Cellular Model of Malignant Transformation of Primary Human Astrocytes Induced by Deadhesion/Readhesion Cycles
Abstract
1. Introduction
2. Results
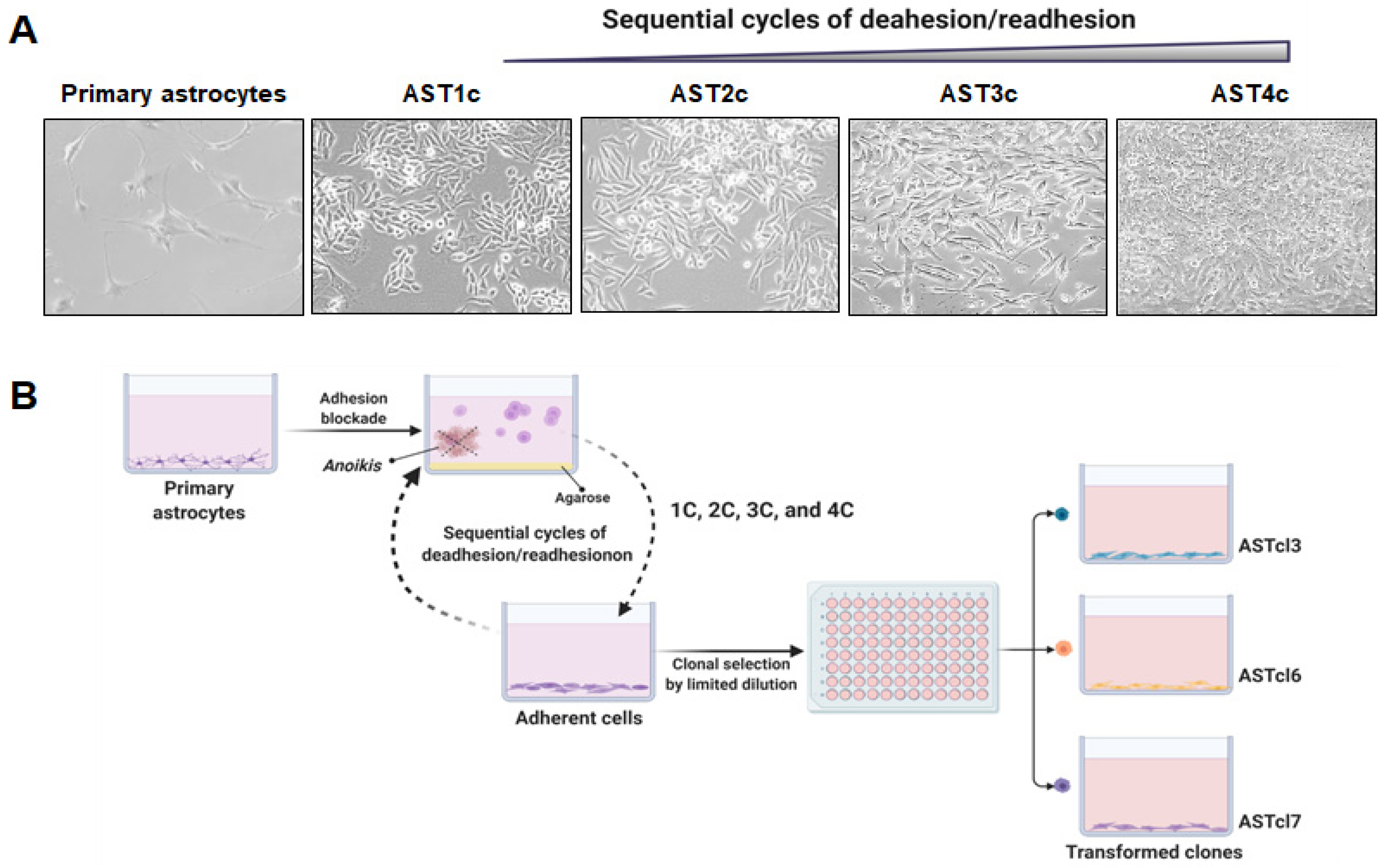
2.1. Malignant Astrocyte Transformation
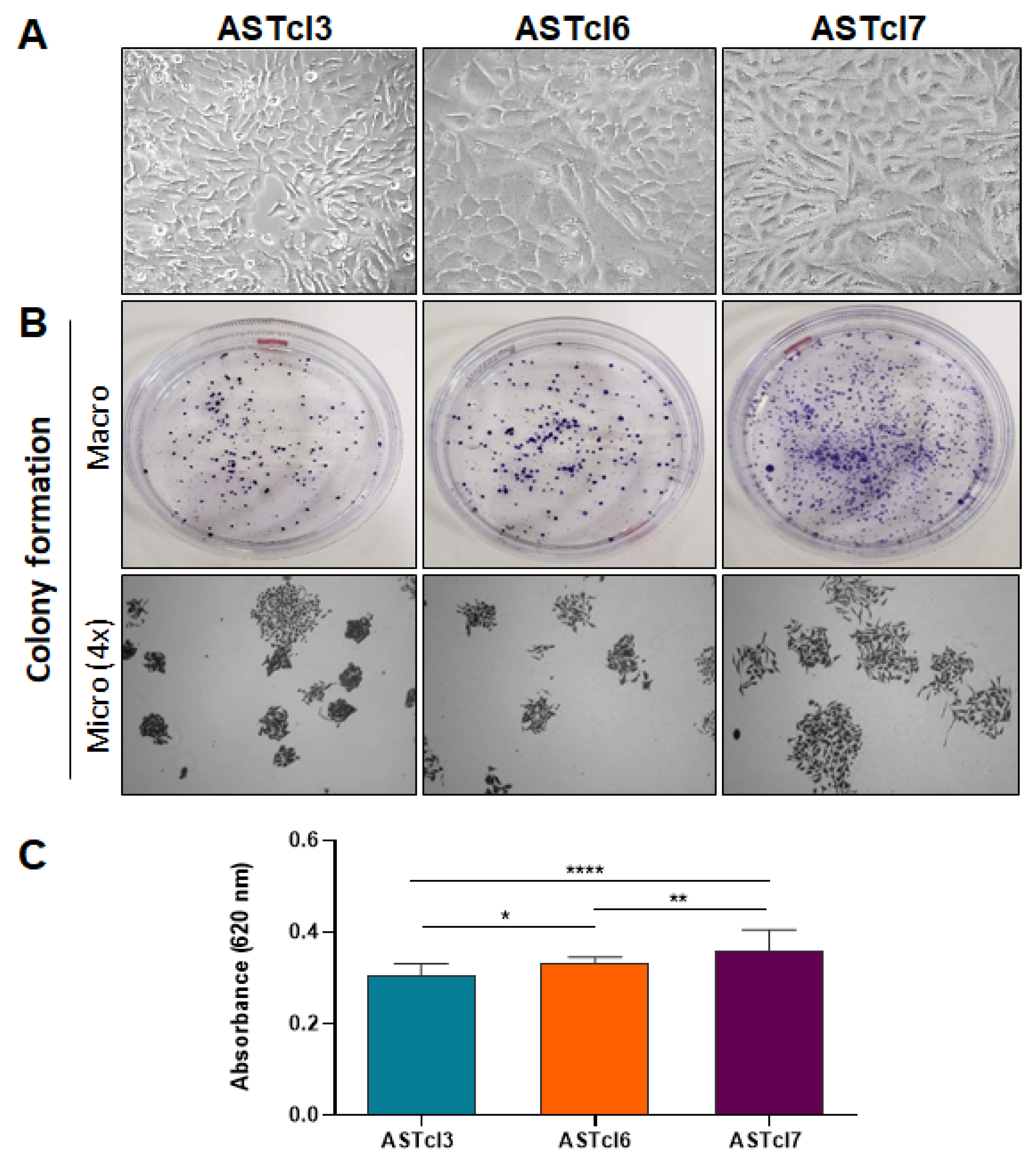
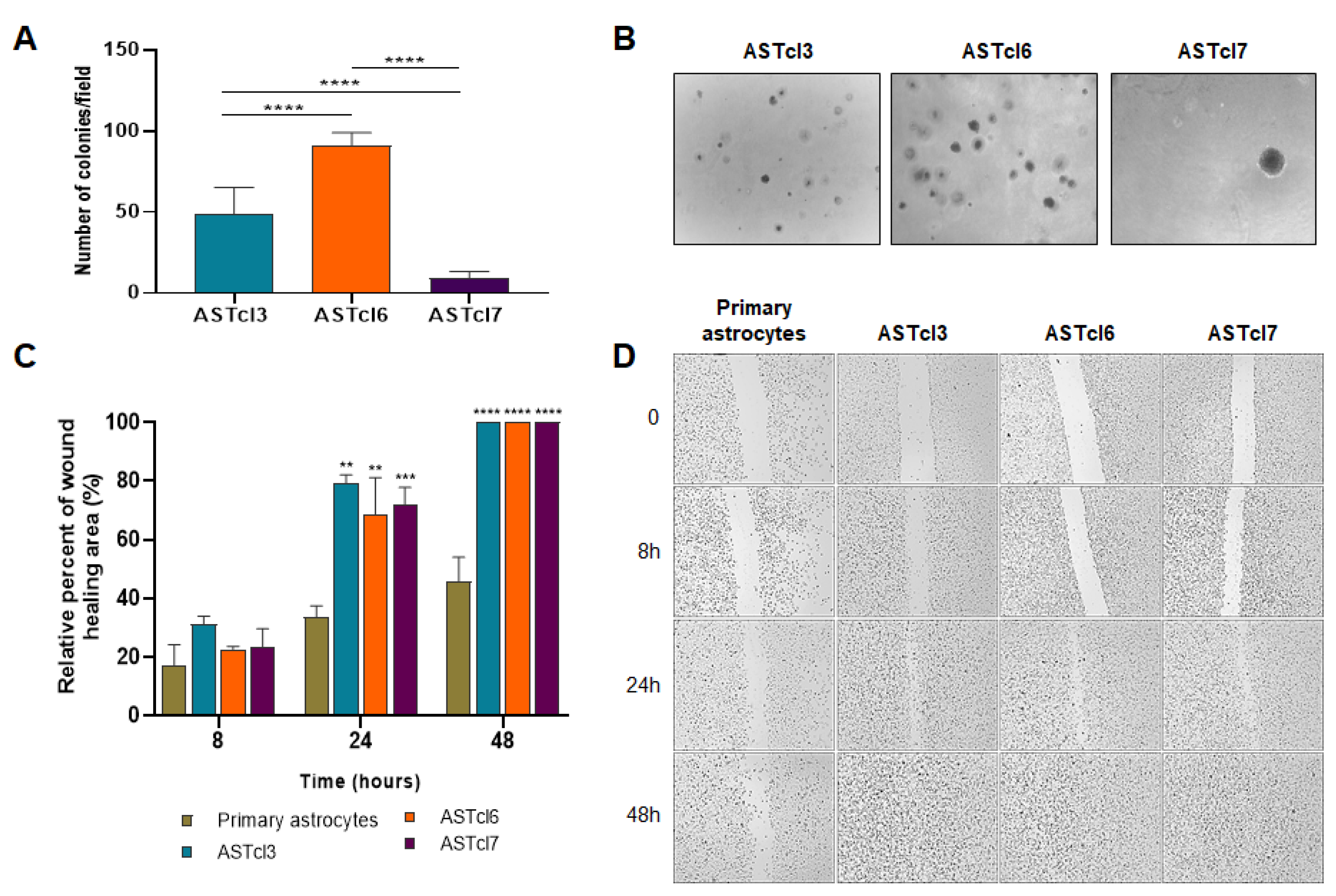
2.2. Astrocyte-Derived Clones Acquired Tumor Cell Traits
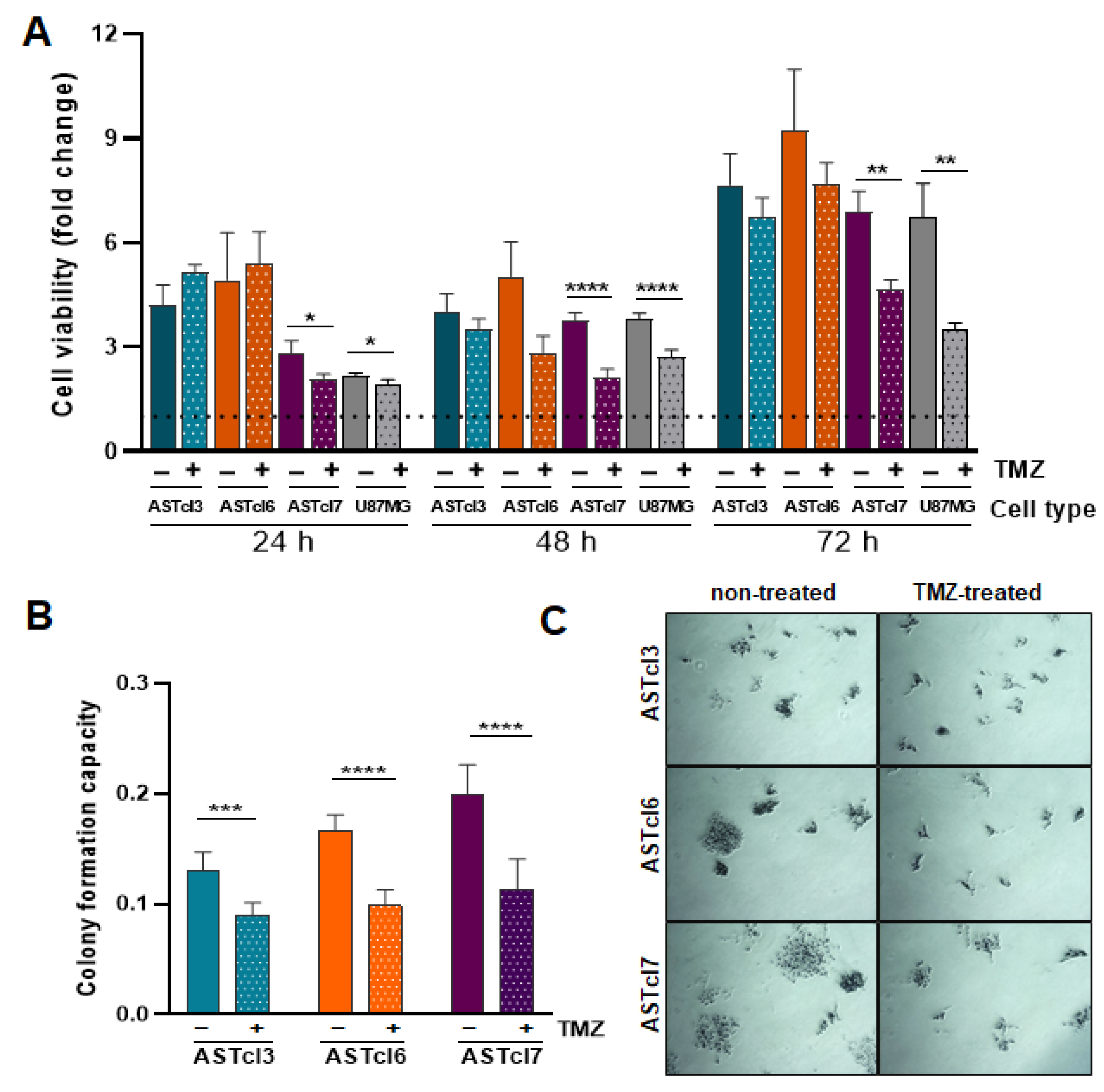
2.3. Astrocyte-Derived Clones’ Response to Temozolomide Treatment Proved Similar to That of U87MG Cells, with Decreased Cell Viability and Clonogenic Capacity
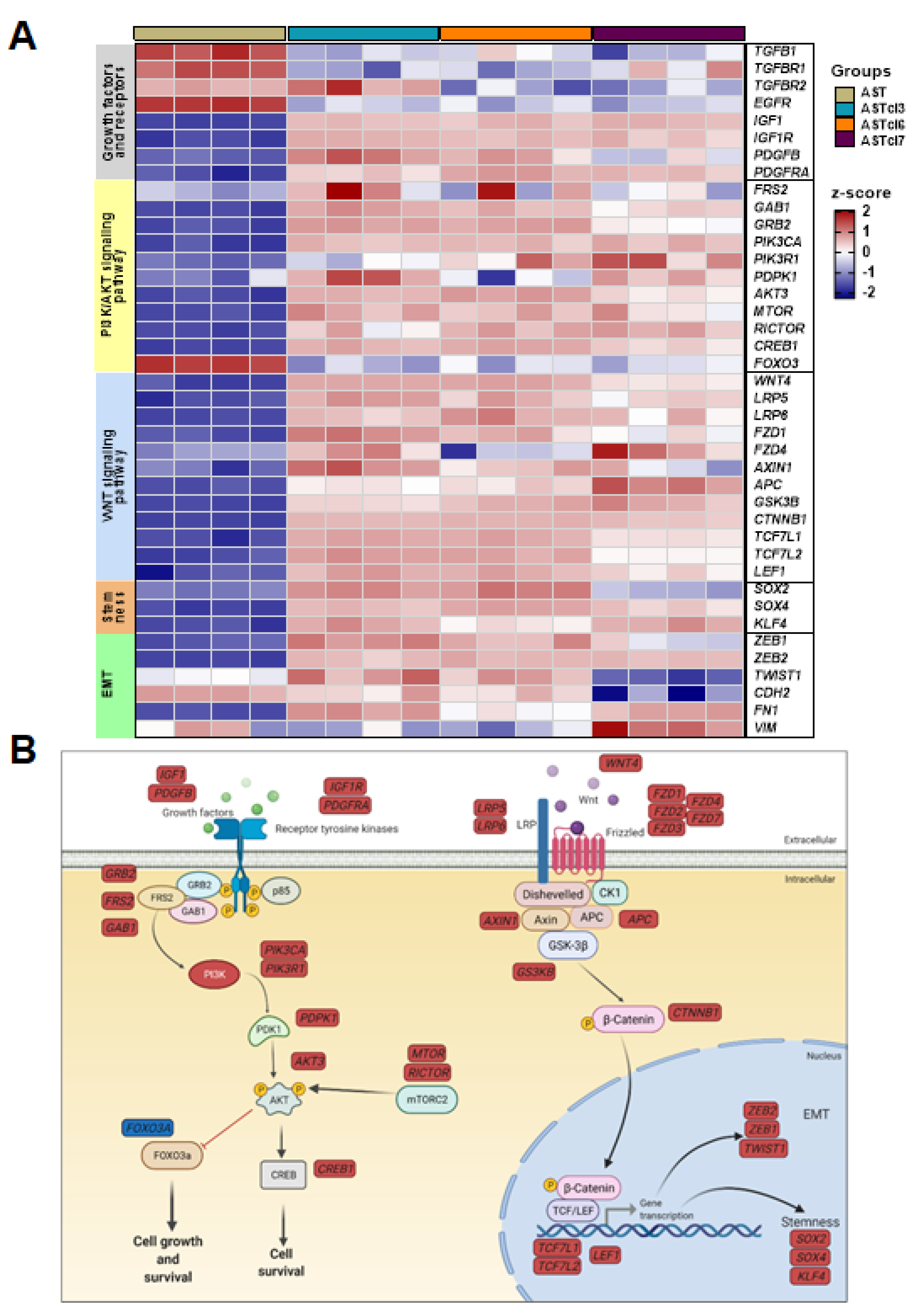
2.4. Astrocyte-Derived Cell Lines Presented Activation of PI3K/AKT and Wnt Signaling Pathways, and Downregulation of Genes Related to Aerobic Respiration
2.5. Astrocyte-Derived Cell Lines Exhibited Increased GFAP and PDGFRA Protein Expression
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Immunofluorescence Analysis
4.3. Cell Adhesion Blockade (Anoikis Resistance)
4.4. DNA and RNA Extraction
4.5. Complementary DNA Synthesis by Reverse Transcription
4.6. DNA and RNA Extraction
4.7. Acquired TMZ Sensitivity
4.8. Colony Formation Assay
4.9. Cell Viability
4.10. Wound-Healing Assay
4.11. High-Throughput Sequencing for Transcriptome Analysis
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyon, K.A.; Allen, N.J. From Synapses to Circuits, Astrocytes Regulate Behavior. Front. Neural Circuits 2022, 15, 786293. [Google Scholar] [CrossRef] [PubMed]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.G.; Karimi-Abdolrezaee, S. Recent insights on astrocyte mechanisms in CNS homeostasis, pathology, and repair. J. Neurosci. Res. 2021, 99, 2427–2462. [Google Scholar] [CrossRef] [PubMed]
