HIV-2 Neutralization Sensitivity in Relation to Co-Receptor Entry Pathways and Env Motifs
Abstract
1. Introduction
2. Results
2.1. Characterisation of HIV-2 Co-Receptor Use in Relation V3 Sequence Motifs
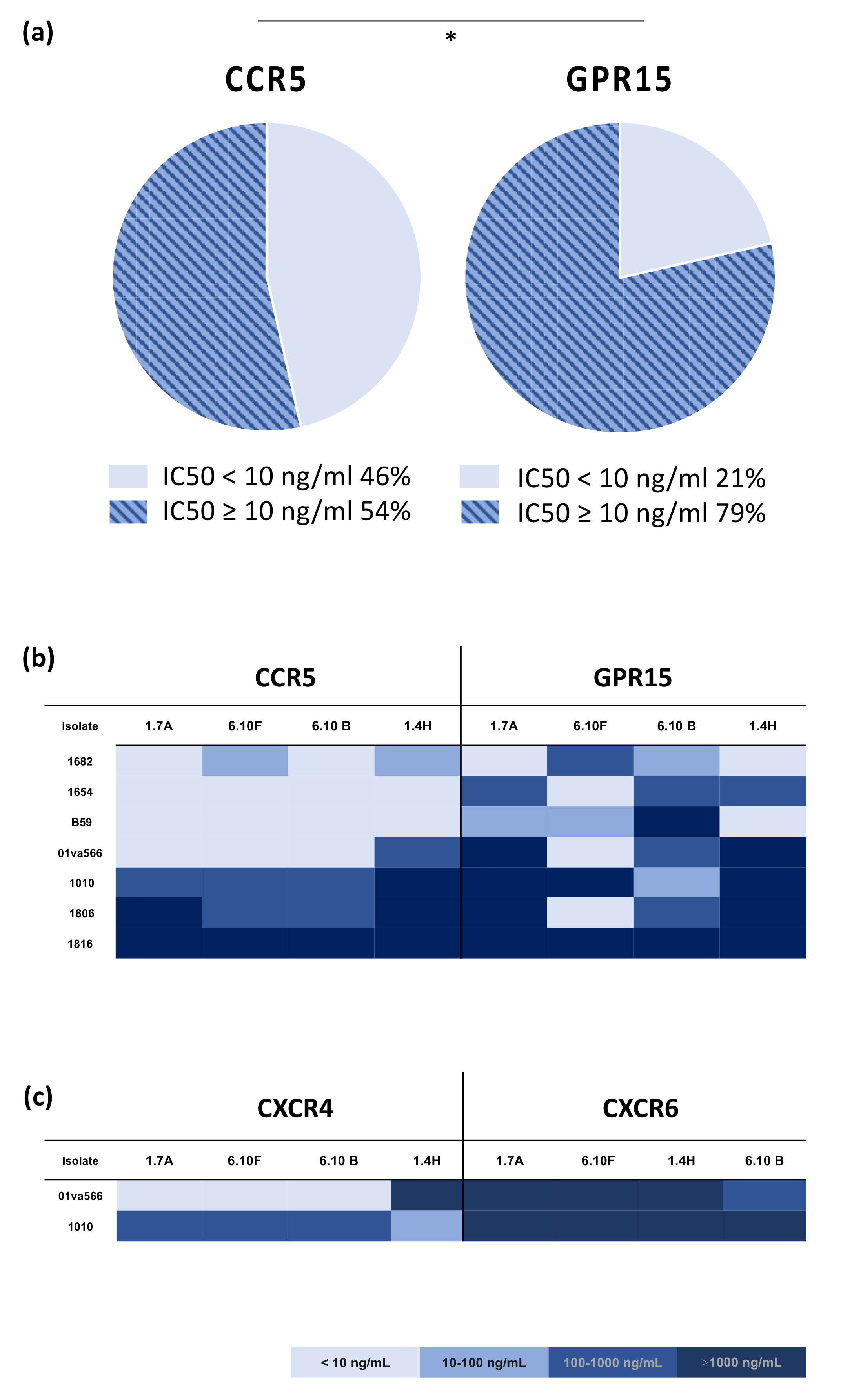
2.2. HIV-2 Sensitivity to Neutralizing Antibodies Is Associated with Co-Receptor Entry-Pathway
2.3. Alterations in the HIV-2 Env V1/V2 Regions in Relation to Neutralization Sensitivity
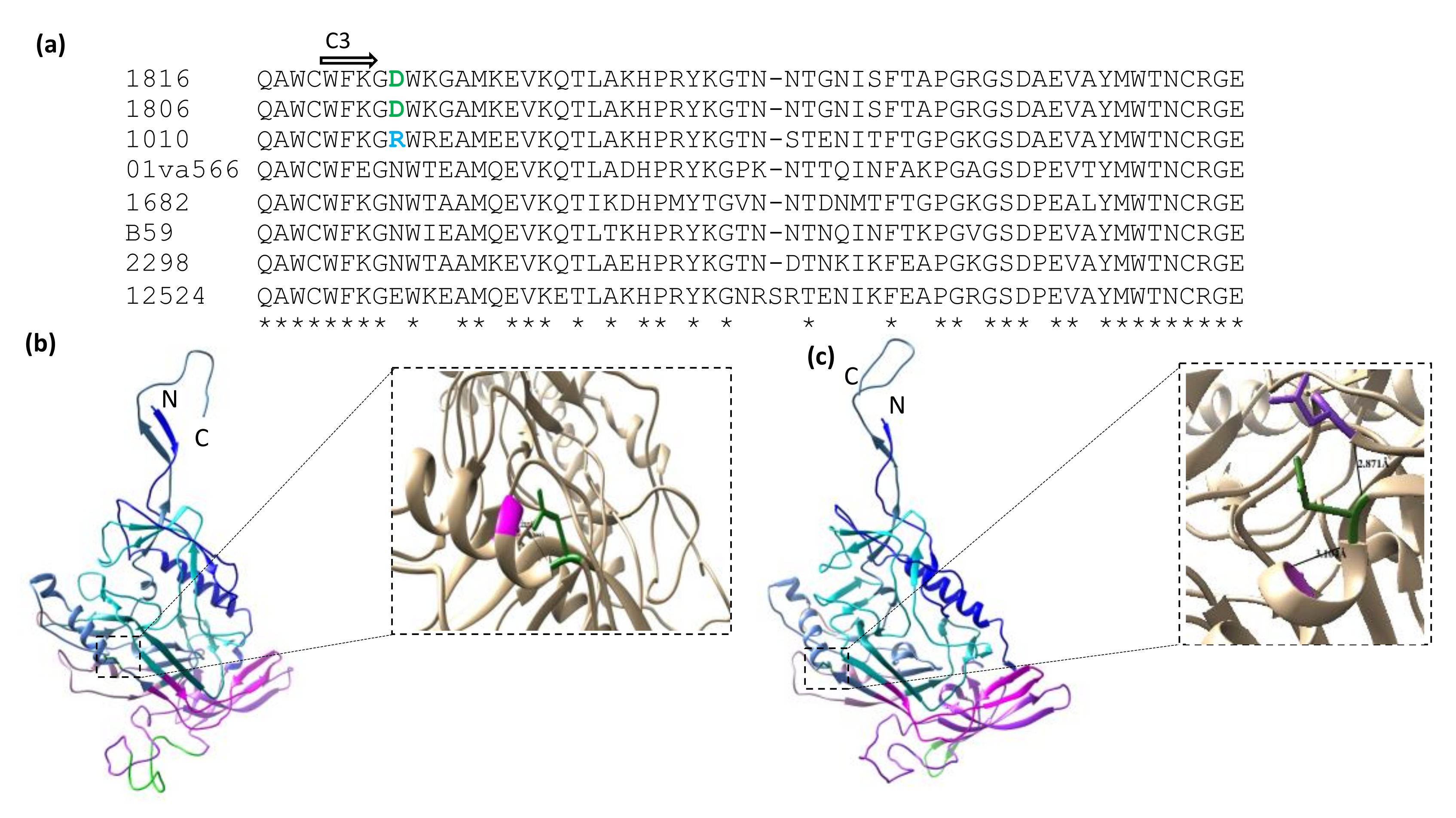
2.4. Alterations in the HIV-2 Env C3 Region in Relation to Neutralization Sensitivity
3. Discussion
4. Materials and Methods
4.1. Panel of HIV-2 Primary Isolates
4.2. PCR Amplification and Sequencing of HIV-2 Env Regions
4.3. Analysis of Globally Accessible HIV-2 Env Sequences
4.4. Modelling and In Silico Predictions of HIV-2 Env Motifs and Structures
4.5. Production and Purification of Monoclonal Antibodies
4.6. HIV-2 Neutralization Assay Using Co-Receptor Indicator Cell Lines
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azevedo-Pereira, J.M.; Santos-Costa, Q. HIV Interaction with Human Host: HIV-2 as a Model of a Less Virulent Infection. Aids Rev. 2016, 18, 44–53. [Google Scholar] [PubMed]
- Gottlieb, G.S.; Raugi, D.N.; Smith, R.A. 90-90-90 for HIV-2? Ending the HIV-2 epidemic by enhancing care and clinical management of patients infected with HIV-2. Lancet HIV 2018, 5, e390–e399. [Google Scholar] [CrossRef]
- Esbjörnsson, J.; Månsson, F.; Kvist, A.; da Silva, Z.J.; Andersson, S.; Fenyö, E.M.; Isberg, P.-E.; Biague, A.J.; Lindman, J.; Palm, A.A.; et al. Long-term follow-up of HIV-2-related AIDS and mortality in Guinea-Bissau: A prospective open cohort study. Lancet HIV 2018, 6, e25–e31. [Google Scholar] [CrossRef]
- Andersson, S.; Norrgren, H.; da Silva, Z.; Biague, A.; Bamba, S.; Kwok, S.; Christopherson, C.; Biberfeld, G.; Albert, J. Plasma viral load in HIV-1 and HIV-2 singly and dually infected individuals in Guinea-Bissau, West Africa: Significantly lower plasma virus set point in HIV-2 infection than in HIV-1 infection. Arch. Intern. Med. 2000, 160, 3286–3293. [Google Scholar] [CrossRef] [PubMed]
- Damond, F.; Gueudin, M.; Pueyo, S.; Farfara, I.; Robertson, D.L.; Descamps, D.; Chène, G.; Matheron, S.; Campa, P.; Brun-Vézinet, F.; et al. Plasma RNA Viral Load in Human Immunodeficiency Virus Type 2 Subtype A and Subtype B Infections. J. Clin. Microbiol. 2002, 40, 3654–3659. [Google Scholar] [CrossRef]
