The Role of Systemic Filtrating Organs in Aging and Their Potential in Rejuvenation Strategies
Abstract
1. Introduction
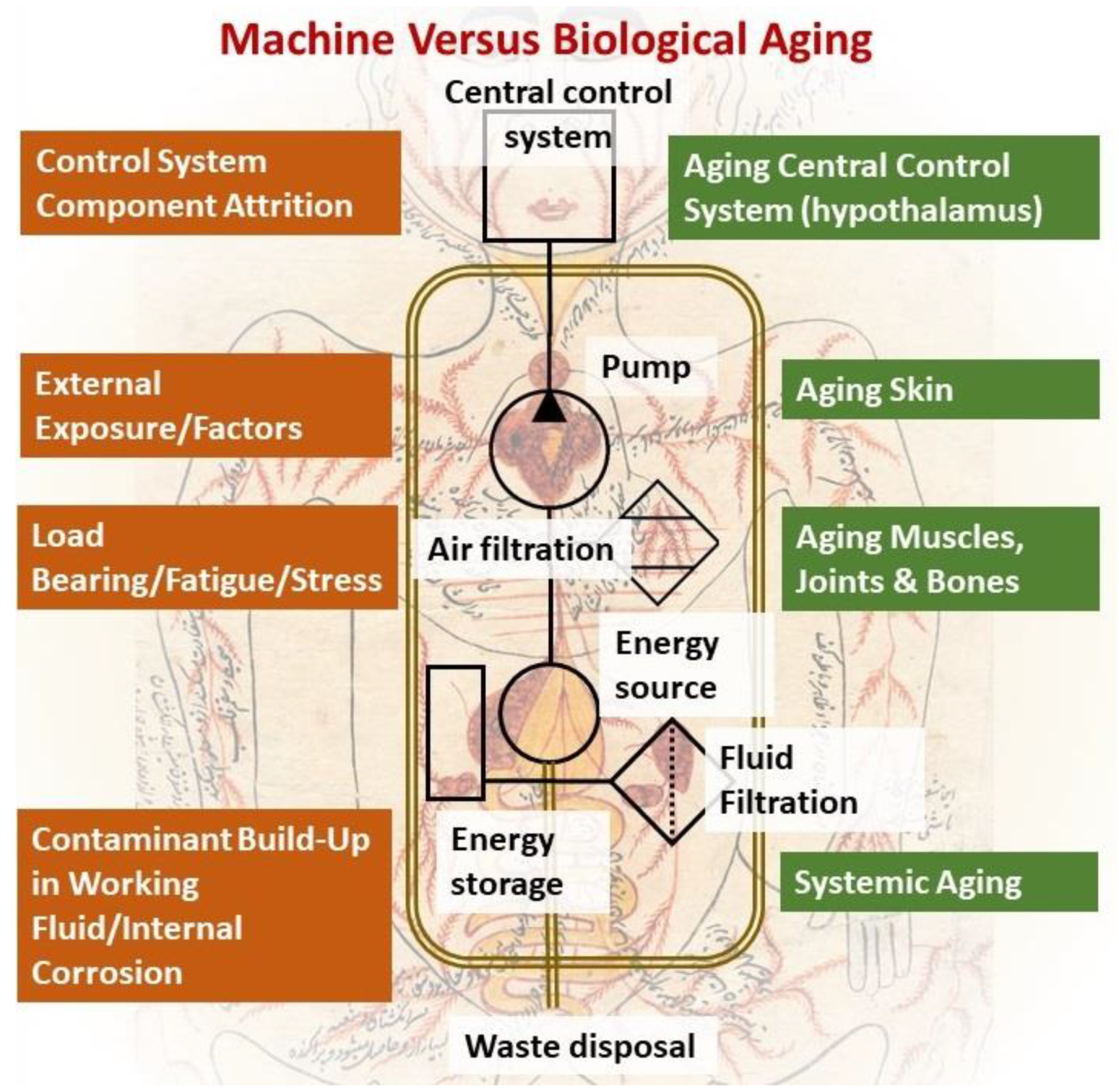
2. An Engineering Perspective on Biological Aging
3. Hallmarks of Aging: The Complexity of Cause and Effect
4. Circulating Factors of Aging and the Kidney
4.1. Metabolic by-Product Clearance and Kidney-Associated Protein Metabolism
4.2. Urine Metal Concentration and Aging
4.3. Circulating Factors and Plasma Protein Clearance by the Kidney
5. Rejuvenation Strategies: Systemic versus Targeted
5.1. Targeting Nutrient-Sensing Pathways
5.2. Senolytic Drugs, the New Pharmacological Focus of Rejuvenation
5.3. Cellular Reprogramming and Genetic Rejuvenation
6. Future Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmoudi, S.; Xu, L.; Brunet, A. Turning back time with emerging rejuvenation strategies. Nat. Cell Biol. 2019, 21, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Katcher, H.L. Towards an evidence-based model of aging. Curr. Aging Sci. 2015, 8, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.M. Lifestyle (Medicine) and Healthy Aging. Clin. Geriatr. Med. 2020, 36, 645–653. [Google Scholar] [CrossRef]
- Sellami, M.; Bragazzi, N.L.; Slimani, M.; Hayes, L.; Jabbour, G.; De Giorgio, A.; Dugué, B. The effect of exercise on glucoregulatory hormones: A countermeasure to human aging: Insights from a comprehensive review of the literature. Int. J. Environ. Res. Public Health 2019, 16, 1709. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Tarnopolsky, M.A. Mitochondria and aging—The role of exercise as a countermeasure. Biology 2019, 8, 40. [Google Scholar] [CrossRef]
- Watanabe, M. Smoking: Additional burden on aging and death. Genes Environ. 2016, 38, 3. [Google Scholar] [CrossRef]
- Ottinger, M.A. A Comparative Approach to Metabolic Aspects of Aging: Conserved Mechanisms and Effects of Calorie Restriction and Environment. Prog. Mol. Biol. Transl. Sci. 2018, 155, 109–127. [Google Scholar]
- Broskey, N.T.; Marlatt, K.L.; Most, J.; Erickson, M.L.; Irving, B.A.; Redman, L.M. The Panacea of Human Aging: Calorie Restriction Versus Exercise. Exerc. Sport Sci. Rev. 2019, 47, 169–175. [Google Scholar] [CrossRef]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The gut microbiome, aging, and longevity: A systematic review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Ottens, F.; Franz, A.; Hoppe, T. Build-UPS and break-downs: Metabolism impacts on proteostasis and aging. Cell Death Differ. 2021, 28, 505–521. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Hillson, O.; Gonzalez, S.; Rallis, C. Prospects of Pharmacological Interventions to Organismal Aging. Biomol. Concepts 2018, 9, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Ros, M.; Carrascosa, J.M. Current nutritional and pharmacological anti-aging interventions. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165612. [Google Scholar] [CrossRef]
- Kheifets, V.; Braithwaite, S.P. Plasma-Based Strategies for Therapeutic Modulation of Brain Aging. Neurotherapeutics 2019, 16, 675–684. [Google Scholar] [CrossRef]
- Goya, R.G.; Lehmann, M.; Chiavellini, P.; Canatelli-Mallat, M.; Hereñú, C.B.; Brown, O.A. Rejuvenation by cell reprogramming: A new horizon in gerontology. Stem Cell Res. Ther. 2018, 9, 349. [Google Scholar] [CrossRef]
- Singh, P.B.; Newman, A.G. Age reprogramming and epigenetic rejuvenation. Epigenetics Chromatin 2018, 11, 73. [Google Scholar] [CrossRef]
- Lehmann, M.; Canatelli-Mallat, M.; Chiavellini, P.; Cónsole, G.M.; Gallardo, M.D.; Goya, R.G. Partial Reprogramming As An Emerging Strategy for Safe Induced Cell Generation and Rejuvenation. Curr. Gene Ther. 2019, 19, 248–254. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Pilling, L.C.; Liu, Z.; Atkins, J.L.; Levine, M.E.; Levine, M.; Kuo, C.-L. Genetic associations for two biological age measures point to distinct aging phenotypes. Aging Cell 2021, 20, e13376. [Google Scholar] [CrossRef]
- Guerville, F.; De Souto Barreto, P.; Ader, I.; Andrieu, S.; Casteilla, L.; Dray, C.; Fazilleau, N.; Guyonnet, S.; Langin, D.; Liblau, R.; et al. Revisiting the Hallmarks of Aging to Identify Markers of Biological Age. J. Prev. Alzheimer’s Dis. 2020, 7, 56–64. [Google Scholar] [CrossRef]
- Vandenberk, B.; Brouwers, B.; Hatse, S.; Wildiers, H. P16INK4a: A central player in cellular senescence and a promising aging biomarker in elderly cancer patients. J. Geriatr. Oncol. 2011, 2, 259–269. [Google Scholar] [CrossRef]