- Esemen, Y.; Awan, M.; Parwez, R.; Baig, A.; Rahman, S.; Masala, I.; Franchini, S.; Giakoumettis, D. Molecular Pathogenesis of Glioblastoma in Adults and Future Perspectives: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 2607. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23, iii1–iii105. [Google Scholar] [CrossRef]
- Lee, C.Y. Strategies of temozolomide in future glioblastoma treatment. OncoTargets Ther. 2017, 10, 265–270. [Google Scholar] [CrossRef]
- Coleman, W.B.; Tsongalis, G.J. Molecular mechanisms of human carcinogenesis. Cancer Cell Struct. Carcinog. Genom. Instab. 2006, 96, 321–349. [Google Scholar] [CrossRef]
- Oba-Shinjo, S.M.; Correa, M.; Ricca, T.I.; Molognoni, F.; Pinhal, M.A.; Neves, I.A.; Marie, S.K.; Sampaio, L.O.; Nader, H.B.; Chammas, R.; et al. Melanocyte transformation associated with substrate adhesion impediment. Neoplasia 2006, 8, 231–241. [Google Scholar] [CrossRef]
- Frisch, S.M.; Screaton, R.A. Anoikis mechanisms. Curr. Opin. Cell Biol. 2001, 13, 555–562. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta 2013, 1833, 3481–3498. [Google Scholar] [CrossRef]
- Balani, S.; Nguyen, L.V.; Eaves, C.J. Modeling the process of human tumorigenesis. Nat. Commun. 2017, 8, 15422. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.; Dongre, H.; Singh, A.K.; Joshi, S.; Costea, D.E.; Mahadik, S.; Ahire, C.; Makani, V.; Dange, P.; Sharma, S.; et al. Establishment of 3D Co-Culture Models from Different Stages of Human Tongue Tumorigenesis: Utility in Understanding Neoplastic Progression. PLoS ONE 2016, 11, e0160615. [Google Scholar] [CrossRef] [PubMed]
