Application of Ultrasound Combined with Microbubbles for Cancer Therapy
Abstract
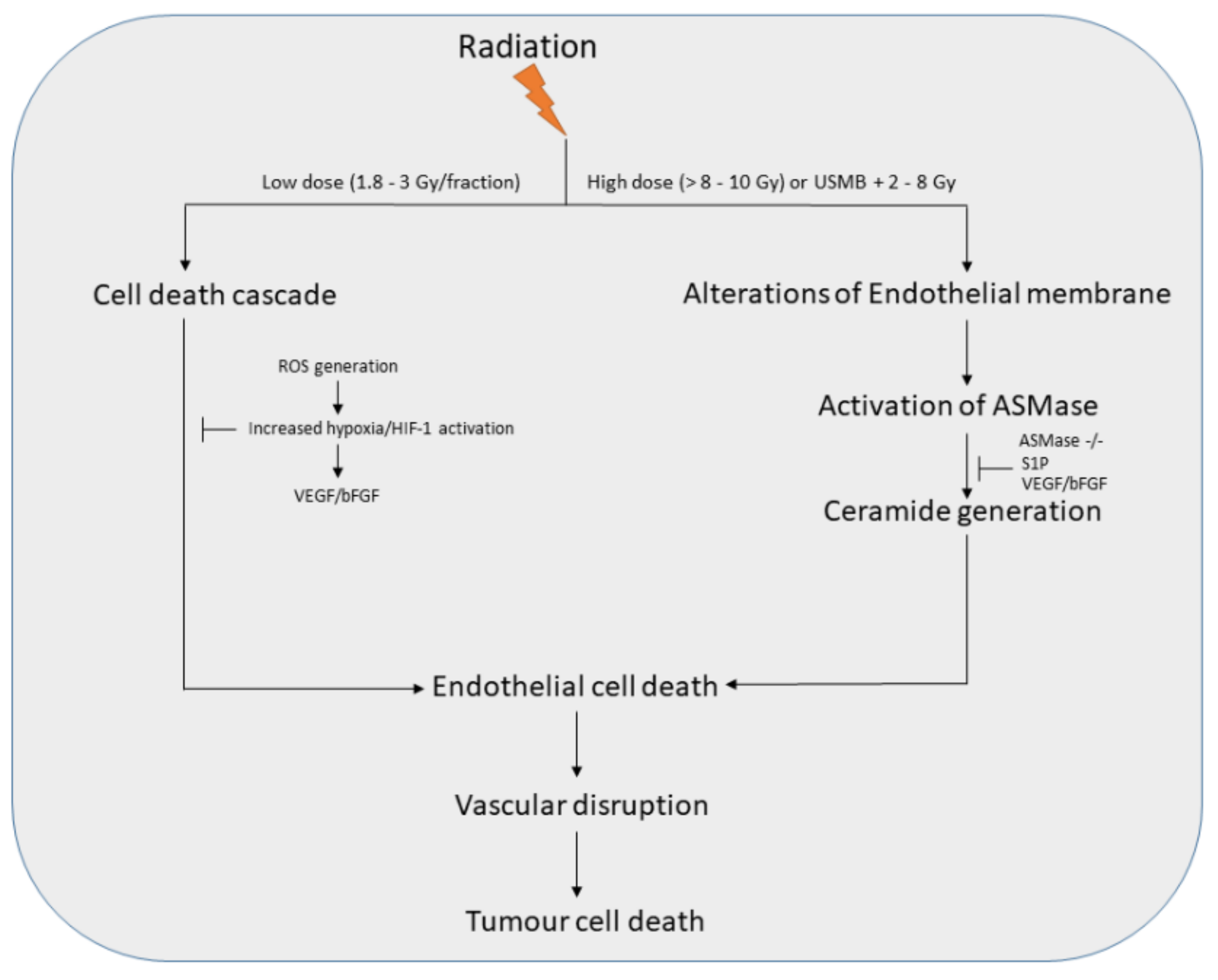
:1. Introduction
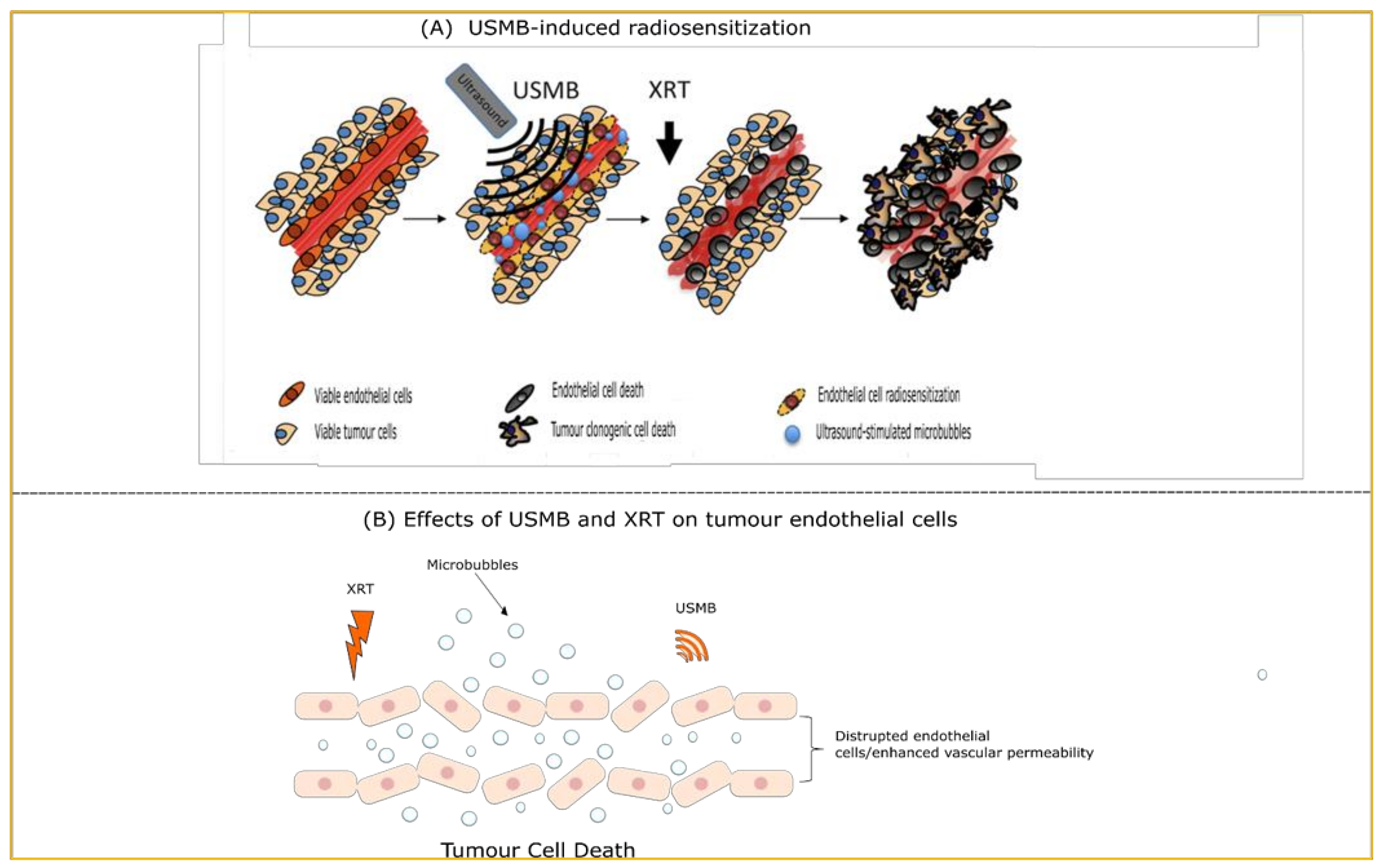
2. USMB and Radiotherapy
3. USMB and Chemotherapy
4. Ultrasound-Stimulated Microbubbles-Mediated Drug Delivery
4.1. Co-Administration of Microbubbles and Chemotherapeutic Drugs
4.2. Drug-Loaded Microbubbles Combined with Ultrasound
5. Effect of USMB and Chemotherapy on 3D Cultures (Spheroids)
6. USMB-Mediated BBB Disruption for Targeted Drug Delivery
7. Clinical Applications of USMB
7.1. USMB and Radiotherapy
7.2. USMB and Chemotherapy
8. Conclusions and Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernot, S.; Klibanov, A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1153–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stride, E.P.; Coussios, C.C. Cavitation and contrast: The use of bubbles in ultrasound imaging and therapy. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2009, 224, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, F.; Fokong, S.; Koczera, P.; Lederle, W.; Lammers, T. Ultrasound Microbubbles for Molecular Diagnosis, Therapy, and Theranostics. J. Nucl. Med. 2012, 53, 345–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, S.M.; Lee, T.; Willmann, J.K. Ultrasound-guided drug delivery in cancer. Ultrasonography 2017, 36, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, H.; Han, H.; Lee, M.; Lee, S.; Yoo, H.; Chang, J.H.; Kim, H. Microbubbles used for contrast enhanced ultrasound and theragnosis: A review of principles to applications. Biomed. Eng. Lett. 2017, 7, 59–69. [Google Scholar] [CrossRef]
- Dijkmans, P.; Juffermans, L.; Musters, R.; van Wamel, A.; Cate, F.T.; van Gilst, W.; Visser, C.; De Jong, N.; Kamp, O. Microbubbles and ultrasound: From diagnosis to therapy. Eur. J. Echocardiogr. 2004, 5, 245–256. [Google Scholar] [CrossRef]
- Lee, P.J.H.; Matkar, P.N.; Kuliszewski, M.A.; Leong-Poi, H. Ultrasound-targeted cardiovascular gene therapy. In Cardiac Regeneration and Repair; Woodhead Publishing: Sawston, UK, 2014; pp. 380–407. [Google Scholar] [CrossRef]
- Sirsi, S.; Borden, M. Microbubble Compositions, Properties and Biomedical Applications. Bubble Sci. Eng. Technol. 2010, 1, 3–17. [Google Scholar] [CrossRef]
- Chong, W.K.; Papadopoulou, V.; Dayton, P.A. Imaging with ultrasound contrast agents: Current status and future. Abdom. Radiol. 2018, 43, 762–772. [Google Scholar] [CrossRef]
- Al-Jawadi, S.; Thakur, S.S. Ultrasound-responsive lipid microbubbles for drug delivery: A review of preparation techniques to optimise formulation size, stability and drug loading. Int. J. Pharm. 2020, 585, 119559. [Google Scholar] [CrossRef]
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble Agents: New Directions. Ultrasound Med. Biol. 2020, 46, 1326–1343. [Google Scholar] [CrossRef]
- Lacerda, Q.; Tantawi, M.; Leeper, D.B.; Wheatley, M.A.; Eisenbrey, J.R. Emerging Applications of Ultrasound-Contrast Agents in Radiation Therapy. Ultrasound Med. Biol. 2021, 47, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Ma, Y.; Guo, Y.; Zhang, C.; Guo, X.; Tu, J.; Yu, A.C.; Wu, J.; Zhang, D. Enhanced porosity and permeability of three-dimensional alginate scaffolds via acoustic microstreaming induced by low-intensity pulsed ultrasound. Ultrason. Sonochem. 2017, 37, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Neppiras, E.A. Acoustic cavitation. Phys. Rep. 1980, 61, 159–251. [Google Scholar] [CrossRef]
- Leighton, T. Bubble population phenomena in acoustic cavitation. Ultrason. Sonochem. 1995, 2, S123–S136. [Google Scholar] [CrossRef]
- Ibsen, S.; Schutt, C.E.; Esener, S. Microbubble-mediated ultrasound therapy: A review of its potential in cancer treatment. Drug Des. Dev. Ther. 2013, 7, 375. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Giles, A.; Hashim, A.; Yip, J.; Ji, Y.; Do, N.N.A.; Sebastiani, J.; Tran, W.T.; Farhat, G.; Oelze, M.; et al. Ultrasound microbubble potentiated enhancement of hyperthermia-effect in tumours. PLoS ONE 2019, 14, e0226475. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Cartar, H.; Law, N.; Giles, A.; Farhat, G.; Oelze, M.; Czarnota, G.J. Optimization of microbubble enhancement of hyperthermia for cancer therapy in an in vivo breast tumour model. PLoS ONE 2020, 15, e0237372. [Google Scholar] [CrossRef]
- Snipstad, S.; Vikedal, K.; Maardalen, M.; Kurbatskaya, A.; Sulheim, E.; Davies, C.D.L. Ultrasound and microbubbles to beat barriers in tumors: Improving delivery of nanomedicine. Adv. Drug Deliv. Rev. 2021, 177, 113847. [Google Scholar] [CrossRef]
- Tharkar, P.; Varanasi, R.; Wong, W.S.F.; Jin, C.; Chrzanowski, W. Nano-Enhanced Drug Delivery and Therapeutic Ultrasound for Cancer Treatment and Beyond. Front. Bioeng. Biotechnol. 2019, 7, 324. [Google Scholar] [CrossRef] [Green Version]
- Czarnota, G.J.; Karshafian, R.; Burns, P.N.; Wong, S.; Al Mahrouki, A.; Lee, J.W.; Caissie, A.; Tran, W.; Kim, C.; Furukawa, M.; et al. Tumor radiation response enhancement by acoustical stimulation of the vasculature. Proc. Natl. Acad. Sci. USA 2012, 109, E2033–E2041. [Google Scholar] [CrossRef] [Green Version]
- Al-Mahrouki, A.A.; Iradji, S.; Tran, W.T.; Czarnota, G.J. Cellular characterization of ultrasound-stimulated microbubble radiation enhancement in a prostate cancer xenograft model. DMM Dis. Model. Mech. 2014, 7, 363–372. [Google Scholar] [PubMed] [Green Version]
