Structural and Functional Support by Left Atrial Appendage Transplant to the Left Ventricle after a Myocardial Infarction
Abstract
:1. Introduction
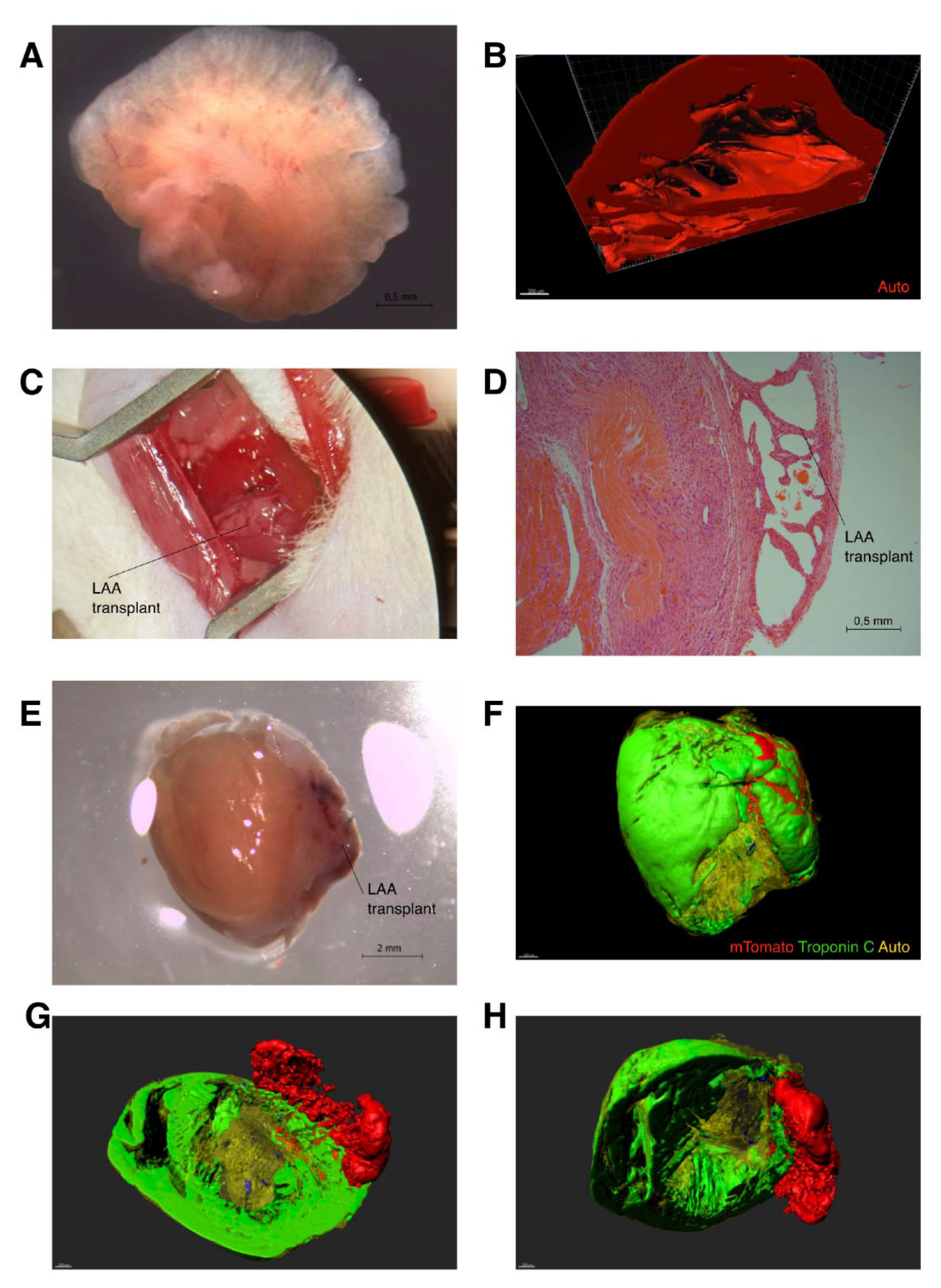
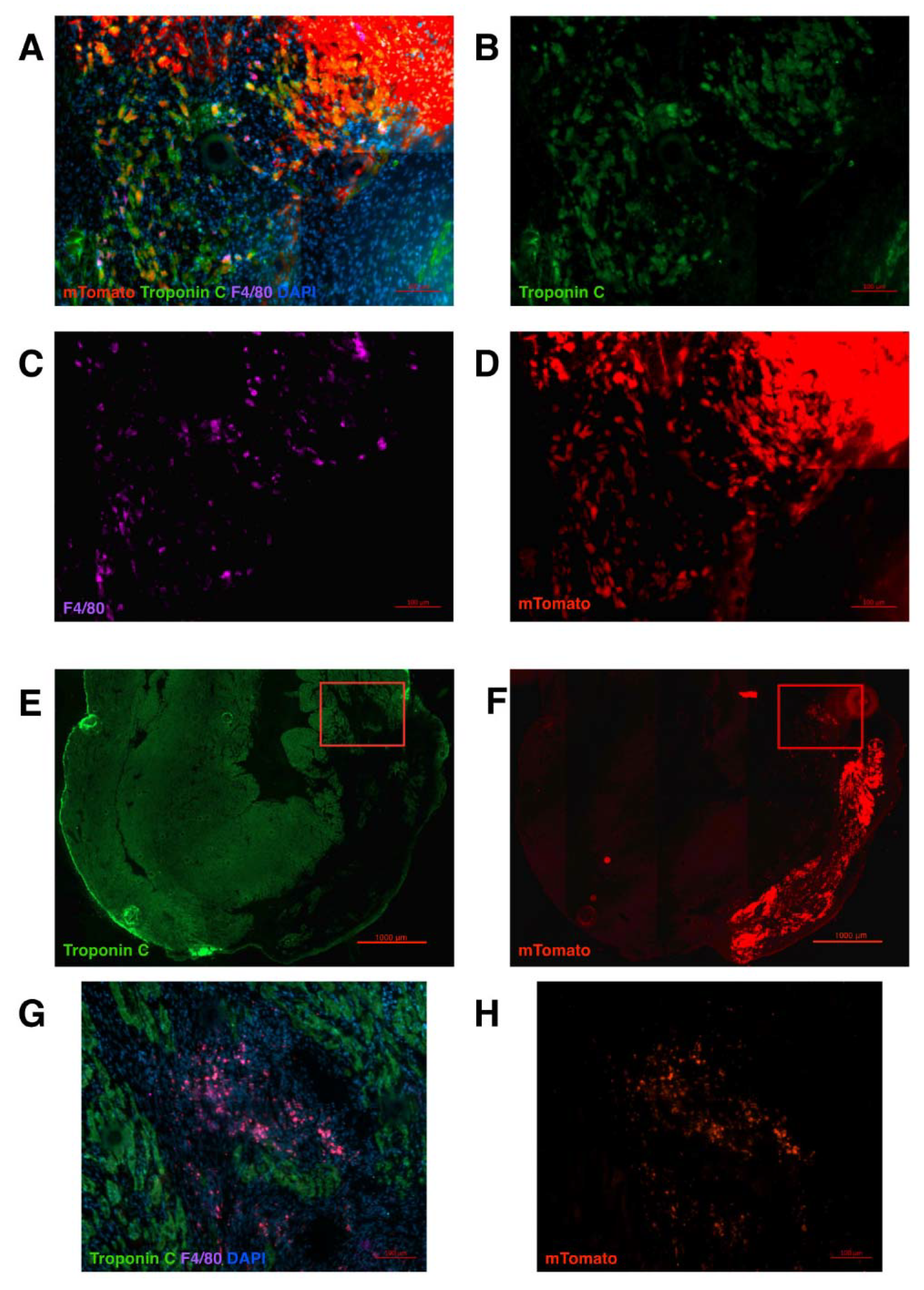
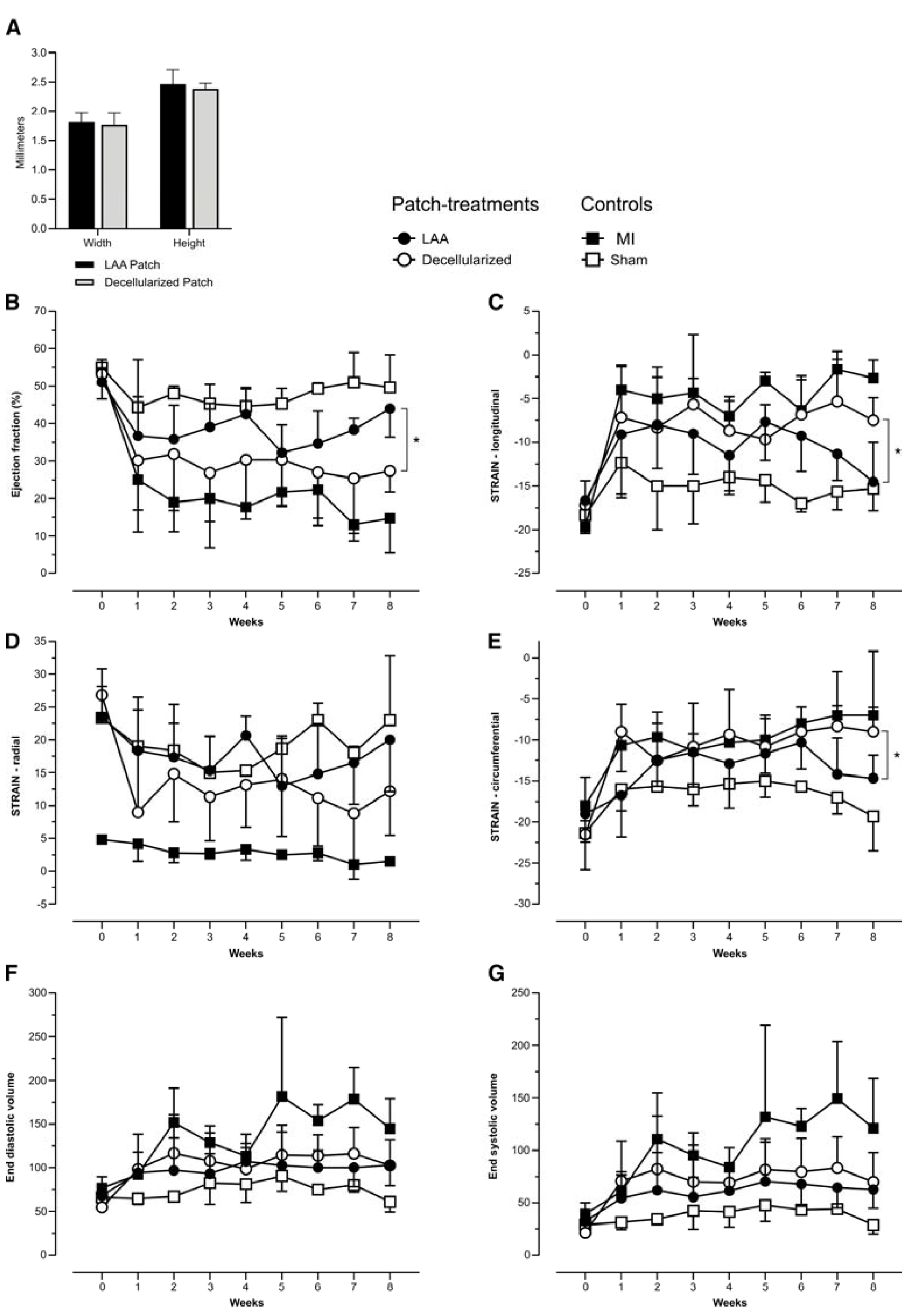
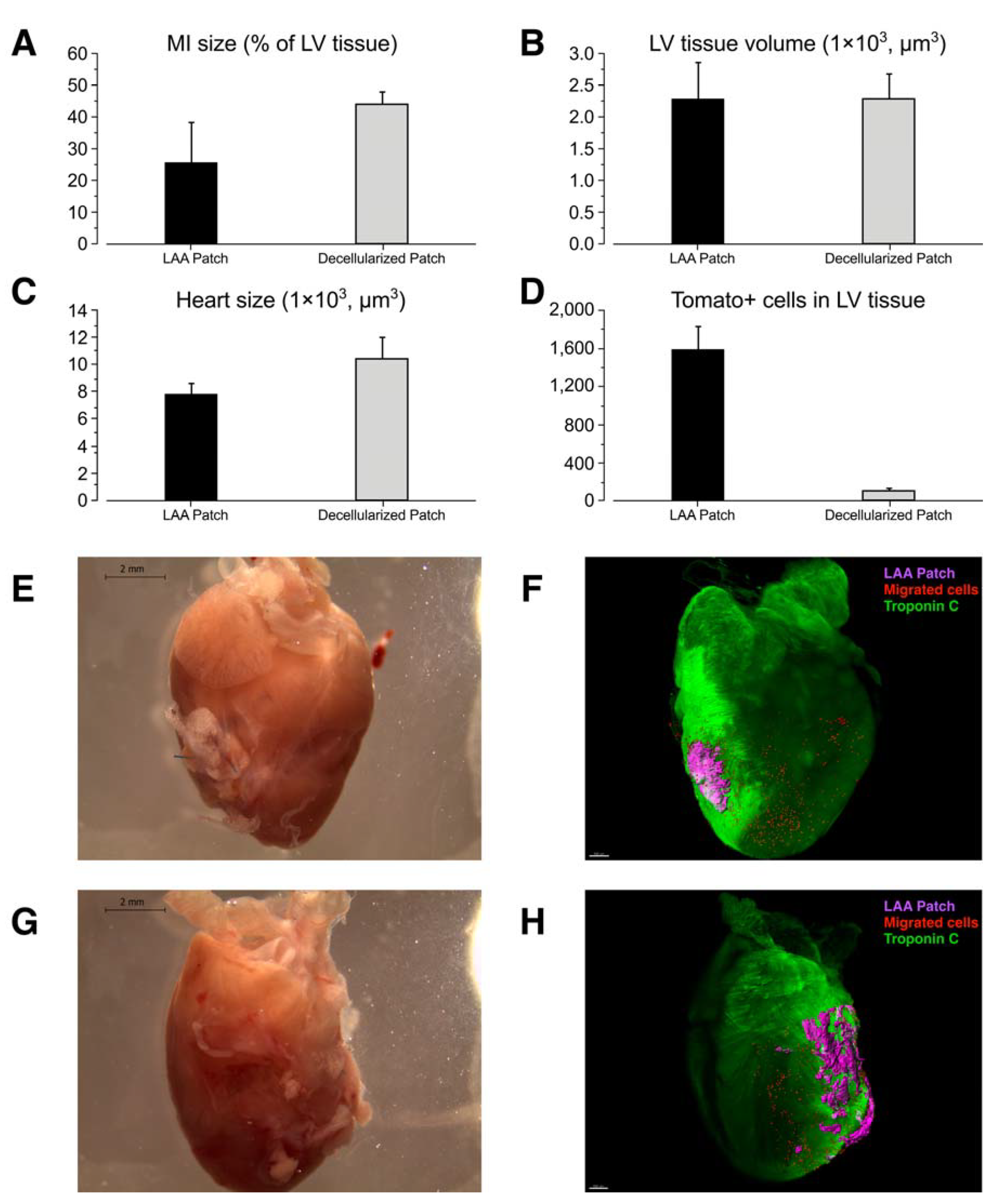
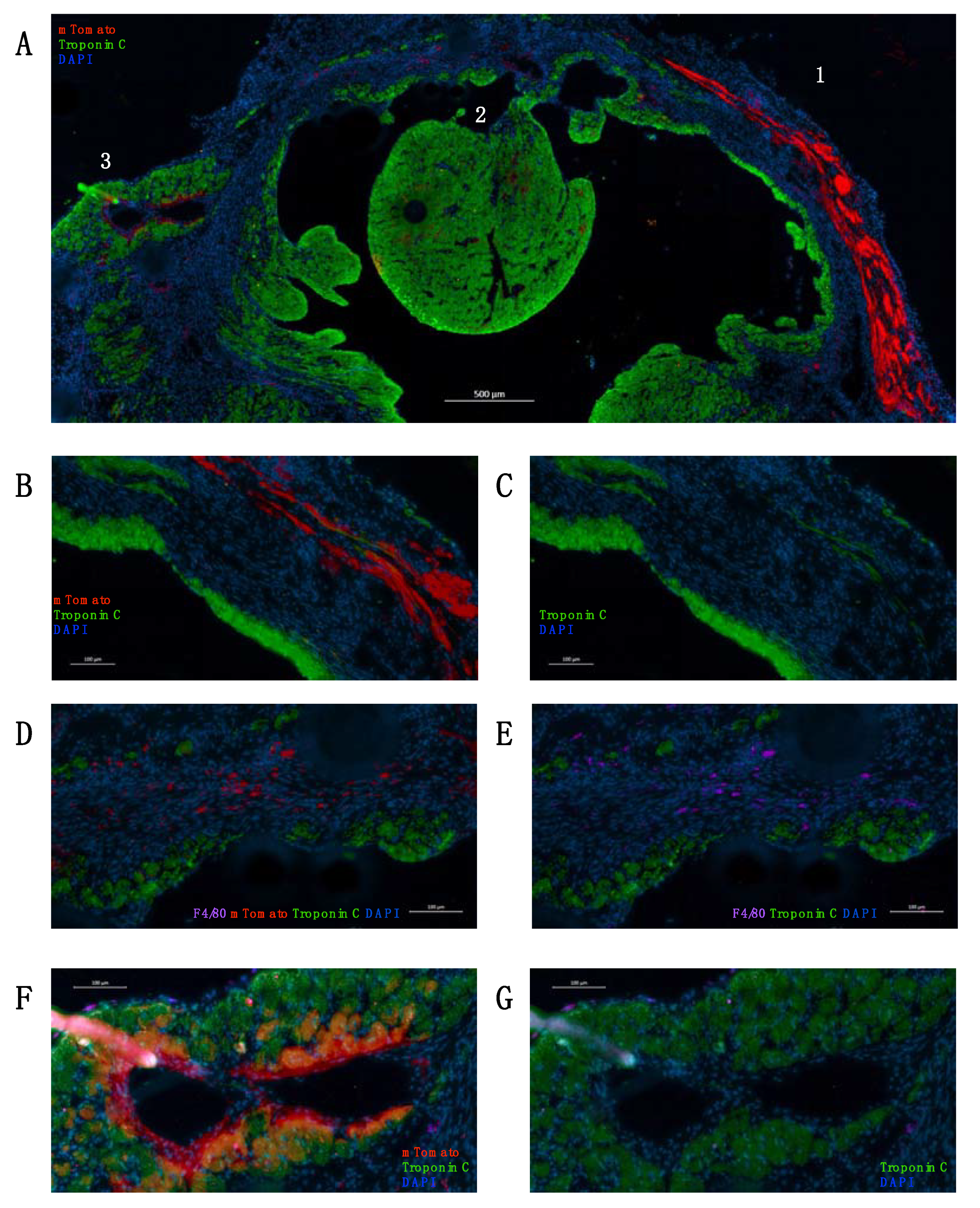
2. Results
3. Discussion
4. Materials and Methods
4.1. Mouse Strains and Patch Transplantation
4.2. Ethics
4.3. Left Atrial Appendage Decellularization
4.4. Cardiac Ultrasound and Analysis
4.5. 3D Imaging (iDisco)
4.6. Immunohistochemistry
4.7. Antibodies
4.8. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Vivien, C.J.; Hudson, J.E.; Porrello, E.R. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. npj Regen. Med. 2016, 1, 16012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.-I.; Penninger, J.M. Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ. Res. 2016, 118, 216–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Termglinchan, V.; Karakikes, I. Concise Review: Mending a Broken Heart: The Evolution of Biological Therapeutics. Stem Cells 2017, 35, 1131–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwetsloot, P.P.; Végh, A.M.D.; Lorkeers, S.J.J.O.; van Hout, G.P.; Currie, G.L.; Sena, E.S.; Gremmels, H.; Buikema, J.W.; Goumans, M.