- Chekhonin, I.V.; Chistiakov, D.A.; Grinenko, N.F.; Gurina, O.I. Glioma Cell and Astrocyte Co-cultures As a Model to Study Tumor-Tissue Interactions: A Review of Methods. Cell Mol. Neurobiol. 2018, 38, 1179–1195. [Google Scholar] [CrossRef] [PubMed]
- Kronemberger, G.S.; Miranda, G.; Tavares, R.S.N.; Montenegro, B.; Kopke, U.D.; Baptista, L.S. Recapitulating Tumorigenesis in vitro: Opportunities and Challenges of 3D Bioprinting. Front. Bioeng. Biotechnol. 2021, 9, 682498. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.C.; Flaherty, S.J.; Somasundaram, V.; Scheiblin, D.A.; Lockett, S.J.; Wink, D.A.; Heinz, W.F. An in vitro tumorigenesis model based on live-cell-generated oxygen and nutrient gradients. Commun. Biol. 2021, 4, 477. [Google Scholar] [CrossRef]
- Chiarugi, P.; Giannoni, E. Anoikis: A necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 2008, 76, 1352–1364. [Google Scholar] [CrossRef]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Tomlinson, I.P. Mutations in normal breast tissue and breast tumours. Breast Cancer Res. 2001, 3, 299–303. [Google Scholar] [CrossRef][Green Version]
- Nørøxe, D.S.; Poulsen, H.S.; Lassen, U. Hallmarks of glioblastoma: A systematic review. ESMO Open 2017, 1, e000144. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Gil del Alcazar, C.R.; Hardebeck, M.C.; Mukherjee, B.; Tomimatsu, N.; Gao, X.; Yan, J.; Xie, X.J.; Bachoo, R.; Li, L.; Habib, A.A.; et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin. Cancer Res. 2014, 20, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Tchokonte-Nana, V.; Lotfi, M.; Lotfi, M.; Ghorbani, A.; Sadeghnia, H.R. Wnt/beta-catenin and PI3K/Akt/mTOR Signaling Pathways in Glioblastoma: Two Main Targets for Drug Design: A Review. Curr. Pharm. Des. 2020, 26, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Agani, F.; Jiang, B.H. Oxygen-independent