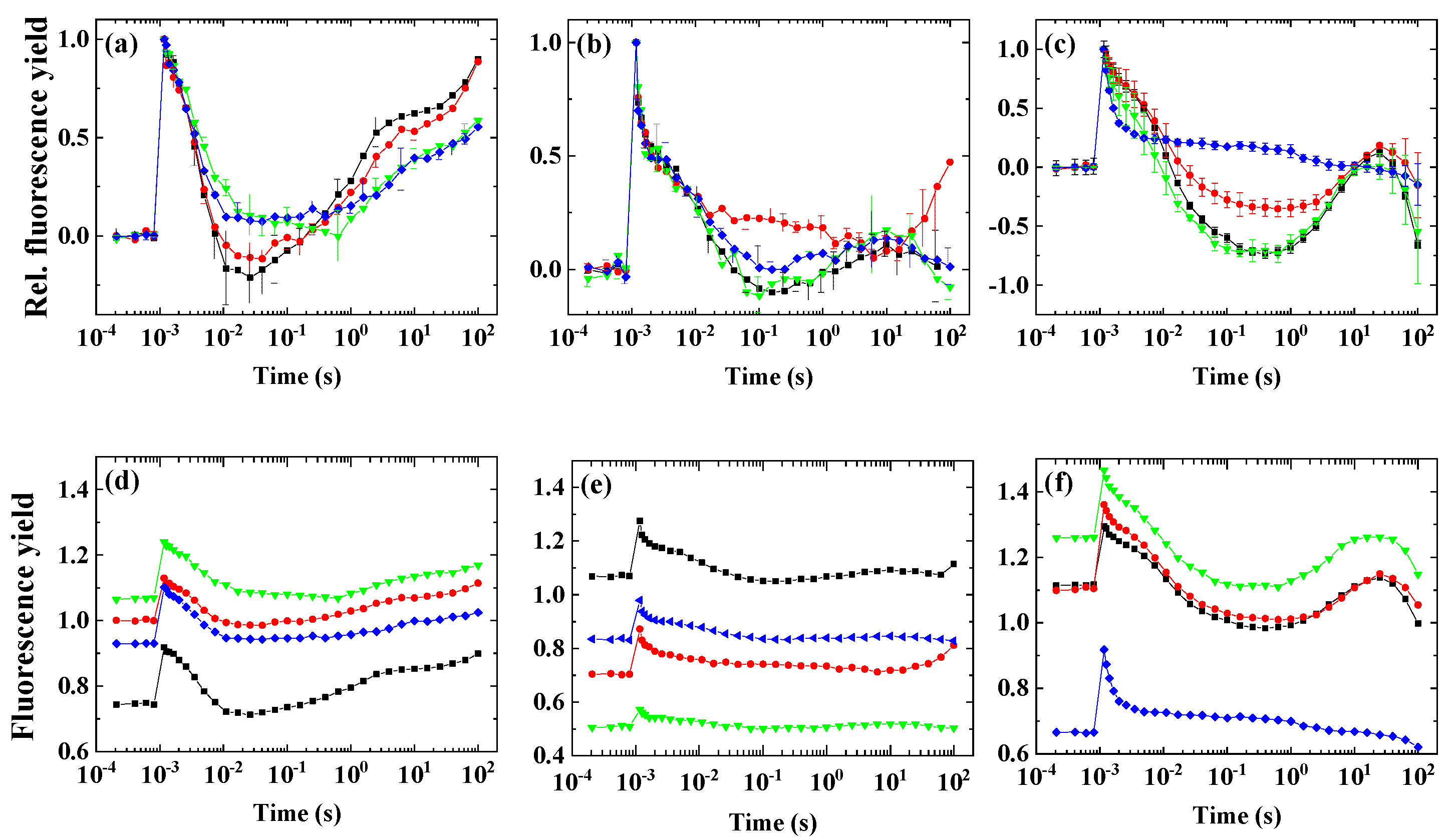
2. Results
2.1. Flash-Induced Fluorescence Relaxation under Microaerobic Conditions
2.2. Flash-Induced Fluorescence Relaxation under Partially Inhibited PSII Activity
2.3. Flash-Induced Fluorescence Relaxation under Partially Inhibited PSII Activity Combined with Microaerobic Condition
2.4. Variability of the Flash-Induced Fluorescence Wave Pattern in the Different Species
2.5. The Effect of Inhibitors of Cyclic Electron Flow on the Fluorescence Wave Phenomenon
3. Discussion
3.1. Significance of the Wave Phenomenon in Flash-Induced Chlorophyll Fluorescence Relaxation
3.2. The Role of Impaired PSII Activity and Microaerobic Conditions in the Wave Phenomenon in Microalgae
3.3. Different Conditions Are Required to Induce the Wave Phenomenon in Different Species
3.4. Impact of Inhibitors of Cyclic Electron Flow on the Fluorescence Wave Phenomenon in Microalgae
4. Materials and Methods
4.1. Algal Cultures
4.2. Experimental Procedure
4.3. Flash-Induced Chlorophyll Fluorescence Relaxation Kinetics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Formighieri, C.; Bassi, R.; Algae, A. New Biomass Resource. In Renewable Energy Systems; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–26. [Google Scholar]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging technologies in algal biotechnology: Toward the establishment of a sustainable, algae-based bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [Green Version]
- Cardol, P.; Forti, G.; Finazzi, G. Regulation of electron transport in microalgae. Biochim. Biophys. Acta-Bioenerg. 2011, 1807, 912–918. [Google Scholar] [CrossRef] [Green Version]
- Nawrocki, W.J.; Bailleul, B.; Picot, D.; Cardol, P.; Rappaport, F.; Wollman, F.A.; Joliot, P. The mechanism of cyclic electron flow. Biochim. Biophys. Acta-Bioenerg. 2019, 1860, 433–438. [Google Scholar] [CrossRef]
- Peltier, G.; Aro, E.-M.; Shikanai, T. NDH-1 and NDH-2 plastoquinone reductases in oxygenic photosynthesis. Annu. Rev. Plant Biol. 2016, 67, 55–80. [Google Scholar] [CrossRef]
- Vass, I.; Kirilovsky, D.; Etienne, A.-L. UV-B radiation-induced donor- and acceptor-side modifications of Photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. Biochemistry 1999, 38, 12786–12794. [Google Scholar] [CrossRef]
- Deák, Z.; Sass, L.; Kiss, É.; Vass, I. Characterization of wave phenomena in the relaxation of flash-induced chlorophyll fluorescence yield in cyanobacteria. Biochim. Biophys. Acta-Bioenerg. 2014, 1837, 1522–1532. [Google Scholar] [CrossRef] [Green Version]
- Krishna, P.S.; Morello, G.; Mamedov, F. Characterization of the transient fluorescence wave phenomenon that occurs during H2 production in Chlamydomonas reinhardtii. J. Exp. Bot. 2019, 70, 6321–6336. [Google Scholar] [CrossRef]