-J.; Macleod, M.R.; et al. Cardiac Stem Cell Treatment in Myocardial Infarction: A Systematic Review and Meta-Analysis of Preclinical Studies. Circ. Res. 2016, 118, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-L.; Li, Q.; Rokosh, G.; Sanganalmath, S.K.; Chen, N.; Ou, Q.; Stowers, H.; Hunt, G.; Bolli, R. Long-Term Outcome of Administration of c-kit POS Cardiac Progenitor Cells After Acute Myocardial Infarction: Transplanted Cells Do not Become Cardiomyocytes, but Structural and Functional Improvement and Proliferation of Endogenous Cells Persist for at Least One Year. Circ. Res. 2016, 118, 1091–1105. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Mishra, R.; Bigham, G.E.; Wehman, B.; Khan, M.M.; Xu, H.; Saha, P.; Goo, Y.A.; Datla, S.R.; Chen, L.; et al. A Deep Proteome Analysis Identifies the Complete Secretome as the Functional Unit of Human Cardiac Progenitor Cells. Circ. Res. 2017, 120, 816–834. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases—Cell Types, Mechanisms and Improvement Strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, D.; Liang, P.; Li, Y.; Cao, F. The Current Dilemma and Breakthrough of Stem Cell Therapy in Ischemic Heart Disease. Front. Cell Dev. Biol. 2021, 9, 636136. [Google Scholar] [CrossRef]
