Toxic Effects on Thyroid Gland of Male Adult Lizards (Podarcis Siculus) in Contact with PolyChlorinated Biphenyls (PCBs)-Contaminated Soil
Abstract
1. Introduction
2. Results
2.1. Signs of Toxicity, Animal Mortality and Body Weight
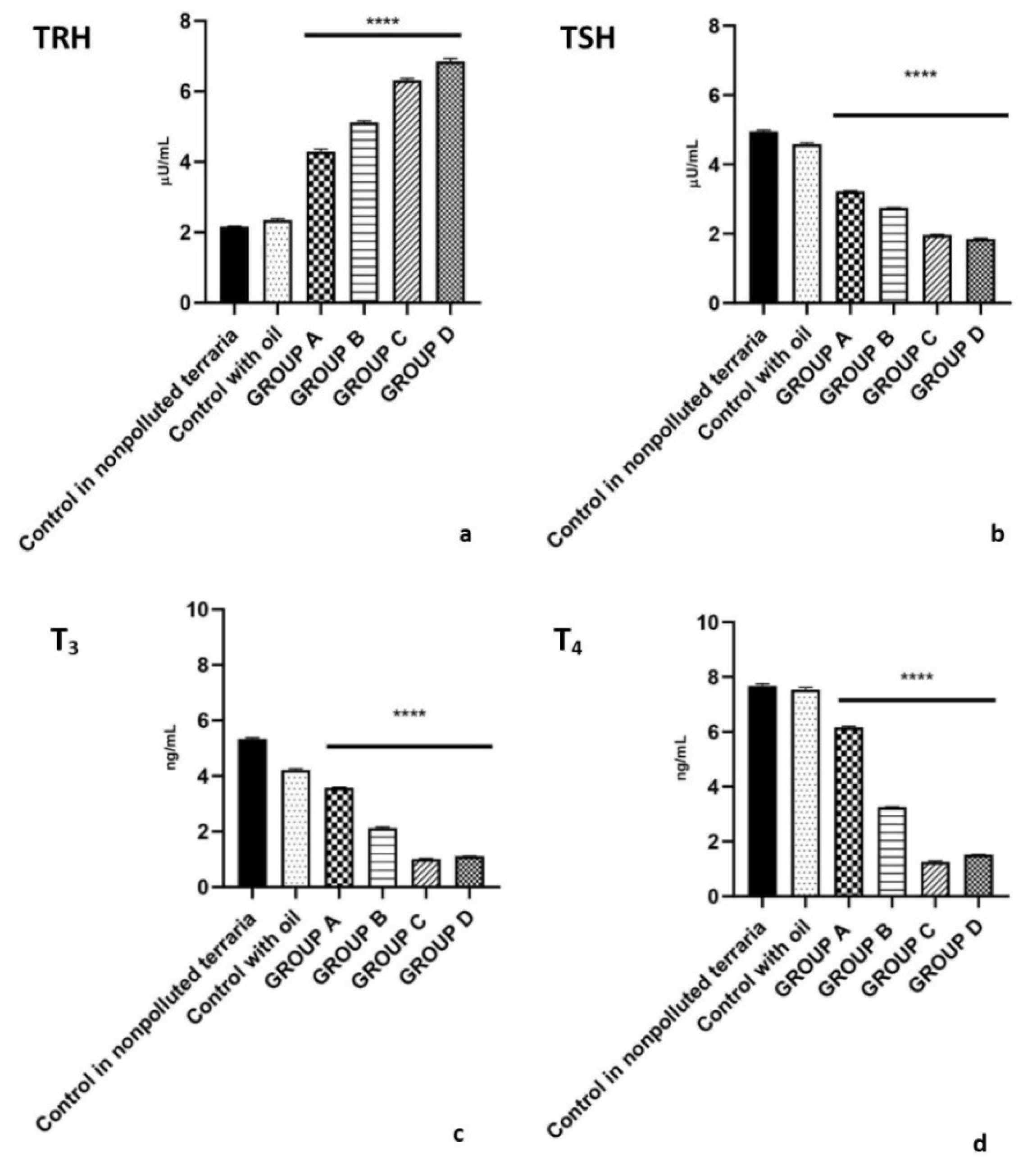
2.2. Plasma Levels of Hormones Belonging to the HPT Axis
2.3. Hepatic Thyroid Hormones Content and 5′ORD (Type II) Monodeiodinase Activity
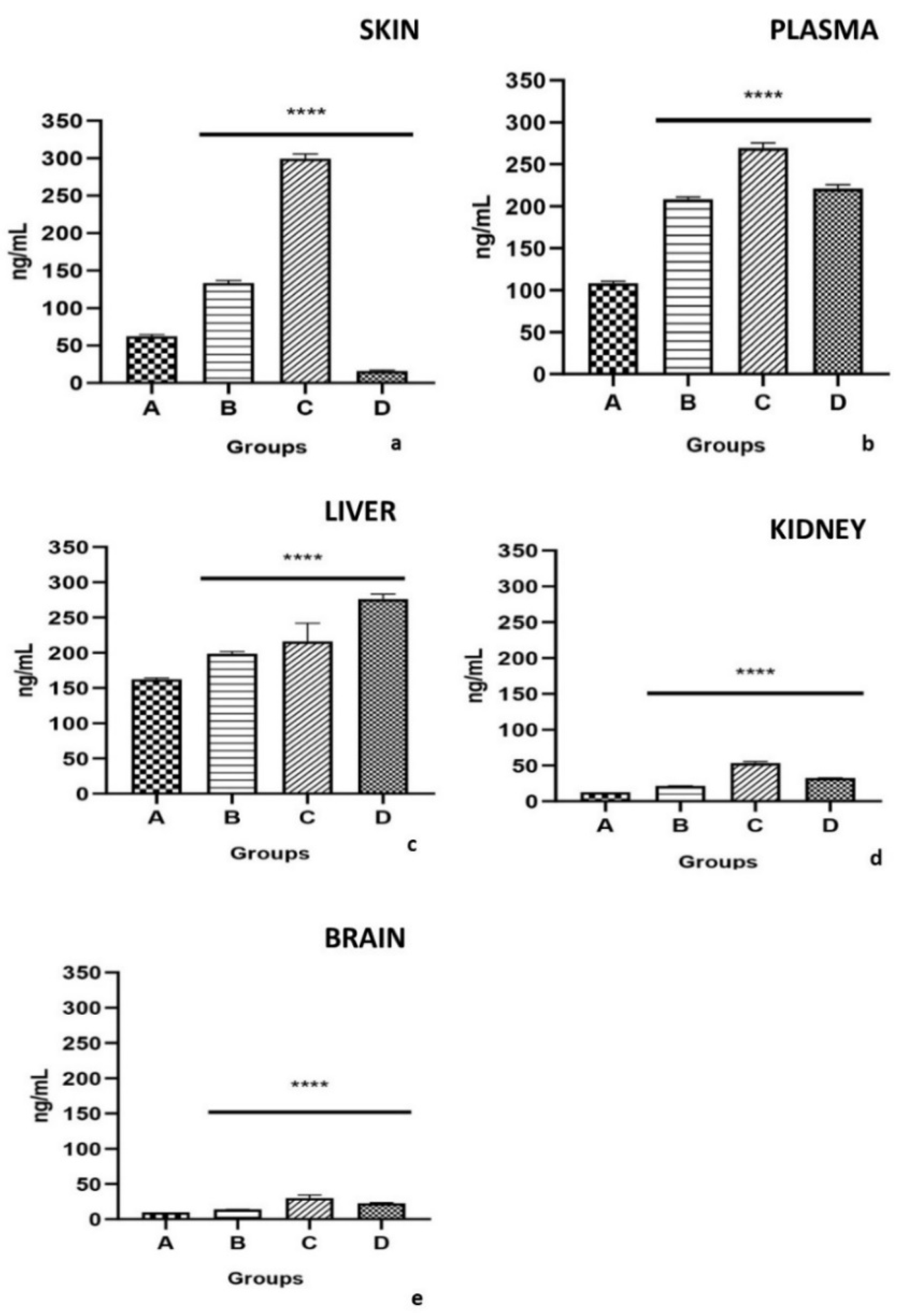
2.4. Determination of PCBs in Plasma and Tissues (Liver, Skin, Brain, and Kidney)
2.5. Thyroid Glands Histology
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Biochemical Analysis
4.2.1. Determination of 31 Congeners in Plasma and Tissues (Liver, Kidney, Brain, and Skin)
4.2.2. Plasma Thyroid Hormones and TSH and TRH Assays
4.2.3. Hepatic Thyroid Hormones and 5′ORD (Type II) Monodeiodinase
4.3. Histological Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ATSDR Agency for Toxic Substances and Disease Registry. Toxicological Profile for Selected Polychlorinated Biphenyls; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2000.
