Candida albicans Potassium Transporters
Abstract
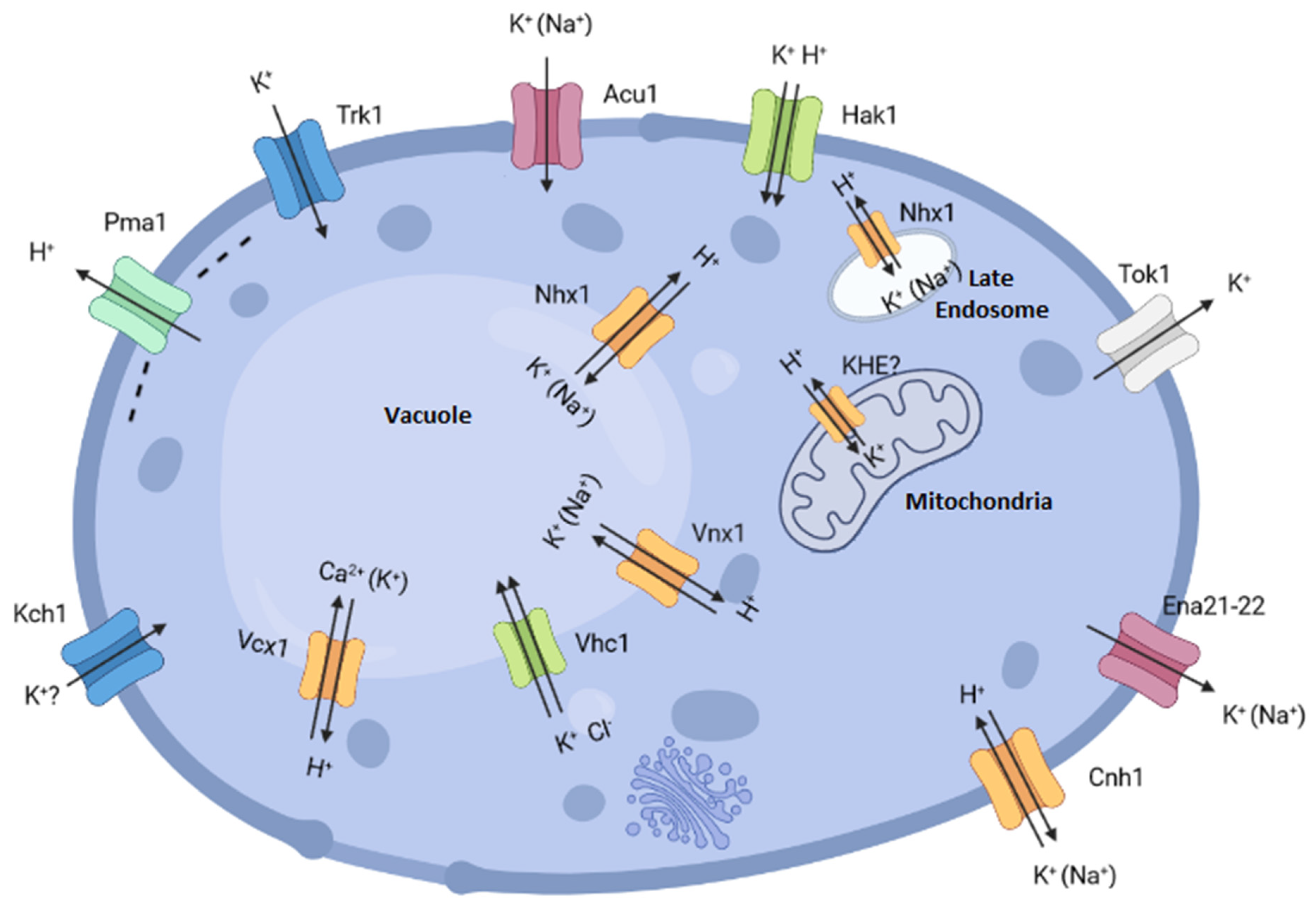
1. Introduction
2. Potassium Transporters in the Plasma Membrane
2.1. Trk1
2.2. Acu1
2.3. Hak1
2.4. Tok1
2.5. Cnh1
2.6. Ena21-22
2.7. Kch1
3. Intracellular Potassium Transporters
3.1. Nhx1 (Na+/H+ Exchanger)
3.2. Vhc1 (Vacuolar Protein Homologous to CCC Family)
3.3. Vnx1 (Vacuolar Na+/H+ Exchanger)
3.4. Vcx1 (Vacuolar Ca2+/H+ Exchanger)
3.5. KHE (K+/H+ Exchanger)
4. Potassium Homeostasis and Pathogenicity
4.1. Pma1
4.2. Trk1
4.3. Tok1
4.4. ENA
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yenush, L. Potassium and Sodium Transport in Yeast. In Yeast Membrane Transport; Ramos, J., Sychrová, H., Kschischo, M., Eds.; Springer: Cham, Switzerlands, 2016; Volume 892, pp. 187–228. ISBN 978-3-319-25302-2. [Google Scholar]
- Ariño, J.; Ramos, J.; Sychrova, H. Monovalent cation transporters at the plasma membrane in yeasts. Yeast 2019, 36, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Do, E.A.; Gries, C.M. Beyond Homeostasis: Potassium and Pathogenesis during Bacterial Infections. Infect Immun. 2021, 89, e0076620. [Google Scholar] [CrossRef] [PubMed]
- Stautz, J.; Hellmich, Y.; Fuss, M.F.; Silberberg, J.M.; Devlin, J.R.; Stockbridge, R.B.; Hänelt, I. Molecular Mechanisms for Bacterial Potassium Homeostasis. J. Mol. Biol. 2021, 433, 166968. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Tcherkez, G. Potassium dependency of enzymes in plant primary metabolism. Plant Physiol. Biochem. 2021, 166, 522–530. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef]
- Ariño, J.; Ramos, J.; Sychrová, H. Alkali metal cation transport and homeostasis in yeasts. Microbiol. Mol. Biol. Rev. 2010, 74, 95–120. [Google Scholar] [CrossRef]
- Herrera, R.; Álvarez, M.C.; Gelis, S.; Ramos, J. Subcellular potassium and sodium distribution in Saccharomyces cerevisiae wild-type and vacuolar mutants. Biochem. J. 2013, 454, 525–532. [Google Scholar] [CrossRef]
- Ene, I.V.; Bennett, R.J.; Anderson, M.Z. Mechanisms of genome evolution in Candida albicans. Curr. Opin. Microbiol. 2019, 52, 47–54. [Google Scholar] [CrossRef]
- Jones, T.; Federspiel, N.A.; Chibana, H.; Dungan, J.; Kalman, S.; Magee, B.B.; Newport, G.; Thorstenson, Y.R.; Agabian, N.; Magee, P.T.; et al. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA 2004, 101, 7329–7334. [Google Scholar] [CrossRef]
- Candida Genome Database. Available online: http://www.candidagenome.org/ (accessed on 23 April 2022).
- Ropars, J.; Maufrais, C.; Diogo, D.; Marcet-Houben, M.; Perin, A.; Sertour, N.; Mosca, K.; Permal, E.; Laval, G.; Bouchier, C.; et al. Gene flow contributes to diversification of the major fungal pathogen Candida Albicans. Nat Commun. 2018, 9, 2253. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.H.; Bennett, R.J. The Impact of Gene Dosage and Heterozygosity on The Diploid Pathobiont Candida Albicans. J. Fungi 2019, 6, 10. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I.; Eze, E.; Anyaegbunam, Z. Genome plasticity in Candida albicans: A cutting-edge strategy for evolution, adaptation, and survival. Infect. Genet. Evol. 2022, 105256. Advance Online Publication. [Google Scholar] [CrossRef] [PubMed]