- El Kaffas, A.; Al-Mahrouki, A.; Hashim, A.; Law, N.; Giles, A.; Czarnota, G.J. Role of acid sphingomyelinase and ceramide in mechano-acoustic enhancement of tumor radiation responses. J. Natl. Cancer Inst. 2018, 110, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Tran, W.T.; Iradji, S.; Sofroni, E.; Giles, A.; Eddy, D.M.; Czarnota, G.J. Microbubble and ultrasound radioenhancement of bladder cancer. Br. J. Cancer 2012, 107, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, P.; Tarapacki, C.; Tran, W.T.; El Kaffas, A.; Lee, J.; Hupple, C.; Iradji, S.; Giles, A.; Al-Mahrouki, A.; Czarnota, G.J. Breast tumor response to ultrasound mediated excitation of microbubbles and radiation therapy in vivo. Oncoscience 2016, 3, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mahrouki, A.A.; Karshafian, R.; Giles, A.; Czarnota, G.J. Bioeffects of Ultrasound-Stimulated Microbubbles on Endothelial Cells: Gene Expression Changes Associated with Radiation Enhancement In Vitro. Ultrasound Med. Biol. 2012, 38, 1958–1969. [Google Scholar] [CrossRef]
- Park, J.; Aryal, M.; Vykhodtseva, N.; Zhang, Y.-Z.; McDannold, N. Evaluation of permeability, doxorubicin delivery, and drug retention in a rat brain tumor model after ultrasound-induced blood-tumor barrier disruption. J. Control. Release 2016, 250, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wan, J.M.; Yu, A.C.H. Membrane Perforation and Recovery Dynamics in Microbubble-Mediated Sonoporation. Ultrasound Med. Biol. 2013, 39, 2393–2405. [Google Scholar] [CrossRef]
- Fan‡, Z.; E Kumon‡, R.; Deng, C.X. Mechanisms of microbubble-facilitated sonoporation for drug and gene delivery. Ther. Deliv. 2014, 5, 467–486. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Hwang, J.H. Ultrasound-targeted microbubble destruction for chemotherapeutic drug delivery to solid tumors. J. Ther. Ultrasound 2013, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Meairs, S. Facilitation of Drug Transport across the Blood–Brain Barrier with Ultrasound and Microbubbles. Pharmaceutics 2015, 7, 275–293. [Google Scholar] [CrossRef] [Green Version]
- I Kovacs, Z.; Tu, T.-W.; Sundby, M.; Qureshi, F.; Lewis, B.K.; Jikaria, N.; Burks, S.R.; A Frank, J. MRI and histological evaluation of pulsed focused ultrasound and microbubbles treatment effects in the brain. Theranostics 2018, 8, 4837–4855. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343.e2. [Google Scholar] [CrossRef] [PubMed]
- Bredel, M. Anticancer drug resistance in primary human brain tumors. Brain Res. Rev. 2001, 35, 161–204. [Google Scholar] [CrossRef]
- Bredel, M.; Zentner, J. Brain-tumour drug resistance: The bare essentials. Lancet Oncol. 2002, 3, 397–406. [Google Scholar] [CrossRef]
- Regina, A.; Demeule, M.; Laplante, A.; Jodoin, J.; Dagenais, C.; Berthelet, F.; Moghrabi, A.; Béliveau, R. Multidrug Resistance in Brain Tumors: Roles of the Blood–brain Barrier. Cancer Metastasis Rev. 2001, 20, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Lalier, L.; Salaud, C.; Heymann, D.; Cartron, P.F.; Vallette, F.M. Drug resistance in glioblastoma: Are persisters the key to therapy? Cancer Drug Resist. 2020, 3, 287–301. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-T.; Wei, K.-C.; Liu, H.-L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front. Pharmacol. 2019, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, M.A.; Hynynen, K. Ultrasound enhanced drug delivery to the brain and central nervous system. Int. J. Hyperth. 2012, 28, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Reyngold, M.; Parikh, P.; Crane, C.H. Ablative radiation therapy for locally advanced pancreatic cancer: Techniques and results. Radiat. Oncol. 2019, 14, 95. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Santivasi, W.L.; Xia, F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid. Redox Signal. 2014, 21, 251–259. [Google Scholar] [CrossRef]
- Sancar, A.; Lindsey-Boltz, L.A.; Ünsal-Kaçmaz, K.; Linn, S. Molecular Mechanisms of Mammalian DNA Repair and the DNA Damage Checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitter, K.L.; Casey, D.L.; Lu, Y.C.; Hannum, M.; Zhang, Z.; Song, X.; Pecorari, I.; Mcmillan, B.; Ma, J.; Samstein, R.M.; et al. Pathogenic ATM Mutations in Cancer and a Genetic Basis for Radiotherapeutic Efficacy. J. Natl. Cancer Inst. 2021, 113, 266–273. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Schultz, C.W.; Azimi-Sadjadi, A.; Brody, J.R.; Pishvaian, M.J. ATM Dysfunction in Pancreatic Adenocarcinoma and Associated Therapeutic Implications. Mol. Cancer Ther. 2019, 18, 1899–1908. [Google Scholar] [CrossRef] [Green Version]
- Gachechiladze, M.; Skarda, J.; Bouchalova, K.; Soltermann, A.; Joerger, M. Predictive and Prognostic Value of DNA Damage Response Associated Kinases in Solid Tumors. Front. Oncol. 2020, 10, 2346. [Google Scholar] [CrossRef]
- Jette, N.R.; Kumar, M.; Radhamani, S.; Arthur, G.; Goutam, S.; Yip, S.; Kolinsky, M.; Williams, G.J.; Bose, P.; Lees-Miller, S.P. ATM-Deficient Cancers Provide New Opportunities for Precision Oncology. Cancers 2020, 12, 687. [Google Scholar] [CrossRef] [Green Version]