- Mensà, E.; Latini, S.; Ramini, D.; Storci, G.; Bonafè, M.; Olivieri, F. The telomere world and aging: Analytical challenges and future perspectives. Ageing Res. Rev. 2019, 50, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Fasching, C.L. Telomere length measurement as a clinical biomarker of aging and disease. Crit. Rev. Clin. Lab. Sci. 2018, 55, 443–465. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.; Fiorito, G.; Hernandez, B.; Polidoro, S.; O’Halloran, A.M.; Hever, A.; Ni Cheallaigh, C.; Lu, A.T.; Horvath, S.; Vineis, P.; et al. GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. J. Gerontol. Ser. A 2020, 76, 741–749. [Google Scholar] [CrossRef]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Bonewald, L. Use it or lose it to age: A review of bone and muscle communication. Bone 2019, 120, 212–218. [Google Scholar] [CrossRef]
- Carina, V.; Della Bella, E.; Costa, V.; Bellavia, D.; Veronesi, F.; Cepollaro, S.; Fini, M.; Giavaresi, G. Bone’s Response to Mechanical Loading in Aging and Osteoporosis: Molecular Mechanisms. Calcif. Tissue Int. 2020, 107, 301–318. [Google Scholar] [CrossRef]
- Sierra, F.; Kohanski, R. Advances in Geroscience; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Alexander, J.W.; Bennett, L.E.; Breen, T.J. Effect of donor age on outcome of kidney transplantation. A two-year analysis of transplants reported to the United Network for Organ Sharing Registry. Transplantation 1994, 57, 871–876. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Larsson, T.E. Chronic Kidney Disease: A Clinical Model of Premature Aging. Am. J. Kidney Dis. 2013, 62, 339–351. [Google Scholar] [CrossRef]
- Langen, R.C.J.; Gosker, H.R.; Remels, A.H.V.; Schols, A.M.W.J. Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease. Int. J. Biochem. Cell Biol. 2013, 45, 2245–2256. [Google Scholar] [CrossRef]
- Crowson, C.S.; Liang, K.P.; Therneau, T.M.; Kremers, H.M.; Gabriel, S.E. Could accelerated aging explain the excess mortality in patients with seropositive rheumatoid arthritis? Arthritis Rheum. 2010, 62, 378–382. [Google Scholar] [CrossRef]
- Amann, K.; Ritz, E. Cardiovascular abnormalities in ageing and in uraemia--only analogy or shared pathomechanisms? Nephrol. Dial. Transplant 1998, 13 (Suppl. 7), 6–11. [Google Scholar] [CrossRef][Green Version]
- Pathai, S.; Lawn, S.D.; Gilbert, C.E.; McGuinness, D.; McGlynn, L.; Weiss, H.A.; Port, J.; Christ, T.; Barclay, K.; Wood, R.; et al. Accelerated biological ageing in HIV-infected individuals in South Africa: A case-control study. AIDS 2013, 27, 2375–2384. [Google Scholar] [CrossRef]
- Kooman, J.P.; Broers, N.J.H.; Usvyat, L.; Thijssen, S.; van der Sande, F.M.; Cornelis, T.; Levin, N.W.; Leunissen, K.M.L.; Kotanko, P. Out of control: Accelerated aging in uremia. Nephrol. Dial. Transplant. 2013, 28, 48–54. [Google Scholar] [CrossRef]
- Kooman, J.P.; Kotanko, P.; Schols, A.M.W.J.; Shiels, P.G.; Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014, 10, 732–742. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Aunan, J.R.; Watson, M.M.; Hagland, H.R.; Søreide, K. Molecular and biological hallmarks of ageing. Br. J. Surg. 2016, 103, e29–e46. [Google Scholar] [CrossRef]
- Yates, D. Blood-derived rejuvenation. Nat. Rev. Neurosci. 2014, 15, 352–353. [Google Scholar] [CrossRef]
- Conboy, I.M.; Rando, T.A. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle 2012, 11, 2260–2267. [Google Scholar] [CrossRef]
- Conboy, M.J.; Conboy, I.M.; Rando, T.A. Heterochronic parabiosis: Historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 2013, 12, 525–530. [Google Scholar] [CrossRef]
- Portugal-Nunes, C.; Costa Castanho, T.; Amorim, L.; Silva Moreira, P.; Mariz, J.; Marques, F.; Sousa, N.; Correia Santos, N.; Almeida Palha, J. Iron status is associated with mood, cognition, and functional ability in older adults: A cross-sectional study. Nutrients 2020, 12, 3594. [Google Scholar] [CrossRef]
- Rył, A.; Miazgowski, T.; Szylińska, A.; Turoń-Skrzypińska, A.; Jurewicz, A.; Bohatyrewicz, A.; Rotter, I. Bone health in aging men: Does zinc and cuprum level matter? Biomolecules 2021, 11, 237. [Google Scholar] [CrossRef]
- Parmalee, N.L.; Aschner, M. Manganese and aging. Neurotoxicology 2016, 56, 262–268. [Google Scholar] [CrossRef]
- Gorell, J.M.; Johnson, C.C.; Rybicki, B.A.; Peterson, E.L.; Kortsha, G.X.; Brown, G.G.; Richardson, R.J. Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology 1997, 48, 650–658. [Google Scholar] [CrossRef]
