Neuronal and Brain Maturation
Funding
Conflicts of Interest
References
- Urbàn, N.; Guillemot, F. Neurogenesis in the embryonic and adult brain: Same regulators, different roles. Front. Cell. Neurosci. 2014, 8, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipp, H.-P.; Bonfanti, L. Adult Neurogenesis in Mammals: Variations and Confusions. Brain Behav. Evol. 2016, 87, 205–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, J.S. Recalibrating the Relevance of Adult Neurogenesis. Trends Neurosci. 2019, 42, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Bradke, F.; Di Giovanni, S.; Fawcett, J. Neuronal Maturation: Challenges and Opportunities in a Nascent Field. Trends Neurosci. 2020, 43, 360–362. [Google Scholar] [CrossRef]
- König, R.; Benedetti, B.; Rotheneichner, P.; O’ Sullivan, A.; Kreutzer, C.; Belles, M.; Nacher, J.; Weiger, T.M.; Aigner, L.; Couillard-Després, S. Distribution and Fate of DCX/PSA-NCAM Expressing Cells in the Adult Mammalian Cortex: A Local Reservoir for Adult Cortical Neuroplasticity? Front. Biol. 2016, 11, 193–213. [Google Scholar] [CrossRef]
- Bonfanti, L.; Seki, T. The PSA-NCAM-Positive “Immature” Neurons: An Old Discovery Providing New Vistas on Brain Structural Plasticity. Cells 2021, 10, 2542. [Google Scholar] [CrossRef]
- Bonfanti, L.; Charvet, C.J. Brain Plasticity in Humans and Model Systems: Advances, Challenges, and Future Directions. Int. J. Mol. Sci. 2021, 22, 9358. [Google Scholar] [CrossRef]
- Benedetti, B.; Dannehl, D.; Janssen, J.M.; Corcelli, C.; Couillard-Després, S.; Engelhardt, M. Structural and Functional Maturation of Rat Primary Motor Cortex Layer V Neurons. Int. J. Mol. Sci. 2020, 21, 6101. [Google Scholar] [CrossRef]
- Coviello, S.; Benedetti, B.; Jakubecova, D.; Belles, M.; Klimczak, P.; Gramuntell, Y.; Couillard-Despres, S.; Nacher, J. PSA Depletion Induces the Differentiation of Immature Neurons in the Piriform Cortex of Adult Mice. Int. J. Mol. Sci. 2021, 22, 5733. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Paredes, M. Implications of Extended Inhibitory Neuron Development. Int. J. Mol. Sci. 2021, 22, 5113. [Google Scholar] [CrossRef]
- Annamneedi, A.; del Angel, M.; Gundelfinger, E.; Stork, O.; Çalışkan, G. The Presynaptic Scaffold Protein Bassoon in Forebrain Excitatory Neurons Mediates Hippocampal Circuit Maturation: Potential Involvement of TrkB Signalling. Int. J. Mol. Sci. 2021, 22, 7944. [Google Scholar] [CrossRef] [PubMed]
- Chareyron, L.J.; Banta Lavenex, P.; Amaral, D.G.; Lavenex, P. Life and Death of Immature Neurons in the Juvenile and Adult Primate Amygdala. Int. J. Mol. Sci. 2021, 22, 6691. [Google Scholar] [CrossRef] [PubMed]