- Bradford, L.L.; Ravel, J. The vaginal mycobiome: A contemporary perspective on fungi in women’s health and diseases. Virulence 2017, 8, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Nikou, S.A.; Kichik, N.; Brown, R.; Ponde, N.O.; Ho, J.; Naglik, J.R.; Richardson, J.P. Candida albicans Interactions with Mucosal Surfaces during Health and Disease. Pathogens 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.J.; Pereira, M.L.; Araujo, R.; Ramalho, C.; Zaura, E.; Sampaio-Maia, B. Influence of delivery and feeding mode in oral fungi colonization-a systematic review. Microb. Cell 2020, 7, 36–45. [Google Scholar] [CrossRef]
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Alonso-Monge, R.; Gresnigt, M.S.; Román, E.; Hube, B.; Pla, J. Candida albicans colonization of the gastrointestinal tract: A double-edged sword. PLoS Pathog. 2021, 17, e1009710. [Google Scholar] [CrossRef]
- Tong, Y.; Tang, J. Candida albicans infection and intestinal immunity. Microbiol. Res. 2017, 198, 27–35. [Google Scholar] [CrossRef]
- Baldewijns, S.; Sillen, M.; Palmans, I.; Vandecruys, P.; Van Dijck, P.; Demuyser, L. The Role of Fatty Acid Metabolites in Vaginal Health and Disease: Application to Candidiasis. Front. Microbiol. 2021, 12, 705779. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.S.; Jayaraman, S.; Pandiyan, P. Fungal Colonization and Infections-Interactions with Other Human Diseases. Pathogens 2022, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Mukaremera, L.; Lee, K.K.; Mora-Montes, H.M.; Gow, N. Candida albicans Yeast, Pseudohyphal, and Hyphal Morphogenesis Differentially Affects Immune Recognition. Front. Immunol. 2017, 8, 629. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Desai, J.V. Candida albicans Hyphae: From Growth Initiation to Invasion. J. Fungi 2018, 4, 10. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, T.; Min, D.; Soteyome, T.; Lan, H.; Hong, W.; Peng, F.; Fu, X.; Peng, G.; Liu, J.; et al. Regulatory network controls microbial biofilm development, with Candida albicans as a representative: From adhesion to dispersal. Bioengineered 2022, 13, 253–267. [Google Scholar] [CrossRef]
- Lu, Y.; Su, C.; Liu, H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef]
- Flanagan, P.R.; Liu, N.N.; Fitzpatrick, D.J.; Hokamp, K.; Köhler, J.R.; Moran, G.P. The Candida albicans TOR-Activating GTPases Gtr1 and Rhb1 Coregulate Starvation Responses and Biofilm Formation. mSphere 2017, 2, e00477-17. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Lu, C.; Gong, Y.; Li, M.; Sun, S. Promising Antifungal Targets Against Candida albicans Based on Ion Homeostasis. Front. Cell. Infect. Microbiol. 2018, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020, 18, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.; Atamas, A.; Tsarenko, A.; Rogachev, A.; Guskov, A. Cation Transporters of Candida albicans-New Targets to Fight Candidiasis? Biomolecules 2021, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Prigitano, A.; Morroni, G.; Brescini, L.; Barchiesi, F. Candidemia: Evolution of Drug Resistance and Novel Therapeutic Approaches. Infect. Drug Resist. 2021, 14, 5543–5553. [Google Scholar] [CrossRef]
- Wijnants, S.; Vreys, J.; Van Dijck, P. Interesting antifungal drug targets in the central metabolism of Candida albicans. Trends Pharmacol. Sci. 2022, 43, 69–79. [Google Scholar] [CrossRef]