- Epelman, S.; Liu, P.P.; Mann, D. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015, 15, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.B.; Olson, E.N. Immune Modulation of Stem Cells and Regeneration. Cell Stem Cell 2014, 15, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Aurora, A.B.; Porrello, E.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 2014, 124, 1382–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavine, K.J.; Epelman, S.; Uchida, K.; Weber, K.J.; Nichols, C.G.; Schilling, J.D.; Ornitz, D.M.; Randolph, G.J.; Mann, D.L. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 2014, 111, 16029–16034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molawi, K.; Wolf, Y.; Kandalla, P.K.; Favret, J.; Hagemeyer, N.; Frenzel, K.; Pinto, A.R.; Klapproth, K.; Henri, S.; Malissen, B.; et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 2014, 211, 2151–2158. [Google Scholar] [CrossRef]
- Lörchner, H.; Pöling, J.; Gajawada, P.; Hou, Y.; Polyakova, V.; Kostin, S.; Adrian-Segarra, J.M.; Boettger, T.; Wietelmann, A.; Warnecke, H.; et al. Myocardial healing requires Reg3β-dependent accumulation of macrophages in the ischemic heart. Nat. Med. 2015, 21, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Leid, J.; Carrelha, J.; Boukarabila, H.; Epelman, S.; Jacobsen, S.E.W.; LaVine, K.J. Primitive Embryonic Macrophages are Required for Coronary Development and Maturation. Circ. Res. 2016, 118, 1498–1511. [Google Scholar] [CrossRef]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522.e20. [Google Scholar] [CrossRef] [Green Version]
