Transcriptomic and Proteomic Analysis of CRISPR/Cas9-Mediated ARC-Knockout HEK293 Cells
Abstract
:1. Introduction
2. Results
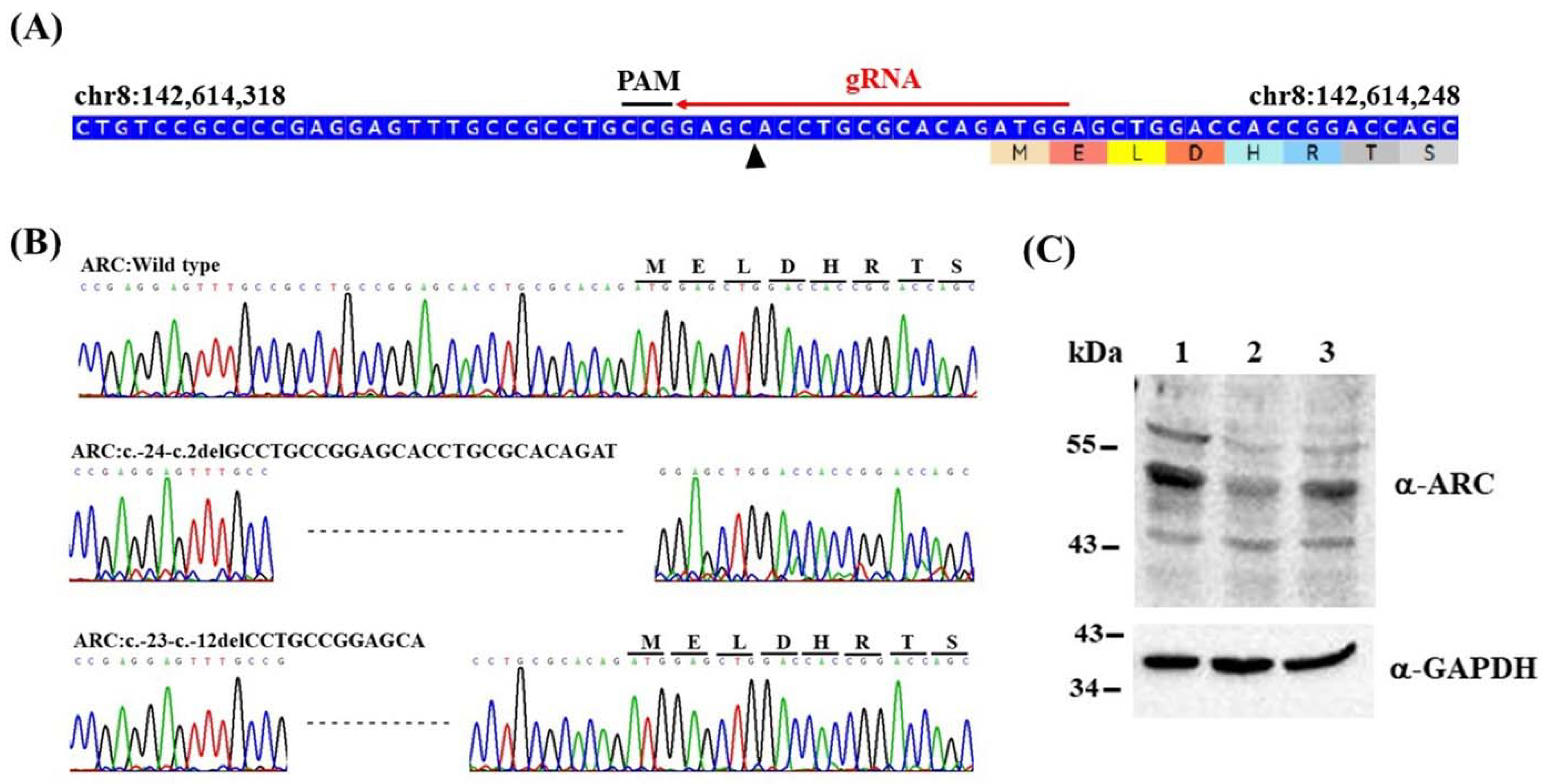
2.1. Generation of Isogenic ARC-KO HEK293 Cell Lines via CRISPR/Cas9 Editing
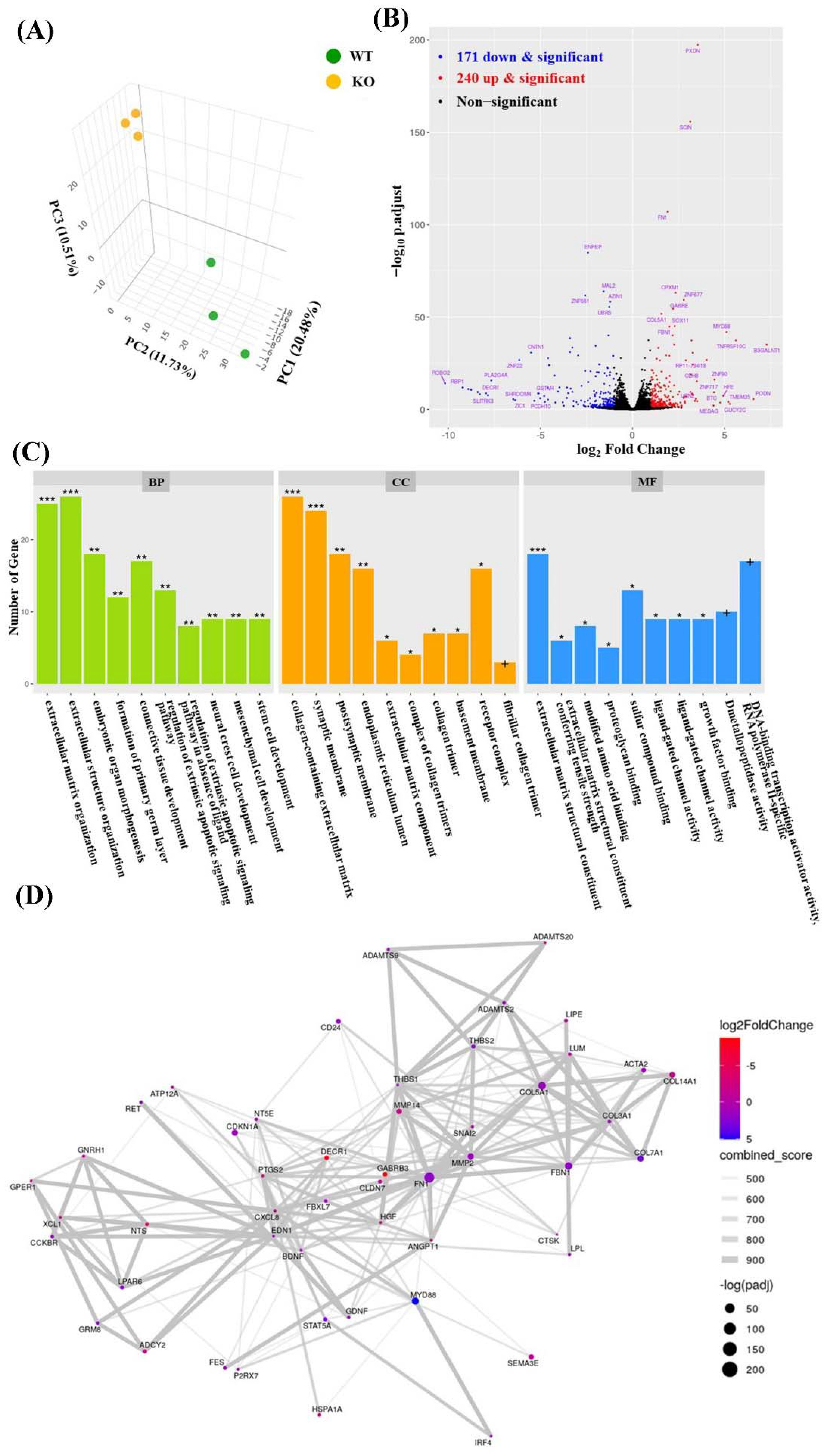
2.2. RNA Sequencing of the ARC-KO HEK293 Cell Line
2.3. Proteomes of ARC-KO HEK293 Cells Identified Using Label-Free Mass Spectrometry-Based Shotgun Proteomics
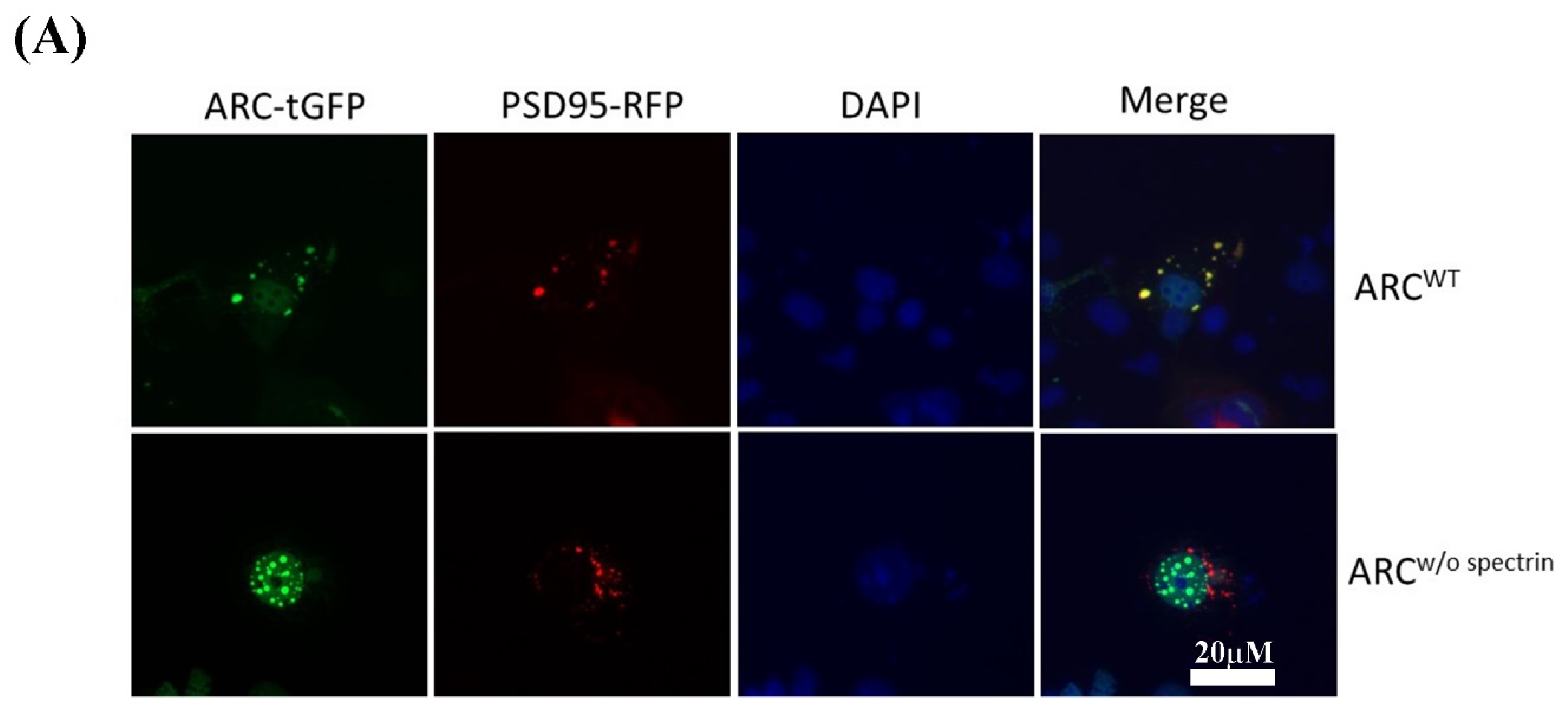
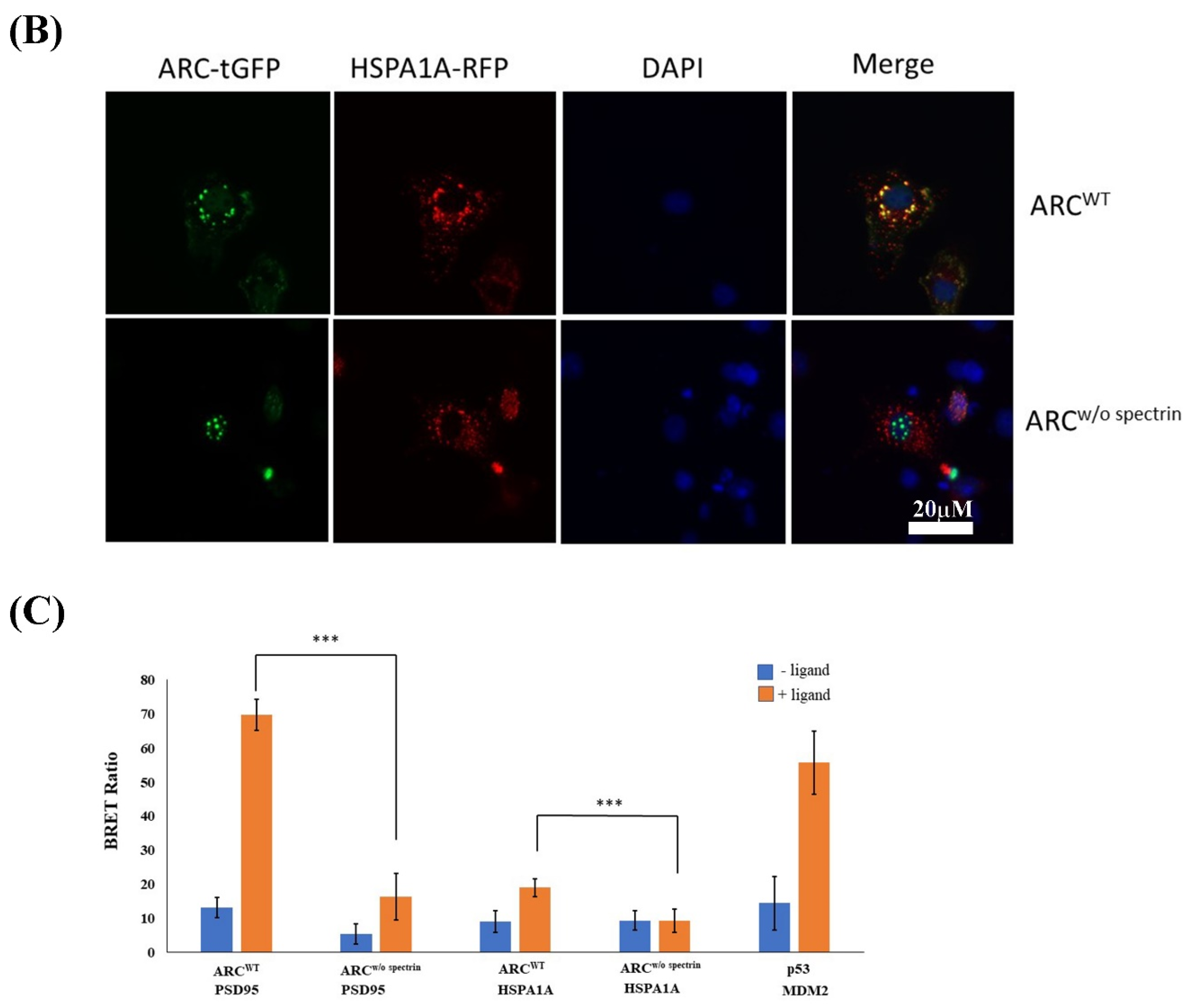
2.4. Immunocytochemistry and Protein–Protein Interactions
3. Discussion
3.1. Genetic Deletion of the Human ARC Gene Disturbs Several Signaling Pathways
3.2. ECM Abnormalities in ARC-KO HEK293 Cells
3.3. Synaptic Membrane Genes in ARC-KO HEK293 Cells
3.4. Interaction between ARC and HSPA1A
3.5. Limitations
4. Materials and Methods
4.1. CRISPR/Cas9-Directed Genome Editing of Isogenic HEK293 Cell Lines
4.2. Single CRISPR/Cas9-Edited Cell Isolation
4.3. Total RNA Preparation, Transcriptome Sequencing, and RT-qPCR
4.4. Protein Sample Preparation and LC-MS/MS Analysis
4.5. Immunoblotting Assay
4.6. Construction of Expression Plasmids, Transfection, and Immunocytochemistry
4.7. BRET Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lyford, G.L.; Yamagata, K.; Kaufmann, W.E.; Barnes, C.A.; Sanders, L.K.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Lanahan, A.A.; Worley, P.F. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 1995, 14, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Link, W.; Konietzko, U.; Kauselmann, G.; Krug, M.; Schwanke, B.; Frey, U.; Kuhl, D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc. Natl. Acad. Sci. USA 1995, 92, 5734–5738. [Google Scholar] [CrossRef] [Green Version]
- Bramham, C.R.; Alme, M.N.; Bittins, M.; Kuipers, S.D.; Nair, R.R.; Pai, B.; Panja, D.; Schubert, M.; Soule, J.; Tiron, A.; et al. The Arc of synaptic memory. Exp. Brain Res. 2009, 200, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Nikolaienko, O.; Patil, S.; Eriksen, M.S.; Bramham, C.R. Arc protein: A flexible hub for synaptic plasticity and cognition. Semin. Cell Dev. Biol. 2018, 77, 33–42. [Google Scholar] [CrossRef]
