Abstract
The contributory roles of vitamin D in ocular and visual health have long been discussed, with numerous studies pointing to the adverse effects of vitamin D deficiency. In this paper, we provide a systematic review of recent findings on the association between vitamin D and different ocular diseases, including myopia, age-related macular degeneration (AMD), glaucoma, diabetic retinopathy (DR), dry eye syndrome (DES), thyroid eye disease (TED), uveitis, retinoblastoma (RB), cataract, and others, from epidemiological, clinical and basic studies, and briefly discuss vitamin D metabolism in the eye. We searched two research databases for articles examining the association between vitamin D deficiency and different ocular diseases. One hundred and sixty-two studies were found. There is evidence on the association between vitamin D and myopia, AMD, DR, and DES. Overall, 17 out of 27 studies reported an association between vitamin D and AMD, while 48 out of 54 studies reported that vitamin D was associated with DR, and 25 out of 27 studies reported an association between vitamin D and DES. However, the available evidence for the association with other ocular diseases, such as glaucoma, TED, and RB, remains limited.
1. Introduction
Vitamin D has diverse functions in maintaining human health, including regulating gene expression, immune system, inflammation, cell proliferation and differentiation, apoptosis, and angiogenesis [,]. Vitamin D3, or cholecalciferol, is produced from its precursor, 7-dehydrocholesterol, in the epidermal layer of skin under exposure to sunlight, or is obtained from the diet. It is metabolized in the liver and kidneys to its biologically active forms, 25-hydroxyvitamin D (25(OH)D3) and 1,25-dihydroxyvitamin D (1,25(OH)2D3), respectively. The latter is also known as potent steroid hormone calcitriol. Reduced sun exposure will lead to vitamin D deficiency [,]. Low vitamin D levels have been associated with many diseases, including cardiovascular diseases [,], hypertension [], diabetes mellitus [,], and cancers [].
The vitamin D status of an individual is usually determined by serum 25(OH)D3 instead of 1,25(OH)2D3 because of its longer circulating half-life and higher concentration in circulation []. Besides, 1,25(OH)2D3 levels are affected by calcium levels [,]. Even though a range of thresholds is used between various scientific societies, having blood levels lower than 12 ng/mL of 25(OH)D3 represents deficiency, 12–20 ng/mL represents insufficiency, 20–100 ng/mL represents sufficiency, and >100 ng/mL indicates a risk of toxicity [].
2. Metabolism of Vitamin D
Vitamin D is synthesized and activated in three steps (Figure 1). Cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2) are the two major biologically inert precursors of vitamin D. For the former, 7-dehydrocholesterol in the skin produces previtamin D3 under exposure to ultraviolet B radiation (UVB, λ = 290–315 nm), which then thermally isomerizes to Vitamin D3 in the skin; in contrast, vitamin D2 is derived from plants and obtained from the diet. After its production, vitamin D3 attaches to vitamin D-binding protein (DBP) in the liver, where it is activated to produce 25(OH)D3, the primary circulating form of vitamin D, by 25-hydroxylases, CYP2R1 and CYP27A1. Then, 25(OH)D3 is converted to 1,25(OH)2D3, the active form of vitamin D, by 1α-hydroxylase, CYP27B1. In contrast, vitamin D2 is derived from plants and obtained from the diet. CYP27A1 does not hydroxylate vitamin D2 at the 25 positions. Lastly, vitamin D metabolite levels are downregulated by CYP24A1, which catalyzes the 24-hydroxylation of both 25(OH)D3 and 1,25(OH)2D3 []. The genetic variation in the metabolic enzyme would affect the regulation of vitamin D levels.
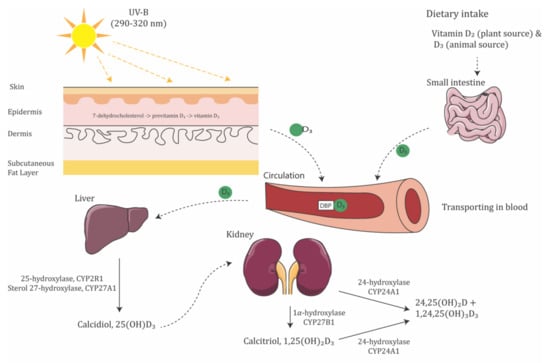
Figure 1.
Schematic illustration of vitamin D synthesis pathway. Moreover, 7-dehydrocholesterol in the epidermis layer of skin absorbs UV-B radiation and is converted to pre-vitamin D3. Vitamin D3, by either the isomerization of pre-vitamin D3 in the epidermal basal layers or intestinal absorption from the diet, binds to vitamin D-binding protein (DBP) in the bloodstream, transported to the liver. Vitamin D3 is hydroxylated by 25-hydroxylase or sterol 27-hydroxylase. The resultant calcidiol (25(OH)D3) is 1α-hydroxylated in the kidney by 1α-hydroxylase, yielding biologically active vitamin D (1,25(OH)2D3).
Moreover, 1,25(OH)2D3 can penetrate the cell membranes, either as a free molecule or in DBP-1,25(OH)2D3 complexes. It then binds to the vitamin D receptor (VDR), facilitating the interaction of VDR with the retinoic X receptor (RXR) []. This VDR-RXR heterodimer binds to both positive and negative vitamin D response elements in target genes, influencing gene transcription []. Hence, the presence of VDR suggests the local activity of vitamin D []. In particular, VDR has been detected in different parts of the eye, including the epithelium and endothelium of the cornea, lens, ciliary body, retinal ganglion cells (RGCs), inner nuclear layer, photoreceptors, and retinal pigment epithelium (RPE) [,]. Genetic alternations of the VDR gene could lead to defects in gene function, calcium metabolism, cell proliferation, and immune function. DBP is mainly responsible for the transportation of vitamin D and its metabolites.
Levels of active vitamin D in the body are regulated by the enzymes 25-hydroxylase, 1α-hydroxylase, and 24-hydroxylase []. In a recent study, the 25(OH)D3 and 1,25(OH)2D3 generating enzymes 25-hydroxylase (CYP2R1 and CYP27A1) and 1α-hydroxylase (CYP27B1), as well as the deactivating enzyme 24-hydroxylase (CYP24A1), were found to be strongly localized at the complementary regions of the ciliary body, RPE, neural retina, corneal epithelium and endothelium, and scleral fibroblast, suggesting that vitamin D in the eye is locally produced, activated, and regulated [,]. Moreover, vitamin D-dependent calcium binding protein calbindin, a vitamin D metabolizing protein, was shown to be expressed throughout the human retina []. Some of the cohort studies reported the correlation of metabolic enzymes in ocular diseases. In diabetic patients, retinal CYP27B1 was found to correlate strongly with VEGF-A in the eyes []. In a cohort of patients with Vogt-Koyanagi-Harada disease, a non-synonymous variant of CYP2R1 was found in 17 of 39 patients, suggesting that the variant in CYP2R1 may play a role in VKH pathogenesis [].
Some of the vitamin D regulating proteins, such as ferredoxin reductase participating in the activation of vitamin D in the kidney, are metalloproteins. Vitamin D is able to interact with the matrix metalloproteinase. Metal deficiency may affect ocular condition. However, only one study found significantly lower serum calcium levels in blepharospasm patients, but no significant difference in magnesium, phosphorus, or vitamin D [].
Therefore, the potential of vitamin D to regulate various processes of potential relevance to ocular diseases has been acknowledged. Studies investigating the roles of vitamin D in ocular tissues and ocular disease pathogenic pathways have been carried out and will continue to contribute towards our understanding of ocular disease mechanisms and help establish effective intervention.
3. Vitamin D and Ocular Diseases
The potential effect of vitamin D deficiency on human health is a big concern. Recently, especially over the past few years, since the last published review articles related to vitamin D and ocular diseases, more and more studies investigating the relationship between serum vitamin D level and ocular diseases were published, including some prospective studies examining this relationship and therapeutic effects of vitamin D. Currently, review articles related to vitamin D and ocular disease are available [,]. To update this and reach a comprehensive understanding, hence, we performed a systematic review here to summarize the evidence revealing the association between vitamin D and ocular diseases.
3.1. Method of Literature Search
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines []. The protocol is described as follows.