- Eriksen, B.O.; Palsson, R.; Ebert, N.; Melsom, T.; van der Giet, M.; Gudnason, V.; Indridasson, O.S.; Inker, L.A.; Jenssen, T.G.; Levey, A.S.; et al. GFR in healthy aging: An individual participant data meta-analysis of iohexol clearance in european population-based cohorts. J. Am. Soc. Nephrol. 2020, 31, 1602–1615. [Google Scholar] [CrossRef]
- Cirillo, M.; Laurenzi, M.; Mancini, M.; Zanchetti, A.; Lombardi, C.; De Santo, N.G. Low glomerular filtration in the population: Prevalence, associated disorders, and awareness. Kidney Int. 2006, 70, 800–806. [Google Scholar] [CrossRef]
- Mahbub, M.H.; Yamaguchi, N.; Takahashi, H.; Hase, R.; Yamamoto, H.; Kikuchi, S.; Tanabe, T. Relationship of reduced glomerular filtration rate with alterations in plasma free amino acids and uric acid evaluated in healthy control and hypertensive subjects. Sci. Rep. 2019, 9, 10252. [Google Scholar] [CrossRef]
- Gibson, T. Hyperuricemia, gout and the kidney. Curr. Opin. Rheumatol. 2012, 24, 127–131. [Google Scholar] [CrossRef]
- Nashaat, E.H.; Mohamed, M.M.; Aziz, T.M.; Nakhla, M.W. Serum Beta 2-Microglobulin as a Biomarker of Activity in Ulcerative Colitis. QJM Int. J. Med. 2020, 113, 10916. [Google Scholar] [CrossRef]
- Shi, F.; Sun, L.; Kaptoge, S. Association of beta-2-microglobulin and cardiovascular events and mortality: A systematic review and meta-analysis. Atherosclerosis 2021, 320, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Hyung, J.; Hong, J.Y.; Kim, S.; Ryu, J.S.; Huh, J.; Suh, C. Beta-2 microglobulin as a prognostic factor of primary central nervous system lymphoma. Blood Res. 2019, 54, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.H.; Roumelioti, M.E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.K.; Giles, D.A.; Segal, B.M.; Irani, D.N. An emerging role for eotaxins in neurodegenerative disease. Clin. Immunol. 2018, 189, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.J.; Griffiths-Johnson, D.A.; Collins, P.D.; Walsh, D.T.; Moqbel, R.; Totty, N.F.; Truong, O.; Hsuan, J.J.; Williams, T.J. Eotaxin: A potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J. Exp. Med. 1994, 179, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Andres-Hernando, A.; Dursun, B.; Altmann, C.; Ahuja, N.; He, Z.; Bhargava, R.; Edelstein, C.E.; Jani, A.; Hoke, T.S.; Klein, C.; et al. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol. Dial. Transplant. 2012, 27, 4339–4347. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Teh, J.P.Y.; Cheon, B.K.; Yang, Y.; Schlundt, J.; Wang, Y.; Conway, P.L. Comparative Blood and Urine Metabolomics Analysis of Healthy Elderly and Young Male Singaporeans. J. Proteome Res. 2020, 19, 3264–3275. [Google Scholar] [CrossRef]
- Garibotto Giacomo, G.; Sofia, A.; Saffioti, S.; Bonanni, A.; Mannucci, I.; Verzola, D. Amino acid and protein metabolism in the human kidney and in patients with chronic kidney disease. Clin. Nutr. 2010, 29, 424–433. [Google Scholar] [CrossRef]
- Teruya, T.; Goga, H.; Yanagida, M. Aging markers in human urine: A comprehensive, non-targeted LC-MS study. FASEB BioAdvances 2020, 2, 720–733. [Google Scholar] [CrossRef]
- Rodwell, G.E.J.; Sonu, R.; Zahn, J.M.; Lund, J.; Wilhelmy, J.; Wang, L.; Xiao, W.; Mindrinos, M.; Crane, E.; Segal, E.; et al. A Transcriptional Profile of Aging in the Human Kidney. PLOS Biol. 2004, 2, e427. [Google Scholar] [CrossRef]
- Imelda, M.; Szilvia, A.; Barna, B. Epidemiology of anemia. Orv. Hetil. 2020, 161, 1569–1573. [Google Scholar] [CrossRef]
- Sharif, R.; Thomas, P.; Zalewski, P.; Fenech, M. The role of zinc in genomic stability. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2012, 733, 111–121. [Google Scholar] [CrossRef]
- Pfrimer, K.; Micheletto, R.F.; Marchini, J.S.; Padovan, G.J.; Moriguti, J.C.; Ferriolli, E. Impact of aging on urinary excretion of iron and zinc. Nutr. Metab. Insights 2014, 7, 47–50. [Google Scholar] [CrossRef]
- Nwanaji-Enwerem, J.C.; Colicino, E.; Specht, A.J.; Gao, X.; Wang, C.; Vokonas, P.; Weisskopf, M.G.; Boyer, E.W.; Baccarelli, A.A.; Schwartz, J. Individual species and cumulative mixture relationships of 24-hour urine metal concentrations with DNA methylation age variables in older men. Environ. Res. 2020, 186, 109573. [Google Scholar] [CrossRef]
- Guo, J.; Xie, J.; Zhou, B.; Găman, M.A.; Kord-Varkaneh, H.; Clark, C.C.T.; Salehi-Sahlabadi, A.; Li, Y.; Han, X.; Hao, Y.; et al. The influence of zinc supplementation on IGF-1 levels in humans: A systematic review and meta-analysis. J. King Saud Univ.-Sci. 2020, 32, 1824–1830. [Google Scholar] [CrossRef]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and respiratory tract infections: Perspectives for COVID’19 (Review). Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kulakov, L.; Opländer, C.; Kolb-Bachofen, V.; Kröncke, K.D.; Suschek, C.V. Zinc regulates iNOS-derived nitric oxide formation in endothelial cells. Redox Biol. 2014, 2, 945–954. [Google Scholar] [CrossRef]