- Steward, O.; Worley, P.F. Selective Targeting of Newly Synthesized Arc mRNA to Active Synapses Requires NMDA Receptor Activation. Neuron 2001, 30, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, J.; Rumbaugh, G.; Wu, J.; Chowdhury, S.; Plath, N.; Kuhl, D.; Huganir, R.L.; Worley, P.F. Arc/Arg3.1 Mediates Homeostatic Synaptic Scaling of AMPA Receptors. Neuron 2006, 52, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.; Shepherd, J.; Okuno, H.; Lyford, G.; Petralia, R.S.; Plath, N.; Kuhl, D.; Huganir, R.L.; Worley, P.F. Arc/Arg3.1 Interacts with the Endocytic Machinery to Regulate AMPA Receptor Trafficking. Neuron 2006, 52, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, E.; Kanhema, T.; Soulé, J.; Tiron, A.; Dagyte, G.; Da Silva, B.; Bramham, C.R. Sustained Arc/Arg3.1 Synthesis Controls Long-Term Potentiation Consolidation through Regulation of Local Actin Polymerization in the Dentate Gyrus In Vivo. J. Neurosci. 2007, 27, 10445–10455. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Chotiner, J.K.; Steward, O. Actin Polymerization and ERK Phosphorylation Are Required for Arc/Arg3.1 mRNA Targeting to Activated Synaptic Sites on Dendrites. J. Neurosci. 2007, 27, 9054–9067. [Google Scholar] [CrossRef] [Green Version]
- Plath, N.; Ohana, O.; Dammermann, B.; Errington, M.L.; Schmitz, D.; Gross, C.; Mao, X.; Engelsberg, A.; Mahlke, C.; Welzl, H.; et al. Arc/Arg3.1 Is Essential for the Consolidation of Synaptic Plasticity and Memories. Neuron 2006, 52, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Penrod, R.D.; Kumar, J.; Smith, L.N.; McCalley, D.; Nentwig, T.; Hughes, B.; Barry, G.; Glover, K.; Taniguchi, M.; Cowan, C.W. Activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) regulates anxiety- and novelty-related behaviors. Genes Brain Behav. 2019, 18, e12561. [Google Scholar] [CrossRef]
- Managò, F.; Mereu, M.; Mastwal, S.; Mastrogiacomo, R.; Scheggia, D.; Emanuele, M.; De Luca, M.A.; Weinberger, D.R.; Wang, K.H.; Papaleo, F. Genetic Disruption of Arc/Arg3.1 in Mice Causes Alterations in Dopamine and Neurobehavioral Phenotypes Related to Schizophrenia. Cell Rep. 2016, 16, 2116–2128. [Google Scholar] [CrossRef] [Green Version]