- Colazzo, F.; Castiglioni, L.; Sironi, L.; Fontana, L.; Nobili, E.; Franzosi, M.; Guerrini, U. Murine Left Atrium and Left Atrial Appendage Structure and Function: Echocardiographic and Morphologic Evaluation. PLoS ONE 2015, 10, e0125541. [Google Scholar] [CrossRef]
- van den Berg, C.W.; Okawa, S.; Chuva de Sousa Lopes, S.M.; van Iperen, L.; Passier, R.; Braam, S.R.; Tertoolen, L.G.; del Sol, A.; Davis, R.P.; Mummery, C.L. Transcriptome of human foetal heart compared with cardiomyocytes from pluripotent stem cells. Development 2015, 142, 3231–3238. [Google Scholar] [CrossRef] [Green Version]
- Beigel, R.; Wunderlich, N.C.; Ho, S.Y.; Arsanjani, R.; Siegel, R.J. The Left Atrial Appendage: Anatomy, Function, and Noninvasive Evaluation. JACC Cardiovasc. Imaging 2014, 7, 1251–1265. [Google Scholar] [CrossRef] [Green Version]
- Leinonen, J.V.; Emanuelov, A.K.; Platt, Y.; Helman, Y.; Feinberg, Y.; Lotan, C.; Beeri, R. Left Atrial Appendages from Adult Hearts Contain a Reservoir of Diverse Cardiac Progenitor Cells. PLoS ONE 2013, 8, e59228. [Google Scholar] [CrossRef]
- Leinonen, J.V.; Korkus-Emanuelov, A.; Wolf, Y.; Milgrom-Hoffman, M.; Lichtstein, D.; Hoss, S.; Lotan, C.; Tzahor, E.; Jung, S.; Beeri, R. Macrophage precursor cells from the left atrial appendage of the heart spontaneously reprogram into a C-kit+/CD45− stem cell-like phenotype. Int. J. Cardiol. 