- Rychlik, M.; Mlyniec, K. Zinc-mediated Neurotransmission in Alzheimer’s Disease: A Potential Role of the GPR39 in Dementia. Curr. Neuropharmacol. 2019, 18, 2–13. [Google Scholar] [CrossRef]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef]
- Tanaka, T.; Biancotto, A.; Moaddel, R.; Moore, A.Z.; Gonzalez-Freire, M.; Aon, M.A.; Candia, J.; Zhang, P.; Cheung, F.; Fantoni, G.; et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018, 17, e12799. [Google Scholar] [CrossRef]
- Katsimpardi, L.; Litterman, N.K.; Schein, P.A.; Miller, C.M.; Loffredo, F.S.; Wojtkiewicz, G.R.; Chen, J.W.; Lee, R.T.; Wagers, A.J.; Rubin, L.L. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 2014, 344, 630–634. [Google Scholar] [CrossRef]
- Elabd, C.; Cousin, W.; Upadhyayula, P.; Chen, R.Y.; Chooljian, M.S.; Li, J.; Kung, S.; Jiang, K.P.; Conboy, I.M. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Commun. 2014, 5, 4082. [Google Scholar] [CrossRef]
- Castellano, J.M.; Mosher, K.I.; Abbey, R.J.; McBride, A.A.; James, M.L.; Berdnik, D.; Shen, J.C.; Zou, B.; Xie, X.S.; Tingle, M.; et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 2017, 544, 488–492. [Google Scholar] [CrossRef]
- Thornton, P.L.; Ingram, R.L.; Sonntag, W.E. Chronic [D-Ala2]-growth hormone-releasing hormone administrationattenuates age-related deficits in spatial memory. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, B106–B112. [Google Scholar] [CrossRef]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradère, J.P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- Yang, Y.R.; Kabir, M.H.; Park, J.H.; Park, J.I.; Kang, J.S.; Ju, S.; Shin, Y.J.; Lee, S.M.; Lee, J.; Kim, S.; et al. Plasma proteomic profiling of young and old mice reveals cadherin-13 prevents age-related bone loss. Aging (Albany N. Y.) 2020, 12, 8652–8668. [Google Scholar] [CrossRef]
- Yoshida, M.; Satoh, A.; Lin, J.B.; Mills, K.F.; Sasaki, Y.; Rensing, N.; Wong, M.; Apte, R.S.; Imai, S. ichiro Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab. 2019, 30, 329–342.e5. [Google Scholar] [CrossRef]
- Gan, K.J.; Südhof, T.C. Specific factors in blood from young but not old mice directly promote synapse formation and NMDA-receptor recruitment. Proc. Natl. Acad. Sci. USA 2019, 116, 12524–12533. [Google Scholar] [CrossRef]
- Khrimian, L.; Obri, A.; Ramos-Brossier, M.; Rousseaud, A.; Moriceau, S.; Nicot, A.S.; Mera, P.; Kosmidis, S.; Karnavas, T.; Saudou, F.; et al. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 2017, 214, 2859–2873. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; He, Y.; Park, J.S.; Bieri, G.; Snethlage, C.E.; Lin, K.; Gontier, G.; Wabl, R.; Plambeck, K.E.; Udeochu, J.; et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 2015, 21, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Pastushkova, L.K.; Kononikhin, A.S.; Tiys, E.S.; Dobrokhotov, I.V.; Ivanisenko, V.A.; Nikolaev, E.N.; Larina, I.M.; Popov, I.A. Characteristics of age-dependent changes in urine proteome in healthy men. Adv. Gerontol. 2016, 6, 123–128. [Google Scholar] [CrossRef]
- Kuipers, A.L.; Zhang, Y.; Yu, S.; Kammerer, C.M.; Nestlerode, C.S.; Chu, Y.; Bunker, C.H.; Patrick, A.L.; Wheeler, V.W.; Miljkovic, I.; et al. Relative influence of heritability, environment and genetics on serum sclerostin. Osteoporos. Int. 2014, 25, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Kindstedt, E.; Holm, C.K.; Sulniute, R.; Martinez-Carrasco, I.; Lundmark, R.; Lundberg, P. CCL11, a novel mediator of inflammatory bone resorption. Sci. Rep. 2017, 7, 5334. [Google Scholar] [CrossRef]
- Andreasová, T.; Vránová, J.; Vondráková, D.; Sedláčková, L.; Zákostelská, Z.J.; Neužil, P.; Málek, F. Role of biomarkers of cardiac remodeling, myofibrosis, and inflammation in assessment of disease severity in euvolemic patients with chronic stable heart failure. J. Int. Med. Res. 2020, 48, 0300060520947869. [Google Scholar] [CrossRef]
- Romanova, Y.; Laikov, A.; Markelova, M.; Khadiullina, R.; Makseev, A.; Hasanova, M.; Rizvanov, A.; Khaiboullina, S.; Salafutdinov, I. Proteomic analysis of human serum from patients with chronic kidney disease. Biomolecules 2020, 10, 257. [Google Scholar] [CrossRef]