3.1.1. Search Strategy
A systematic search on PubMed (Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?DB5pubmed, accessed on 18 March 2022) and Web of Science (Available online: https://www.webofscience.com/wos/woscc/basic-search, accessed on 18 March 2022) with coverage up to 18 March 2022 was conducted initially using the following keywords: vitamin D in combinations with eye (PubMED: 544; WOS: 1909), eye disease (PubMed: 824; WOS: 854), ocular (PubMed: 183; WOS: 534), cataract (PubMed: 158; WOS: 355), lens opacity (PubMed: 118; WOS: 51), glaucoma (PubMed: 51; WOS: 151), intraocular pressure (PubMed: 24; WOS: 68), maculopathy (PubMed: 85; WOS: 97), diabetic retinopathy (PubMed: 126; WOS: 228), hypertensive retinopathy (PubMed: 2; WOS: 2), retinal arterial occlusion (PubMed: 0; WOS: 0), retinal venous occlusion (PubMed: 1; WOS:0). The search results from both databases were exported and imported in Covidence, which is a software for literature screening in systematic reviews. Among these 6813 results, the system detects that 3533 results were duplicated. They have been removed prior to the screening of the articles (Figure 2).
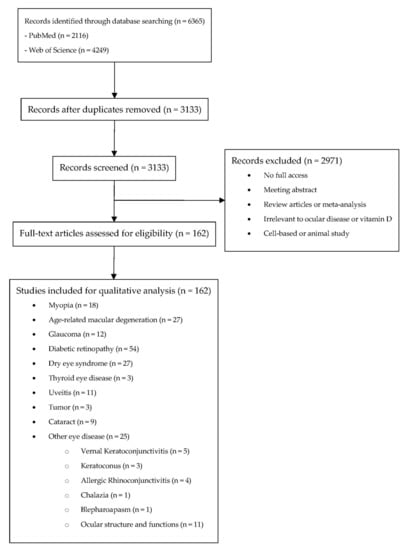
Figure 2.
Systematic review flow diagram.
3.1.2. Inclusion and Exclusion Criteria
The inclusion criteria for studies were: (1) written in English; (2) evaluating the association between blood vitamin D and different ocular diseases in a randomized controlled trial, prospective study, cross-sectional study, or case-control study. After a review of abstracts, relevant articles were retrieved and reviewed. Bibliographies of these articles provided further references. All retrieved records were reviewed by two independent reviewers (HNC and XL). Uncertainties were resolved via discussion with another reviewer (XJZ).
3.1.3. Risk of Bias Assessment
Included interventional studies (both randomized controlled trials and clinical controlled trials) were assessed for quality according to the RoB tool for randomized trials from the Effective Practice and Organisation of Care (EPOC) Group. The assessment for the clinical controlled trial was assessed according to the suggestion from previous literature that both “random sequence generation” and “allocation concealment” were scored as “high risk”, while grading the remaining items as RCT []. We further modified the RoB tool by allocating 1 point to “low risk”, 0.5 point to “unclear risk” and 0 points to “high risk”. There are a total of 9 items to be assessed using the RoB tool, and hence, the total number of points for the RoB tool is 9 points, while those cohort, case-control, cross-sectional studies were assessed for quality according to the LEGEND (Let Evidence Guide Every New Decision) System designed for Cincinnati Children’s Hospital [].
3.2. Myopia
Myopia is an important public health problem worldwide []. The etiology of myopia is complex, with both genetic and environmental risk factors [,,,]. Epidemiologic evidence indicates that time spent outdoors is a protective factor against myopia development [,,,,], yet the underlying mechanism is unclear. Since the main source of vitamin D is sunlight exposure, vitamin D is linked to myopia, hypothesizing that a vitamin D pathway may mediate the protective effect of time spent outdoors on myopia. Evidence from studies on the relationship of vitamin D and myopia is summarized in Table 1.

Table 1.
Summary of studies related to myopia included.
As demonstrated in Table 1, the association between vitamin D and myopia is controversial in cross-sectional studies. Many studies suggest that the serum 25(OH)D3 level shows an inverse association with myopia and may have a protective effect on myopia [,,,,,,,,,]. However, several case-control studies from Australia [], Denmark [], and the US [] found that the risks of myopia are not related to their neonatal vitamin D levels.
Nevertheless, it is important to distinguish the causation between vitamin D and myopia. A large longitudinal cohort study found that 25(OH)D3 levels correlated with self-reported time spent outdoors; however, no evidence suggested that the participants’ serum vitamin D levels were independently associated with myopia []. Another study of preterm children also suggested that more time spent outdoors was associated with a lower risk of myopia, despite serum 25(OH)D3 concentrations not being shown to relate to myopia []. However, an Australian perspective study showed that, in young adults, myopia was most strongly associated with recent 25(OH)D3 concentrations, which is a marker of time spent outdoors [].
Our meta-analysis found that the risk of myopia is inversely associated with blood 25(OH)D3 concentration after adjusting for sunlight exposure or time spent outdoors. However, this relationship was not significant among individuals under 18 years of age []. Polymorphisms in the vitamin D pathway genes may affect the development of myopia. One study reported the association of VDR polymorphisms, rs2853559, with myopia []. However, the results of other studies suggested that the true contribution of the vitamin D pathway to myopia could be negligible [,,]. Our meta-analysis suggested that polymorphisms in the VDR gene are not associated with myopia []. On the other hand, animal studies proved that violet light (VL, λ = 360–400 nm) can suppress myopia progression, whereas no therapeutic effects were observed with UVB radiation (λ = 290–315 nm) [], suggesting that UVB exposure and its dependent vitamin D synthetic pathway may not have a protective effect on myopia progression.
In conclusion, from the literature evidence, we know that, although blood 25(OH)D3 concentration is inversely associated with the risk of myopia, it seems unlikely that vitamin D has a direct protective effect on myopia progression. Instead, vitamin D levels may only serve as a biomarker for outdoor exposure.
3.3. Age-Related Macular Degeneration
As a chronic, progressive, degenerative disease, age-related macular degeneration (AMD) is a major cause of central blindness among people aged 60 years or over worldwide [,]. Oxidation, inflammation, and angiogenesis contribute to the pathogenesis of AMD, resulting in the dysfunction of RPE [], Bruch’s membrane, and choriocapillaries []. In an aging retina, the complement cascade [,] and the tissue resident macrophage (retinal microglia) activation pathway [] ultimately cause protein damage and aggregation, and degeneration of the RPE []. Angiogenesis, often caused by oxidative stress and inflammatory reactions, plays a major role in the development and progression of exudative AMD, potentially leading to severe and permanent visual impairment.
The results of studies on cell lines and animal models have shown that vitamin D can protect cells or reduce oxidative stress [,,]. Vitamin D has an anti-inflammatory role in chronic inflammatory diseases by decreasing the proliferation of T-cells and the production of pro-inflammatory agents [,]. On the other hand, vitamin D exerted an inhibitory effect on the angiogenesis signaling pathway [,], which may play a protective role in exudative AMD development and/or progression. Morrison et al. studied the variants in the vitamin D catabolizing enzyme, CYP24A1, and reported that variants (rs1570669, rs1570670, rs2274130, rs2296239, and rs4809957) were associated with reduced risk for AMD [].
Table 2 summarized the studies on vitamin D and AMD. Case-control studies with small sample sizes suggest that AMD patients always have relatively low levels of serum vitamin D [,,,,,,,,,,], except in a Iranian study, which did not find any significant correlation between serum vitamin D level and AMD []. However, this association seems to change in cross-sectional studies with larger sample sizes. Population-based studies held in France [], the United States [,], and Israel [] did not support a specific role for vitamin D in AMD, but vitamin D may work in some specific populations. An analysis of a sample of 1313 US participants indicated that high serum 25(OH)D3 concentrations may protect against early AMD in women less than 75 years old [], while another US study supported the fact that levels of serum vitamin D were inversely associated with early AMD but not advanced AMD []. A Korean study had 17,045 participants and found that a high level of vitamin D was inversely associated with late AMD in men but not women []. Vitamin D deficiency in the European population was found to be associated with nvAMD, but the adjusted OR was small, and cannot exclude residual confounding [].

Table 2.
Summary of studies related to age-related macular degeneration included.
Prospective studies, however, have not found a consistent association between vitamin D and the risk of developing AMD. In a large prospective cohort study of 2146 participants with a mean follow-up time of over 9 years, high dietary intake of vitamin D was significantly associated with a 40% lower risk of progression to advanced AMD []. However, recently, a nationwide, placebo-controlled, randomized clinical trial found that supplementing vitamin D had no significant overall effect on AMD incidence or progression in healthy people []. For this trial, 25,871 participants with a median age of 67.1 years were divided into four groups, receiving vitamin D supplements (2000 IU/day), ω-3 fatty acids (1 g/day), a combination of both, and placebo, respectively. After a median follow-up period of 5.3 years, no significant differences were found in the incidence or progression of AMD when compared with baseline []. This study suffered from a lack of stratification by clinical manifestations of AMD, a relatively short follow-up period for chronic disease, and a reliance on self-reported AMD diagnosis, leading to inconsistencies with the previous two studies [,].