2016, 209, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Fanton, Y.; Robic, B.; Rummens, J.-L.; Daniëls, A.; Windmolders, S.; Willems, L.; Jamaer, L.; Dubois, J.; Bijnens, E.; Heuts, N.; et al. Cardiac atrial appendage stem cells engraft and differentiate into cardiomyocytes in vivo: A new tool for cardiac repair after MI. Int. J. Cardiol. 2015, 201, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lampinen, M.; Nummi, A.; Nieminen, T.; Harjula, A.; Kankuri, E.; AADC Consortium. Intraoperative processing and epicardial transplantation of autologous atrial tissue for cardiac repair. J. Heart Lung Transplant. 2017, 36, 1020–1022. [Google Scholar] [CrossRef] [Green Version]
- Nummi, A.; Nieminen, T.; Pätilä, T.; Lampinen, M.; Lehtinen, M.L.; Kivistö, S.; Holmström, M.; Wilkman, E.; Teittinen, K.; Laine, M.; et al. Epicardial delivery of autologous atrial appendage micrografts during coronary artery bypass surgery—safety and feasibility study. Pilot Feasibility Stud. 2017, 3, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Lampinen, M.; Takala, J.; Sikorski, V.; Soliymani, R.; Tarkia, M.; Lalowski, M.; Mervaala, E.; Kupari, M.; Zheng, Z.; et al. Epicardial transplantation of atrial appendage micrograft patch salvages myocardium after infarction. J. Heart Lung Transplant. 2020, 39, 707–718. [Google Scholar] [CrossRef]