- Morsiani, C.; Bacalini, M.G.; Santoro, A.; Garagnani, P.; Collura, S.; D’Errico, A.; de Eguileor, M.; Grazi, G.L.; Cescon, M.; Franceschi, C.; et al. The peculiar aging of human liver: A geroscience perspective within transplant context. Ageing Res. Rev. 2019, 51, 24–34. [Google Scholar] [CrossRef]
- Rhinn, M.; Ritschka, B.; Keyes, W.M. Cellular senescence in development, regeneration and disease. Development 2019, 146, dev151837. [Google Scholar] [CrossRef]
- Krimpenfort, P.; Quon, K.C.; Mooi, W.J.; Loonstra, A.; Berns, A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 2001, 413, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Schmitt, C.A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019, 21, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E.; Bardeesy, N.; Lee, K.H.; Carrasco, D.; Castrillon, D.H.; Aguirre, A.J.; Wu, E.A.; Horner, J.W.; DePinho, R.A. Loss of p16Ink4a with retention of p19 predisposes mice to tumorigenesis. Nature 2001, 413, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, H.; Mavrogonatou, E.; Kletsas, D. Scarless wound healing: From development to senescence. Adv. Drug Deliv. Rev. 2019, 146, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16 Ink4a-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Gallagher, J.C. Vitamin D and Aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef]
- Kumar, R.; Tebben, P.J.; Thompson, J.R. Vitamin D and the kidney. Arch. Biochem. Biophys. 2012, 523, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Álvarez, S.; Guerra-Varela, J.; Sobrido-Cameán, D.; Quelle, A.; Barreiro-Iglesias, A.; Sánchez, L.; Collado, M. Cell senescence contributes to tissue regeneration in zebrafish. Aging Cell 2020, 19, e13052. [Google Scholar] [CrossRef]
- Beckett, E. More Than Bone Health: The Many Roles for Vitamin D. Nutrients 2020, 12, 2388. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Horowitz, A.M. Therapeutic potential of systemic brain rejuvenation strategies for neurodegenerative disease. F1000Research 2017, 6, 1291. [Google Scholar] [CrossRef]
- Kang, J.S.; Yang, Y.R. Circulating plasma factors involved in rejuvenation. Aging (Albany N. Y.) 2020, 12, 23394–23408. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.J.; Zhang, Q.; Gong, C.X.; Wang, F.X.; Huang, J.C.; Yang, G.Q.; Liu, L.; Zhou, K.; Xu, R.; Chen, Q.; et al. Young plasma ameliorates aging-related acute brain injury after intracerebral hemorrhage. Biosci. Rep. 2019, 39, BSR20190537. [Google Scholar] [CrossRef]
- Tripathi, S.S.; Kumar, R.; Arya, J.; Rizvi, S.I. Plasma from young rats injected into old rats induce anti-aging effects. Rejuvenation Res. 2020, 24, 206–212. [Google Scholar] [CrossRef]
- Sha, S.J.; Deutsch, G.K.; Tian, L.; Richardson, K.; Coburn, M.; Gaudioso, J.L.; Marcal, T.; Solomon, E.; Boumis, A.; Bet, A.; et al. Safety, Tolerability, and Feasibility of Young Plasma Infusion in the Plasma for Alzheimer Symptom Amelioration Study: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 35–40. [Google Scholar] [CrossRef] [PubMed]
- VandeVrede, L.; Dale, M.L.; Fields, S.; Frank, M.; Hare, E.; Heuer, H.W.; Keith, K.; Koestler, M.; Ljubenkov, P.A.; McDermott, D.; et al. Open-Label Phase 1 Futility Studies of Salsalate and Young Plasma in Progressive Supranuclear Palsy. Mov. Disord. Clin. Pract. 2020, 7, 440–447. [Google Scholar] [CrossRef]
- Ma, J.; Gao, B.; Zhang, K.; Zhang, Q.; Jia, G.; Li, J.; Li, C.; Yan, L.J.; Cai, Z. Circulating factors in young blood as potential therapeutic agents for age-related neurodegenerative and neurovascular diseases. Brain Res. Bull. 2019, 153, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Rybtsova, N.; Berezina, T.; Kagansky, A.; Rybtsov, S. Can Blood-Circulating Factors Unveil and Delay Your Biological Aging? Biomedicines 2020, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Rebo, J.; Mehdipour, M.; Gathwala, R.; Causey, K.; Liu, Y.; Conboy, M.J.; Conboy, I.M. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat. Commun. 2016, 7, 13363. [Google Scholar] [CrossRef]
- Mehdipour, M.; Skinner, C.; Wong, N.; Lieb, M.; Liu, C.; Etienne, J.; Kato, C.; Kiprov, D.; Conboy, M.J.; Conboy, I.M. Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin. Aging (Albany N. Y.) 2020, 12, 8790–8819. [Google Scholar] [CrossRef] [PubMed]