regulation of HIF-1: Novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr. Cancer Drug Targets 2013, 13, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.H.; Jain, S.; Aghi, M.K. Metabolic Drivers of Invasion in Glioblastoma. Front. Cell Dev. Biol. 2021, 9, 683276. [Google Scholar] [CrossRef]
- Kodama, M.; Nakayama, K.I. A second Warburg-like effect in cancer metabolism: The metabolic shift of glutamine-derived nitrogen: A shift in glutamine-derived nitrogen metabolism from glutaminolysis to de novo nucleotide biosynthesis contributes to malignant evolution of cancer. BioEssays 2020, 42, e2000169. [Google Scholar] [CrossRef]
- Yang, R.; Wang, M.; Zhang, G.; Li, Y.; Wang, L.; Cui, H. POU2F2 regulates glycolytic reprogramming and glioblastoma progression via PDPK1-dependent activation of PI3K/AKT/mTOR pathway. Cell Death Dis. 2021, 12, 433. [Google Scholar] [CrossRef]
- Daniel, P.M.; Filiz, G.; Tymms, M.J.; Ramsay, R.G.; Kaye, A.H.; Stylli, S.S.; Mantamadiotis, T. Intratumor MAPK and PI3K signaling pathway heterogeneity in glioblastoma tissue correlates with CREB signaling and distinct target gene signatures. Exp. Mol. Pathol. 2018, 105, 23–31. [Google Scholar] [CrossRef]
- Chen, Y.F.; Pandey, S.; Day, C.H.; Chen, Y.F.; Jiang, A.Z.; Ho, T.J.; Chen, R.J.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. Synergistic effect of HIF-1α and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J. Cell Physiol. 2018, 233, 3660–3671. [Google Scholar] [CrossRef]