In summary, cross-sectional studies suggest that vitamin D may have a protective effect on AMD formation, but this effect is small or may only work in a specific population. Furthermore, evidence from prospective cohort studies showed that continuously supplementing vitamin D may not reduce the risks of AMD over a period of several years.
3.4. Glaucoma
A leading cause of irreversible blindness, glaucoma is a group of optic neuropathies involving the death of retinal ganglion cells (RGCs) and the loss of their axons [,]. Two cross-sectional studies in South Korea reported that vitamin D deficiency is associated with glaucoma [,]. Similarly, a Chinese study found that the vitamin D deficiency, along with the presence of the BsmI ‘B’ allele and TaqI ‘t’ allele of the VDR gene, are relevant risk factors for glaucoma development []. Other studies in France [], Croatia [], the United States [,], and Turkey [] have demonstrated that glaucoma patients have lower serum vitamin D levels compared to normal controls. However, another Turkish case-control study found no statistically significant difference in serum vitamin D levels between glaucoma patients and control subjects []. Similarly, a recent large-sample study in the United States showed that dietary intake, supplements, and serum levels of vitamin D are not significantly related to the risk of glaucoma []. Notably, ethnicity may contribute to the pathogenesis of glaucoma, giving rise to different conclusions among these studies []. Most of the literature reported the association between vitamin D and glaucoma and that a lower vitamin D concentration was found in glaucoma patients when compared with the control group [,,,,], however, there were no findings on the association between vitamin D and the severity. Increases in vitamin D were associated with lower risks of having glaucoma (fourth quintile versus first quintile, OR 0.713, 95% confidence interval, 0.520 to 0.979) []. Only a limited study reported no statistically significant difference between the glaucoma group and the control group []; significantly lower vitamin D can only be found in advanced glaucoma patients [] (Table 3).

Table 3.
Summary of studies related to glaucoma included.
High intraocular pressure (IOP) is an important risk factor for glaucoma. In an animal study on non-human primates, vitamin D treatment modulated the expression of IOP–regulating genes, with IOP falling in a dose-dependent manner []. However, a human study found no association between serum 25(OH)D3 levels and IOP, nor significant changes in participants’ IOP levels after receiving 6 months of oral vitamin D supplements (20,000 IU twice weekly) compared to the placebo group []. This contradiction may be due to the oral intake of vitamin D, which may lower the availability of vitamin D in the eye. Patients with glaucoma were found to have lower 25(OH)D concentrations in aqueous humor [], and the IOP values were higher in cases of vitamin D deficiency []. Further studies are required to determine if vitamin D can be a potential intervention for glaucoma, especially through testing different supplement approaches.
Some studies have identified vitamin D as an independent risk factor for glaucoma; however, the role that vitamin D plays in relation to glaucoma remains uncertain. Apart from the elevated IOP pathway, vitamin D may participate in the oxidative stress pathway due to its anti-oxidation and anti-inflammatory abilities. In an in vivo study, 1,25(OH)2D3 ameliorated the effects of oxidative stress from hydrogen peroxide-induced toxicity in human RPE cells through antioxidant signaling pathways, leading to lower levels of reactive oxygen species (ROS), cytokines, and vascular endothelial growth factor (VEGF) []. Another study demonstrated that vitamin D significantly altered the inflammatory-related genes in glaucoma, suppressing the expression of the angiotensin I–converting enzyme (ACE), carbonic anhydrase (CA), and Ras homologue gene family member A (RhoA), while significantly increasing the expression of the cytokine A20 precursor (CCL20) in the small intestines of rats []. ACE inhibitors are neuroprotective for cultured retinal neurons and can lower IOP in humans [,], while CA inhibitors can lower IOP and increase blood flow in the retinal vasculature and optic nerve []. The suppression of RhoA through subsequent vitamin D treatment can reduce aqueous outflow resistance and enhance fluid outflow [,]. Lastly, CCL2, an intraocular pressure responsive cytokine, possesses a potential role in intraocular pressure regulation [].
In summary, all reported studies are cross-sectional studies (case-control studies and population surveys) and suggested the protective associations of vitamin D on glaucoma. Future studies should employ randomized clinical trial designs to investigate the causal relationship between glaucoma and low vitamin D levels or calcitriol deficiency.
3.5. Diabetic Retinopathy
Because of its ability to inhibit neovascularization, vitamin D has been studied in the development of diabetic retinopathy (DR). Many observational studies have examined the relationship between vitamin D levels and the prevalence or severity of DR, with most identifying an inverse association with both type 1 and 2 diabetes [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. However, a Chinese study has reported a lack of association between vitamin D deficiency and DR after adjusting for all potential covariates, such as demographics, physical measurements, laboratory measurements, related complications, comorbidities, and medications []. Another Indian study suggested a possible association of vitamin D deficiency with type 2 diabetes, but not specifically with DR []. As demonstrated in Table 4, in general, some of the studies reported an inverse correlation between the serum vitamin D and severity of retinopathy [,,,,,,,,,,,,]; similar findings were also reported, for example, the co-existence of low vitamin D and microvascular complications [] or the association between the severity of DR and the prevalence of vitamin D deficiency [,,] (Table 4); while some studies reported either no association or no significant difference between DR patients and healthy controls [,,,]. The agreement of the association between vitamin D deficiency and neuropathy is lower when compared with retinopathy. While some studies report that the risk for having diabetic neuropathy is higher in those with vitamin D deficiency [,], there is limited research on contrasting findings []. Further investigations are warranted.

Table 4.
Summary of studies related to diabetic retinopathy included.
Besides cross-sectional studies, a population-based prospective study also showed that a high level of vitamin D was associated with a lower risk of DR after 3 years []. A double-blind, placebo-controlled trial found that low blood 25(OH)D3 levels were associated with an increased risk of macrovascular and microvascular disease events among type 2 diabetics [].
DR is a serious microvascular complication of diabetes. The characteristics of early DR include the loss of pericytes from retinal capillaries, the appearance of acellular capillaries and microaneurysms, and the breakdown of the blood-retinal barrier. In the proliferative phase of DR, neovascularization in the retina may occur, which significantly increases the probability of vision loss [,]. Potential mechanisms that explain how vitamin D can prevent DR include insulin resistance, immune regulation, anti-inflammation, and anti-angiogenesis. Animal studies have shown that vitamin D is important for insulin synthesis and can improve the body’s sensitivity to insulin, reducing the risk of insulin resistance [,]. Other studies have found that vitamin D treatment decreased the retinal expression of VEGF and the transforming growth factor TGF-β1 in rats [], which may have protective effects on the retina. VDR has also been implicated in the pathogenesis of DR []. A meta-analysis of seven studies evaluating the association of the VDR gene polymorphisms with DR found that the FokI polymorphism of the VDR gene has a significant association with DR susceptibility []. Apart from the VDR polymorphism, other studies have proposed different protective mechanisms of vitamin D on DR, including protecting the vasculature [,,], reducing oxidative stress [,], modulating inflammation and immune responses [,,,], inhibiting the renin-angiotensin aldosterone system [,], reducing the effects of advanced glycation end products [,], reducing endoplasmic reticulum stress [,], regulating endothelial cells apoptosis [], and regulating diabetic leukostasis []. Further studies are needed to determine the exact mechanisms of vitamin D on DR.
In summary, even though there are no consistent associations between vitamin D level and DR in observational studies, more than 30 reports suggested an inverse relationship. The same conclusion is made in perspective studies, although the causal relationship has not been identified. Further studies should investigate whether vitamin D supplementation can reduce the risk of DR.
3.6. Dry Eye Syndrome
Dry eye syndrome (DES), or dry eye disease [], is a common eye disease affecting about 12% of the world’s population; prevalence was lowest in North America (4.6%) and highest in Africa (47.9%) []. Many factors are related to DES, including hormonal alterations, environmental changes, and aging []. DES is accompanied by the inflammation of the ocular surface, which may cause visual disturbances, tear film instability, and potential damage []. Whereas an increasing number of studies have shown that a relationship exists between vitamin D and DES, their findings remained controversial. Some cross-sectional studies suggested an inverse correlation between vitamin D levels and ocular surface disease index (OSDI) scores or DES incidence [,,,,,,,,,,,,,,,], while three others have not reached a significant conclusion [,,,], as demonstrated in Table 5.