- Drylewicz, J.; Matheron, S.; Lazaro, E.; Damond, F.; Bonnet, F.; Simon, F.; Dabis, F.; Brun-Vezinet, F.; Chêne, G.; Thiébaut, R. Comparison of viro-immunological marker changes between HIV-1 and HIV-2-infected patients in France. AIDS 2008, 22, 457–468. [Google Scholar] [CrossRef]
- Angin, M.; Wong, G.; Papagno, L.; Versmisse, P.; David, A.; Bayard, C.; Muylder, B.C.-D.; Besseghir, A.; Thiébaut, R.; Boufassa, F.; et al. Preservation of Lymphopoietic Potential and Virus Suppressive Capacity by CD8+ T Cells in HIV-2–Infected Controllers. J. Immunol. 2016, 197, 2787–2795. [Google Scholar] [CrossRef]
- De Silva, T.I.; Aasa-Chapman, M.; Cotten, M.; Hué, S.; Robinson, J.; Bibollet-Ruche, F.; Sarge-Njie, R.; Berry, N.; Jaye, A.; Aaby, P.; et al. Potent Autologous and Heterologous Neutralizing Antibody Responses Occur in HIV-2 Infection across a Broad Range of Infection Outcomes. J. Virol. 2012, 86, 930–946. [Google Scholar] [CrossRef]
- Duvall, M.G.; Precopio, M.L.; Ambrozak, D.A.; Jaye, A.; McMichael, A.J.; Whittle, H.C.; Roederer, M.; Rowland-Jones, S.L.; Koup, R.A. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur. J. Immunol. 2008, 38, 350–363. [Google Scholar] [CrossRef]
- Gillespie, G.M.A.; Pinheiro, S.; Sayeid-Al-Jamee, M.; Alabi, A.; Kaye, S.; Sabally, S.; Sarge-Njie, R.; Njai, H.; Joof, K.; Jaye, A.; et al. CD8+ T?cell responses to human immunodeficiency viruses type?2 (HIV-2) and type?1 (HIV-1) gag proteins are distinguishable by magnitude and breadth but not cellular phenotype. Eur. J. Immunol. 2005, 35, 1445–1453. [Google Scholar] [CrossRef]
- Kong, R.; Li, H.; Georgiev, I.; Changela, A.; Bibollet-Ruche, F.; Decker, J.M.; Rowland-Jones, S.L.; Jaye, A.; Guan, Y.; Lewis, G.K.; et al. Epitope Mapping of Broadly Neutralizing HIV-2 Human Monoclonal Antibodies. J. Virol. 2012, 86, 12115–12128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leligdowicz, A.; Onyango, C.; Yindom, L.-M.; Peng, Y.; Cotten, M.; Jaye, A.; McMichael, A.; Whittle, H.; Dong, T.; Rowland-Jones, S. Highly avid, oligoclonal, early-differentiated antigen-specific CD8+ T cells in chronic HIV-2 infection. Eur. J. Immunol. 2010, 40, 1963–1972. [Google Scholar] [CrossRef]
- Şahin, G.; Holmgren, B.; da Silva, Z.; Nielsen, J.; Nowroozalizadeh, S.; Esbjörnsson, J.; Månsson, F.; Andersson, S.; Norrgren, H.; Aaby, P.; et al. Potent Intratype Neutralizing Activity Distinguishes Human Immunodeficiency Virus Type 2 (HIV-2) from HIV-1. J. Virol. 2012, 86, 961–971. [Google Scholar] [CrossRef] [PubMed]