- Kirov, G.; Pocklington, A.; Holmans, P.; Ivanov, D.; Ikeda, M.; Ruderfer, D.; Moran, J.; Chambert, K.; Toncheva, D.; Georgieva, L.; et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol. Psychiatry 2011, 17, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Guillozet-Bongaarts, A.L.; Hyde, T.M.; Dalley, R.A.; Hawrylycz, M.J.; Henry, A.; Hof, P.R.; Hohmann, J.; Jones, A.R.; Kuan, C.L.; Royall, J.; et al. Altered gene expression in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol. Psychiatry 2014, 19, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Alhowikan, A.M. Activity-Regulated Cytoskeleton-Associated Protein Dysfunction May Contribute to Memory Disorder and Earlier Detection of Autism Spectrum Disorders. Med. Princ. Pract. 2016, 25, 350–354. [Google Scholar] [CrossRef]
- Park, S.; Park, J.M.; Kim, S.; Kim, J.-A.; Shepherd, J.D.; Smith-Hicks, C.L.; Chowdhury, S.; Kaufmann, W.; Kuhl, D.; Ryazanov, A.G.; et al. Elongation Factor 2 and Fragile X Mental Retardation Protein Control the Dynamic Translation of Arc/Arg3.1 Essential for mGluR-LTD. Neuron 2008, 59, 70–83. [Google Scholar] [CrossRef] [Green Version]
- Landgren, S.; Von Otter, M.; Palmér, M.S.; Zetterström, C.; Nilsson, S.; Skoog, I.; Gustafson, D.R.; Minthon, L.; Wallin, A.; Andreasen, N.; et al. A novel ARC gene polymorphism is associated with reduced risk of Alzheimer’s disease. J. Neural Transm. 2012, 119, 833–842. [Google Scholar] [CrossRef]
- Purcell, S.M.; Moran, J.; Fromer, M.; Ruderfer, D.; Solovieff, N.; Roussos, P.; O’Dushlaine, C.; Chambert, K.; Bergen, S.; Kähler, A.; et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature 2014, 506, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Fromer, M.; Pocklington, A.J.; Kavanagh, D.H.; Williams, H.J.; Dwyer, S.; Gormley, P.; Georgieva, L.; Rees, E.; Palta, P.; Ruderfer, D.M.; et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 2014, 506, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Petralia, R.S.; Kurushima, H.; Patel, H.; Jung, M.-Y.; Volk, L.; Chowdhury, S.; Shepherd, J.; Dehoff, M.; Li, Y.; et al. Arc/Arg3.1 Regulates an Endosomal Pathway Essential for Activity-Dependent β-Amyloid Generation. Cell 2011, 147, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Okuno, H.; Akashi, K.; Ishii, Y.; Yagishita-Kyo, N.; Suzuki, K.; Nonaka, M.; Kawashima, T.; Fujii, H.; Takemoto-Kimura, S.; Abe, M.; et al. Inverse Synaptic Tagging of Inactive Synapses via Dynamic Interaction of Arc/Arg3.1 with CaMKIIβ. Cell 2012, 149, 886–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabb, A.; Je, H.S.; Wall, M.; Robinson, C.; Larsen, R.S.; Qiang, Y.; Corrêa, S.A.; Ehlers, M.D. Triad3A Regulates Synaptic Strength by Ubiquitination of Arc. Neuron 2014, 82, 1299–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, Y.