- Nummi, A.; Mulari, S.; Stewart, J.A.; Kivistö, S.; Teittinen, K.; Nieminen, T.; Lampinen, M.; Pätilä, T.; Sintonen, H.; Juvonen, T.; et al. Epicardial Transplantation of Autologous Cardiac Micrografts During Coronary Artery Bypass Surgery. Front. Cardiovasc. Med. 2021, 8, 726889. [Google Scholar] [CrossRef]
- Zimmermann, W.-H.; Melnychenko, I.; Wasmeier, G.H.; Didié, M.; Naito, H.; Nixdorff, U.; Hess, A.; Budinsky, L.; Brune, K.; Michaelis, B.; et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006, 12, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Riegler, J.; Tiburcy, M.; Ebert, A.D.; Tzatzalos, E.; Raaz, U.; Abilez, O.J.; Shen, Q.; Kooreman, N.G.; Neofytou, E.; Chen, V.C.; et al. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ. Res. 2015, 117, 720–730. [Google Scholar] [CrossRef] [Green Version]
- Lesman, A.; Habib, M.; Caspi, O.; Gepstein, A.; Arbel, G.; Levenberg, S.; Gepstein, L. Transplantation of a Tissue-Engineered Human Vascularized Cardiac Muscle. Tissue Eng. Part A 2010, 16, 115–125. [Google Scholar] [CrossRef]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Hou, J.; Zheng, S.; Zheng, Z.; Hu, S. Vascularized Atrial Tissue Patch Cardiomyoplasty With Omentopexy Improves Cardiac Performance After Myocardial Infarction. Ann. Thorac. Surg. 2011, 92, 1435–1442. [Google Scholar] [CrossRef]