- Baschnagel, A.M.; Elnaggar, J.H.; VanBeek, H.J.; Kromke, A.C.; Skiba, J.H.; Kaushik, S.; Abel, L.; Clark, P.A.; Longhurst, C.A.; Nickel, K.P.; et al. ATR Inhibitor M6620 (VX-970) Enhances the Effect of Radiation in Non–Small Cell Lung Cancer Brain Metastasis Patient-Derived Xenografts. Mol. Cancer Ther. 2021, 20, 2129–2139. [Google Scholar] [CrossRef]
- Tang, S.; Li, Z.; Yang, L.; Shen, L.; Wang, Y. A potential new role of ATM inhibitor in radiotherapy: Suppressing ionizing Radiation-Activated EGFR. Int. J. Radiat. Biol. 2020, 96, 461–468. [Google Scholar] [CrossRef]
- Riches, L.C.; Trinidad, A.G.; Hughes, G.; Jones, G.N.; Hughes, A.M.; Thomason, A.G.; Gavine, P.; Cui, A.; Ling, S.; Stott, J.; et al. Pharmacology of the ATM inhibitor AZD0156: Potentiation of irradiation and olaparib responses pre-clinically. Mol. Cancer Ther. 2020, 19, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goon, P.; Lip, G.; Boos, C.; Stonelake, P.; Blann, A. Circulating Endothelial Cells, Endothelial Progenitor Cells, and Endothelial Microparticles in Cancer. Neoplasia 2006, 8, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial Apoptosis as the Primary Lesion Initiating Intestinal Radiation Damage in Mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef]
- Garcia-Barros, M.; Paris, F.; Cordon-Cardo, C.; Lyden, D.; Rafii, S.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Tumor Response to Radiotherapy Regulated by Endothelial Cell Apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef] [Green Version]
- Sathishkumar, S.; Boyanovski, B.; A Karakashian, A.; Rozenova, K.A.; Giltiay, N.V.; Kudrimoti, M.; Mohiuddin, M.; Ahmed, M.M.; Nikolova-Karakashian, M. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: Implications for endothelial apoptosis. Cancer Biol. Ther. 2005, 4, 979–986. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, H.; Lee, Y.-J.; Sonn, J.K.; Lim, Y.-B. Ionizing Radiation Regulates Vascular Endothelial Growth Factor-A Transcription in Cultured Human Vascular Endothelial Cells Via the PERK/eIF2α/ATF4 Pathway. Int. J. Radiat. Oncol. 2020, 107, 563–570. [Google Scholar] [CrossRef]
- Ferranti, C.S.; Cheng, J.; Thompson, C.; Zhang, J.; Rotolo, J.A.; Buddaseth, S.; Fuks, Z.; Kolesnick, R.N. Fusion of lysosomes to plasma membrane initiates radiation-induced apoptosis. J. Cell Biol. 2020, 219, e201903176. [Google Scholar] [CrossRef] [Green Version]
- Santana-Delgado, P.; A Peña, L.; Haimovitz-Friedman, A.; Martin, S.; Green, D.; McLoughlin, M.; Cordon-Cardo, C.; Schuchman, E.H.; Fuks, Z.; Kolesnick, R. Acid Sphingomyelinase–Deficient Human Lymphoblasts and Mice Are Defective in Radiation-Induced Apoptosis. Cell 1996, 86, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Peña, L.A.; Fuks, Z.; Kolesnick, R.N. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000, 60, 321–327. [Google Scholar] [PubMed]
- Fuks, Z.; Kolesnick, R. Engaging the vascular component of the tumor response. Cancer Cell 2005, 8, 89–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truman, J.P.; García-Barros, M.; Kaag, M.; Hambardzumyan, D.; Stancevic, B.; Chan, M.; Fuks, Z.; Kolesnick, R.; Haimovitz-Friedman, A. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS ONE 2010, 5, e12310. [Google Scholar] [CrossRef]
- Bodo, S.; Campagne, C.; Thin, T.H.; Higginson, D.; Vargas, H.A.; Hua, G.; Fuller, J.D.; Ackerstaff, E.; Russell, J.; Zhang, Z.; et al. Single-dose radiotherapy disables tumor cell homologous recombination via ischemia/reperfusion injury. J. Clin. Investig. 2019, 129, 786–801. [Google Scholar] [CrossRef] [Green Version]
- Tarapacki, C.; Kuebler, W.M.; Tabuchi, A.; Karshafian, R. Reversible and irreversible vascular bioeffects induced by ultrasound and microbubbles in chorioallantoic membrane model. AIP Conf. Proc. 2017, 1821, 170002. [Google Scholar] [CrossRef] [Green Version]
- Nofiele, J.I.T.; Karshafian, R.; Furukawa, M.; Al Mahrouki, A.; Giles, A.; Wong, S.; Czarnota, G.J. Ultrasound-Activated Microbubble Cancer Therapy: Ceramide Production Leading to Enhanced Radiation Effect in vitro. Technol. Cancer Res. Treat. 2013, 12, 53–60. [Google Scholar] [CrossRef]
- Li, N.; Tang, J.; Yang, J.; Zhu, B.; Wang, X.; Luo, Y.; Yang, H.; Jang, F.; Zou, J.; Liu, Z.; et al. Tumor perfusion enhancement by ultrasound stimulated microbubbles potentiates PD-L1 blockade of MC38 colon cancer in mice. Cancer Lett. 2020, 498, 121–129. [Google Scholar] [CrossRef]
- Shi, J.; Fu, C.; Su, X.; Feng, S.; Wang, S. Ultrasound-Stimulated Microbubbles Inhibit Aggressive Phenotypes and Promotes Radiosensitivity of esophageal squamous cell carcinoma. Bioengineered 2021, 12, 3000–3013. [Google Scholar] [CrossRef]
- Jang, K.W.; Seol, D.; Ding, L.; Lim, T.-H.; Frank, J.A.; Martin, J. Ultrasound-Mediated Microbubble Destruction Suppresses Melanoma Tumor Growth. Ultrasound Med. Biol. 2018, 44, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Al-Mahrouki, A.A.; Wong, E.; Czarnota, G.J. Ultrasound-stimulated microbubble enhancement of radiation treatments: Endothelial cell function and mechanism. Oncoscience 2015, 2, 944–957. [Google Scholar] [CrossRef] [Green Version]