- De Silva, T.I.; Peng, Y.; Leligdowicz, A.; Zaidi, I.; Li, L.; Griffin, H.; Blais, M.E.; Vincent, T.; Saraiva, M.; Yindom, L.M.; et al. Correlates of T-cell-mediated viral control and phenotype of CD8(+) T cells in HIV-2, a naturally contained human retroviral infection. Blood 2013, 121, 4330–4339. [Google Scholar] [CrossRef] [PubMed]
- Şahin, G.; Holmgren, B.; Sheik-Khalil, E.; da Silva, Z.; Nielsen, J.; Nowroozalizadeh, S.; Månsson, F.; Norrgren, H.; Aaby, P.; Fenyö, E.M.; et al. Effect of Complement on HIV-2 Plasma Antiviral Activity Is Intratype Specific and Potent. J. Virol. 2013, 87, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.K.; Sarr, A.D.; MacNeil, A.; Thakore-Meloni, S.; Gueye-Ndiaye, A.; Traoré, I.; Dia, M.C.; Mboup, S.; Kanki, P.J. Comparison of Heterologous Neutralizing Antibody Responses of Human Immunodeficiency Virus Type 1 (HIV-1)- and HIV-2-Infected Senegalese Patients: Distinct Patterns of Breadth and Magnitude Distinguish HIV-1 and HIV-2 Infections. J. Virol. 2007, 81, 5331–5338. [Google Scholar] [CrossRef][Green Version]
- Björling, E.; Scarlatti, G.; Von Gegerfelt, A.; Albert, J.; Biberfeld, G.; Chiodi, F.; Norrby, E.; Fenyö, E.M. Autologous Neutralizing Antibodies Prevail in HIV-2 but Not in HIV-1 Infection. Virology 1993, 193, 528–530. [Google Scholar] [CrossRef]
- Shi, Y.; Brandin, E.; Vincic, E.; Jansson, M.; Blaxhult, A.; Gyllensten, K.; Moberg, L.; Broström, C.; Fenyö, E.M.; Albert, J. Evolution of human immunodeficiency virus type 2 coreceptor usage, autologous neutralization, envelope sequence and glycosylation. J. Gen. Virol. 2005, 86, 3385–3396. [Google Scholar] [CrossRef]
- Reeves, J.D.; Hibbitts, S.; Simmons, G.; McKnight, A.; Azevedo-Pereira, J.M.; Moniz-Pereira, J.; Clapham, P.R. Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: Comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo. J. Virol. 1999, 73, 7795–7804. [Google Scholar] [CrossRef]
- Morner, A.; Bjorndal, A.; Albert, J.; Kewalramani, V.N.; Littman, D.R.; Inoue, R.; Thorstensson, R.; Fenyo, E.M.; Bjorling, E. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 1999, 73, 2343–2349. [Google Scholar] [CrossRef]
- Santos-Costa, Q.; Lopes, M.M.; Calado, M.; Azevedo-Pereira, J.M. HIV-2 interaction with cell coreceptors: Amino acids within the V1/V2 region of viral envelope are determinant for CCR8, CCR5 and CXCR4 usage. Retrovirology 2014, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Mörner, A.; Björndal, A.; Leandersson, A.-C.; Albert, J.; Björling, E.; Jansson, M. CCR5 or CXCR4 Is Required for Efficient Infection of Peripheral Blood Mononuclear Cells by Promiscuous Human Immunodeficiency Virus Type 2 Primary Isolates. AIDS Res. Hum. Retrovir. 