- Elicharová, H.; Hušeková, B.; Sychrová, H. Three Candida albicans potassium uptake systems differ in their ability to provide Saccharomyces cerevisiae trk1trk2 mutants with necessary potassium. FEMS Yeast Res. 2016, 16, fow039. [Google Scholar] [CrossRef]
- Ruiz-Castilla, F.J.; Bieber, J.; Caro, G.; Michán, C.; Sychrova, H.; Ramos, J. Regulation and activity of CaTrk1, CaAcu1 and CaHak1, the three plasma membrane potassium transporters in Candida albicans. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183486. [Google Scholar] [CrossRef]
- Benito, B.; Garciadeblás, B.; Schreier, P.; Rodríguez-Navarro, A. Novel p-type ATPases mediate high-affinity potassium or sodium uptake in fungi. Eukaryot. Cell 2004, 3, 359–368. [Google Scholar] [CrossRef]
- Ruiz-Castilla, F.J.; Rodríguez-Castro, E.; Michán, C.; Ramos, J. The Potassium Transporter Hak1 in Candida Albicans, Regulation and Physiological Effects at Limiting Potassium and under Acidic Conditions. J. Fungi 2021, 7, 362. [Google Scholar] [CrossRef]
- Bañuelos, M.A.; Klein, R.D.; Alexander-Bowman, S.J.; Rodríguez-Navarro, A. A potassium transporter of the yeast Schwanniomyces occidentalis homologous to the Kup system of Escherichia coli has a high concentrative capacity. EMBO J. 1995, 14, 3021–3027. [Google Scholar] [CrossRef]
- Baev, D.; Rivetta, A.; Li, X.S.; Vylkova, S.; Bashi, E.; Slayman, C.L.; Edgerton, M. Killing of Candida albicans by human salivary histatin 5 is modulated, but not determined, by the potassium channel TOK1. Infect. Immun. 2003, 71, 3251–3260. [Google Scholar] [CrossRef] [PubMed]
- Soong, T.W.; Yong, T.F.; Ramanan, N.; Wang, Y. The Candida albicans antiporter gene CNH1 has a role in Na+ and H+ transport, salt tolerance, and morphogenesis. Microbiology 2000, 146, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Kinclova-Zimmermannova, O.; Sychrová, H. Plasma-membrane Cnh1 Na+/H+ antiporter regulates potassium homeostasis in Candida albicans. Microbiology 2007, 153, 2603–2612. [Google Scholar] [CrossRef]
- Enjalbert, B.; Moran, G.P.; Vaughan, C.; Yeomans, T.; Maccallum, D.M.; Quinn, J.; Coleman, D.C.; Brown, A.J.; Sullivan, D.J. Genome-wide gene expression profiling and a forward genetic screen show that differential expression of the sodium ion transporter Ena21 contributes to the differential tolerance of Candida albicans and Candida dubliniensis to osmotic stress. Mol. Microbiol. 2009, 72, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Stefan, C.P.; Cunningham, K.W. Kch1 family proteins mediate essential responses to endoplasmic reticulum stresses in the yeasts Saccharomyces cerevisiae and Candida albicans. J. Biol. Chem. 2013, 288, 34861–34870. [Google Scholar] [CrossRef] [PubMed]
- Felcmanova, K.; Neveceralova, P.; Sychrova, H.; Zimmermannova, O. Yeast Kch1 and Kch2 membrane proteins play a pleiotropic role in membrane potential establishment and monovalent cation homeostasis regulation. FEMS Yeast Res. 2017, 17, fox053. [Google Scholar] [CrossRef]
- Nass, R.; Cunningham, K.W.; Rao, R. Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J. Biol. Chem. 1997, 272, 26145–26152. [Google Scholar] [CrossRef]
- André, B.; Scherens, B. The yeast YBR235w gene encodes a homolog of the mammalian electroneutral Na+-K+-Cl− cotransporter family. Biochem. Biophys. Res. Commun. 1995, 217, 150–153. [Google Scholar] [CrossRef]