-A.; Hu, T.-M.; Chen, C.-H.; Hsu, S.-H.; Tsai, H.-Y.; Cheng, M.-C. Rare mutations and hypermethylation of the ARC gene associated with schizophrenia. Schizophr. Res. 2016, 176, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rees, E.; Carrera, N.; Morgan, J.; Hambridge, K.; Escott-Price, V.; Pocklington, A.J.; Richards, A.L.; Pardiñas, A.F.; McDonald, C.; Donohoe, G.; et al. Targeted Sequencing of 10,198 Samples Confirms Abnormalities in Neuronal Activity and Implicates Voltage-Gated Sodium Channels in Schizophrenia Pathogenesis. Biol. Psychiatry 2019, 85, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Dityatev, A.; Schachner, M. The extracellular matrix and synapses. Cell Tissue Res. 2006, 326, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Pantazopoulos, H.; Katsel, P.; Haroutunian, V.; Chelini, G.; Klengel, T.; Berretta, S. Molecular signature of extracellular matrix pathology in schizophrenia. Eur. J. Neurosci. 2020, 53, 3960–3987. [Google Scholar] [CrossRef]
- Rodrigues-Amorim, D.; Rivera-Baltanás, T.; Fernández-Palleiro, P.; Iglesias-Martínez-Almeida, M.; Freiría-Martínez, L.; Jarmardo-Rodriguez, C.; Vallejo-Curto, M.D.C.; Álvarez-Ariza, M.; López-García, M.; Heras, E.D.L.; et al. Changes in the Brain Extracellular Matrix Composition in schizophrenia: A Pathophysiological Dysregulation and a Potential Therapeutic Target. Cell. Mol. Neurobiol. 2021, 1–12. [Google Scholar] [CrossRef]
- Berretta, S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology 2012, 62, 1584–1597. [Google Scholar] [CrossRef] [Green Version]
- Sethi, M.K.; Zaia, J. Extracellular matrix proteomics in schizophrenia and Alzheimer’s disease. Anal. Bioanal. Chem. 2016, 409, 379–394. [Google Scholar] [CrossRef] [Green Version]
- Tonge, D.A.; de Burgh, H.T.; Docherty, R.; Humphries, M.J.; Craig, S.E.; Pizzey, J. Fibronectin supports neurite outgrowth and axonal regeneration of adult brain neurons in vitro. Brain Res. 2012, 1453, 8–16. [Google Scholar] [CrossRef]