- Boada, M.; López, O.; Núñez, L.; Szczepiorkowski, Z.M.; Torres, M.; Grifols, C.; Páez, A. Plasma exchange for Alzheimer’s disease Management by Albumin Replacement (AMBAR) trial: Study design and progress. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Relkin, N. Clinical Trials of Intravenous Immunoglobulin for Alzheimer’s Disease. J. Clin. Immunol. 2014, 34, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Fahy, G.M.; Brooke, R.T.; Watson, J.P.; Good, Z.; Vasanawala, S.S.; Maecker, H.; Leipold, M.D.; Lin, D.T.S.; Kobor, M.S.; Horvath, S. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 2019, 18, e13028. [Google Scholar] [CrossRef]
- Sogabe, Y.; Seno, H.; Yamamoto, T.; Yamada, Y. Unveiling epigenetic regulation in cancer, aging, and rejuvenation with in vivo reprogramming technology. Cancer Sci. 2018, 109, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Shanley, D.P.; Kirkwood, T.B.L. Calorie restriction and aging: A life-history analysis. Evolution 2000, 54, 740–750. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. MTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Pan, H.; Finkel, T. Key proteins and pathways that regulate lifespan. J. Biol. Chem. 2017, 292, 6452–6460. [Google Scholar] [CrossRef]
- Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Interacting NAD + and Cell Senescence Pathways Complicate Antiaging Therapies. Rejuvenation Res. 2019, 22, 261–266. [Google Scholar] [CrossRef]
- Ehninger, D.; Neff, F.; Xie, K. Longevity, aging and rapamycin. Cell. Mol. Life Sci. 2014, 71, 4325–4346. [Google Scholar] [CrossRef] [PubMed]
- Zajda, A.; Huttunen, K.M.; Sikora, J.; Podsiedlik, M.; Markowicz-Piasecka, M. Is metformin a geroprotector? A peek into the current clinical and experimental data. Mech. Ageing Dev. 2020, 191, 111350. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Wahlqvist, M.L.; Lee, M.S.; Tsai, H.N. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimer’s Dis. 2011, 24, 485–493. [Google Scholar] [CrossRef]
- Chin-Hsiao, T. Metformin and the risk of dementia in type 2 diabetes patients. Aging and Disease. 2019, 10, 37. [Google Scholar] [CrossRef]
- Yang, W.; Cai, X.; Wu, H.; Ji, L. Associations between metformin use and vitamin B12 levels, anemia, and neuropathy in patients with diabetes: A meta-analysis. J. Diabetes 2019, 11, 729–743. [Google Scholar] [CrossRef]
- Lalau, J.D.; Kajbaf, F.; Protti, A.; Christensen, M.M.; De Broe, M.E.; Wiernsperger, N. Metformin-associated lactic acidosis (MALA): Moving towards a new paradigm. Wiley Online Libr. 2017, 19, 1502–1512. [Google Scholar] [CrossRef]
- Salvatore, T.; Pafundi, P.C.; Marfella, R.; Sardu, C.; Rinaldi, L.; Monaco, L.; Ricozzi, C.; Imbriani, S.; Nevola, R.; Adinolfi, L.E.; et al. Metformin lactic acidosis: Should we still be afraid? Diabetes Res. Clin. Pract. 2019, 157, 107879. [Google Scholar] [CrossRef] [PubMed]
- Bonkowski, M.S.; Sinclair, D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef]
- Liao, C.Y.; Rikke, B.A.; Johnson, T.E.; Diaz, V.; Nelson, J.F. Genetic variation in the murine lifespan response to dietary restriction: From life extension to life shortening. Aging Cell 2010, 9, 92–95. [Google Scholar] [CrossRef]
- Hunt, N.J.; McCourt, P.A.G.; Le Couteur, D.G.; Cogger, V.C. Novel targets for delaying aging: The importance of the liver and advances in drug delivery. Adv. Drug Deliv. Rev. 2018, 135, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Zhou, Y.M.; Li, D.; Lun, Y.Z. Dietary methyl-consuming compounds and metabolic syndrome. Hypertens. Res. 2011, 34, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Schmeer, C.; Kretz, A.; Wengerodt, D.; Stojiljkovic, M.; Witte, O.W. Dissecting Aging and Senescence-Current Concepts and Open Lessons. Cells 2019, 8, 1446. [Google Scholar] [CrossRef]
- Austad, S.N.; Hoffman, J.M. Is antagonistic pleiotropy ubiquitous in aging biology? Evol. Med. Public Health 2018, 2018, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Yoo, A.S.; Sun, A.X.; Li, L.; Shcheglovitov, A.; Portmann, T.; Li, Y.; Lee-Messer, C.; Dolmetsch, R.E.; Tsien, R.W.; Crabtree, G.R. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 2011, 476, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Huh, C.J.; Zhang, B.; Victor, M.B.; Dahiya, S.; Batista, L.F.Z.; Horvath, S.; Yoo, A.S. Maintenance of age in human neurons generated by microRNA-based neuronal conversion of fibroblasts. eLife 2016, 5, e18648. [Google Scholar] [CrossRef]
- Kanwal, S.; Guo, X.; Ward, C.; Volpe, G.; Qin, B.; Esteban, M.A.; Bao, X. Role of Long Non-coding RNAs in Reprogramming to Induced Pluripotency. Genom. Proteom. Bioinform. 2020, 18, 16–25. [Google Scholar] [CrossRef]