- Patil, P.P.; Mohammad Aslam, S.; Vass, I.; Szabó, M. Characterization of the wave phenomenon of flash-induced chlorophyll fluorescence in Chlamydomonas reinhardtii. Photosynth. Res. 2022. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Trejo, A.; Huss, V.A.R.; Hernandez, J.-P.; Bashan, Y. Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresour. Technol. 2008, 99, 4980–4989. [Google Scholar] [CrossRef]
- Leu, S.; Boussiba, S. Advances in the production of high-value products by microalgae. Ind. Biotechnol. 2014, 10, 169–183. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Borowitzka, L.J.; Kessly, D. Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J. Appl. Phycol. 1990, 2, 111–119. [Google Scholar] [CrossRef]
- Pick, U.; Zarka, A.; Boussiba, S.; Davidi, L. A hypothesis about the origin of carotenoid lipid droplets in the green algae Dunaliella and Haematococcus. Planta 2019, 249, 31–47. [Google Scholar] [CrossRef]
- Krienitz, L.; Hepperle, D.; Stich, H.-B.; Weiler, W. Nannochloropsis limnetica (Eustigmatophyceae), a new species of picoplankton from freshwater. Phycologia 2000, 39, 219–227. [Google Scholar] [CrossRef]
- Houille-Vernes, L.; Rappaport, F.; Wollman, F.-A.; Alric, J.; Johnson, X. Plastid terminal oxidase 2 (PTOX2) is the major oxidase involved in chlororespiration in Chlamydomonas. Proc. Natl. Acad. Sci. USA 2011, 108, 20820–20825. [Google Scholar] [CrossRef] [Green Version]
- Johnson, X.; Alric, J. Central carbon metabolism and electron transport in Chlamydomonas reinhardtii: Metabolic constraints for carbon partitioning between oil and starch. Eukaryot. Cell 2013, 12, 776–793. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, M.; Huokko, T.; Richaud, P.; Bersanini, L.; Howe, C.J.; Lea-Smith, D.J.; Peltier, G.; Allahverdiyeva, Y. Distinguishing the roles of thylakoid respiratory terminal oxidases in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 2016, 171, 1307–1319. [Google Scholar] [CrossRef]
- Havurinne, V.; Tyystjärvi, E. Photosynthetic sea slugs induce protective changes to the light reactions of the chloroplasts they steal from algae. eLife 2020, 9, e57389. [Google Scholar] [CrossRef]
- Volgusheva, A.; Styring, S.; Mamedov, F. Increased photosystem II stability promotes H2 production in sulfur-deprived Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2013, 110, 7223–7228. [Google Scholar] [CrossRef] [Green Version]
- Volgusheva, A.; Kruse, O.; Styring, S.; Mamedov, F. Changes in the Photosystem II complex associated with hydrogen formation in sulfur deprived Chlamydomonas reinhardtii. Algal Res. 2016, 18, 296–304. [Google Scholar] [CrossRef]
- Cheniae, G.M.; Martin, I.F. Effects of hydroxylamine on Photosystem II: I. Factors affecting the decay of O2 evolution 1. Plant Physiol. 1971, 47, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Tóth, S.Z.; Schansker, G.; Garab, G.; Strasser, R.J. Photosynthetic electron transport activity in heat-treated barley leaves: The role of internal alternative electron donors to photosystem II. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Bennoun, P. Evidence for a respiratory chain in the chloroplast. Proc. Natl. Acad. Sci. USA 1982, 79, 4352–4356. [Google Scholar] [CrossRef] [Green Version]