- El Kaffas, A.; Nofiele, J.; Giles, A.; Cho, S.; Liu, S.K.; Czarnota, G.J. Dll4-notch signalling blockade synergizes combined ultrasound-stimulated microbubble and radiation therapy in human colon cancer xenografts. PLoS ONE 2014, 9, e93888. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.J.; El Kaffas, A.; Lai, P.; Al Mahrouki, A.; Lee, J.; Iradji, S.; Tran, W.T.; Giles, A.; Czarnota, G.J. Ultrasound-Mediated Microbubble Enhancement of Radiation Therapy Studied Using Three-Dimensional High-Frequency Power Doppler Ultrasound. Ultrasound Med. Biol. 2013, 39, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Briggs, K.; Al Mahrouki, A.; Nofiele, J.; El-Falou, A.; Stanisz, M.; Kim, H.C.; Kolios, M.C.; Czarnota, G.J. Non-invasive Monitoring of Ultrasound-Stimulated Microbubble Radiation Enhancement Using Photoacoustic Imaging. Technol. Cancer Res. Treat. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.C.; Al-Mahrouki, A.; Gorjizadeh, A.; Karshafian, R.; Czarnota, G.J. Effects of Biophysical Parameters in Enhancing Radiation Responses of Prostate Tumors with Ultrasound-Stimulated Microbubbles. Ultrasound Med. Biol. 2013, 39, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahrouki, A.; Giles, A.; Hashim, A.; Kim, H.C.; El-Falou, A.; Rowe-Magnus, D.; Farhat, G.; Czarnota, G.J. Microbubble-based enhancement of radiation effect: Role of cell membrane ceramide metabolism. PLoS ONE 2017, 12, e0181951. [Google Scholar] [CrossRef] [Green Version]
- Daecher, A.; Stanczak, M.; Liu, J.-B.; Zhang, J.; Du, S.; Forsberg, F.; Leeper, D.B.; Eisenbrey, J.R. Localized microbubble cavitation-based antivascular therapy for improving HCC treatment response to radiotherapy. Cancer Lett. 2017, 411, 100–105. [Google Scholar] [CrossRef]
- Deng, H.; Cai, Y.; Feng, Q.; Wang, X.; Tian, W.; Qiu, S.; Wang, Y.; Li, Z.; Wu, J. Ultrasound-Stimulated Microbubbles Enhance Radiosensitization of Nasopharyngeal Carcinoma. Cell. Physiol. Biochem. 2018, 48, 1530–1542. [Google Scholar] [CrossRef]
- Klein, J.; Tran, W.; Lai, P.; Al-Mahrouki, A.; Giles, A.; Czarnota, G.J. Effect of Treatment Sequencing on the Tumor Response to Combined Treatment With Ultrasound-Stimulated Microbubbles and Radiotherapy. J. Ultrasound. Med. 2020, 39, 2415–2425. [Google Scholar] [CrossRef]
- McNabb, E.; Al-Mahrouki, A.; Law, N.; McKay, S.; Tarapacki, C.; Hussein, F.; Czarnota, G.J. Ultrasound-stimulated microbubble radiation enhancement of tumors: Single-dose and fractionated treatment evaluation. PLoS ONE 2020, 15, e0239456. [Google Scholar] [CrossRef]
- Peng, C.; Wu, Y.; Yang, Y.; Li, N.; Chen, X.; Gu, L.; Xu, D.; Yang, C. Using ultrasound-targeted microbubble destruction to enhance radiotherapy of glioblastoma. J. Cancer Res. Clin. Oncol. 2021, 147, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Shi, J.; Su, X.; Feng, S.; Wang, S. Ultrasound-stimulated microbubbles contributes to radiotherapy in esophageal carcinoma. Biochem. Cell Biol. 2021, 99, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Cochran, M.C.; Eisenbrey, J.R.; Soulen, M.C.; Schultz, S.M.; Ouma, R.O.; White, S.B.; Furth, E.E.; Wheatley, M.A. Disposition of Ultrasound Sensitive Polymeric Drug Carrier in a Rat Hepatocellular Carcinoma Model. Acad. Radiol. 2011, 18, 1341–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorace, A.G.; Saini, R.; Mahoney, M.; Hoyt, K. Molecular ultrasound imaging using a targeted contrast agent for assessing early tumor response to antiangiogenic therapy. J. Ultrasound Med. 2012, 31, 1543–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goertz, D.E.; Todorova, M.; Mortazavi, O.; Agache, V.; Chen, B.; Karshafian, R.; Hynynen, K. Antitumor Effects of Combining Docetaxel (Taxotere) with the Antivascular Action of Ultrasound Stimulated Microbubbles. PLoS ONE 2012, 7, e52307. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-L.; Lin, C.-Y.; Tseng, H.-C.; Shiu, H.-R.; Wu, M.-F. Ultrasound sonication with microbubbles disrupts blood vessels and enhances tumor treatments of anticancer nanodrug. Int. J. Nanomed. 2012, 7, 2143–2152. [Google Scholar] [CrossRef] [Green Version]
- Todorova, M.; Agache, V.; Mortazavi, O.; Chen, B.; Karshafian, R.; Hynynen, K.; Man, S.; Kerbel, R.S.; Goertz, D.E. Antitumor effects of combining metronomic chemotherapy with the antivascular action of ultrasound stimulated microbubbles. Int. J. Cancer 2012, 132, 2956–2966. [Google Scholar] [CrossRef]
- Kotopoulis, S.; Stigen, E.; Popa, M.; Safont, M.M.; Healey, A.; Kvåle, S.; Sontum, P.; Gjertsen, B.T.; Gilja, O.H.; McCormack, E. Sonoporation with Acoustic Cluster Therapy (ACT®) induces transient tumour volume reduction in a subcutaneous xenograft model of pancreatic ductal adenocarcinoma. J. Control. Release 2017, 245, 70–80. [Google Scholar] [CrossRef]
- Wu, M.; Song, Z.; Zhang, S.; Dan, Q.; Tang, C.; Peng, C.; Liang, Y.; Zhang, L.; Wang, H.; Li, Y. Local tumor ischemia-reperfusion mediated by ultrasound-targeted microbubble destruction enhances the anti-tumor efficacy of doxorubicin chemotherapy. Cancer Manag. Res. 2019, 11, 9387–9395. [Google Scholar] [CrossRef] [Green Version]
- Xiao, N.; Liu, J.; Liao, L.; Sun, J.; Jin, W.; Shu, X. Improved delivery of doxorubicin by altering the tumor microenvironment using ultrasound combined with microbubbles and chemotherapy. J. Buon Off. J. Balk. Union Oncol. 2019, 24, 844–852. [Google Scholar]