- Miyamae, Y.; Nakamura, Y.; Kashiwagi, Y.; Tanaka, T.; Kudo, T.; Takeda, M. Altered adhesion efficiency and fibronectin content in fibroblasts from schizophrenic patients. Psychiatry Clin. Neurosci. 1998, 52, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Mahadik, S.P.; Mukherjee, S.; Wakade, C.G.; Laev, H.; Reddy, R.R.; Schnur, D.B. Decreased adhesiveness and altered cellular distribution of fibronectin in fibroblasts from schizophrenic patients. Psychiatry Res. 1994, 53, 87–97. [Google Scholar] [CrossRef]
- Peykov, S.; Berkel, S.; Schoen, M.; Weiss, K.M.; Degenhardt, F.; Strohmaier, J.; Weiss, B.; Proepper, C.; Schratt, G.; Nöthen, M.; et al. Identification and functional characterization of rare SHANK2 variants in schizophrenia. Mol. Psychiatry 2015, 20, 1489–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Lin, Y.; Takasaki, Y.; Wang, C.; Kimura, H.; Xing, J.; Ishizuka, K.; Toyama, M.; Kushima, I.; Mori, D.; et al. Rare loss of function mutations in N-methyl-d-aspartate glutamate receptors and their contributions to schizophrenia susceptibility. Transl. Psychiatry 2018, 8, 12. [Google Scholar] [CrossRef]
- Frajman, A.; Maggio, N.; Muler, I.; Haroutunian, V.; Katsel, P.; Yitzhaky, A.; Weiser, M.; Hertzberg, L. Gene expression meta-analysis reveals the down-regulation of three GABA receptor subunits in the superior temporal gyrus of patients with schizophrenia. Schizophr. Res. 2020, 220, 29–37. [Google Scholar] [CrossRef]
- Li, W.; Ju, K.; Li, Z.; He, K.; Chen, J.; Wang, Q.; Yang, B.; An, L.; Feng, G.; Sun, W.; et al. Significant association of GRM7 and GRM8 genes with schizophrenia and major depressive disorder in the Han Chinese population. Eur. Neuropsychopharmacol. 2016, 26, 136–146. [Google Scholar] [CrossRef]
- Fernández, E.; Collins, M.; Frank, R.; Zhu, F.; Kopanitsa, M.V.; Nithianantharajah, J.; Lemprière, S.; Fricker, D.; Elsegood, K.A.; McLaughlin, C.; et al. Arc Requires PSD95 for Assembly into Postsynaptic Complexes Involved with Neural Dysfunction and Intelligence. Cell Rep. 2017, 21, 679–691. [Google Scholar] [CrossRef]
- Kiang, J.G.; Tsokos, G.C. Heat shock protein 70 kda: Molecular biology, biochemistry, and physiology. Pharmacol. Ther. 1998, 80, 183–201. [Google Scholar] [CrossRef]