- La Rocca, C.; Mantovani, A. From Environment to Food: The Case of PCB. Ann.-Ist. Super. Di Sanita 2006, 42, 410–416. [Google Scholar]
- El Majidi, N.; Bouchard, M.; Carrier, G. Systematic analysis of the relationship between standardized biological levels of polychlorinated biphenyls and thyroid function in pregnant women and newborns. Chemosphere 2014, 98, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Thambirajah, A.A.; Wade, M.G.; Verreault, J.; Buisine, N.; Alves, V.A.; Langlois, V.S.; Helbing, C.C. Disruption by stealth-Interference of endocrine disrupting chemicals on hormonal crosstalk with thyroid axis function in humans and other animals. Environ. Res. 2022, 203, 111906. [Google Scholar] [CrossRef] [PubMed]
- Zani, C.; Magoni, M.; Speziani, F.; Leonardi, L.; Orizio, G.; Scarcella, C.; Gaia, A.; Donato, F. Polychlorinated biphenyl serum levels, thyroid hormones and endocrine and metabolic diseases in people living in a highly polluted area in North Italy: A population-based study. Heliyon 2019, 5, e01870. [Google Scholar] [CrossRef] [PubMed]
- Takaguchi, K.; Nishikawa, H.; Mizukawa, H.; Tanoue, R.; Yokoyama, N.; Ichii, O.; Takiguchi, M.; Nakayama, S.M.M.; Ikenaka, Y.; Kunisue, T.; et al. Effects of PCB exposure on serum thyroid hormone levels in dogs and cats. Sci. Total Environ. 2019, 688, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, A.B.; Antonijevic, E.; Curcic, M.; Milovanovic, V.; Antonijevic, B. Endocrine-disrupting mechanisms of polychlorinated biphenyls. Curr. Opin. Toxicol. 2020, 19, 42–49. [Google Scholar] [CrossRef]
- Schnitzler, J.G.; Celis, N.; Klaren, P.H.M.; Blust, R.; Dirtu, A.C.; Covaci, A. Thyroid dysfunction in sea bass (Dicentrarchus labrax): Underlying mechanisms and effects of polychlorinated biphenyls on thyroid hormone physiology and metabolism. Aquat. Toxicol. 2011, 105, 438–447. [Google Scholar] [CrossRef]
- Schnitzler, J.G.; Klaren, P.H.M.; Bouquegneau, J.M.; Das, K. Environmental factors affecting thyroid function of wild sea bass (Dicentrarchus labrax) from European coasts. Chemosphere 2012, 87, 1009–1017. [Google Scholar] [CrossRef]
- Jarque, S.; Piña, B. Deiodinases and thyroid metabolism disruption in teleost fish. Environ. Res. 2014, 135, 361–375. [Google Scholar] [CrossRef]
- Dong, Y.; Tian, H.; Wang, W.; Zhang, X.; Liu, J. Disruption of the Thyroid System by the Thyroid-Disrupting Compound Aroclor 1254 in Juvenile Japanese Flounder (Paralichthys olivaceus). PLoS ONE 2014, 9, e104196. [Google Scholar] [CrossRef]
- Couderc, M.; Marchand, J.; Zalouk-Vergnoux, A.; Kamari, A.; Moreau, B.; Blanchet-Letrouvé, I.; Le Bizec, B.; Mouneyrac, C.; Poirier, L. Thyroid endocrine status of wild European eels (Anguilla anguilla) in the Loire (France). Relationships with organic contaminant body burdens. Sci. Total Environ. 2016, 550, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Klaassen, C.D. Differential effects of polychlorinated biphenyl congeners on serum thyroid hormone levels in rats. Toxicol. Sci. 2010, 117, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.A.; Wilson, D.T.; Reuhl, K.R.; Gallo, M.A.; Klaassen, C.D. Polychlorinated biphenyl congeners that increase the glucuronidation and biliary excretion of thyroxine are distinct from the congeners that enhance the serum disappearance of thyroxine. Drug. Metab. Dispos. 2012, 40, 588–595. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Denslow, N.D.; Orlando, E.F.; Gutierrez-Villagomez, J.M.; Trudeau, V.L. Neuroendocrine disruption of organizational and activational hormone programming in poikilothermic vertebrates. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 276–304. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Barra, T.; Rosati, L.; Valiante, S.; Capaldo, A.; De Falco, M.; Laforgia, V. Adrenal gland response to endocrine disrupting chemicals in fshes, amphibians and reptiles: A comparative overview. Gen. Comp. Endocrinol. 2020, 297, 113550. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Sciarrillo, R.; Rosati, L.; Sellitti, A.; Barra, T.; De Luca, A.; Laforgia, V.; De Falco, M. Effects of alkylphenols mixture on the adrenal gland of the lizard Podarcis sicula. Chemosphere 2020, 258, 127239. [Google Scholar] [CrossRef]