- Xiao, N.; Liu, J.; Liao, L.; Sun, J.; Jin, W.; Shu, X. Ultrasound Combined With Microbubbles Increase the Delivery of Doxorubicin by Reducing the Interstitial Fluid Pressure. Ultrasound Q. 2018, 35, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bellary, A.; Villarreal, A.; Eslami, R.; Undseth, Q.J.; Lec, B.; Defnet, A.M.; Bagrodia, N.; Kandel, J.J.; Borden, M.A.; Shaikh, S.; et al. Perfusion-guided sonopermeation of neuroblastoma: A novel strategy for monitoring and predicting liposomal doxorubicin uptake in vivo. Theranostics 2020, 10, 8143–8161. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Qiao, W.; Tang, J.; Yu, Y.; Gao, S.; Liu, Z.; Zhu, X. Chemotherapy Augmentation Using Low-Intensity Ultrasound Combined with Microbubbles with Different Mechanical Indexes in a Pancreatic Cancer Model. Ultrasound Med. Biol. 2021, 47, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Dong, X.-H.; Zhu, Q.; Xu, Y.-L.; Chen, M.-L.; Liu, Z. Ultrasound-triggered microbubble destruction enhances the radiosensitivity of glioblastoma by inhibiting PGRMC1-mediated autophagy in vitro and in vivo. Mil. Med Res. 2022, 9, 1–20. [Google Scholar] [CrossRef]
- Geis, H.A.K.A.R.B.N.A.; Katus, H.; Bekeredjian, R. Microbubbles as a Vehicle for Gene and Drug Delivery: Current Clinical Implications and Future Perspectives. Curr. Pharm. Des. 2012, 18, 2166–2183. [Google Scholar] [CrossRef]
- Lammertink, B.H.A.; Bos, C.; Deckers, R.; Storm, G.; Moonen, C.T.W.; Escoffre, J.M. Sonochemotherapy: From bench to bedside. Front. Pharmacol. 2015, 6, 138. [Google Scholar] [CrossRef] [Green Version]
- Lentacker, I.; De Smedt, S.C.; Sanders, N.N. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter 2009, 5, 2161–2170. [Google Scholar] [CrossRef]
- Derieppe, M.; Rojek, K.; Escoffre, J.-M.; De Senneville, B.D.; Moonen, C.; Bos, C. Recruitment of endocytosis in sonopermeabilization-mediated drug delivery: A real-time study. Phys. Biol. 2015, 12, 046010. [Google Scholar] [CrossRef]
- Fekri, F.; Santos, R.C.D.; Karshafian, R.; Antonescu, C.N. Ultrasound Microbubble Treatment Enhances Clathrin-Mediated Endocytosis and Fluid-Phase Uptake through Distinct Mechanisms. PLoS ONE 2016, 11, e0156754. [Google Scholar] [CrossRef] [Green Version]
- Fekri, F.; Abousawan, J.; Bautista, S.; Orofiamma, L.; Dayam, R.M.; Antonescu, C.N.; Karshafian, R. Targeted enhancement of flotillin-dependent endocytosis augments cellular uptake and impact of cytotoxic drugs. Sci. Rep. 2019, 9, 17768. [Google Scholar] [CrossRef] [Green Version]
- Roovers, S.; Lajoinie, G.; De Cock, I.; Brans, T.; Dewitte, H.; Braeckmans, K.; Versluis, M.; De Smedt, S.C.; Lentacker, I. Sonoprinting of nanoparticle-loaded microbubbles: Unraveling the multi-timescale mechanism. Biomaterials 2019, 217, 119250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karshafian, R.; Almasri, F.; Giles, A.; Czarnota, G.J. Enhancing chemotherapy by ultrasound and microbubbles: Effect of acoustic pressure and treatment order in in vitro suspension of reast and prostate cancer cells. In Proceedings of the 2010 IEEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010; pp. 1582–1585. [Google Scholar] [CrossRef]
- Lee, N.G.; Berry, J.L.; Lee, T.C.; Wang, A.T.; Honowitz, S.; Murphree, A.L.; Varshney, N.; Hinton, D.R.; Fawzi, A.A. Sonoporation Enhances Chemotherapeutic Efficacy in Retinoblastoma Cells In Vitro. Investig. Opthalmol. Vis. Sci. 2011, 52, 3868–3873. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.M.; Piron, J.; Novell, A.; Bouakaz, A. Doxorubicin Delivery into Tumor Cells with Ultrasound and Microbubbles. Mol. Pharm. 2011, 8, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Lammertink, B.H.; Bos, C.; van der Wurff-Jacobs, K.M.; Storm, G.; Moonen, C.T.; Deckers, R. Increase of intracellular cisplatin levels and radiosensitization by ultrasound in combination with microbubbles. J. Control. Release 2016, 238, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Mariglia, J.; Momin, S.; Coe, I.; Karshafian, R. Analysis of the cytotoxic effects of combined ultrasound, microbubble and nucleoside analog combinations on pancreatic cells in vitro. Ultrasonics 2018, 89, 110–117. [Google Scholar] [CrossRef]
- Heath, C.H.; Sorace, A.; Knowles, J.; Rosenthal, E.; Hoyt, K. Microbubble Therapy Enhances Anti-tumor Properties of Cisplatin and Cetuximab In Vitro and In Vivo. Otolaryngol. Neck Surg. 2012, 146, 938–945. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Lu, C.T.; Zhou, Z.C.; Jin, Z.; Zhang, L.; Sun, C.Z.; Xu, Y.Y.; Gao, H.S.; Tian, J.L.; Gao, F.H.; et al. Enhancing chemotherapeutic drug inhibition on tumor growth by ultrasound: An in vivo experiment. J. Drug Target 2011, 19, 154–160. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Luo, Y.-K.; Lu, C.-T.; Xu, J.-F.; Tang, J.; Zhang, M.; Zhang, Y.; Liang, H.-D. Phospholipids-based microbubbles sonoporation pore size and reseal of cell membrane cultured in vitro. J. Drug Target. 2008, 16, 18–25. [Google Scholar] [CrossRef]
- Shen, Z.; Shao, J.; Zhang, J.; Qu, W. Ultrasound cavitation enhanced chemotherapy: In vivo research and clinical application. Exp. Biol. Med. 2020, 245, 1200–1212. [Google Scholar] [CrossRef]
- Delaney, L.J.; Eisenbrey, J.R.; Brown, D.; Brody, J.R.; Jimbo, M.; Oeffinger, B.E.; Stanczak, M.; Forsberg, F.; Liu, J.-B.; Wheatley, M.A. Gemcitabine-loaded microbubble system for ultrasound imaging and therapy. Acta Biomater. 2021, 130, 385–394. [Google Scholar] [CrossRef]