- Bechtold, D.A.; Rush, S.J.; Brown, I.R. Localization of the Heat-Shock Protein Hsp70 to the Synapse Following Hyperthermic Stress in the Brain. J. Neurochem. 2001, 74, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Karunanithi, S.; Barclay, J.W.; Brown, I.R.; Robertson, M.; Atwood, H.L. Enhancement of presynaptic performance in transgenicDrosophila overexpressing heat shock protein Hsp70. Synapse 2002, 44, 8–14. [Google Scholar] [CrossRef]
- Park, A.Y.; Park, Y.S.; So, D.; Song, I.-K.; Choi, J.-E.; Kim, H.-J.; Lee, K.-J. Activity-Regulated Cytoskeleton-Associated Protein (Arc/Arg3.1) is Transiently Expressed after Heat Shock Stress and Suppresses Heat Shock Factor. Sci. Rep. 2019, 9, 2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamne, M.; Wood, J.; Chowdari, K.; Watson, A.M.; Celik, C.; Mansour, H.; Klei, L.; Gur, R.C.; Bradford, L.D.; Calkins, M.E.; et al. Evaluation of HLA Polymorphisms in Relation to Schizophrenia Risk and Infectious Exposure. Schizophr. Bull. 2012, 38, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Lee, S.J.; Toh, K.Y.; Lee, C.U.; Lee, C.; Paik, I.H. Identification of antibodies to heat shock proteins 90 kDa and 70 kDa in patients with schizophrenia. Schizophr. Res. 2001, 52, 127–135. [Google Scholar] [CrossRef]
- Schwarz, M.J.; Riedel, M.; Gruber, R.; Ackenheil, M.; Müller, N. Antibodies to heat shock proteins in schizophrenic patients: Implications for the mechanism of the disease. Am. J. Psychiatry 1999, 156, 1103–1104. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Kucia, K.; Owczarek, A.; Suchanek-Raif, R.; Merk, W.; Paul-Samojedny, M.; Kowalski, J. Association Studies of HSPA1A and HSPA1L Gene Polymorphisms with Schizophrenia. Arch. Med. Res. 2018, 49, 342–349. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Owczarek, A.; Suchanek, R.; Paul-Samojedny, M.; Fila-Danilow, A.; Borkowska, P.; Kucia, K.; Kowalski, J. Heat shock protein 70 gene polymorphisms are associated with paranoid schizophrenia in the Polish population. Cell Stress Chaperones 2013, 19, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.J.; Mandelli, L.; Lim, S.; Lim, H.K.; Kwon, O.J.; Pae, C.U.; Serretti, A.; Nimgaonkar, V.L.; Paik, I.-H.; Jun, T.-Y. Association analysis of heat shock protein 70 gene polymorphisms in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 239–244. [Google Scholar] [CrossRef]