- Iwamoto, K.; Bundo, M.; Ueda, J.; Oldham, M.C.; Ukai, W.; Hashimoto, E.; Saito, T.; Geschwind, D.H.; Kato, T. Neurons show distinctive DNA methylation profile and higher interindividual variations compared with non-neurons. Genome Res. 2011, 21, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Samoylova, E.M.; Baklaushev, V.P. Cell Reprogramming Preserving Epigenetic Age: Advantages and Limitations. Biochemistry 2020, 85, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Lo Sardo, V.; Ferguson, W.; Erikson, G.A.; Topol, E.J.; Baldwin, K.K.; Torkamani, A. Influence of donor age on induced pluripotent stem cells. Nat. Biotechnol. 2017, 35, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, A.; Reddy, P.; Martinez-Redondo, P.; Platero-Luengo, A.; Hatanaka, F.; Hishida, T.; Li, M.; Lam, D.; Kurita, M.; Beyret, E.; et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell 2016, 167, 1719–1733.e12. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Galiano, R.D.; Capla, J.M.; Kalka, C.; Gagne, P.J.; Jacobowitz, G.R.; Levine, J.P.; Gurtner, G.C. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002, 106, 2781–2786. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Son, M.-Y. Current Challenges Associated with the Use of Human Induced Pluripotent Stem Cell-Derived Organoids in Regenerative Medicine. Int. J. Stem Cells 2021, 14, 9–20. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef]
- Messina, A.; Luce, E.; Hussein, M.; Dubart-Kupperschmitt, A. Pluripotent-Stem-Cell-Derived Hepatic Cells: Hepatocytes and Organoids for Liver Therapy and Regeneration. Cells 2020, 9, 420. [Google Scholar] [CrossRef]
- Li, P.; Lee, G.-H.; Kim, S.Y.; Kwon, S.Y.; Kim, H.-R.; Park, S. From Diagnosis to Treatment: Recent Advances in Patient-Friendly Biosensors and Implantable Devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef]
- French, P. In-Vivo Microsystems: A Review. Sensors 2020, 20, 4953. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, B.; Ouyang, H.; Fan, Y.; Wang, Z.L.; Chen, Z.M.; Li, Z. A 25-year bibliometric study of implantable energy harvesters and self-powered implantable medical electronics researches. Mater. Today Energy 2020, 16, 100386. [Google Scholar] [CrossRef]
- Singh, R.; Bathaei, M.J.; Istif, E.; Beker, L. A Review of Bioresorbable Implantable Medical Devices: Materials, Fabrication, and Implementation. Adv. Healthc. Mater. 2020, 9, 2000790. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Ethridge, E.C. Biomaterials-the interfacial problem. Adv. Biomed. Eng. 1975, 5, 35–150. [Google Scholar] [CrossRef]
- Dinis, H.; Mendes, P.M. A comprehensive review of powering methods used in state-of-the-art miniaturized implantable electronic devices. Biosens. Bioelectron. 2021, 172, 112781. [Google Scholar] [CrossRef] [PubMed]
- Teshome, A.K.; Kibret, B.; Lai, D.T.H. A Review of Implant Communication Technology in WBAN: Progress and Challenges. IEEE Rev. Biomed. Eng. 2018, 12, 88–99. [Google Scholar] [CrossRef]
- Masliukov, P.M.; Nozdrachev, A.D. Hypothalamic Regulatory Mechanisms of Aging. J. Evol. Biochem. Physiol. 2021, 57, 473–491. [Google Scholar] [CrossRef]
- Kim, K.; Choe, H.K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 2019, 177, 74–79. [Google Scholar] [CrossRef]

| Top 10 Plasma Protein Pathways Increasing with Age | Top 10 Plasma Proteins Pathways Decreasing with Age | |||
|---|---|---|---|---|
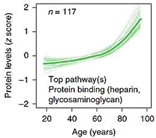 | 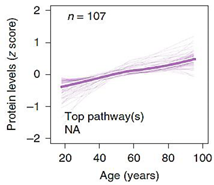 | 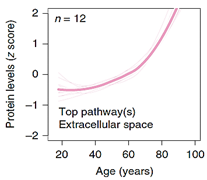 |  |  |
| Extracellular Region | Chondroitin Sulfate Biosynthesis | Extracellular Space | Extracellular Space | Transmembrane Receptor Activity |
| ADAMTS5/ASPN/C1QTNF1/…/ TREML4/TTN/VEGFA/VIT | CHST9/DCN/BGN/CHST11 | CCDC80/FSTL3/GDF15/MMP12/NPPB/PTN/SVEP1/WFDC2 | ACE2/ADAMTS3/AHSG/AIMP1/…/SPINT1/TF/TNXB/UBB | EGFR/KDR/RET |
| Extracellular Region Part | Heparan Sulfate Metabolism | Fibronectin Binding | Blood Microparticle | Tyrosine Kinase Activity |
| ADAMTS5/ASPN/…/TNFSF15/TTN/VEGFA/VIT | DCN/BGN/GPC6/GLCE/HS3ST3A1 | CCDC80/FSTL3 | AHSG/APOL1/CFB/…/IGLL1/ITIH1/PLG/SERPINC1/TF | EGFR/KDR |
| Extracellular Space | Chondroitin Sulfate Metabolism | Extracellular Region | Fibrillar Collagen Trimer | Integrin Binding |
| ADAMTS5/C1QTNF1/…/TNFRSF11B/TNFSF15/VEGFA | CHST9/DCN/BGN/GPC6/CHST11 | CCDC80/CHRDL1/FSTL3/…/PTN/RNASE1/SVEP1/WFDC2 | COL11A2/COL1A1/TNXB | ADAMTS13/EGFR/KDR |
| Proteinaceous Extracellular Matrix | Regulation of IFNG Signaling | Extracellular Region Part | Banded Collagen Fibril | Protein Kinase Activity |