Table 5.
Summary of studies related to dry eye syndrome included.
Several clinical trials investigated the treatment effects of vitamin D supplementation on DES symptoms, as demonstrated. Some of them only involved DES patients [,,], while four other studies set healthy controls [,,,]. All these studies concluded that vitamin D can improve the DES symptoms of tear quality and ocular surface conditions. However, these studies may suffer from a small sample size or lack of a placebo-control group. Further well-design clinical trials with more samples are required to better understand the relationship between vitamin D and DES.
The key mechanism of vitamin D on DES may involve its antioxidation, anti-inflammatory, and immune-regulatory effects [,,]. Vitamin D deficiency may cause the inflammation of the ocular surface and ultimately DES []. Conversely, vitamin D may relieve DES through inhibiting the interleukin-6 (IL-6) inhibitor [], the key mediator of localized inflammation []. Moreover, vitamin D can suppress the release of inflammatory cytokines and stimulate the release of antioxidant cytokines in tears. Lastly, vitamin D can improve corneal epithelial barrier functions [], which may improve ocular conditions. Apart from the role of vitamin D in the pathogenesis of DES, a study reported that the expression of VDR and CYP27B1 (vitamin D metabolism enzyme) was significantly decreased in DED patients, suggesting the possible involvement of the vitamin D regulatory enzyme in protecting the human eye from dry eye []. A study on SNPs of the VDR gene Apa-1, Bsm-1, Fok-1 and Taq-1, reported the association of Apa-1 and Taq-1 with the risk of DES [].
In conclusion, the exact mechanisms of vitamin D on DES are unclear, but evidence from cross-sectional studies seems to suggest that vitamin D has a protective effect against DES. Limit evidence from clinical trials also suggests that vitamin D supplementation could help to improve DES symptoms, but a further placebo RCT is needed to verify this treatment effect.
3.7. Thyroid Eye Diseases
Thyroid eye disease (TED), also known as Graves’ ophthalmopathy (GO), is an autoimmune inflammatory disorder. Few studies have examined the relationship between vitamin D and TED (Table 6). A pilot study in Texas, United States found prevalence rates of 20% and 31% for vitamin D deficiency and insufficiency among TED patients, respectively []. Another retrospective case-control study comparing vitamin D levels between Graves’ disease patients and TED patients found that low serum vitamin D was associated with TED []. Assessing and supplementing vitamin D levels may be an important addition to the early management strategies of GD patients. Since vitamin D can regulate immune responses and reduce inflammation, there is a definite need to further evaluate the role of vitamin D deficiency in TED patients. The current evidence for TED suggests that vitamin D would be associated with TED, however, the evidence is limited. It is worthwhile to further investigate the effect of vitamin D on TED.

Table 6.
Summary of studies related to thyroid eye disease included.
3.8. Uveitis
Uveitis is the inflammation of the uvea driven by the T-cells []. It can be described as a failure of the ocular immune system, with the disease resulting from inflammation and tissue destruction. Since vitamin D can inhibit inflammation, influence T-cell responses, and regulate the immune system, it is necessary to examine its involvement in the development of uveitis. In an experimental autoimmune uveitis model, the oral administration of calcitriol was found to prevent and reverse the progression of uveitis by reducing the immunological response [].
Although in a population-based study, none of the 25 uveitis patients were found to have vitamin D deficiency [], two large retrospective case-control studies in the United States found an association of lower vitamin D levels with uveitis and scleritis, respectively [,]. Other case-control studies have been conducted on patients with specific types of uveitis, such as anterior uveitis (AAU) or non-infectious anterior uveitis [,,], Vogt-Koyanagi-Harada (VKH) disease [], sarcoidosis-associated uveitis [], and juvenile idiopathic arthritis (JIA)-associated uveitis [] (Table 7). All these studies suggested that the vitamin D deficiency is associated with uveitis development. A recent prospective case-control study consistently reported the association of vitamin D levels with active and inactive non-infectious uveitis patients. Vitamin D levels are related to uveitis severity [].

Table 7.
Summary of studies related to uveitis included.
There are reported associations between VDR polymorphisms and uveitis. Single-nucleotide polymorphisms (SNPs) of the CYP2R1, CYP27B1, CYP24A1 and DHCR7 genes are linked with lower circulating vitamin D levels [,,,]. A study in a Chinese cohort found that the frequencies of the genotype TT and T allele of DHCR7 rs12785878 were both significantly higher among ocular Behçet disease (BD) patients compared with healthy controls; however, similar associations were not found for the VKH, AAU with ankylosing spondylitis (AS), or pediatric uveitis []. Another cross-sectional study found that gene variants involved in vitamin D anabolism and catabolism may be important for VKH pathology [].
In summary, much evidence has shown that the onset and activity of uveitis are associated with vitamin D level, but most of the above studies were observational and did not provide evidence for causality. In the future, randomized-controlled studies are required to evaluate whether vitamin D can be an option for treating uveitis.
3.9. Retinoblastoma
Vitamin D has antineoplastic functions against many types of cancers through influencing cell differentiation, apoptosis regulation, anti-angiogenesis, and cell cycle arrest in various tumors []. Animal studies suggested that vitamin D analogues inhibited retinoblastoma (RB) tumor growth in athymic mice by increasing apoptosis, which is associated with the upregulation of both the p53 and p21 proteins [,]. However, due to the extremely low incidence rate of RB, few clinical studies have been conducted regarding the effect of vitamin D on RB. Two recent studies in Mexico found that sun exposure in early childhood protects against RB and may decrease the degree of intraocular spread in children with bilateral RB [,] (Table 8). However, RB is a childhood cancer, which usually presents at the age of 1 to 2 for bilateral and unilateral RB. Further studies are needed to confirm and understand the association between vitamin D levels and RB.

Table 8.
Summary of studies related to tumor included.
3.10. Cataract
For the past 20 years, the prevalence of cataracts has declined due to the advancement of surgical technology. However, in middle-income and low-income countries, cataract is still the most common cause of visual loss, accounting for 50% blindness []. Cataract is caused by losing lens transparency when the lens becomes opaque []. Epidemiology studies have shown that ultraviolet radiation is an important factor in increasing the risk of cataract [,,]. Since the natural source of vitamin D is sunlight exposure, vitamin D may be involved in the pathophysiology of cataract. The opacity of the lens is a result of oxidative stress []. Since vitamin D can protect cells or reduce oxidative stress [,,], it can be protective against cataract and play a role in lens metabolism.
There are studies attempting to reveal the association between vitamin D and cataract (Table 9). A large cross-sectional study in South Korea, with 16,086 participants aged 40 years or older, revealed that serum 25(OH)D levels were inversely associated with the risk of nuclear cataract []. Similarly, another South Korean study on 18,804 subjects also found that cataract risk decreased in men with higher serum 25-hydroxyvitamin D levels compared with those with lower serum 25(OH)D levels, but this association is not significant in women []. Other studies in Egypt [], Turkey [,], and the UK [] also showed that cataract patients often have a low level of serum vitamin D. However, one study [] reported that serum 25(OH)D levels were not related to nuclear opacities.

Table 9.
Summary of studies related to cataract included.
In summary, all the current studies are cross-sectional or retrospective in design, so the causal relationship between vitamin D and cataract needs to be proved by further large-prospective cohort or RCT. Cataract is essentially a treatable disease. Future study design should aim to find out whether vitamin D supplementation could protect elders against cataract development.
3.11. Other Ocular Diseases
Apart from the diseases described earlier, some studies reported on the association of vitamin D with curable or rare ocular diseases (Table 10). Vernal keratoconjunctivitis patients were found to be at a significantly lower level of serum vitamin D in case-control studies held in Turkey [,], Italy [], and Iran []. Deficiency of vitamin D is also more frequently found in patients with keratoconus [,,], retinal venous occlusions [,], and optic neuritis [,,]. However, in children with allergic conjunctivitis, the results were contradictory: two studies [,] found significantly higher vitamin D levels in patients, while the other studies reported complete opposite conclusion [,].