- Zorzin, S.; Corsi, A.; Ciarpella, F.; Bottani, E.; Dolci, S.; Malpeli, G.; Pino, A.; Amenta, A.; Fumagalli, G.F.; Chiamulera, C.; et al. Environmental Enrichment Induces Meningeal Niche Remodeling through TrkB-Mediated Signaling. Int. J. Mol. Sci. 2021, 22, 10657. [Google Scholar] [CrossRef] [PubMed]
- Gribaudo, S.; Saraulli, D.; Nato, G.; Bonzano, S.; Gambarotta, G.; Luzzati, F.; Costanzi, M.; Peretto, P.; Bovetti, S.; De Marchis, S. Neurogranin Regulates Adult-Born Olfactory Granule Cell Spine Density and Odor-Reward Associative Memory in Mice. Int. J. Mol. Sci. 2021, 22, 4269. [Google Scholar] [CrossRef] [PubMed]
- Butt, U.; Hassouna, I.; Garcia-Agudo, L.F.; Steixner-Kumar, A.; Depp, C.; Barnkothe, N.; Zillmann, M.; Ronnenberg, A.; Bonet, V.; Goebbels, S.; et al. CaMKIIα Expressing Neurons to Report Activity-Related Endogenous Hypoxia upon Motor-Cognitive Challenge. Int. J. Mol. Sci. 2021, 22, 3164. [Google Scholar] [CrossRef]
- Gostomska-Pampuch, K.; Drulis-Fajdasz, D.; Gizak, A.; Wiśniewski, J.; Rakus, D. Absolute Proteome Analysis of Hippocampus, Cortex and Cerebellum in Aged and Young Mice Reveals Changes in Energy Metabolism. Int. J. Mol. Sci. 2021, 22, 6188. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, D.; Luhmann, H.; Kirischuk, S. Effects of Mutations in TSC Genes on Neurodevelopment and Synaptic Transmission. Int. J. Mol. Sci. 2021, 22, 7273. [Google Scholar] [CrossRef]
- Trova, S.; Bovetti, S.; Bonzano, S.; De Marchis, S.; Peretto, P. Sex Steroids and the Shaping of the Peripubertal Brain: The Sexual-Dimorphic Set-Up of Adult Neurogenesis. Int. J. Mol. Sci. 2021, 22, 7984. [Google Scholar] [CrossRef]
- Limoni, G. Modelling and Refining Neuronal Circuits with Guidance Cues: Involvement of Semaphorins. Int. J. Mol. Sci. 2021, 22, 6111. [Google Scholar] [CrossRef]
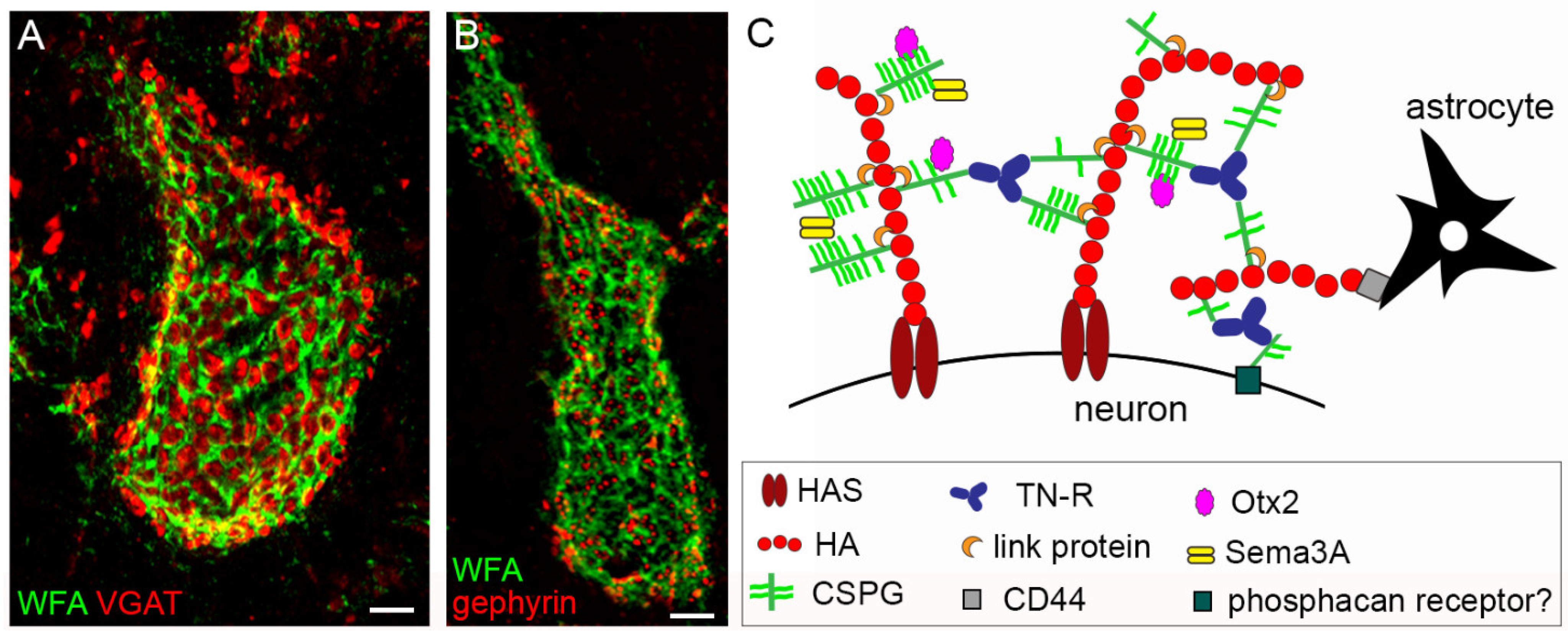
- Carulli, D.; Verhaagen, J. An Extracellular Perspective on CNS Maturation: Perineuronal Nets and the Control of Plasticity. Int. J. Mol. Sci. 2021, 22, 2434. [Google Scholar] [CrossRef]
- Semenov, M. Proliferative Capacity of Adult Mouse Brain. Int. J. Mol. Sci. 2021, 22, 3449. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonfanti, L.; Couillard-Després, S. Neuronal and Brain Maturation. Int. J. Mol. Sci. 2022, 23, 4400. https://doi.org/10.3390/ijms23084400
Bonfanti L, Couillard-Després S. Neuronal and Brain Maturation. International Journal of Molecular Sciences. 2022; 23(8):4400. https://doi.org/10.3390/ijms23084400
Chicago/Turabian StyleBonfanti, Luca, and Sébastien Couillard-Després. 2022. "Neuronal and Brain Maturation" International Journal of Molecular Sciences 23, no. 8: 4400. https://doi.org/10.3390/ijms23084400
APA StyleBonfanti, L., & Couillard-Després, S. (2022). Neuronal and Brain Maturation. International Journal of Molecular Sciences, 23(8), 4400. https://doi.org/10.3390/ijms23084400