- Pae, C.-U.; Drago, A.; Kim, J.-J.; Mandelli, L.; De Ronchi, D.; Serretti, A. The Impact of Heat Shock Protein 70 Gene Variations on Clinical Presentation and Outcome in Schizophrenic Inpatients. Neuropsychobiology 2009, 59, 135–141. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Kucia, K.; Owczarek, A.; Suchanek-Raif, R.; Merk, W.; Fila-Danilow, A.; Paul-Samojedny, M.; Choręza, P.; Kowalski, J. Association of HSPA1B Polymorphisms with Paranoid Schizophrenia in a Polish Population. NeuroMol. Med. 2020, 22, 159–169. [Google Scholar] [CrossRef] [Green Version]


| Ontology | GO Accession | GO Term | Gene Count | Gene IDs | Adjusted p-Value |
|---|---|---|---|---|---|
| MF | GO:0005201 | extracellular matrix structural constituent | 18 | COL19A1, SRPX, SRPX2, COL7A1, FN1, COL21A1, PXDN, COL5A1, THBS1, LUM, ECM1, SBSPON, FBN1, COL3A1, PODN, THBS2, COL14A1, ZP3 | 2.89 × 10−6 |
| CC | GO:0062023 | collagen-containing extracellular matrix | 26 | COL19A1, ADAMTS2, MMP2, SRPX, SRPX2, SMOC2, COL7A1, FN1, COL21A1, PXDN, COL5A1, THBS1, LUM, ECM1, FLG, LAD1, ADAMTS9, SBSPON, FBN1, COL3A1, ADAMTS20, PODN, THBS2, COL14A1, ZP3, L1CAM | 7.29 × 10−6 |
| BP | GO:0030198 | extracellular matrix organization | 25 | COL19A1, ADAMTS2, MMP2, ICAM2, NR2E1, SMOC2, COL7A1, FN1, PXDN, COL5A1, SPINK5, THBS1, LUM, CTSK, SCUBE3, GAS2, MMP14, CREB3L1, ELF3, ADAMTS9, FBN1, COL3A1, ADAMTS20, FOXC2, COL14A1 | 7.29 × 10−6 |
| BP | GO:0043062 | extracellular structure organization | 26 | COL19A1, ADAMTS2, MMP2, ICAM2, NR2E1, SMOC2, COL7A1, FN1, PXDN, COL5A1, SPINK5, THBS1, LUM, CTSK, SCUBE3, GAS2, MMP14, CREB3L1, ELF3, ADAMTS9, FBN1, COL3A1, ADAMTS20, LPL, FOXC2, COL14A1 | 2.13 × 10−5 |
| CC | GO:0097060 | synaptic membrane | 24 | CNTN1, LRRC7, LZTS1, SYT1, GABRE, SRPX2, DRP2, LIN7A, SLITRK3, SSPN, SYT11, CHRM3, FAIM2, CDH8, LHFPL4, GRIN2C, SHANK2, GPER1, BAALC, GABRB3, KCNJ4, NSG1, GRM8, GABRQ | 0.000229 |
| Ontology | GO Accession | GO Term | Protein Count | Protein ID | Benjamini p |
|---|---|---|---|---|---|
| CC | GO:0005829 | cytosol | 54 | OGFOD1, HMGCS1, GFUS, LSM1, RAD23A, SKP1, SEC31A, SET, UAP1, GALE, VPS29, ALDH1B1, ANXA1, ANXA5, ASNS, CAPN1, CAPZA2, CA2, CSE1L, CLTC, CPNE3, CAND1, CDK5, DCTPP1, ENO1, ENO2, EIF4G3, FSCN1, FABP5, FLNC, HSPA1A, HSPH1, PPA1, LONP1, MSN, MYH9, NUTF2, NUDT5, PGM3, PREP, PSMB3, PSMB6, PSMD5, PTMA, RPS18, RPS4X, RPLP1, RPLP2, SNRPD1, TLN1, TXNRD1, TUBB, YWHAB, VCP | 1.06 × 10−14 |