It is notable that vitamin D is associated with most ocular diseases, from anterior segment to retina. One possible explanation is that vitamin D or its metabolite plays a role in maintaining the stability of ocular metabolism and structure. Higher 25(OH)D levels in aqueous humor may have an influence on ocular disease [,]. The change of ocular structure and function, including spatial contrast [], contrast sensitivity [], choroidal thickness [,,], corneal endothelial [], and macular thickness [], may be affected by serum levels of vitamin D. On the other hand, this association is not a causality. Vitamin D may work as a marker of health status. People with poor health or low vision will have little outdoor activities and consequentially less exposure to sunlight. Some studies found a positive association or no association between vitamin D levels and pterygium [,,,,]. Outdoor occupation is a major risk factor for the development of pterygium [,].

Table 10.
Summary of studies related to other ocular diseases included.
Table 10.
Summary of studies related to other ocular diseases included.
| First Author | Years | Country | Disease | Study-Design | Sample Size | Main Finding | Rate # |
|---|---|---|---|---|---|---|---|
| Gonul Karatas Durusoy [] | 2020 | Turkey | VKC | Case-control study | 46 VKC patients and 40 healthy controls | Children with VKC has a lower serum 25(OH)D3 levels when compared with healthy controls. | 4b |
| Anna Maria Zicari [] | 2016 | Italy | VKC | Prospective | 47 VKC patients and 63 healthy controls | Children with VKC has a lower serum 25(OH)D3 levels when compared with healthy controls. And the vitamin D level was significantly correlated with the severity. | 3b |
| Banu Bozkurt [] | 2016 | Turkey | VKC | Case-control study | 29 VKC patients and 62 healthy controls | Serum 25(OH)D3 levels in VKC children was significantly lower than those healthy control. 48.3% of VKC children and 22.6% healthy children were found to have severe vitamin D deficiency. | 4b |
| Rana Sorkhabi [] | 2021 | Iran | VKC | Case-control study | 39 VKC patients and 32 healthy controls | Serum 25(OH)D3 levels in VKC patients were significantly lower than healthy controls. A statically insignificant reverse correlation of the serum vitamin D levels and the severity were found. | 4a |
| Daniele Giovanni Ghiglioni [] | 2019 | Italy | VKC | Prospective | 71 VKC patients (mixed, 46; tarsal, 19; and limbal, 6) | There was a significant different in serum 25(OH)D3 levels in children with limbal VKC and tarsal VKC. The ocular treatment with immunomodulator eye drops allow the improvement in serum 25(OH)D3 levels. | 3b |
| Mehmet Gökhan Aslan [] | 2021 | Italy | KCN | Case-control study | 28 progressive KCN patients, 27 nonprogressive KCN patients and 30 healthy controls | Serum 25(OH)D3 levels in KCN were significantly lower than healthy controls. Decreased vitamin D levels significantly increased nonprogressive KCN and progressive KCN probability 1.23 and 1.29 times, respectively. | 4a |
| Serkan Akkaya [] | 2020 | Turkey | KCN | Case-control study | 100 KCN patients and 100 healthy controls | Serum 25(OH)D3 levels were significantly lower in KCN group than healthy control group, but no significant difference in the distribution of vitamin D levels among KCN groups of different severity. | 4a |
| Siamak Zarei-Ghanavati [] | 2020 | Iran | KCN | Cross-sectional | 100 KCN patients and 100 healthy controls | A lower serum 25(OH)D3 level was found in the KCN group compared to the control group, but insignificant differences were found among different KCN stage groups. | 4a |
| Sevil Bilir Goksugur [] | 2015 | Turkey | ARC | Case-control study | 22 ARC patients and 31 healthy controls | Serum 25(OH)D3 levels were associated with ARC in children. | 4a |
| Alper Yenign [] | 2015 | Turkey | ARC | Prospective cross-sectional study | 42 ARC patients and 35 healthy controls | Plasma 25(OH)D3 levels in ARC patients were significantly lower than the control group. | 4a |
| Zeynep Dadaci [] | 2014 | Turkey | Seasonal ARC | Case-control study | 49 seasonal ARC patients and 44 healthy controls | Plasma 25(OH)D3 levels of seasonal ARC were significantly lower than control group. | 4a |
| Farhan Khashim Alswailmi [] | 2021 | Saudi Arabi | Seasonal ARC | Cross-sectional case-control study | 26 seasonal ARC patients and 26 healthy controls | Mean vitamin D level was significantly higher in seasonal ARC patients. Higher serum vitamin D levels may be linked with seasonal ARC. | 4b |
| Lin Chen [] | 2014 | China | Chalazia | Prospective case-control study | 88 chalazia patients and 72 healthy controls | No significant differences in vitamin D3 | 4b |
| Kubra Serefoglu Cabuk [] | 2020 | Turkey | Blepharospasm | Prospective case-control study | 50 Blepharospasm patients and 22 healthy controls | Serum 25(OH)D3 levels were moderately negatively correlated with Blepharospasm severity (Jankovic severity score). | 4b |
| Emrah Utku Kabatas [] | 2017 | Turkey | Retinopathy | Prospective | 97 premature infants patients | Serum 25(OH)D3 levels were significantly lower in infants with retinopathy of prematurity than those without retinopathy of prematurity. | 3a |
| Sedat Arikan [] | 2020 | Turkey | Spatial contrast | Prospective | 41 VDD patients and 30 without VDD controls | Vitamin D deficiency cause a decrease in contrast sensitivity function. | 3a |
| Emrah Ozturk [] | 2020 | Turkey | Contrast sensitivity | Prospective | 42 VDD patients and 34 normal levels control | VDD had negative effects on contrast sensitivity function and also caused thickness difference in certain segments of retinal layers. | 3a |
| Aydemir Gozde Aksoy [] | 2022 | Turkey | Choroidal thickness | Case-control study | 46 DM with VDD patients, 42 DM with normal vitamin D level patients, and 73 healthy controls | No difference in retinal nerve fibre layer (RNFL) between three groups. VDD has no effect on the RNFL. However, a positive correlation existed between the macular choroidal thickness (CT) and the vitamin D levels in DM patients with VDD. | 4a |
| Esra Vural [] | 2020 | Turkey | Choroidal thickness | Prospective case-control study | 30 VDD patients and 30 normal level controls | A positive correlation was found between vitamin D levels and subfoveal choroidal thickness and inferior and nasal peripapillary choroidal thickness in all participants. | 4a |
| Hasan Oncul [] | 2020 | Turkey | Choroidal thickness | Prospective case-control study | 65 VDD patients and 60 normal level controls | VDD patients have a thinner choroid and the choroidal thickness increased after vitamin D replacement therapy. | 4a |
| Cem Cankaya [] | 2018 | United States | Corneal endothelial | Case-control study | 58 VDD patients and 40 normal level controls | The mean corneal endothelial cell density and mean hexagonal cell ratio in VDD patients were lower than healthy controls. The mean coefficient of variation in VDD patients were higher than healthy controls. VDD may affect the corneal endothelial layer. | 4a |
| Alix Graffe [] | 2014 | France | Macular thickness | Cross-sectional | 62 patients (17 and 45 with vitamin D insufficiency and sufficiency, respectively) | Vitamin D insufficiency was associated with reduced macular thickness with no patent macular dysfunction. | 4a |
| Unal Mutlu [] | 2016 | Netherlands | Retinal microvascular | Prospective population-based study | 5675 subjects (sample) and 2973 subjects (subsample) | Individuals with lower vitamin D levels were more likely to have retinopathy. Lower vitamin D levels were associated with wider venular calibers. | 3a |
| Hatice Daldal [] | 2021 | Turkey | Ocular findings | Prospective | 98 patients (41, 45 and 12 were vitamin D severe deficient, deficient and insufficient, respectively) | Vitamin D may be related to thinning in macular and nasal of RNFL. | 3a |
| Karabulut Mujdat [] | 2022 | Turkey | Retinal microvascularity | Case-control study | 98 VDD patients and 96 healthy controls | There was a strong negative correlation between the serum vitamin D level and vessel density in the whole image, parafoveal, and perifoveal regions of the deep capillary plexus in the study group (Spearman’s rho = −0.71, p = 0.043; Spearman’s rho = −0.79, p = 0.011; and Spearman’s rho = −0.74, p = 0.032; respectively). | 4a |
VKC: vernal keratoconjunctivitis; KCN: keratoconus; ARC: allergic rhinoconjunctivitis; VDD: vitamin D deficiency; # LEGEND for case-control, cohort, and cross-sectional studies, rating of the studies follow the guidelines from LEGEND.
4. Perspective
In this systematic review, we summarize the evidence of vitamin D’s effect on different ocular diseases. However, there are significant limitations in many studies, and the interpretation of results should be within these limitations.