- Chen, C.; Pande, K.; French, S.D.; Tuch, B.B.; Noble, S.M. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell. Host. Microbe 2011, 10, 118–135. [Google Scholar] [CrossRef]
- Ariño, J.; Ramos, J. Cation Fungal Homeostasis. Ref. Modul. Life Sci. 2017, 1–7. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Jacobsen, I.D.; Miramón, P.; Große, K.; Hube, B. The novel Candida albicans transporter Dur31 Is a multi-stage pathogenicity factor. PLoS Pathog. 2012, 8, e1002592. [Google Scholar] [CrossRef] [PubMed]
- Cagnac, O.; Aranda-Sicilia, M.N.; Leterrier, M.; Rodriguez-Rosales, M.P.; Venema, K. Vacuolar cation/H+ antiporters of Saccharomyces cerevisiae. J. Biol. Chem. 2010, 285, 33914–33922. [Google Scholar] [CrossRef] [PubMed]
- Cabezón, V.; Llama-Palacios, A.; Nombela, C.; Monteoliva, L.; Gil, C. Analysis of Candida albicans plasma membrane proteome. Proteomics 2009, 9, 4770–4786. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.V.; Bruno, V.M.; Ganguly, S.; Stamper, R.J.; Mitchell, K.F.; Solis, N.; Hill, E.M.; Xu, W.; Filler, S.G.; Andes, D.R.; et al. Regulatory role of glycerol in Candida albicans biofilm formation. mBio 2013, 4, e00637-12. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.; Ramos, J. Dual system for potassium transport in Saccharomyces cerevisiae. J. Bacteriol. 1984, 159, 940–945. [Google Scholar] [CrossRef]
- Ramos, J.; Contreras, P.; Rodríguez-Navarro, A. A potassium transport mutant of Saccharomyces cerevisiae. Arch. Microbiol. 1985, 143, 88–93. [Google Scholar] [CrossRef]
- Gaber, R.F.; Styles, C.A.; Fink, G.R. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Moll. Cell. Biol. 1988, 8, 2848–2859. [Google Scholar] [CrossRef]
- Durell, S.R.; Hao, Y.; Nakamura, T.; Bakker, E.P.; Guy, H.R. Evolutionary relationship between K+ channels and symporters. Biophys. J. 1999, 77, 775–788. [Google Scholar] [CrossRef]
- Zayats, V.; Stockner, T.; Pandey, S.K.; Wörz, K.; Ettrich, R.; Ludwig, J. A refined atomic scale model of the Saccharomyces cerevisiae K+-translocation protein Trk1p combined with experimental evidence confirms the role of selectivity filter glycines and other key residues. Biochim. Biophys. Acta 2015, 1848, 1183–1195. [Google Scholar] [CrossRef][Green Version]
- Kale, D.; Spurny, P.; Shamayeva, K.; Spurna, K.; Kahoun, D.; Ganser, D.; Zayats, V.; Ludwig, J. The S. cerevisiae cation translocation protein Trk1 is functional without its “long hydrophilic loop” but LHL regulates cation translocation activity and selectivity. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1476–1488. [Google Scholar] [CrossRef]
- Shamayeva, K.; Spurna, K.; Kulik, N.; Kale, D.; Munko, O.; Spurny, P.; Zayats, V.; Ludwig, J. MPM motifs of the yeast SKT protein Trk1 can assemble to form a functional K+-translocation system. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183513. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Navarro, A.; Blatt, M.R.; Slayman, C.L. A potassium-proton symport in Neurospora Crassa. J. Gen. Physyol. 1986, 87, 649–674. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L.; Sychrova, H.; Ramos, J. Monovalent cations regulate expression and activity of the Hak1 potassium transporter in Debaryomyces hansenii. Fungal. Genet. Biol. 2011, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Bihler, H.; Bashi, E.; Slayman, C.L.; Rivetta, A. Chloride channel function in the yeast TRK-potassium transporters. J. Membr. Biol. 2004, 198, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Bashi, E.; Vylkova, S.; Edgerton, M.; Slayman, C.; Rivetta, A. Conservation and dispersion of sequence and function in fungal TRK potassium transporters: Focus on Candida albicans. FEMS Yeast Res. 2009, 9, 278–292. [Google Scholar] [CrossRef][Green Version]