- Takebe, N.; Harris, P.J.; Warren, R.Q.; Ivy, S.P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 2011, 8, 97–106. [Google Scholar] [CrossRef]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Mikheeva, S.A.; Mikheev, A.M.; Petit, A.; Beyer, R.; Oxford, R.G.; Khorasani, L.; Maxwell, J.P.; Glackin, C.A.; Wakimoto, H.; González-Herrero, I.; et al. TWIST1 promotes invasion through mesenchymal change in human glioblastoma. Mol. Cancer 2010, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Song, Y.; Yokomizo, A.; Kiyoshima, K.; Tada, Y.; Uchino, H.; Uchiumi, T.; Inokuchi, J.; Oda, Y.; Kuroiwa, K.; et al. Foxo3a suppression of urothelial cancer invasiveness through Twist1, Y-box-binding protein 1, and E-cadherin regulation. Clin. Cancer Res. 2010, 16, 5654–5663. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Song, Y.; Peng, Y.; Wang, H.; Long, H.; Yu, X.; Li, Z.; Fang, L.; Wu, A.; Luo, W.; et al. ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion, and apoptosis in glioma. PLoS ONE 2012, 7, e38842. [Google Scholar] [CrossRef] [PubMed]
- Vivinetto, A.L.; Cave, J.W. Zeb2 directs EMT-like processes that underlies the glial response to injury. Neural Reg. Res. 2021, 16, 1788–1790. [Google Scholar] [CrossRef]
- Epifanova, E.; Babaev, A.; Newman, A.G.; Tarabykin, V. Role of Zeb2/Sip1 in neuronal development. Brain Res. 2019, 1705, 24–31. [Google Scholar] [CrossRef]
- Iwadate, Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol. Lett. 2016, 11, 1615–1620. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, H.; Li, H.; Zhang, J.; Wang, S. Roles of sex-determining region Y-box 2 in cell pluripotency and tumor-related signaling pathways. Mol. Clin. Oncol. 2015, 3, 1203–1207. [Google Scholar] [CrossRef]
- Feng, R.; Wen, J. Overview of the roles of Sox2 in stem cell and development. Biol. Chem. 2015, 396, 883–891. [Google Scholar] [CrossRef]