- Solovchenko, A.E. Recent breakthroughs in the biology of astaxanthin accumulation by microalgal cell. Photosynth. Res. 2015, 125, 437–449. [Google Scholar] [CrossRef]
- Chekanov, K.; Schastnaya, E.; Neverov, K.; Leu, S.; Boussiba, S.; Zarka, A.; Solovchenko, A. Non-photochemical quenching in the cells of the carotenogenic chlorophyte Haematococcus lacustris under favorable conditions and under stress. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 1429–1442. [Google Scholar] [CrossRef]
- Scibilia, L.; Girolomoni, L.; Berteotti, S.; Alboresi, A.; Ballottari, M. Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res. 2015, 12, 170–181. [Google Scholar] [CrossRef]
- Zhang, L.; Su, F.; Zhang, C.; Gong, F.; Liu, J. Changes of photosynthetic behaviors and photoprotection during cell transformation and astaxanthin accumulation in Haematococcus pluvialis grown outdoors in tubular photobioreactors. Int. J. Mol. Sci. 2017, 18, 33. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yu, W.; Zhang, L.; Liu, J. Chlororespiration protects the photosynthetic apparatus against photoinhibition by alleviating inhibition of photodamaged-PSII repair in Haematococcus pluvialis at the green motile stage. Algal Res. 2021, 54, 102140. [Google Scholar] [CrossRef]
- Tan, S.; Cunningham, F.X., Jr.; Youmans, M.; Grabowski, B.; Sun, Z.; Gantt, E. Cytochrome f loss in astaxanthin-accumulating red cells of Haematococcus pluvialis (Chlorophyceae): Comparison of photosynthetic activity, photosynthetic enzymes, and thylakoid membrane polypeptides in red and green cells. J. Phycol. 1995, 31, 897–905. [Google Scholar] [CrossRef]
- Gu, W.; Li, H.; Zhao, P.; Yu, R.; Pan, G.; Gao, S.; Xie, X.; Huang, A.; He, L.; Wang, G. Quantitative proteomic analysis of thylakoid from two microalgae (Haematococcus pluvialis and Dunaliella salina) reveals two different high light-responsive strategies. Sci. Rep. 2014, 4, 6661. [Google Scholar] [CrossRef]
- Einali, A.; Shariati, M.; Sato, F.; Endo, T. Cyclic electron transport around photosystem I and its relationship to non-photochemical quenching in the unicellular green alga Dunaliella salina under nitrogen deficiency. J. Plant Res. 2013, 126, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Hu, J.; Qiao, Y.; Chen, W.; Rong, J.; Zhang, Y.; He, C.; Wang, Q. Ca2+-regulated cyclic electron flow supplies ATP for nitrogen starvation-induced lipid biosynthesis in green alga. Sci. Rep. 2015, 5, 15117. [Google Scholar] [CrossRef] [PubMed]