2002, 18, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, K.S.; Elliott, S.T.; Collman, R.G. SIV Coreceptor Specificity in Natural and Non-Natural Host Infection: Implications for Cell Targeting and Differential Outcomes from Infection. Curr. HIV Res. 2018, 16, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.; Stalhandske, P.; Marquina, S.; Karis, J.; Fouchier, R.; Norrby, E.; Chiodi, F. Biological Phenotype of HIV Type 2 Isolates Correlates with V3 Genotype. AIDS Res. Hum. Retrovir. 1996, 12, 821–828. [Google Scholar] [CrossRef]
- Isaka, Y.; Sato, A.; Miki, S.; Kawauchi, S.; Sakaida, H.; Hori, T.; Uchiyama, T.; Adachi, A.; Hayami, M.; Fujiwara, T.; et al. Small amino acid changes in the V3 loop of human immunodeficiency virus type 2 determines the coreceptor usage for CXCR4 and CCR5. Virology 1999, 264, 237–243. [Google Scholar] [CrossRef]
- Döring, M.; Borrego, P.; Büch, J.; Martins, A.; Friedrich, G.; Camacho, R.J.; Eberle, J.; Kaiser, R.; Lengauer, T.; Taveira, N.; et al. A genotypic method for determining HIV-2 coreceptor usage enables epidemiological studies and clinical decision support. Retrovirology 2016, 13, 85. [Google Scholar] [CrossRef]
- Marcelino, J.M.; Borrego, P.; Nilsson, C.; Família, C.; Barroso, H.; Maltez, F.; Doroana, M.; Antunes, F.; Quintas, A.; Taveira, N. Resistance to antibody neutralization in HIV-2 infection occurs in late stage disease and is associated with X4 tropism. AIDS 2012, 26, 2275–2284. [Google Scholar] [CrossRef]
- Groenink, M.; Andeweg, A.C.; Fouchier, R.A.; Broersen, S.; van der Jagt, R.C.; Schuitemaker, H.; de Goede, R.E.; Bosch, M.L.; Huisman, H.G.; Tersmette, M. Phenotype-associated env gene variation among eight related human immunodeficiency virus type 1 clones: Evidence for in vivo recombination and determinants of cytotropism outside the V3 domain. J. Virol. 1992, 66, 6175–6180. [Google Scholar] [CrossRef]
- Albert, J.; Nauclér, A.; Böttiger, B.; Broliden, P.-A.; Albino, P.; Ouattara, S.A.; Björkegren, C.; Valentin, A.; Biberfeld, G.; Fenyö, E.M. Replicative capacity of HIV-2, like HIV-1, correlates with severity of immunodeficiency. AIDS 1990, 4, 291–296. [Google Scholar] [CrossRef]
- Nabatov, A.A.; Pollakis, G.; Linnemann, T.; Kliphius, A.; Chalaby, M.I.M.; Paxton, W.A. Intrapatient Alterations in the Human Immunodeficiency Virus Type 1 gp120 V1V2 and V3 Regions Differentially Modulate Coreceptor Usage, Virus Inhibition by CC/CXC Chemokines, Soluble CD4, and the b12 and 2G12 Monoclonal Antibodies. J. Virol. 2004, 78, 524–530. [Google Scholar] [CrossRef]