- Pouremamali, F.; Vahedian, V.; Hassani, N.; Mirzaei, S.; Pouremamali, A.; Kazemzadeh, H.; Faridvand, Y.; Jafari-Gharabaghlou, D.; Nouri, M.; Maroufi, N.F. The role of SOX family in cancer stem cell maintenance: With a focus on SOX2. Pathol. Res. Pract. 2022, 231, 153783. [Google Scholar] [CrossRef]
- Serres, E.; Debarbieux, F.; Stanchi, F.; Maggiorella, L.; Grall, D.; Turchi, L.; Burel-Vandenbos, F.; Figarella-Branger, D.; Virolle, T.; Rougon, G.; et al. Fibronectin expression in glioblastomas promotes cell cohesion, collective invasion of basement membrane in vitro and orthotopic tumor growth in mice. Oncogene 2014, 33, 3451–3462. [Google Scholar] [CrossRef] [PubMed]
- Trombetta-Lima, M.; Rosa-Fernandes, L.; Angeli, C.B.; Moretti, I.F.; Franco, Y.M.; Mousessian, A.S.; Wakamatsu, A.; Lerario, A.M.; Oba-Shinjo, S.M.; Pasqualucci, C.A.; et al. Extracellular Matrix Proteome Remodeling in Human Glioblastoma and Medulloblastoma. J. Proteome Res. 2021, 20, 4693–4707. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.X.; Zhang, Z.P.; Zhao, J.; Liu, J.P. Effects of Fibronectin 1 on Cell Proliferation, Senescence and Apoptosis of Human Glioma Cells Through the PI3K/AKT Signaling Pathway. Cell Physiol. Biochem. 2018, 48, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Xue, Y.; Liu, J.; Xi, Z.; Li, Z.; Liu, Y. Fibronectin Promotes the Malignancy of Glioma Stem-Like Cells Via Modulation of Cell Adhesion, Differentiation, Proliferation and Chemoresistance. Ront. Mol. Neurosci. 2018, 11, 130. [Google Scholar] [CrossRef]