First, the definition of vitamin D deficiency is not consistent in different studies, which affects the objective comparison of results from different studies. Until 1998, vitamin D deficiency was defined as a blood level of 25(OH)D, which represents a total concentration of both 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 of less than 10 ng/mL (25 nmol/L). However, this definition was redefined in 1998 as a blood level of 25(OH)D < 20 ng/mL (50 nmol/L). One reason for the change in the involvement of PTH: adults may require a serum 25(OH)D of at least 50 nmol/L to achieve optimum PTH levels []. Moreover, some studies use quintiles or quartiles to perform data analysis. We suggest that future studies should use the common classification to analyze serum vitamin D levels.
Second, vitamin D levels are related to different socioeconomic, lifestyle, and dietary factors. These factors are very important when considering the onset or development of ocular diseases. Future studies should determine these factors and consider them as confounders in statistical analysis.
Finally, few studies provided causal evidence on whether supplementing vitamin D can reduce the prevalence of ocular diseases. Currently, available evidence is insufficient to confirm how vitamin D works in ocular diseases. Moreover, investigations of those metabolic enzymes are also important to understand why significantly low vitamin D is found in patients with ocular diseases. In summary, among all ocular diseases, the association between vitamin D and AMD was unclear, while clinical trials in DES or DR required bigger sample sizes in different ethnic populations. Other ocular diseases such as cataract, myopia, and uveitis, no RCTs or perspective cohorts have been reported, so their associations with vitamin D are still unclear.
5. Vitamin D and Eye Care
Vitamin D can be a potential intervention for different ocular diseases. Although findings for certain ocular diseases are inconsistent, they can still serve as references for further studies that examine the therapeutic effects of vitamin D on ocular diseases, considering that vitamin D deficiency is a common health issue worldwide, and vitamin D has wide safety doses and rare side effects. Even though more evidence from the randomized controlled trial is needed to confirm the effect of vitamin D on various ocular diseases, it is recommended to maintain blood 25(OH)D3 at a desirable level (25–50 nmol/L) by spending a short period of time outdoors, baring skin to the sun, and boosting vitamin D intake by a daily supplement of 400–800 international units (10 to 20 μg).
The human body can also obtain vitamin D naturally from sunlight exposure, however, both extended exposure to unprotected sunshine, which also increases the risk for cataracts, and AMD and completely avoiding sunlight by applying UV B sunscreen should be avoided. Several factors affect vitamin D production, and a more efficient production of vitamin D can be achieved when someone is closer to the equator, has a lighter skin color, and/or exposes larger surfaces of skin during summer midday without sunscreen. Generally, 5–30 min of sun exposure on the unprotected face, arms, legs, or back between 10 a.m. and 3 p.m. twice to three times a week is enough for sufficient vitamin D. Even though vitamin D can be beneficial to our ocular health, long term prolonged sun exposure is also associated with corneal sunburn, tissue growths on sclera, cataracts, and macular degeneration, and wearing hats, and sunglasses are recommended to protect the eyes from UV damage.
6. Conclusions
Various epidemiological and clinical studies have demonstrated a connection between vitamin D deficiency and ocular diseases, such as myopia, age-related macular degeneration, glaucoma, diabetic retinopathy, dry eye syndrome, thyroid eye disease, uveitis, retinoblastoma, and cataract, among others. While vitamin D is associated with potential pathways related to these respective diseases, their pathogeneses are complicated, and current understandings of the underlying mechanisms remain limited. Vitamin D not only affects mineral metabolism homeostasis, but also possesses antioxidation and anti-inflammatory properties. It also plays a role in anti-angiogenesis, modulating cell cycle including cell proliferation, differentiation, and apoptosis. VDR and vitamin D regulatory enzymes are present in ocular tissues, and studies have demonstrated that ocular tissues can activate and regulate vitamin D, suggesting the importance of vitamin D in maintaining ocular health.
Author Contributions
Conceptualization, H.-N.C., X.-J.Z., J.C.Y., C.-P.P.; methodology, H.-N.C. and X.-J.Z.; validation, H.-N.C., X.-J.Z. and X.-T.L.; formal analysis, H.-N.C., X.-J.Z. and X.-T.L.; investigation, H.-N.C. and X.-J.Z.; writing—original draft preparation, H.-N.C., X.-J.Z., X.-T.L. and C.H.-T.B.; writing—review and editing, Y.-M.W., P.I., W.-K.C., L.-J.C., C.C.T., J.C.Y. and C.-P.P.; supervision, J.C.Y. and C.-P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the General Research Fund (GRF), Research Grants Council, Hong Kong (14111515 and 14103419 [JCY]); Collaborative Research Fund (C7149-20G [JCY]); Health and Medical Research Fund (HMRF), Hong Kong (5160836, [LJC] and 07180826 [XJZ]), and the Direct Grants of the Chinese University of Hong Kong, (4054193 [LJC] and 4054121 & 4054199 [JCY] and 4054634 [XJZ]), the Innovation and Technology Fund (7010590 [JCY]), the UBS Optimus Foundation Grant 8984 (JCY); the Centaline Myopia Fund [JCY]; the CUHK Jockey Club Children’s Eye Care Programme; and the CUHK Jockey Club Myopia Prevention Programme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef]
- Zmijewski, M.A. Vitamin D and Human Health. Int. J. Mol. Sci. 2019, 20, 10145. [Google Scholar] [CrossRef]
- Zhang, R.; Naughton, D.P. Vitamin D in health and disease: Current perspectives. Nutr. J. 2010, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Kubatka, P.; Brozmanova, M.; Gazdikova, K.; Caprnda, M.; Büsselberg, D. Health implication of vitamin D on the cardiovascular and the renal system. Arch. Physiol. Biochem. 2021, 127, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Agrawal, D.K. Role of Vitamin D in Cardiovascular Diseases. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef]
- Adamczak, M.; Surma, S.; Więcek, A. Vitamin D and Arterial Hypertension: Facts and Myths. Curr. Hypertens. Rep. 2020, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, K.G.; Tamilselvan, B.; Rajendran, A. Role of Vitamin D in Diabetes. J. Endocrinol. Metab. 2011, 1, 47–56. [Google Scholar] [CrossRef][Green Version]
- Jani, R.; Mhaskar, K.; Tsiampalis, T.; Kassaw, N.A.; González, M.Á.M.; Panagiotakos, D.B. Circulating 25-hydroxy-vitamin D and the risk of cardiovascular diseases. Systematic review and meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3282–3304. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Gamba, G.; Riccardi, D.; Lombardi, M.; Butters, R.; Kifor, O.; Sun, A.; Hediger, M.A.; Lytton, J.; Hebert, S.C. Cloning and characterization of an extracellular Ca(2+)–sensing receptor from bovine parathyroid. Nature 1993, 366, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Kim, S.; Shevde, N.K.; Pike, J.W. 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA–binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J. Bone Miner. Res. 2005, 20, 305–317. [Google Scholar] [CrossRef]
- Skowron, K.; Pawlicka, I.; Gil, K. The role of vitamin D in the pathogenesis of ocular diseases. Folia Med. Cracov. 2018, 58, 103–118. [Google Scholar]
- Jamali, N.; Wang, S.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Vitamin D receptor expression is essential during retinal vascular development and attenuation of neovascularization by 1, 25(OH)2D3. PLoS ONE 2017, 12, e0190131. [Google Scholar] [CrossRef]