- Erdős, G.; Pajkos, M.; Dosztányi, Z. IUPred3: Prediction of protein disorder enhanced with unambiguous experimental annotation and visualization of evolutionary conservation. Nucleic Acids Res. 2021, 49, W297–W303. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Paliwal, K.K.; Litfin, T.; Zhou, Y. SPOT-Disorder2: Improved Protein Intrinsic Disorder Prediction by Ensembled Deep Learning. Genom. Proteom. Bioinform. 2019, 17, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.K.; Yue, L.; Pan, R.; Boliar, S.; Sethi, A.; Tian, J.; Pfafferot, K.; Karita, E.; Allen, S.A.; Cormier, E.; et al. Viral Escape from Neutralizing Antibodies in Early Subtype A HIV-1 Infection Drives an Increase in Autologous Neutralization Breadth. PLOS Pathog. 2013, 9, e1003173. [Google Scholar] [CrossRef] [PubMed]
- Farzan, M.; Choe, H.; Martin, K.; Marcon, L.; Hofmann, W.; Karlsson, G.; Sun, Y.; Barrett, P.; Marchand, N.; Sullivan, N.; et al. Two Orphan Seven-Transmembrane Segment Receptors Which Are Expressed in CD4-positive Cells Support Simian Immunodeficiency Virus Infection. J. Exp. Med. 1997, 186, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Kõks, S.; Kõks, G. Activation of GPR15 and its involvement in the biological effects of smoking. Exp. Biol. Med. 2017, 242, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Blaak, H.; Boers, P.H.M.; Gruters, R.; Schuitemaker, H.; van der Ende, M.E.; Osterhaus, A.D.M.E. CCR5, GPR15, and CXCR6 Are Major Coreceptors of Human Immunodeficiency Virus Type 2 Variants Isolated from Individuals with and without Plasma Viremia. J. Virol. 2005, 79, 1686–1700. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Unutmaz, D.; KewalRamani, V.N.; Littman, D.R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 1997, 388, 296–300. [Google Scholar] [CrossRef]
- Wetzel, K.S.; Yi, Y.; Yadav, A.; Bauer, A.M.; Bello, E.A.; Romero, D.C.; Bibollet-Ruche, F.; Hahn, B.; Paiardini, M.; Silvestri, G.; et al. Loss of CXCR6 coreceptor usage characterizes pathogenic lentiviruses. PLOS Pathog. 2018, 14, e1007003. [Google Scholar] [CrossRef]
- Hlmann, S.P.; Krumbiegel, M.; Kirchhoff, F. Coreceptor usage of BOB/GPR15 and Bonzo/STRL33 by primary isolates of human immunodeficiency virus type 1. J. Gen. Virol. 1999, 80, 1241–1251. [Google Scholar] [CrossRef]