- Shyh-Chang, N.; Ng, H.H. The metabolic programming of stem cells. Genes Dev. 2017, 31, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Duan, Y.; Li, Y.; Sun, Y.; Sun, H.; Yu, X.; Gao, X.; Zhang, C.; Zhang, H.; et al. Metabolic Regulation: A Potential Strategy for Rescuing Stem Cell Senescence. Stem Cell Rev. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, V.; Tomassoni, D.; Avitabile, M.; Amenta, F.; Avola, R. Biomarkers of glial cell proliferation and differentiation in culture. Front. Biosci. 2010, 2, 558–570. [Google Scholar] [CrossRef]
- Rodnight, R.; Gonçalves, C.A.; Wofchuk, S.T.; Leal, R. Control of the phosphorylation of the astrocyte marker glial fibrillary acidic protein (GFAP) in the immature rat hippocampus by glutamate and calcium ions: Possible key factor in astrocytic plasticity. Braz. J. Med. Biol. Res. 1997, 30, 325–338. [Google Scholar] [CrossRef]
- Wlodarczyk, A.; Grot, D.; Stoczynska-Fidelus, E.; Rieske, P. Gaps and Doubts in Search to Recognize Glioblastoma Cellular Origin and Tumor Initiating Cells. J. Oncol. 2020, 2020, 6783627. [Google Scholar] [CrossRef]
- Sommerlath, V.N.; Buergy, D.; Etminan, N.; Brehmer, S.; Reuss, D.; Sarria, G.R.; Guiot, M.C.; Hänggi, D.; Wenz, F.; Petrecca, K.; et al. Molecular features of glioblastomas in long-term survivors compared to short-term survivors—A matched-pair analysis. Radiat. Oncol. 2022, 17, 15. [Google Scholar] [CrossRef]
- Barzegar Behrooz, A.; Talaie, Z.; Jusheghani, F.; Łos, M.J.; Klonisch, T.; Ghavami, S. Wnt and PI3K/Akt/mTOR Survival Pathways as Therapeutic Targets in Glioblastoma. Int. J. Mol. Sci. 2022, 23, 1353. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Saito, T. The Role of Nephronectin on Proliferation and Differentiation in Human Dental Pulp Stem Cells. Stem Cells Int. 2017, 2017, 2546261. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.P.S.; Dias, A.A.M. ImageJ macros for the user-friendly analysis of soft-agar and wound-healing assays. BioTechniques 2017, 62, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Gingeras, T.R. Optimizing RNA-Seq Mapping with STAR. Methods Mol. Biol. 2016, 1415, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da S. Soares, R.; de S. Laurentino, T.; da Silva, C.T.; Gonçalves, J.D.; Lerario, A.M.; Marie, S.K.N.; Oba-Shinjo, S.M.; Jasiulionis, M.G. Cellular Model of Malignant Transformation of Primary Human Astrocytes Induced by Deadhesion/Readhesion Cycles. Int. J. Mol. Sci. 2022, 23, 4471. https://doi.org/10.3390/ijms23094471
da S. Soares R, de S. Laurentino T, da Silva CT, Gonçalves JD, Lerario AM, Marie SKN, Oba-Shinjo SM, Jasiulionis MG. Cellular Model of Malignant Transformation of Primary Human Astrocytes Induced by Deadhesion/Readhesion Cycles. International Journal of Molecular Sciences. 2022; 23(9):4471. https://doi.org/10.3390/ijms23094471
Chicago/Turabian Styleda S. Soares, Roseli, Talita de S. Laurentino, Camila T. da Silva, Jéssica D. Gonçalves, Antonio M. Lerario, Suely K. N. Marie, Sueli M. Oba-Shinjo, and Miriam G. Jasiulionis. 2022. "Cellular Model of Malignant Transformation of Primary Human Astrocytes Induced by Deadhesion/Readhesion Cycles" International Journal of Molecular Sciences 23, no. 9: 4471. https://doi.org/10.3390/ijms23094471
APA Styleda S. Soares, R., de S. Laurentino, T., da Silva, C. T., Gonçalves, J. D., Lerario, A. M., Marie, S. K. N., Oba-Shinjo, S. M., & Jasiulionis, M. G. (2022). Cellular Model of Malignant Transformation of Primary Human Astrocytes Induced by Deadhesion/Readhesion Cycles. International Journal of Molecular Sciences, 23(9), 4471. https://doi.org/10.3390/ijms23094471