- Reins, R.Y.; McDermott, A.M. Vitamin D: Implications for ocular disease and therapeutic potential. Exp. Eye Res. 2015, 134, 101–110. [Google Scholar] [CrossRef]
- Alsalem, J.A.; Patel, D.; Susarla, R.; Coca-Prados, M.; Bland, R.; Walker, E.A.; Rauz, S.; Wallace, G.R. Characterization of vitamin D production by human ocular barrier cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Pintea, V.; Lin, Y.; Hammock, B.D.; Watsky, M.A. Vitamin D enhances corneal epithelial barrier function. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7359–7364. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, A.; Parmentier, M.; Chirnoaga, M.; Lawson, D.E.; Pasteels, J.L.; Pochet, R. Vitamin D–dependent calcium binding protein immunoreactivity in human retina. Ophthalmic Res. 1986, 18, 209–214. [Google Scholar] [CrossRef]
- Rullo, J.; Pennimpede, T.; Mehraban Far, P.; Strube, Y.N.; Irrcher, I.; Urton, T.; Bona, M.; Gonder, T.; Campbell, R.J.; Ten Hove, M.; et al. Intraocular calcidiol: Uncovering a role for vitamin D in the eye. J. Steroid Biochem. Mol. Biol. 2020, 197, 105536. [Google Scholar] [CrossRef] [PubMed]
- Al-Barry, M.A.; Albalawi, A.M.; Sayf, M.A.; Badawi, A.; Afzal, S.; Latif, M.; Samman, M.I.; Basit, S. Sequence analysis of four vitamin D family genes (VDR, CYP24A1, CYP27B1 and CYP2R1) in Vogt-Koyanagi-Harada (VKH) patients: Identification of a potentially pathogenic variant in CYP2R1. BMC Ophthalmol. 2016, 16, 172. [Google Scholar] [CrossRef]
- Serefoglu Cabuk, K.; Tunc, U.; Ozturk Karabulut, G.; Fazil, K.; Karaagac Gunaydin, Z.; Asik Nacaroglu, S.; Taskapili, M. Serum calcium, magnesium, phosphorus, and vitamin D in benign essential blepharospasm. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1293–1297. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta–Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar]
- Clark, E.; Burkett, K.; Stanko-Lopp, D. Let Evidence Guide Every New Decision (LEGEND): An evidence evaluation system for point-of-care clinicians and guideline development teams. J. Eval. Clin. Pract. 2009, 15, 1054–1060. [Google Scholar] [CrossRef]
- Dolgin, E. The myopia boom. Nature 2015, 519, 276–278. [Google Scholar] [CrossRef]
- Zadnik, K.; Satariano, W.A.; Mutti, D.O.; Sholtz, R.I.; Adams, A.J. The effect of parental history of myopia on children’s eye size. JAMA 1994, 271, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.S.; Fan, D.S.; Lam, R.F.; Rao, S.K.; Chong, K.S.; Lau, J.T.; Lai, R.Y.; Cheung, E.Y. The effect of parental history of myopia on children’s eye size and growth: Results of a longitudinal study. Investig. Ophthalmol. Vis. Sci. 2008, 49, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Verhoeven, V.J.; Wojciechowski, R.; Barathi, V.A.; Hysi, P.G.; Guggenheim, J.A.; Hohn, R.; Vitart, V.; Khawaja, A.P.; Yamashiro, K.; et al. Meta–analysis of gene–environment–wide association scans accounting for education level identifies additional loci for refractive error. Nat. Commun. 2016, 7, 11008. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.A.; Morgan, I.G.; Ip, J.; Kifley, A.; Huynh, S.; Smith, W.; Mitchell, P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008, 115, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Dirani, M.; Tong, L.; Gazzard, G.; Zhang, X.; Chia, A.; Young, T.L.; Rose, K.A.; Mitchell, P.; Saw, S.M. Outdoor activity and myopia in Singapore teenage children. Br. J. Ophthalmol. 2009, 93, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Sinnott, L.T.; Mutti, D.O.; Mitchell, G.L.; Moeschberger, M.L.; Zadnik, K. Parental history of myopia, sports and outdoor activities, and future myopia. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3524–3532. [Google Scholar] [CrossRef] [PubMed]
- Low, W.; Dirani, M.; Gazzard, G.; Chan, Y.H.; Zhou, H.J.; Selvaraj, P.; Au Eong, K.G.; Young, T.L.; Mitchell, P.; Wong, T.Y.; et al. Family history, near work, outdoor activity, and myopia in Singapore Chinese preschool children. Br. J. Ophthalmol. 2010, 94, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xiang, F.; Zeng, Y.; Mai, J.; Chen, Q.; Zhang, J.; Smith, W.; Rose, K.; Morgan, I.G. Effect of Time Spent Outdoors at School on the Development of Myopia Among Children in China: A Randomized Clinical Trial. JAMA 2015, 314, 1142–1148. [Google Scholar] [CrossRef]
- Choi, J.A.; Han, K.; Park, Y.M.; La, T.Y. Low serum 25-hydroxyvitamin D is associated with myopia in Korean adolescents. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2041–2047. [Google Scholar] [CrossRef]
- Guggenheim, J.A.; Williams, C.; Northstone, K.; Howe, L.D.; Tilling, K.; St. Pourcain, B.; McMahon, G.; Lawlor, D.A. Does vitamin D mediate the protective effects of time outdoors on myopia? Findings from a prospective birth cohort. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8550–8558. [Google Scholar] [CrossRef]
- Mutti, D.O.; Marks, A.R. Blood levels of vitamin D in teens and young adults with myopia. Optom. Vis. Sci. 2011, 88, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Tideman, J.W.; Polling, J.R.; Voortman, T.; Jaddoe, V.W.; Uitterlinden, A.G.; Hofman, A.; Vingerling, J.R.; Franco, O.H.; Klaver, C.C. Low serum vitamin D is associated with axial length and risk of myopia in young children. Eur. J. Epidemiol. 2016, 31, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Yazar, S.; Hewitt, A.W.; Black, L.J.; McKnight, C.M.; Mountain, J.A.; Sherwin, J.C.; Oddy, W.H.; Coroneo, M.T.; Lucas, R.M.; Mackey, D.A. Myopia is associated with lower vitamin D status in young adults. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4552–4559. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.W.; Choi, J.A.; La, T.Y.; Epidemiologic Survey Committee of the Korean Ophthalmological Society. Serum 25-hydroxyvitamin D level is associated with myopia in the Korea national health and nutrition examination survey. Medicine 2016, 95, 5012. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.M.; Bentham, G.C.; Young, I.S.; McGinty, A.; McKay, G.J.; Hogg, R.; Hammond, C.J.; Chakravarthy, U.; Rahu, M.; Seland, J.; et al. Association Between Myopia, Ultraviolet B Radiation Exposure, Serum Vitamin D Concentrations, and Genetic Polymorphisms in Vitamin D Metabolic Pathways in a Multicountry European Study. JAMA Ophthalmol. 2017, 135, 47–53. [Google Scholar] [CrossRef]
- Specht, I.O.; Jacobsen, N.; Frederiksen, P.; Heitmann, B.L. Neonatal vitamin D status and myopia in young adult men. Acta Ophthalmol. 2020, 98, 500–505. [Google Scholar] [CrossRef]
- Jung, B.J.; Jee, D. Association between serum 25-hydroxyvitamin D levels and myopia in general Korean adults. Indian J. Ophthalmol. 2020, 68, 15–22. [Google Scholar]
- Chou, H.D.; Yao, T.C.; Huang, Y.S.; Huang, C.Y.; Yang, M.L.; Sun, M.H.; Chen, H.C.; Liu, C.H.; Chu, S.M.; Hsu, J.F.; et al. Myopia in school-aged children with preterm birth: The roles of time spent outdoors and serum vitamin D. Br. J. Ophthalmol. 2021, 105, 468–472. [Google Scholar] [CrossRef]
- Lingham, G.; Mackey, D.A.; Zhu, K.; Lucas, R.M.; Black, L.J.; Oddy, W.H.; Holt, P.; Walsh, J.P.; Sanfilippo, P.G.; Chan She Ping-Delfos, W.; et al. Time spent outdoors through childhood and adolescence–assessed by 25-hydroxyvitamin D concentration–and risk of myopia at 20 years. Acta Ophthalmol. 2021, 99, 679–687. [Google Scholar] [CrossRef]
- Han, S.B.; Jang, J.; Yang, H.K.; Hwang, J.M.; Park, S.K. Prevalence and risk factors of myopia in adult Korean population: Korea national health and nutrition examination survey 2013–2014 (KNHANES VI). PLoS ONE 2019, 14, e0211204. [Google Scholar] [CrossRef]
- Gao, F.; Li, P.; Liu, Y.Q.; Chen, Y. Association study of the serum 25(OH)D concentration and myopia in Chinese children. Medicine 2021, 100, e26570. [Google Scholar] [CrossRef] [PubMed]