- Dvir, T.; Kedem, A.; Ruvinov, E.; Levy, O.; Freeman, I.; Landa, N.; Holbova, R.; Feinberg, M.S.; Dror, S.; Etzion, Y.; et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc. Natl. Acad. Sci. USA 2009, 106, 14990–14995. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, S.; Nakazawa, G.; Ohno, Y.; Ishihara, M.; Sakai, K.; Nakamura, N.; Murakami, T.; Natsumeda, M.; Kabuki, T.; Shibata, A.; et al. Efficacy of exogenous atrial natriuretic peptide in patients with heart failure with preserved ejection fraction: Deficiency of atrial natriuretic peptide and replacement therapy. ESC Heart Fail. 2020, 7, 4172–4181. [Google Scholar] [CrossRef]
- Fu, S.; Ping, P.; Wang, F.; Luo, L. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J. Biol. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Bhan, A.; Sirker, A.; Zhang, J.; Protti, A.; Catibog, N.; Driver, W.; Botnar, R.; Monaghan, M.J.; Shah, A.M. High-frequency speckle tracking echocardiography in the assessment of left ventricular function and remodeling after murine myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1371–H1383. [Google Scholar] [CrossRef] [Green Version]
- Hoit, B.D. Strain and Strain Rate Echocardiography and Coronary Artery Disease. Circ. Cardiovasc. Imaging 2011, 4, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Renier, N.; Wu, Z.; Simon, D.J.; Yang, J.; Ariel, P.; Tessier-Lavigne, M. iDISCO: A Simple, Rapid Method to Immunolabel Large Tissue Samples for Volume Imaging. Cell 2014, 159, 896–910. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leinonen, J.V.; Leinikka, P.; Tarkia, M.; Lampinen, M.; Emanuelov, A.K.; Beeri, R.; Kankuri, E.; Mervaala, E. Structural and Functional Support by Left Atrial Appendage Transplant to the Left Ventricle after a Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 4661. https://doi.org/10.3390/ijms23094661
Leinonen JV, Leinikka P, Tarkia M, Lampinen M, Emanuelov AK, Beeri R, Kankuri E, Mervaala E. Structural and Functional Support by Left Atrial Appendage Transplant to the Left Ventricle after a Myocardial Infarction. International Journal of Molecular Sciences. 2022; 23(9):4661. https://doi.org/10.3390/ijms23094661
Chicago/Turabian StyleLeinonen, Jussi V., Päivi Leinikka, Miikka Tarkia, Milla Lampinen, Avishag K. Emanuelov, Ronen Beeri, Esko Kankuri, and Eero Mervaala. 2022. "Structural and Functional Support by Left Atrial Appendage Transplant to the Left Ventricle after a Myocardial Infarction" International Journal of Molecular Sciences 23, no. 9: 4661. https://doi.org/10.3390/ijms23094661
APA StyleLeinonen, J. V., Leinikka, P., Tarkia, M., Lampinen, M., Emanuelov, A. K., Beeri, R., Kankuri, E., & Mervaala, E. (2022). Structural and Functional Support by Left Atrial Appendage Transplant to the Left Ventricle after a Myocardial Infarction. International Journal of Molecular Sciences, 23(9), 4661. https://doi.org/10.3390/ijms23094661