- Sciarrillo, R.; Di Lorenzo, M.; Valiante, S.; Rosati, L.; De Falco, M. OctylPhenol (OP) alone and in Combination with NonylPhenol (NP) Alters the Structure and the Function of Thyroid Gland of the Lizard Podarcis siculus. Arch. Environ. Contam. Toxicol. 2021, 80, 567–578. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Mileo, A.; Laforgia, V.; De Falco, M.; Rosati, L. Alkyphenol Exposure Alters Steroidogenesis in Male Lizard Podarcis siculus. Animals 2021, 11, 1003. [Google Scholar] [CrossRef]
- Alexander, G.J.; Horne, D.; Hanrahan, S.A. An evaluation of the effects of deltamethrin on two non-target lizard species in the Karoo, South Africa. J. Arid. Environ. 2002, 50, 121–133. [Google Scholar] [CrossRef]
- Cardone, A. Imidacloprid induces morphological and molecular damages on testis of lizard (Podarcis sicula). Ecotoxicology 2015, 24, 94–105. [Google Scholar] [CrossRef]
- Verderame, M.; Scudiero, R. Health status of the lizard Podarcis siculus (Rafinesque-Schmaltz, 1810) subject to dif-ferent anthropogenic pressures. BioMed. Res. Int. 2019, 342, 81–89. [Google Scholar] [CrossRef]
- Chen, L.; Xu, P.; Diao, J.; Di, S.; Li, R.; Zhou, Z. Distribution, metabolism and toxic effects of beta-cypermethrin in lizards (Eremias argus) following oral administration. J. Hazard. Mater. 2016, 306, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Weir, S.M.; Suski, J.G.; Salice, C.J. Ecological risk of anthropogenic pollutants to reptiles: Evaluating assumptions of sensitivity and exposure. Environ. Pollut. 2010, 158, 3596–3606. [Google Scholar] [CrossRef]
- Weir, S.M.; Talent, L.G.; Anderson, T.A.; Salice, C.J. Unraveling the Relative Importance of Oral and Dermal Contaminant Exposure in Reptiles: Insights from Studies Using the Western Fence Lizard (Sceloporus occidentalis). PLoS ONE 2014, 9, e99666. [Google Scholar] [CrossRef] [PubMed]
- Weir, S.M.; Yu, S.; Talent, L.G.; Maul, J.D.; Anderson, T.A.; Salice, C.J. Improving reptile ecological risk assessment: Oral and dermal toxicity of pesticides to a common lizard species (Sceloporus occidentalis). Environ. Toxicol. Chem. 2015, 34, 1778–1786. [Google Scholar] [CrossRef]
- Weir, S.M.; Talent, L.G.; Anderson, T.A.; Salice, C.J. Insights into reptile dermal contaminant exposure: Reptile skin permeability to pesticides. Chemosphere 2016, 154, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Mestre, A.P.; Amavet, P.S.; Vanzetti, A.I.; Moleón, M.S.; Marcó, M.V.P.; Poletta, G.L.; Siroski, P.A. Effects of cypermethrin (pyrethroid), glyphosate and chlorpyrifos (organophosphorus) on the endocrine and immune system of Salvator merianae (Argentine tegu). Ecotoxicol. Environ. Saf. 2019, 169, 61–67. [Google Scholar] [CrossRef]
- Simbula, G.; Moltedo, G.; Catalano, B. Biological responses in pesticide exposed lizards (Podarcis siculus). Ecotoxicology 2021, 30, 1017–1028. [Google Scholar] [CrossRef]
- Böhm, M.; Collen, B.; Baillie, J.E.M.; Bowles, P.; Chanson, J.; Cox, N.; Hammerson, G.; Hoffmann, M.; Livingstone, S.R.; Ram, M.; et al. The conservation status of the world’s reptiles. Biol. Conserv. 2013, 157, 372–385. [Google Scholar] [CrossRef]