- Baev, D.; Rivetta, A.; Vylkova, S.; Sun, J.N.; Zeng, G.F.; Slayman, C.L.; Edgerton, M. The TRK1 potassium transporter is the critical effector for killing of Candida albicans by the cationic protein, Histatin 5. J. Biol. Chem. 2004, 279, 55060–55072. [Google Scholar] [CrossRef]
- Navarrete, C.; Petrezsélyová, S.; Barreto, L.; Martínez, J.L.; Zahrádka, J.; Ariño, J.; Sychrová, H.; Ramos, J. Lack of main K+ uptake systems in Saccharomyces cerevisiae cells affects yeast performance in both potassium-sufficient and potassium-limiting conditions. FEMS Yeast Res. 2010, 10, 508–517. [Google Scholar] [CrossRef]
- Llopis-Torregrosa, V.; Hušeková, B.; Sychrová, H. Potassium Uptake Mediated by Trk1 Is Crucial for Candida glabrata Growth and Fitness. PLoS ONE 2016, 11, e0153374. [Google Scholar] [CrossRef]
- Caro, G.; Bieber, J.; Ruiz-Castilla, F.J.; Michán, C.; Sychrova, H.; Ramos, J. Trk1, the sole potassium-specific transporter in Candida glabrata, contributes to the proper functioning of various cell processes. World. J. Microbiol. Biotechnol. 2019, 35, 124. [Google Scholar] [CrossRef]
- Segal, E.S.; Gritsenko, V.; Levitan, A.; Yadav, B.; Dror, N.; Steenwyk, J.L.; Silberberg, Y.; Mielich, K.; Rokas, A.; Gow, N.; et al. Gene Essentiality Analyzed by In Vivo Transposon Mutagenesis and Machine Learning in a Stable Haploid Isolate of Candida albicans. mBio 2018, 9, e02048-18. [Google Scholar] [CrossRef]
- Noble, S.M.; French, S.; Kohn, L.A.; Chen, V.; Johnson, A.D. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 2010, 42, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Ariño, J.; Sychrová, H. Alkali-metal-cation influx and efflux systems in nonconventional yeast species. FEMS Microbiol. Lett. 2011, 317, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Haro, R.; Sainz, L.; Rubio, F.; Rodríguez-Navarro, A. Cloning of two genes encoding potassium transporters in Neurospora crassa and expression of the corresponding cDNAs in Saccharomyces cerevisiae. Mol. Microbiol. 1999, 31, 511–520. [Google Scholar] [CrossRef]
- Santa-María, G.E.; Oliferuk, S.; Moriconi, J.I. KT-HAK-KUP transporters in major terrestrial photosynthetic organisms: A twenty years tale. J. Plant. Physiol. 2018, 226, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Prista, C.; González-Hernández, J.C.; Ramos, J.; Loureiro-Dias, M.C. Cloning and characterization of two K+ transporters of Debaryomyces hansenii. Microbiology 2007, 153, 3034–3043. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cabrera, E.; Álvarez, M.C.; Martín, Y.; Siverio, J.M.; Ramos, J. K+ uptake systems in the yeast Hansenula polymorpha. Transcriptional and post-translational mechanisms involved in high-affinity K+ transporter regulation. Fungal. Genet. Biol. 2012, 49, 755–763. [Google Scholar] [CrossRef]