- Simionato, D.; Block, M.A.; La Rocca, N.; Jouhet, J.; Marechal, E.; Finazzi, G.; Morosinotto, T. The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryot. Cell 2013, 12, 665–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavřel, T.; Szabó, M.; Tamburic, B.; Evenhuis, C.; Kuzhiumparambil, U.; Literáková, P.; Larkum, A.W.D.; Raven, J.A.; Červený, J.; Ralph, P.J. Effect of carbon limitation on photosynthetic electron transport in Nannochloropsis oculata. J. Photochem. Photobiol. B Biol. 2018, 181, 31–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, W.; Dächsel, L.; Kandler, O. Effects of DCMU and antimycin A on photoassimilation of glucose in Chlorella. Plant Physiol. 1965, 40, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Larkum, A.W.D.; Szabó, M.; Fitzpatrick, D.; Raven, J.A. Cyclic Electron Flow in Cyanobacteria and Eukaryotic Algae. In Photosynthesis and Bioenergetics; World Scientific Publishing: Singapore, 2017; pp. 305–343. [Google Scholar]
- Nawrocki, W.J.; Bailleul, B.; Cardol, P.; Rappaport, F.; Wollman, F.A.; Joliot, P. Maximal cyclic electron flow rate is independent of PGRL1 in Chlamydomonas. Biochim. Biophys. Acta 2019, 1860, 425–432. [Google Scholar] [CrossRef]
- Mus, F.; Cournac, L.; Cardettini, V.; Caruana, A.; Peltier, G. Inhibitor studies on non-photochemical plastoquinone reduction and H2 photoproduction in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 2005, 1708, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Shoaf, W.T.; Lium, B.W. Improved extraction of chlorophyll a and b from algae using dimethyl sulfoxide. Limnol. Oceanogr. 1976, 21, 926–928. [Google Scholar] [CrossRef]
- Trtílek, M.; Kramer, D.M.; Koblížek, M.; Nedbal, L. Dual-modulation LED kinetic fluorometer. J. Lumin. 1997, 72–74, 597–599. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, P.P.; Vass, I.; Szabó, M. Characterization of the Wave Phenomenon in Flash-Induced Fluorescence Relaxation and Its Application to Study Cyclic Electron Pathways in Microalgae. Int. J. Mol. Sci. 2022, 23, 4927. https://doi.org/10.3390/ijms23094927
Patil PP, Vass I, Szabó M. Characterization of the Wave Phenomenon in Flash-Induced Fluorescence Relaxation and Its Application to Study Cyclic Electron Pathways in Microalgae. International Journal of Molecular Sciences. 2022; 23(9):4927. https://doi.org/10.3390/ijms23094927
Chicago/Turabian StylePatil, Priyanka Pradeep, Imre Vass, and Milán Szabó. 2022. "Characterization of the Wave Phenomenon in Flash-Induced Fluorescence Relaxation and Its Application to Study Cyclic Electron Pathways in Microalgae" International Journal of Molecular Sciences 23, no. 9: 4927. https://doi.org/10.3390/ijms23094927
APA StylePatil, P. P., Vass, I., & Szabó, M. (2022). Characterization of the Wave Phenomenon in Flash-Induced Fluorescence Relaxation and Its Application to Study Cyclic Electron Pathways in Microalgae. International Journal of Molecular Sciences, 23(9), 4927. https://doi.org/10.3390/ijms23094927