- Tinkov, S.; Winter, G.; Coester, C.; Bekeredjian, R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: Part I—Formulation development and in-vitro characterization. J. Control. Release 2010, 143, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, S.; Coester, C.; Serba, S.; Geis, N.A.; Katus, H.A.; Winter, G.; Bekeredjian, R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: In-vivo characterization. J. Control. Release 2010, 148, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Li, L.; Zhan, C.; Zhu, P.; Chang, D.; Jiang, Q.; Ye, S.; Yang, X.; Li, Y.; Xie, L.; et al. Preparation and in vitro evaluation of an ultrasound-triggered drug delivery system: 10-Hydroxycamptothecin loaded PLA microbubbles. Ultrasonics 2012, 52, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.-M.; Mannaris, C.; Geers, B.; Novell, A.; Lentacker, I.; Averkiou, M.; Bouakaz, A. Doxorubicin liposome-loaded microbubbles for contrast imaging and ultrasound-triggered drug delivery. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 60, 78–87. [Google Scholar] [CrossRef]
- Lai, B.; Zhu, P.; Li, H.; Hu, L.; Wang, J. Effect of docetaxel-loaded lipid microbubble in combination with ultrasound-triggered microbubble destruction on the growth of a gastric cancer cell line. Oncol. Lett. 2019, 18, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, L. Effect of drug-loaded microbubbles combined with ultrasound on the apoptosis of cancer cells and the expression of Bax and Bcl-2 in a rabbit VX2 liver tumor model. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Wu, X.; Wang, Z.; Ran, H.; Xu, C.; Wu, J.; Wang, Z.; Zhang, Y. Antitumor Effect of Docetaxel-Loaded Lipid Microbubbles Combined With Ultrasound-Targeted Microbubble Activation on VX2 Rabbit Liver Tumors. J. Ultrasound Med. 2010, 29, 61–70. [Google Scholar] [CrossRef]
- Hu, J.; Zong, Y.; Li, J.; Zhou, X.; Zhang, J.; Zhu, T.; Jiao, M.; Su, H.; Bo, B. In Vitro and In Vivo Evaluation of Targeted Sunitinib-Loaded Polymer Microbubbles Against Proliferation of Renal Cell Carcinoma. J. Ultrasound Med. 2016, 35, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhao, Y.; Liu, C.; Hu, B.; Zhao, M.; Ma, Y.; Jiang, J. Synergistic anti-tumor effect of paclitaxel and miR-34a combined with ultrasound microbubbles on cervical cancer in vivo and in vitro. Clin. Transl. Oncol. 2019, 22, 60–69. [Google Scholar] [CrossRef]
- Cochran, M.C.; Eisenbrey, J.; Ouma, R.O.; Soulen, M.; Wheatley, M.A. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int. J. Pharm. 2011, 414, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.-L.; Browning, R.J.; Yildiz, Y.O.; Gray, M.; Bau, L.; Kamila, S.; Thompson, J.; Elliott, A.; Smart, S.; McHale, A.P.; et al. Ultrasound-Mediated Gemcitabine Delivery Reduces the Normal-Tissue Toxicity of Chemoradiation Therapy in a Muscle-Invasive Bladder Cancer Model. Int. J. Radiat. Oncol. 2021, 109, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-K.; Tsai, C.-L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2021, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Tchoryk, A.; Taresco, V.; Argent, R.H.; Ashford, M.B.; Gellert, P.R.; Stolnik, S.; Grabowska, A.M.; Garnett, M.C. Penetration and Uptake of Nanoparticles in 3D Tumor Spheroids. Bioconj. Chem. 2019, 30, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 1–19. [Google Scholar] [CrossRef]
- Misra, R.; Rajic, M.; Sathiyamoorthy, K.; Karshafian, R. Ultrasound and microbubbles (USMB) potentiated doxorubicin penetration and distribution in 3D breast tumour spheroids. J. Drug Deliv. Sci. Technol. 2020, 61, 102261. [Google Scholar] [CrossRef]
- Roovers, S.; Deprez, J.; Priwitaningrum, D.; Lajoinie, G.; Rivron, N.; Declercq, H.; De Wever, O.; Stride, E.; Le Gac, S.; Versluis, M.; et al. Sonoprinting liposomes on tumor spheroids by microbubbles and ultrasound. J. Control. Release 2019, 316, 79–92. [Google Scholar] [CrossRef]
- Paškevičiūtė, M.; Januškevičienė, I.; Sakalauskienė, K.; Raišutis, R.; Petrikaitė, V. Evaluation of low-intensity pulsed ultrasound on doxorubicin delivery in 2D and 3D cancer cell cultures. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Huang, M.; Gu, X.; Gao, X. Nanotherapeutic strategies for the treatment of neurodegenerative diseases. In Brain Targeted Drug Delivery System; Academic Press: Cambridge, MA, USA, 2018; pp. 321–356. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR Imaging–guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Sheikov, N.A.; Jolesz, F.A.; Vykhodtseva, N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage 2005, 24, 12–20. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Raymond, S.; Jolesz, F.A.; Hynynen, K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med. Biol. 2005, 31, 1527–1537. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Effects of Acoustic Parameters and Ultrasound Contrast Agent Dose on Focused-Ultrasound Induced Blood-Brain Barrier Disruption. Ultrasound Med. Biol. 2008, 34, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Konofagou, E.E. Optimization of the Ultrasound-Induced Blood-Brain Barrier Opening. Theranostics 2012, 2, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Englander, Z.K.; Wei, H.-J.; Pouliopoulos, A.N.; Bendau, E.; Upadhyayula, P.; Jan, C.-I.; Spinazzi, E.F.; Yoh, N.; Tazhibi, M.; McQuillan, N.M.; et al. Focused ultrasound mediated blood–brain barrier opening is safe and feasible in a murine pontine glioma model. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Aryal, M.; Arvanitis, C.D.; Alexander, P.M.; McDannold, N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 2014, 72, 94–109. [Google Scholar] [CrossRef] [Green Version]