- Mingo, V.; Lötters, S.; Wagner, N. Risk of pesticide exposure for reptile species in the European Union. Environ. Pollut. 2016, 215, 164–169. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S.; Winne, C.T. The global decline of reptiles, déjà vu amphibians. BioScience 2000, 50, 653–666. [Google Scholar] [CrossRef]
- Freitas, L.M.; Paranaiba, J.F.F.S.; Perez, A.P.S.; Machado, M.R.F.; Lima, F.C. Toxicity of pesticides in lizards. Hum. Exp. Toxicol. 2020, 39, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Weir, S.M.; Salice, C.J. Investigating potential toxic effects of pollutants on population growth rates and probability of extinction for a representative squamate. Ecotoxicology 2021, 30, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.; Casini, S.; Mori, G.; Ancora, S.; Bianchi, N.; D’Agostino, A.; Ferraro, M.; Fossi, M.C. The Italian wall lizard (Podarcis sicula) as a bioindicator of oil field activity. Sci. Total Environ. 2009, 407, 3597–3604. [Google Scholar] [CrossRef]
- Rosati, L.; Agnese, M.; Di Lorenzo, M.; Barra, T.; Valiante, S.; Prisco, M. Spermatogenesis and regulatory factors in the wall lizard Podarcis sicula. Gen. Comp. Endocrinol. 2020, 298, 113579. [Google Scholar] [CrossRef]
- Sciarrillo, R.; Laforgia, V.; Cavagnuolo, A.; Varano, L.; Virgilio, F. Annual variations of thyroid activity in the lizard Podarcis sicula (squamata, lacertidae). Ital. J. Zool. 2000, 67, 263–267. [Google Scholar] [CrossRef]



| Treatments | Dead Animals (% of Animal Mortality) |
|---|---|
| Control in nonpolluted terraria | 0 |
| Control with oil | 0 |
| GROUP A | 1 (6%) |
| GROUP B | 2 (12%) |
| GROUP C | 5 (33%) |
| GROUP D | 5 (33%) |
| Treatments | Whole Lizard Weight (g) | ||||
|---|---|---|---|---|---|
| Day 0 | Days 30 | Days 60 | Days 90 | Days 120 | |
| Control in nonpolluted terraria | 12 ± 0.01 | 13 ± 0.05 | 15 ± 0.05 | 17 ± 0.05 | 20 ± 0.05 |
| Control with oil | 12 ± 0.01 | 13 ± 0.04 | 15 ± 0.04 | 17 ± 0.05 | 20 ± 0.05 |
| GROUP A | 12 ± 0.02 | 12 ± 0.02 | 12 ± 0.04 | 11 ± 0.01 **** | 10 ± 0.05 **** |
| GROUP B | 12 ± 0.02 | 12 ± 0.05 | 11 ± 0.05 | 10 ± 0.05 **** | 9 ± 0.05 **** |
| GROUP C | 12 ± 0.02 | 11 ± 0.02 | 10 ± 0.05 | 9 ± 0.05 **** | 8 ± 0.05 **** |
| GROUP D | 12 ± 0.05 | 12 ± 0.01 | 10 ± 0.04 | 9 ± 0.05 **** | 8 ± 0.01 **** |
| Treatments | Height of Follicular Epithelium (µm) |
|---|---|
| Control in nonpolluted terraria | 22.1 ± 0.02 |
| Control with oil | 21.3 ± 0.05 |
| Group A | 10.6 ± 0.03 **** |
| Group B | 6.61 ± 0.01 **** |
| Group C | 3.32 ± 0.05 **** |
| Group D | 3.15 ± 0.04 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciarrillo, R.; Falzarano, A.; Gallicchio, V.; Mileo, A.; De Falco, M. Toxic Effects on Thyroid Gland of Male Adult Lizards (Podarcis Siculus) in Contact with PolyChlorinated Biphenyls (PCBs)-Contaminated Soil. Int. J. Mol. Sci. 2022, 23, 4790. https://doi.org/10.3390/ijms23094790
Sciarrillo R, Falzarano A, Gallicchio V, Mileo A, De Falco M. Toxic Effects on Thyroid Gland of Male Adult Lizards (Podarcis Siculus) in Contact with PolyChlorinated Biphenyls (PCBs)-Contaminated Soil. International Journal of Molecular Sciences. 2022; 23(9):4790. https://doi.org/10.3390/ijms23094790
Chicago/Turabian StyleSciarrillo, Rosaria, Alessandra Falzarano, Vito Gallicchio, Aldo Mileo, and Maria De Falco. 2022. "Toxic Effects on Thyroid Gland of Male Adult Lizards (Podarcis Siculus) in Contact with PolyChlorinated Biphenyls (PCBs)-Contaminated Soil" International Journal of Molecular Sciences 23, no. 9: 4790. https://doi.org/10.3390/ijms23094790
APA StyleSciarrillo, R., Falzarano, A., Gallicchio, V., Mileo, A., & De Falco, M. (2022). Toxic Effects on Thyroid Gland of Male Adult Lizards (Podarcis Siculus) in Contact with PolyChlorinated Biphenyls (PCBs)-Contaminated Soil. International Journal of Molecular Sciences, 23(9), 4790. https://doi.org/10.3390/ijms23094790