- Kiene, M.; Marzi, A.; Urbanczyk, A.; Bertram, S.; Fisch, T.; Nehlmeier, I.; Gnirß, K.; Karsten, C.B.; Palesch, D.; Münch, J.; et al. The role of the alternative coreceptor GPR15 in SIV tropism for human cells. Virology 2012, 433, 73–84. [Google Scholar] [CrossRef][Green Version]
- Lauren, A.; Fenyö, E.M. Implications from the SIV model in understanding HIV neutralization. Future HIV Ther. 2008, 2, 9. [Google Scholar] [CrossRef]
- Clayton, F.; Kotler, D.P.; Kuwada, S.K.; Morgan, T.; Stepan, C.; Kuang, J.; Le, J.; Fantini, J. Gp120-Induced Bob/GPR15 Activation: A Possible Cause of Human Immunodeficiency Virus Enteropathy. Am. J. Pathol. 2001, 159, 1933–1939. [Google Scholar] [CrossRef]
- Recordon-Pinson, P.; Gosselin, A.; Ancuta, P.; Routy, J.-P.; Fleury, H. Phylogenetic analysis of HIV-1 archived DNA in blood and gut-associated lymphoid tissue in two patients under antiretroviral therapy. Gut Pathog. 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Van Marle, G.; Church, D.L.; van der Meer, F.; Gill, M.J. Combating the HIV reservoirs. Biotechnol. Genet. Eng. Rev. 2018, 34, 76–89. [Google Scholar] [CrossRef]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Kotler, D.P.; Fantini, J.; Clayton, F. The virotoxin model of HIV-1 enteropathy: Involvement of GPR15/Bob and galactosylceramide in the cytopathic effects induced by HIV-1 gp120 in the HT-29-D4 intestinal cell line. J. Biomed. Sci. 2003, 10, 156–166. [Google Scholar] [CrossRef]
- Fernandes, S.; Pires, A.R.; Matoso, P.; Ferreira, C.; Cabaço, H.N.; Correia, L.; Valadas, E.; Poças, J.; Pacheco, P.; Veiga-Fernandes, H.; et al. HIV-2 infection is associated with preserved GALT homeostasis and epithelial integrity despite ongoing mucosal viral replication. Mucosal Immunol. 2017, 11, 236–248. [Google Scholar] [CrossRef]
- Kim, S.V.; Xiang, W.V.; Kwak, C.; Yang, Y.; Lin, X.W.; Ota, M.; Sarpel, U.; Rifkin, D.B.; Xu, R.; Littman, D.R. GPR15-Mediated Homing Controls Immune Homeostasis in the Large Intestine Mucosa. Science 2013, 340, 1456–1459. [Google Scholar] [CrossRef]
- Hayn, M.; Blötz, A.; Rodríguez, A.; Vidal, S.; Preising, N.; Ständker, L.; Wiese, S.; Stürzel, C.M.; Harms, M.; Gross, R.; et al. Natural cystatin C fragments inhibit GPR15-mediated HIV and SIV infection without interfering with GPR15L signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2023776118. [Google Scholar] [CrossRef]
- Kulkarni, V.; Ruprecht, R.M. Mucosal IgA Responses: Damaged in Established HIV Infection-Yet, Effective Weapon against HIV Transmission. Front. Immunol. 2017, 8, 1581. [Google Scholar] [CrossRef]