| CC | GO:0070062 | extracellular exosome | 34 | GFUS, ANXA1, ANXA5, ANXA6, CAPN1, CAPZA2, CA2, CSE1L, CLTC, CPNE3, CAND1, ENO1, ENO2, FSCN1, FABP5, GLA, HSPA1A, HSPH1, PPA1, MSN, MYH9, NUTF2, NUDT5, PSMB3, PSMB6, RPS18, RPS4X, RPLP2, SERPINB1, TLN1, TXNRD1, TUBB, YWHAB, VCP | 5.61 × 10−12 |
| CC | GO:0005925 | focal adhesion | 16 | ANXA1, ANXA5, ANXA6, CAPN1, CLTC, CPNE3, FLNC, HSPA1A, MSN, MYH9, RPS18, RPS4X, RPLP1, RPLP2, TLN1, YWHAB | 1.17 × 10−9 |
| CC | GO:0005737 | cytoplasm | 41 | OGFOD1, HMGCS1, DDX39A, LSM1, RAD23A, SKP1, SEC31A, SET, ANXA1, ANXA5, ANXA6, CAPN1, CA2, CSE1L, CPSF6, CPNE3, CAND1, CDK5, ENO1, FSCN1, FABP5, FLNC, GLA, HSPA1A, HSPH1, HINT2, PPA1, ISOC1, MSN, MYH9, NOLC1, OAT, PREP, PSMB3, PSMB6, TLN1, TXNRD1, TUBB2B, TUBB, YWHAB, VCP | 6.06 × 10−6 |
| CC | GO:0005654 | nucleoplasm | 32 | OGFOD1, DDX39A, RAD23A, SKP1, SET, UAP1, ALDH1B1, ANXA1, CSE1L, CPSF6, CPNE3, CAND1, CDK5, FABP5, HSPA1A, HSPH1, LONP, NUTF2, NOLC1, OAT, PUF60, PSMB3, PSMB6, PSMD5, PTMA, RPS18, RPS4X, SNRPD1, SF3A3, TXNRD1, TRIM28, VCP | 7.81 × 10−5 |
| MF | GO:0003723 | RNA binding | 20 | DDX39A, LSM1, CLTC, CPSF6, CPNE3, ENO1, EIF4G3, FSCN1, HSPA1A, MYH9, NOLC1, PUF60, RPS18, RPS4X, SERPINH, SNRPD1, SLC25A5, SF3A3, TRIM28, VCP | 2.92 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-Y.; Hsu, S.-H.; Tsai, H.-Y.; Cheng, F.-Y.; Cheng, M.-C. Transcriptomic and Proteomic Analysis of CRISPR/Cas9-Mediated ARC-Knockout HEK293 Cells. Int. J. Mol. Sci. 2022, 23, 4498. https://doi.org/10.3390/ijms23094498
Wang Y-Y, Hsu S-H, Tsai H-Y, Cheng F-Y, Cheng M-C. Transcriptomic and Proteomic Analysis of CRISPR/Cas9-Mediated ARC-Knockout HEK293 Cells. International Journal of Molecular Sciences. 2022; 23(9):4498. https://doi.org/10.3390/ijms23094498
Chicago/Turabian StyleWang, Yu-Yuan, Shih-Hsin Hsu, Hsin-Yao Tsai, Fu-Yu Cheng, and Min-Chih Cheng. 2022. "Transcriptomic and Proteomic Analysis of CRISPR/Cas9-Mediated ARC-Knockout HEK293 Cells" International Journal of Molecular Sciences 23, no. 9: 4498. https://doi.org/10.3390/ijms23094498
APA StyleWang, Y.-Y., Hsu, S.-H., Tsai, H.-Y., Cheng, F.-Y., & Cheng, M.-C. (2022). Transcriptomic and Proteomic Analysis of CRISPR/Cas9-Mediated ARC-Knockout HEK293 Cells. International Journal of Molecular Sciences, 23(9), 4498. https://doi.org/10.3390/ijms23094498