- Tascón, I.; Sousa, J.S.; Corey, R.A.; Mills, D.J.; Griwatz, D.; Aumüller, N.; Mikusevic, V.; Stansfeld, P.J.; Vonck, J.; Hänelt, I. Structural basis of proton-coupled potassium transport in the KUP family. Nat. Commun. 2020, 11, 626. [Google Scholar] [CrossRef]
- Bañuelos, M.A.; Madrid, R.; Rodríguez-Navarro, A. Individual functions of the HAK and TRK potassium transporters of Schwanniomyces occidentalis. Mol. Microbiol. 2000, 37, 671–679. [Google Scholar] [CrossRef]
- Ketchum, K.A.; Joiner, W.J.; Sellers, A.J.; Kaczmarek, L.K.; Goldstein, S.A. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature 1995, 376, 690–695. [Google Scholar] [CrossRef]
- Ahmed, A.; Sesti, F.; Ilan, N.; Shih, T.M.; Sturley, S.L.; Goldstein, S.A. A molecular target for viral killer toxin: TOK1 potassium channels. Cell 1999, 99, 283–291. [Google Scholar] [CrossRef]
- Bertl, A.; Slayman, C.L.; Gradmann, D. Gating and conductance in an outward-rectifying K+ channel from the plasma membrane of Saccharomyces cerevisiae. J. Membr. Biol. 1993, 132, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Fairman, C.; Zhou, X.; Kung, C. Potassium uptake through the TOK1 K+ channel in the budding yeast. J. Membr. Biol. 1999, 168, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Maresova, L.; Urbankova, E.; Gaskova, D.; Sychrova, H. Measurements of plasma membrane potential changes in Saccharomyces cerevisiae cells reveal the importance of the Tok1 channel in membrane potential maintenance. FEMS Yeast Res. 2006, 6, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- BañueIos, M.A.; Sychrová, H.; Bleykasten-Grosshans, C.; Souciet, J.L.; Potier, S. The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology 1998, 144, 2749–2758. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.; Benito, B. Sodium or potassium efflux ATPase a fungal, bryophyte, and protozoal ATPase. Biochim. Biophys. Acta Biomembr. 2010, 1798, 1841–1853. [Google Scholar] [CrossRef]
- Benito, B.; Quintero, F.J.; Rodríguez-Navarro, A. Overexpression of the sodium ATPase of Saccharomyces cerevisiae: Conditions for phosphorylation from ATP and Pi. Biochim. Biophys. Acta Biomembr. 1997, 1328, 214–226. [Google Scholar] [CrossRef]
- Stefan, C.P.; Zhang, N.; Sokabe, T.; Rivetta, A.; Slayman, C.L.; Montell, C.; Cunningham, K.W. Activation of an essential calcium signaling pathway in Saccharomyces cerevisiae by Kch1 and Kch2, putative low-affinity potassium transporters. Eukaryot. Cell 2013, 12, 204–214. [Google Scholar] [CrossRef]
- Brett, C.L.; Tukaye, D.N.; Mukherjee, S.; Rao, R. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol. Biol. Cell 2005, 16, 1396–1405. [Google Scholar] [CrossRef]
- Qiu, Q.S.; Fratti, R.A. The Na+/H+ exchanger Nhx1p regulates the initiation of Saccharomyces cerevisiae vacuole fusion. J. Cell Sci. 2010, 123, 3266–3275. [Google Scholar] [CrossRef]
- Petrezselyova, S.; Kinclova-Zimmermannova, O.; Sychrova, H. Vhc1, a novel transporter belonging to the family of electroneutral cation-Cl− cotransporters, participates in the regulation of cation content and morphology of Saccharomyces cerevisiae vacuoles. Biochim. Biophys. Acta Biomembr. 2013, 1828, 623–631. [Google Scholar] [CrossRef]