- Burton, D.R.; Mascola, J.R. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat. Immunol. 2015, 16, 571–576. [Google Scholar] [CrossRef]
- Plantier, J.-C.; Damond, F.; Souquières, S.; Brun-Vézinet, F.; Simon, F.; Barin, F. V3 Serological Subtyping of Human Immunodeficiency Virus Type 2 Infection Is Not Relevant. J. Clin. Microbiol. 2001, 39, 3803–3807. [Google Scholar] [CrossRef] [PubMed]
- Serra, P.; Taveira, N.; Guedes, R. Computational Modulation of the V3 Region of Glycoprotein gp125 of HIV-2. Int. J. Mol. Sci. 2021, 22, 1948. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.; Thali, M.; Furman, C.; Ho, D.D.; Sodroski, J. Effect of amino acid changes in the V1/V2 region of the human immunodeficiency virus type 1 gp120 glycoprotein on subunit association, syncytium formation, and recognition by a neutralizing antibody. J. Virol. 1993, 67, 3674–3679. [Google Scholar] [CrossRef] [PubMed]
- McKnight, A.; Shotton, C.; Cordell, J.; Jones, I.; Simmons, G.; Clapham, P.R. Location, exposure, and conservation of neutralizing and nonneutralizing epitopes on human immunodeficiency virus type 2 SU glycoprotein. J. Virol. 1996, 70, 4598–4606. [Google Scholar] [CrossRef] [PubMed]
- Barroso, H.; Borrego, P.; Bártolo, I.; Marcelino, J.M.; Família, C.; Quintas, A.; Taveira, N. Evolutionary and Structural Features of the C2, V3 and C3 Envelope Regions Underlying the Differences in HIV-1 and HIV-2 Biology and Infection. PLoS ONE 2011, 6, e14548. [Google Scholar] [CrossRef]
- Palm, A.A.; Lemey, P.; Jansson, M.; Månsson, F.; Kvist, A.; Szojka, Z.; Biague, A.; da Silva, Z.J.; Rowland-Jones, S.L.; Norrgren, H.; et al. Low Postseroconversion CD4+ T-cell Level Is Associated with Faster Disease Progression and Higher Viral Evolutionary Rate in HIV-2 Infection. mBio 2019, 10, e01245-18. [Google Scholar] [CrossRef]
- Repits, J.; Öberg, M.; Esbjörnsson, J.; Medstrand, P.; Karlsson, A.; Albert, J.; Fenyö, E.M.; Jansson, M. Selection of human immunodeficiency virus type 1 R5 variants with augmented replicative capacity and reduced sensitivity to entry inhibitors during severe immunodeficiency. J. Gen. Virol. 2005, 86, 2859–2869. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Zhang, M.; Gaschen, B.; Blay, W.; Foley, B.; Haigwood, N.; Kuiken, C.; Korber, B. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology 2004, 14, 1229–1246. [Google Scholar] [CrossRef]
- Buchan, D.W.A.; Minneci, F.; Nugent, T.C.O.; Bryson, K.; Jones, D.T. Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res. 2013, 41, W349–W357. [Google Scholar] [CrossRef]