- Karakatsani, M.E.; Downs, M.E.; Buch, A.M.; Sanchez, C.J.S.; Chen, S.; Konofagou, E.E.; Ferrera, V.P.; Teichert, T. Long-Term Safety of Repeated Blood-Brain Barrier Opening via Focused Ultrasound with Microbubbles in Non-Human Primates Performing a Cognitive Task. PLoS ONE 2015, 10, 1–26. [Google Scholar] [CrossRef]
- Chen, H.; Konofagou, E.E. The Size of Blood–Brain Barrier Opening Induced by Focused Ultrasound is Dictated by the Acoustic Pressure. J. Cereb. Blood Flow Metab. 2014, 34, 1197–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treat, L.H.; McDannold, N.; Vykhodtseva, N.; Zhang, Y.; Tam, K.; Hynynen, K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer 2007, 121, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Treat, L.H.; Zhang, Y.; McDannold, N.; Hynynen, K. Impact of focused ultrasound-enhanced drug delivery on survival in rats with glioma. AIP Conf. Proc. 2009, 1113, 443–447. [Google Scholar] [CrossRef]
- Liu, H.-L.; Hua, M.-Y.; Chen, P.-Y.; Chu, P.-C.; Pan, C.-H.; Yang, H.-W.; Huang, C.-Y.; Wang, J.-J.; Yen, T.-C.; Wei, K.-C. Blood-Brain Barrier Disruption with Focused Ultrasound Enhances Delivery of Chemotherapeutic Drugs for Glioblastoma Treatment. Radiology 2010, 255, 415–425. [Google Scholar] [CrossRef]
- Kovacs, Z.; Werner, B.; Rassi, A.; Sass, J.O.; Martin-Fiori, E.; Bernasconi, M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J. Control. Release 2014, 187, 74–82. [Google Scholar] [CrossRef]
- Liu, H.-L.; Huang, C.-Y.; Chen, J.-Y.; Wang, H.-Y.J.; Chen, P.-Y.; Wei, K.-C. Pharmacodynamic and Therapeutic Investigation of Focused Ultrasound-Induced Blood-Brain Barrier Opening for Enhanced Temozolomide Delivery in Glioma Treatment. PLoS ONE 2014, 9, e114311. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.-Y.; Fan, C.-H.; Liu, H.-L.; Huang, C.-Y.; Hsieh, H.-Y.; Yen, T.-C.; Wei, K.-C.; Yeh, C.-K. Concurrent blood–brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 2012, 33, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-H.; Ting, C.-Y.; Liu, H.-L.; Huang, C.-Y.; Hsieh, H.-Y.; Yen, T.-C.; Wei, K.-C.; Yeh, C.-K. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials 2013, 34, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-H.; Ting, C.-Y.; Lin, H.-J.; Wang, C.-H.; Liu, H.-L.; Yen, T.-C.; Yeh, C.-K. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials 2013, 34, 3706–3715. [Google Scholar] [CrossRef]
- Eisenbrey, J.R.; Forsberg, F.; Wessner, C.E.; Delaney, L.J.; Bradigan, K.; Gummadi, S.; Tantawi, M.; Lyshchik, A.; O’Kane, P.; Liu JBin Intenzo, C.; et al. US-triggered Microbubble Destruction for Augmenting Hepatocellular Carcinoma Response to Transarterial Radioembolization: A Randomized Pilot Clinical Trial. Radiology 2021, 298, 450–457. [Google Scholar] [CrossRef]
- Galeaz, C.; Totis, C.; Bisio, A. Radiation Resistance: A Matter of Transcription Factors. Front. Oncol. 2021, 11, 2055. [Google Scholar] [CrossRef]
- Kotopoulis, S.; Dimcevski, G.; Gilja, O.H.; Hoem, D.; Postema, M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: A clinical case study. Med Phys. 2013, 40, 072902. [Google Scholar] [CrossRef]
- Dimcevski, G.; Kotopoulis, S.; Bjånes, T.; Hoem, D.; Schjøtt, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; Mc Cormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 2016, 243, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Y.; Yan, K.; Shen, L.; Yang, W.; Gong, J.; Ding, K. Clinical study of ultrasound and microbubbles for enhancing chemotherapeutic sensitivity of malignant tumors in digestive system. Chin. J. Cancer Res. 2018, 30, 553–563. [Google Scholar] [CrossRef]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rezai, A.R.; Ranjan, M.; D’Haese, P.-F.; Haut, M.W.; Carpenter, J.; Najib, U.; Mehta, R.I.; Chazen, J.L.; Zibly, Z.; Yates, J.R.; et al. Noninvasive hippocampal blood−brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl. Acad. Sci. USA 2020, 117, 9180–9182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, J.; Zhang, H.; Yu, J.; Liufu, C.; Chen, Z. Ultrasound-mediated microbubble destruction: A new method in cancer immunotherapy. OncoTargets Ther. 2018, 11, 5763–5775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Nanayakkara, G.K.; Drummer, C.; Sun, Y.; Johnson, C.; Cueto, R.; Fu, H.; Shao, Y.; Wang, L.; Yang, W.Y.; et al. Low-Intensity Ultrasound-Induced Anti-inflammatory Effects Are Mediated by Several New Mechanisms Including Gene Induction, Immunosuppressor Cell Promotion, and Enhancement of Exosome Biogenesis and Docking. Front. Physiol. 2017, 8, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Shou, W.-D.; Xu, Y.-J.; Bai, W.-K.; Hu, B. Low-frequency ultrasound-induced VEGF suppression and synergy with dendritic cell-mediated anti-tumor immunity in murine prostate cancer cells in vitro. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Banerjee, R. Nanobubble Liposome Complexes for Diagnostic Imaging and Ultrasound-Triggered Drug Delivery in Cancers: A Theranostic Approach. ACS Omega 2019, 4, 15567–15580. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| (A) USMB + XRT | |||
|---|---|---|---|
| Treatment Type | Tumour Model | Tumour Vascular Effects | References |
| USMB + 2 Gy or 8 Gy | Mouse (human prostate cancer PC3) | Reduced blood flow, reduced vessel density, increased cell death, reduced cell proliferation | [21] |