- Froschauer, E.; Nowikovsky, K.; Schweyen, R.J. Electroneutral K+/H+ exchange in mitochondrial membrane vesicles involves Yol027/Letm1 proteins. Biochim. Biophys. Acta Biomembr. 2005, 1711, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Nowikovsky, K.; Froschauer, E.M.; Zsurka, G.; Samaj, J.; Reipert, S.; Kolisek, M.; Wiesenberger, G.; Schweyen, R.J. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J. Biol. Chem. 2004, 279, 30307–30315. [Google Scholar] [CrossRef] [PubMed]
- Frazier, A.E.; Taylor, R.D.; Mick, D.U.; Warscheid, B.; Stoepel, N.; Meyer, H.E.; Ryan, M.T.; Guiard, B.; Rehling, P. Mdm38 interacts with ribosomes and is a component of the mitochondrial protein export machinery. J. Cell. Biol. 2006, 172, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Zotova, L.; Aleschko, M.; Sponder, G.; Baumgartner, R.; Reipert, S.; Prinz, M.; Schweyen, R.J.; Nowikovsky, K. Novel components of an active mitochondrial K+/H+ exchange. J. Biol. Chem. 2010, 285, 14399–14414. [Google Scholar] [CrossRef]
- Oh, J.; Fung, E.; Schlecht, U.; Davis, R.W.; Giaever, G.; St Onge, R.P.; Deutschbauer, A.; Nislow, C. Gene annotation and drug target discovery in Candida albicans with a tagged transposon mutant collection. PLoS Pathog. 2010, 6, e1001140. [Google Scholar] [CrossRef]
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The Still Underestimated Problem of Fungal Diseases Worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef]
- Firacative, C. Invasive fungal disease in humans: Are we aware of the real impact? Mem. Inst. Oswaldo Cruz. 2020, 115, e200430. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef]
- Calahorra, M.; Lozano, C.; Sánchez, N.S.; Peña, A. Ketoconazole and miconazole alter potassium homeostasis in Saccharomyces cerevisiae. Biochim. Biophys. Acta Biomembr. 2011, 1808, 433–445. [Google Scholar] [CrossRef]
- Monk, B.C.; Mason, A.B.; Abramochkin, G.; Haber, J.E.; Seto-Young, D.; Perlin, D.S. The yeast plasma membrane proton pumping ATPase is a viable antifungal target. I. Effects of the cysteine-modifying reagent omeprazole. Biochim. Biophys. Acta Biomembr. 1995, 1239, 81–90. [Google Scholar] [CrossRef]
- Perlin, D.S.; Seto-Young, D.; Monk, B.C. The plasma membrane H+-ATPase of fungi. A candidate drug target? Ann. N. Y. Acad. Sci. 1997, 834, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, M.; Koshlukova, S.E. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv. Dent. Res. 2000, 14, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Chaudhary, M.; Khanna, G.; Rishi, P.; Kaur, I.P. Envisaging Antifungal Potential of Histatin 5: A Physiological Salivary Peptide. J. Fungi 2021, 7, 1070. [Google Scholar] [CrossRef]
- Hušeková, B.; Elicharová, H.; Sychrová, H. Pathogenic Candida species differ in the ability to grow at limiting potassium concentrations. Can. J. Microbiol. 2016, 62, 394–401. [Google Scholar] [CrossRef]
- Llopis-Torregrosa, V.; Vaz, C.; Monteoliva, L.; Ryman, K.; Engstrom, Y.; Gacser, A.; Gil, C.; Ljungdahl, P.O.; Sychrová, H. Trk1-mediated potassium uptake contributes to cell-surface properties and virulence of Candida glabrata. Sci. Rep. 2019, 9, 7529. [Google Scholar] [CrossRef]
- Yatime, L.; Buch-Pedersen, M.J.; Musgaard, M.; Morth, J.P.; Lund Winther, A.M.; Pedersen, B.P.; Olesen, C.; Andersen, J.P.; Vilsen, B.; Schiøtt, B.; et al. P-type ATPases as drug targets: Tools for medicine and science. Biochim. Biophys. Acta Biomembr. 2009, 1787, 207–220. [Google Scholar] [CrossRef]