| ADAMTS5/ASPN/CHI3L1/…/TNFRSF11B/VEGFA/VIT | STAT1/SOCS3/PTPN11 | CCDC80/…/MMP12/NPPB/PTN/RNASE1/SVEP1/WFDC2 | COL11A2/COL1A1/TNXB | EGFR/KDR/RET |
| Glycosaminoglycan binding | Glycosaminoglycan Metabolism | Basement Membrane | Extracellular Region | Midgut Development |
| ADAMTS5/CXCL10/GREM2/…/SFRP1/THBS3/VEGFA/VIT | CHST9/DCN/BGN/GPC6/CHST11/GLCE/HS3ST3A1 | CCDC80/PTN | ACE2/ADAMTS3/AGER/…/TMEM132A/TNR/TNXB/UBB | EGFR/RET |
| Heparin Binding | Interleukin-6 signaling | Extracellular Matrix Component | Extracellular Region Part | Hormone Binding |
| ADAMTS5/CXCL10/GREM2/…/SFRP1/THBS3/VEGFA | STAT1/SOCS3/PTPN11 | CCDC80/PTN | ACE2/ADAMTS3/…/TF/TMEM132A/TNR/TNXB/UBB | EGFR/GHR |
| Sulfur Compound Binding | Defective B4GALT7, Progeroid | Proteinaceous Extracellular Space | Protein Activation Cascade | Protein Complex Binding |
| ADAMTS5/CD34/…/SFRP1/THBS3/VEGFA | DCN/BGN/GPC6 | CCDC80/MMP12/PTN | C1RL/CFB/CFP/…/IGLL1/MASP1/MBL2/SERPINC1 | ADAMTS13/CTSV/EGFR/KDR |
| G-protein Coupled Receptor Binding | Defective B3GAT3 | BMP Signaling Pathway | Complex of Collagen Trimers | Transmembrane Signaling Receptor Activity |
| CCL3/CXCL10/CXCL16/…/NPW/POMC/PPY/RSPO3/SFRP1 | DCN/BGN/GPC6 | CHRDL1/FSTL3/GDF15 | COL11A2/COL1A1/TNXB | EGFR/GHR/KDR/RET |
| Chitin Binding | Defective B3GALT6 | Response to BMP | Extracellular Exosome | Macromolecular Complex Binding |
| CHI3L1/CHIT1/CTBS | DCN/BGN/GPC6 | CHRDL1/FSTL3/GDF15 | ACE2/ADAMTS3/…//ST3GAL6/TF/TMEM132A/TNXB/UBB | EGFR/GHR/KDR/RET |
| Extracellular Matrix | Particulate Exogenous Antigens | Cellular Response to BMP | Extracellular Organelle | Protein Tyrosine Kinase Activity |
| ADAMTS5/ASPN/…/TIMP4/TNFRSF11B/VEGFA/VIT | CD36/ITGAV | CHRDL1/FSTL3/GDF15 | ACE2/ADAMTS3/AHSG/…/TF/TMEM132A/TNXB/UBB | ADAMTS13/CTSV/EGFR/KDR |
| Plasma Proteins that Increase with Age | Abbreviation | Molecular Weight (kda) | Ref. | Plasma Proteins That Decrease with Age | Abbreviation | Molecular Weight (kda) | Ref. |
|---|---|---|---|---|---|---|---|
| Motilin | MLN | 5.19 | [90] | Cadherin-13 | CDH13 | 7.57 | [91] |
| Eotaxin | CCL11 | 10.73 | [92] | Apelin | APLN | 8.57 | [93] |
| C-CMotif Chemokine 19 | CCL19 | 10.99 | [92] | Osteocalcin | OCN(BGLAP) | 10.96 | [94] |
| C-CMotif Chemokine 2 | CCL2 | 11.03 | [92] | Somatoliberin | GHRH | 12.45 | [95] |
| β-2-Microglobulin | B2M | 11.73 | [96] | Oxytocin-Neurophysin 1 | OXT | 12.72 | [97] |
| Natriuretic Peptides B | NPPB | 14.73 | [98] | Granulocyte-Macrophage Colony-Stimulating Factor | CSF2 | 16.3 | [99] |
| Pleiotrophin | PTN | 18.95 | [90,98] | Metalloproteinase Inhibitor 2 | TIMP2 | 24.4 | [99] |
| A Disintegrin And Metalloproteinase with Thrombospondin Motifs 5 | ADAMTS5 | 21.7 | [98] | Growth Differentiation Factor 11 | GDF11 | 45.1 | [100] |
| Sclerostin | SOST | 24.03 | [90,98] | Extracellular Nicotinamide Phosphoribosyltransferase | eNAMPT | 55.53 | [101] |
| Growth Differentiation Factor 15 | GDF15 | 34.15 | [90,98] | Thrombospondin-4 | THBS4 | 96.03 | [102] |
| Cathepsin L2 | CTSV | 37.33 | [98] | Proto-Oncogene Tyrosine-Protein Kinase Receptor Ret | RET | 124.39 | [90] |
| Adp Ribosylation Factor Interacting Protein 2 | ARFIP2 | 37.86 | [90] | Immunoglobulin Superfamily Dcc Subclass Member 4 | IGDCC4 | 134.23 | [90] |
| Haptoglobin | HP | 45.21 | [92] | ||||
| Chordin-Like Protein 1 | CHRDL1 | 51.18 | [98] | ||||
| Macrophage Metalloelastase | MMP12 | 54.01 | [98] | ||||
| Egf-Containing Fibulin-Like Extracellular Matrix Protein 1 | EFEMP1 | 54.65 | [98] | ||||
| Scavenger Receptor Class F Member 2 | SCARF2 | 92.4 | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassab, A.; Rizk, N.; Prakash, S. The Role of Systemic Filtrating Organs in Aging and Their Potential in Rejuvenation Strategies. Int. J. Mol. Sci. 2022, 23, 4338. https://doi.org/10.3390/ijms23084338
Kassab A, Rizk N, Prakash S. The Role of Systemic Filtrating Organs in Aging and Their Potential in Rejuvenation Strategies. International Journal of Molecular Sciences. 2022; 23(8):4338. https://doi.org/10.3390/ijms23084338
Chicago/Turabian StyleKassab, Amal, Nasser Rizk, and Satya Prakash. 2022. "The Role of Systemic Filtrating Organs in Aging and Their Potential in Rejuvenation Strategies" International Journal of Molecular Sciences 23, no. 8: 4338. https://doi.org/10.3390/ijms23084338
APA StyleKassab, A., Rizk, N., & Prakash, S. (2022). The Role of Systemic Filtrating Organs in Aging and Their Potential in Rejuvenation Strategies. International Journal of Molecular Sciences, 23(8), 4338. https://doi.org/10.3390/ijms23084338