- Tiller, T.; Meffre, E.; Yurasov, S.; Tsuiji, M.; Nussenzweig, M.C.; Wardemann, H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods 2008, 329, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Sheik-Khalil, E.; Bray, M.-A.; Şahin, G.; Scarlatti, G.; Jansson, M.; Carpenter, A.E.; Fenyö, E.M. Automated image-based assay for evaluation of HIV neutralization and cell-to-cell fusion inhibition. BMC Infect. Dis. 2014, 14, 472. [Google Scholar] [CrossRef] [PubMed]
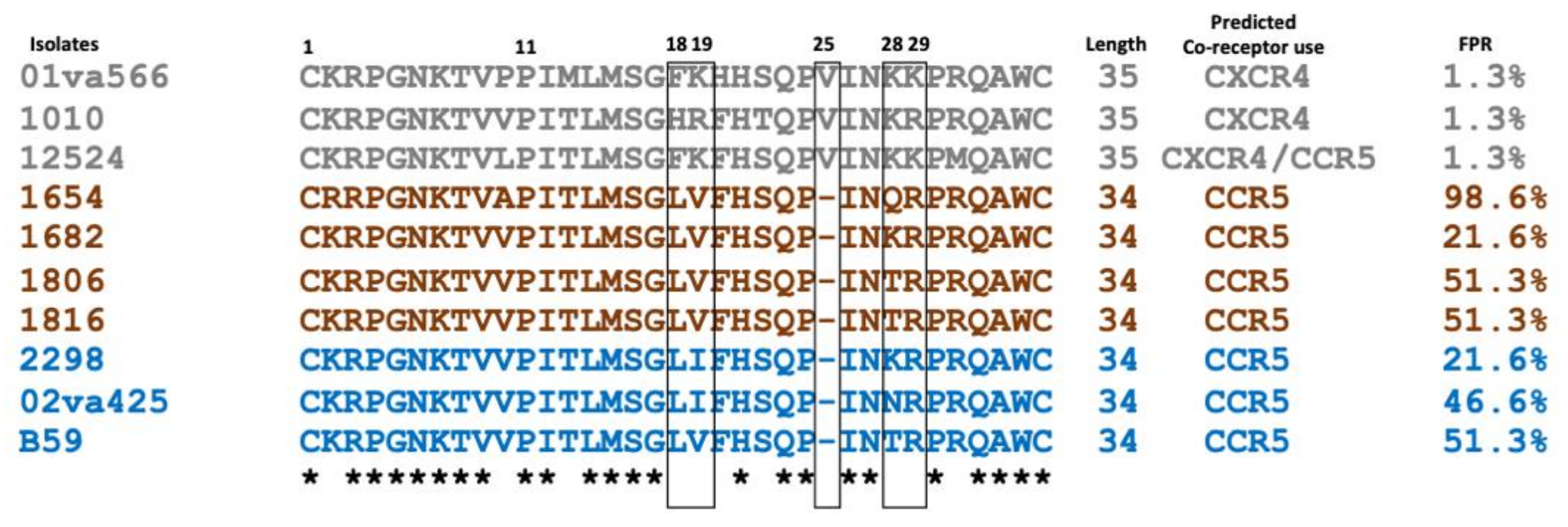

| Isolate c | Disease State | CCR5 | CXCR4 | GPR15 | CXCR6 | 18 | 19 | Insertion 25 | 28 | 29 | Charge |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 01va566 | AIDS | +++++ | +++++ | +++++ | +++++ | F | K | V | K | K | +7 |
| 1010 | AIDS | +++++ | +++++ | +++++ | +++++ | H | R | V | K | R | +7 |
| 12524 | AIDS | +++++ | ++++ | −−−− | −−−− | F | K | V | K | K | +6 |
| 1654 | AIDS | +++++ | −−−− | +++++ | +++ | L | V | −−−− | Q | R | +5 |
| 1682 | AS | ++ | −−−− | ++++ | ++ | L | V | −−−− | K | R | +6 |
| 1806 | AS | +++++ | −−−− | +++++ | +++++ | L | V | −−−− | T | R | +5 |
| 1816 | AS | +++++ | −−−− | +++++ | +/−−−− | L | V | −−−− | T | R | +5 |
| 2298 | AS | ++++ | −−−− | + | −−−− | L | I | −−−− | K | R | +6 |
| 02va425 | AIDS | +++ | −−−− | ++ | +/−−−− | L | I | −−−− | N | R | +5 |
| B59 | AS | +++++ | −−−− | +++ | −−−− | L | V | −−−− | T | R | +5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szojka, Z.I.; Karlson, S.; Johansson, E.; Şahin, G.Ö.; Jansson, M. HIV-2 Neutralization Sensitivity in Relation to Co-Receptor Entry Pathways and Env Motifs. Int. J. Mol. Sci. 2022, 23, 4766. https://doi.org/10.3390/ijms23094766
Szojka ZI, Karlson S, Johansson E, Şahin GÖ, Jansson M. HIV-2 Neutralization Sensitivity in Relation to Co-Receptor Entry Pathways and Env Motifs. International Journal of Molecular Sciences. 2022; 23(9):4766. https://doi.org/10.3390/ijms23094766
Chicago/Turabian StyleSzojka, Zsófia Ilona, Sara Karlson, Emil Johansson, Gülşen Özkaya Şahin, and Marianne Jansson. 2022. "HIV-2 Neutralization Sensitivity in Relation to Co-Receptor Entry Pathways and Env Motifs" International Journal of Molecular Sciences 23, no. 9: 4766. https://doi.org/10.3390/ijms23094766
APA StyleSzojka, Z. I., Karlson, S., Johansson, E., Şahin, G. Ö., & Jansson, M. (2022). HIV-2 Neutralization Sensitivity in Relation to Co-Receptor Entry Pathways and Env Motifs. International Journal of Molecular Sciences, 23(9), 4766. https://doi.org/10.3390/ijms23094766