- Dick, C.F.; Meyer-Fernandes, J.R.; Vieyra, A. The Functioning of Na+-ATPases from Protozoan Parasites: Are These Pumps Targets for Antiparasitic Drugs? Cells 2020, 9, 2225. [Google Scholar] [CrossRef]
- Krauke, Y.; Sychrova, H. Cnh1 Na+/H+ antiporter and Ena1 Na+-ATPase play different roles in cation homeostasis and cell physiology of Candida glabrata. FEMS Yeast Res. 2011, 11, 29–41. [Google Scholar] [CrossRef]
| Name | Orf | Possible Function | Proposed Mechanism | Chromosome | Homology | Specific Experimental Data | Relevant References |
|---|---|---|---|---|---|---|---|
| Trk1 | 19.600 | K+ influx | K+ Uniporter | R | ScTrk1 (32.7%) SpTrk1 (28.7%) | Yes | [38,39] |
| Acu1 | 19.2553 and 19.2552 | K+ (Na+) influx | K+ (Na+) ATPase | R | - * | Yes | [38,39,40] |
| Hak1 | 19.6249 | K+ influx | K+: H+ Symporter | 1 | SchHak1 (56.35%) Dhak1 (52.14 %) HpHak1 (37.91%) | Yes | [38,39,41,42] |
| Tok1 | 19.4175 | K+ efflux | K+ efflux channel | 4 | ScTok1 (32.1%) HsKcnk9 (11.3%) | Yes | [43] |
| Cnh1 | 19.367 | K+ (Na+) efflux. Maintenance of intracellular pH | K+(Na+)/H+ antiporter | 4 | ScNha1 (41.2%) SpSod22 (37.1%) | Yes | [44,45] |
| Ena21 | 19.6070 | K+ (Na+) efflux | P-type ATPase | 1 | ScEna2 (50.8%) SpCta3 (44.4%) | No | [46] |
| Ena22 | 19.5170 | K+ (Na+) efflux | P-type ATPase | 7 | ScEna2 (52.9%) SpCta3 (45.9%) | No | [46] |
| Kch1 | 19.6563 | Unknown | Unknown | 7 | ScKch1 (17.0%) | Yes | [47,48] |
| Nhx1 | 19.4201 | Intracellular K+ (Na+) sequestration | K+ (Na+)/H+ exchanger | 6 | ScNhx1 (58.5%) SpCpa1 (46.0%) HsSlc9a9 (25.6%) | No | [49] |
| Vhc1 | 19.6832 | Vacuolar cation content and morphology | K+-Cl− cotransporter | 3 | ScVhc1 (12.9%) SpVhc1 (9.1%) HsSlc12a9 (3.8%) | No | [50,51] |
| Vnx1 | 19.7670 | Vacuolar K+ (Na+) content and regulation of cytosolic pH | K+ (Na+)/H+ exchanger | R | ScVnx1 (40.8%) SpSst1 (33.8%) | No | [52,53] |
| Vcx1 | 19.405 | Regulation of vacuolar Ca2+ and K+ content | Ca2+ (K+)/H+ exchanger | 1 | ScVcx1 (59.3%) SpVcx1 (47.2%) | No | [54] |
| Mrs7 (KHE) | 19.3321 | Involved in mitochondrial K+ homeostasis | Unknown | 1 | ScMrs7 (40.7%) SpMdm28 (37.5%) HsLetm1 (20.8%) | No | [52,55] |
| C3_01680C (KHE) | 19.1676 | Involved in mitochondrial K+ homeostasis | Unknown | 3 | ScYdl183c (26.0%) SpSPAC23H3.12c (17.8%) | No | [52,56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Castilla, F.J.; Ruiz Pérez, F.S.; Ramos-Moreno, L.; Ramos, J. Candida albicans Potassium Transporters. Int. J. Mol. Sci. 2022, 23, 4884. https://doi.org/10.3390/ijms23094884
Ruiz-Castilla FJ, Ruiz Pérez FS, Ramos-Moreno L, Ramos J. Candida albicans Potassium Transporters. International Journal of Molecular Sciences. 2022; 23(9):4884. https://doi.org/10.3390/ijms23094884
Chicago/Turabian StyleRuiz-Castilla, Francisco J., Francisco S. Ruiz Pérez, Laura Ramos-Moreno, and José Ramos. 2022. "Candida albicans Potassium Transporters" International Journal of Molecular Sciences 23, no. 9: 4884. https://doi.org/10.3390/ijms23094884
APA StyleRuiz-Castilla, F. J., Ruiz Pérez, F. S., Ramos-Moreno, L., & Ramos, J. (2022). Candida albicans Potassium Transporters. International Journal of Molecular Sciences, 23(9), 4884. https://doi.org/10.3390/ijms23094884