| USMB + 2 Gy or 8 Gy | Mouse (human bladder cancer HT-1376) | Reduced blood flow, increased cell death, vascular normalization, increased fibrosis | [24] |
| USMB + 2 Gy, 4 Gy, or 8 Gy | Human umbilical vein endothelial cells (HUVEC), acute myeloid leukemia cells (AML), murine fibrosarcoma cells (KHT-C), prostate cancer cells (PC3), breast cancer cells (MDA-MB-231) and astrocytes cells | Increased nuclear fragmentation, reduced endothelial cell survival | [68] |
| USMB + 2 Gy or 8 Gy | Mouse (human breast cancer MDA-MB-231) | Reduced blood flow, reduced vessel density, increased cell death, inhibited tumour growth | [74] |
| USMB + 2 Gy or 8 Gy | Mouse (human prostate cancer PC3) | Reduced blood flow, reduced oxygen saturation, increased cell death, reduced vessel density | [75] |
| USMB + 2 Gy or 8 Gy | Mouse (human prostate cancer PC3) | Increased blood vessel leakage, reduced vessel density, increased hypoxia, increased cell death, reduced cell proliferation | [22] |
| USMB + 2 Gy or 8 Gy | Mouse (human prostate cancer PC3) | Increased cell disruption and cell death | [76] |
| USMB + 8 Gy | HUVEC cells | Increased cell death, reduced endothelial-cell tube formation | [72] |
| USMB + 2 Gy or 8 Gy | Mouse (human breast cancer MDA-MB-231) | Increased cell death, reduced vessel density, increased vascular leakage, inhibited tumour growth | [25] |
| USMB + 8 Gy | Mouse (human prostate cancer PC3) | Reduced blood flow, reduced oxygen saturation, increased cell death and fibrosis | [77] |
| USMB + 5 Gy | Rat (human hepatocellular carcinoma Hu7.5) | Reduced tumour vascularity, inhibited tumour growth | [78] |
| USMB + 2 Gy or 8 Gy | Human CNE-2 and HUVEC cells, Mouse (human CNE-2) | Reduced tumour cell viability, and formation of endothelial tubule, Reduced blood flow and CD34 expression, increased tumour cell death and increased ANG II and AT1R expression | [79] |
| USMB + 2 Gy or 8 Gy | Mouse (fibrosarcoma MCA/129) | Reduced blood flow and vessel density, increased cell death, inhibited tumour growth | [23] |
| USMB + 8 Gy | Mouse (human prostate cancer PC3) | Increased cell death, reduced vessel density | [80] |
| USMB + 8 Gy | Rabbit (human prostate cancer PC3) | Reduced blood flow, reduced oxygen saturation, increased cell death and fibrosis, reduced vessel density, inhibited tumour growth | [81] |
| USMB + 4 Gy | Human glioblastoma U87-MG cells, Mouse (human glioblastoma U87-MG) | Reduced CD34 expression, increased cell death, inhibited tumour growth | [82] |
| USMB + 2 Gy or 6 Gy | Human esophageal carcinoma cell lines (KYSE-510) and HUVEC cells | Reduced cell viability, reduced colony formation, increased cell death, inhibited angiogenesis, inhibited tumour growth, reduced cell proliferation | [83] |
| (B) USMB/UTMD + Chemotherapy | |||
| Treatment Type | Tumour Model | Tumour Vascular Effects | References |
| USMB/UTMD + doxorubicin (DOX) | Rat (hepatocellular carcinoma 3924a) | Inhibited tumour growth | [84] |
| USMB + bevacizumab | Mouse (human 2LMP breast cancer) | Reduced tumour vascularity | [85] |
| USMB + docetaxel (DTX) | Mouse (human prostate cancer PC3) | Reduced tumour perfusion, increased cell death, inhibited tumour growth | [86] |
| USMB + DOX | Mouse (colorectal adenocarcinoma CT-26) | Disrupted tumour blood vessels, inhibited tumour growth | [87] |
| USMB + Metronomic cyclophosphamide (MCTX) | Mouse (human breast cancer MDA-MB-231) | Reduced tumour perfusion, increased cell death, inhibited tumour growth | [88] |
| USMB + paclitaxel (PTX) | Mouse (MIA PaCa- 2 luc) | Reduced tumour volume, sustained tumour vascularisation | [89] |
| USMB + DOX | Rat (9L gliosarcoma) | Increased Ktrans, vessel damage | [27] |
| USMB + DOX | Mouse (4T1 breast cancer) | Reduced tumour blood perfusion, increased levels of ROS, inhibited tumour growth, increased cell death | [90] |
| USMB + DOX | Rabbit (VX2 tumour) | Increased tumour perfusion, disrupted tumour microvessels, Inhibited tumour growth | [91] |
| USMB + DOX | Rabbit (VX2 tumour) | Increased vascular clearance of particles, reduced interstitial fluid pressure (IFP) | [92] |
| USMB + DOX | Mouse (neuroblastoma) | Increased tumour vascular permeability, reduced pericyte coverage, increased cell death | [93] |
| USMB + DOX | Mouse (human pancreatic carcinoma PANC-1) | Increased tumour blood perfusion | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, D.; Leong, K.X.; Czarnota, G.J. Application of Ultrasound Combined with Microbubbles for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 4393. https://doi.org/10.3390/ijms23084393
Sharma D, Leong KX, Czarnota GJ. Application of Ultrasound Combined with Microbubbles for Cancer Therapy. International Journal of Molecular Sciences. 2022; 23(8):4393. https://doi.org/10.3390/ijms23084393
Chicago/Turabian StyleSharma, Deepa, Kai Xuan Leong, and Gregory J. Czarnota. 2022. "Application of Ultrasound Combined with Microbubbles for Cancer Therapy" International Journal of Molecular Sciences 23, no. 8: 4393. https://doi.org/10.3390/ijms23084393
APA StyleSharma, D., Leong, K. X., & Czarnota, G. J. (2022). Application of Ultrasound Combined with Microbubbles for Cancer Therapy. International Journal of Molecular Sciences, 23(8), 4393. https://doi.org/10.3390/ijms23084393