- Lingham, G.; Yazar, S.; Lucas, R.M.; Walsh, J.P.; Zhu, K.; Hunter, M.; Lim, E.M.; Cooke, B.R.; Mackey, D.A. Low 25-Hydroxyvitamin D Concentration Is Not Associated With Refractive Error in Middle-Aged and Older Western Australian Adults. Transl. Vis. Sci. Technol. 2019, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Chun, M.Y.; Kim, J.S.; Oh, B.; Yoo, S.H.; Cho, B.J. Risk Factors for High Myopia in Koreans: The Korea National Health and Nutrition Examination Survey. Curr. Eye Res. 2018, 43, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Harb, E.N.; Wildsoet, C.F. Nutritional Factors and Myopia: An Analysis of National Health and Nutrition Examination Survey Data. Optom. Vis. Sci. 2021, 98, 458–468. [Google Scholar] [CrossRef]
- Wahyudi, D.; Reiki, W.; Hardhono; Suhartono. The Effect of Vitamin-D and Sunlight to Progressive Myopia in Students with Glasses Correction. Pak. J. Med. Health Sci. 2020, 14, 1588–1591. [Google Scholar]
- Li, X.; Lin, H.; Jiang, L.; Chen, X.; Chen, J.; Lu, F. Low Serum Vitamin D Is Not Correlated with Myopia in Chinese Children and Adolescents. Front. Med. 2022, 9, 809787. [Google Scholar] [CrossRef]
- Tang, S.M.; Lau, T.; Rong, S.S.; Yazar, S.; Chen, L.J.; Mackey, D.A.; Lucas, R.M.; Pang, C.P.; Yam, J.C. Vitamin D and its pathway genes in myopia: Systematic review and meta-analysis. Br. J. Ophthalmol. 2019, 103, 8–17. [Google Scholar] [CrossRef]
- Mutti, D.O.; Cooper, M.E.; Dragan, E.; Jones-Jordan, L.A.; Bailey, M.D.; Marazita, M.L.; Murray, J.C.; Zadnik, K.; Group, C.S. Vitamin D receptor (VDR) and group–specific component (GC, vitamin D–binding protein) polymorphisms in myopia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3818–3824. [Google Scholar] [CrossRef]
- Cuellar-Partida, G.; Williams, K.M.; Yazar, S.; Guggenheim, J.A.; Hewitt, A.W.; Williams, C.; Wang, J.J.; Kho, P.F.; Saw, S.M.; Cheng, C.Y.; et al. Genetically low vitamin D concentrations and myopic refractive error: A Mendelian randomization study. Int. J. Epidemiol. 2017, 46, 1882–1890. [Google Scholar] [CrossRef]
- Torii, H.; Kurihara, T.; Seko, Y.; Negishi, K.; Ohnuma, K.; Inaba, T.; Kawashima, M.; Jiang, X.; Kondo, S.; Miyauchi, M.; et al. Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. eBioMedicine 2017, 15, 210–219. [Google Scholar] [CrossRef]
- Ramin, S.; Soheilian, M.; Habibi, G.; Ghazavi, R.; Gharebaghi, R.; Heidary, F. Age-Related Macular Degeneration: A Scientometric Analysis. Med. Hypothesis Discov. Innov. Ophthalmol. 2015, 4, 39–49. [Google Scholar] [PubMed]
- Apte, R.S. Age-Related Macular Degeneration. N. Engl. J. Med. 2021, 385, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Coppé, A.M.; Ripandelli, G.; Parisi, V.; Varano, M.; Stirpe, M. Prevalence of asymptomatic macular holes in highly myopic eyes. Ophthalmology 2005, 112, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, Y.; Miller, J.W.; Kim, I.K. Age-Related Macular Degeneration: Advances in Management and Diagnosis. J. Clin. Med. 2015, 4, 343–359. [Google Scholar] [CrossRef]
- Khandhadia, S.; Cipriani, V.; Yates, J.R.; Lotery, A.J. Age-related macular degeneration and the complement system. Immunobiology 2012, 217, 127–146. [Google Scholar] [CrossRef]
- Park, Y.G.; Park, Y.S.; Kim, I.B. Complement System and Potential Therapeutics in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 36851. [Google Scholar] [CrossRef]
- Chen, M.; Muckersie, E.; Forrester, J.V.; Xu, H. Immune activation in retinal aging: A gene expression study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5888–5896. [Google Scholar] [CrossRef]
- Layana, A.G.; Minnella, A.M.; Garhofer, G.; Aslam, T.; Holz, F.G.; Leys, A.; Silva, R.; Delcourt, C.; Souied, E.; Seddon, J.M. Vitamin D and Age-Related Macular Degeneration. Nutrients 2017, 9, 1120. [Google Scholar] [CrossRef]
- Peng, X.; Vaishnav, A.; Murillo, G.; Alimirah, F.; Torres, K.E.; Mehta, R.G. Protection against cellular stress by 25-hydroxyvitamin D3 in breast epithelial cells. J. Cell. Biochem. 2010, 110, 1324–1333. [Google Scholar] [CrossRef]
- Diker-Cohen, T.; Koren, R.; Ravid, A. Programmed cell death of stressed keratinocytes and its inhibition by vitamin D: The role of death and survival signaling pathways. Apoptosis 2006, 11, 519–534. [Google Scholar] [CrossRef]
- Tohari, A.M.; Zhou, X.; Shu, X. Protection against oxidative stress by vitamin D in cone cells. Cell Biochem. Funct. 2016, 34, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.K.; Stehle, P.; Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double–blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Rheum. Dis. Clin. N. Am. 2012, 38, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Mantell, D.J.; Owens, P.E.; Bundred, N.J.; Mawer, E.B.; Canfield, A.E. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ. Res. 2000, 87, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol is a potent inhibitor of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef]
- Morrison, M.A.; Silveira, A.C.; Huynh, N.; Jun, G.; Smith, S.E.; Zacharaki, F.; Sato, H.; Loomis, S.; Andreoli, M.T.; Adams, S.M.; et al. Systems biology–based analysis implicates a novel role for vitamin D metabolism in the pathogenesis of age-related macular degeneration. Hum. Genom. 2011, 5, 538–568. [Google Scholar] [CrossRef]
- Song, W.S.; Yoon, W.T.; Kim, Y.K.; Park, S.P. Analysis of 25-Hydroxy Vitamin D in the Aqueous Humor of Age-related Macular Degeneration Patients. J. Korean Ophthalmol. Soc. 2018, 59, 1024–1029. [Google Scholar] [CrossRef]
- Graffe, A.; Milea, D.; Annweiler, C.; Beauchet, O.; Mauget-Faysse, M.; Beauchet, O.; Kodjikian, L.; Milea, D. Association between hypovitaminosis D and late stages of age-related macular degeneration: A case-control study. J. Am. Geriatr. Soc. 2012, 60, 1367–1369. [Google Scholar] [CrossRef]
- Hashemi, R.; Bandarian, M.; Abedi-Taleb, E.; Khojasteh, H.; Khedmat, L.; Asadollahi, E.; Beytollahi, M.; Jelodar, A.M. The association between blood vitamins D and E with age-related macular degeneration: A pilot study. Interv. Med. Appl. Sci. 2018, 10, 127–132. [Google Scholar]
- Kan, E.; Kan, E.K.; Yucel, O.E. The Possible Link Between Vitamin D Levels and Exudative Age-related Macular Degeneration. Oman. Med. J. 2020, 35, e83. [Google Scholar] [CrossRef]
- Kim, K.L.; Park, S.P. Association between serum vitamin D deficiency and age-related macular degeneration in Koreans: Clinical case-control pilot study. Medicine 2018, 97, e11908. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Falk, M.K.; Subhi, Y.; Sorensen, T.L. The association between plasma 25-hydroxyvitamin D and subgroups in age-related macular degeneration: A cross-sectional study. PLoS ONE 2013, 8, e70948. [Google Scholar] [CrossRef] [PubMed]
- Kabatas, N.; Dogan, A.S.; Yilmaz, M.; Kabatas, E.U.; Bicer, T.; Caliskan, S.; Celikay, O.; Ucar, F.; Gurdal, C. Association between age-related macular degeneration and 25(OH) vitamin D levels in the Turkish population. Arq. Bras. Oftalmol. 2022, 85, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Reynolds, R.; Shah, H.R.; Rosner, B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: Epigenetic implications. Ophthalmology 2011, 118, 1386–1394. [Google Scholar] [CrossRef]
- Uro, M.; Beauchet, O.; Cherif, M.; Graffe, A.; Milea, D.; Annweiler, C. Age-Related Vitamin D Deficiency Is Associated with Reduced Macular Ganglion Cell Complex: A Cross-Sectional High–Definition Optical Coherence Tomography Study. PLoS ONE 2015, 10, e0130879. [Google Scholar] [CrossRef]
- Mahgoub, M.Y.; Abou Ghanima, A.T.; Elmohamady, M.N.; Abdul Basset, S. Age-Related Macular Degeneration in Primary Osteoarthritis Egyptian Patients. Open Access Rheumatol. 2020, 12, 35–40. [Google Scholar] [CrossRef]
- Millen, A.E.; Nie, J.; Mares, J.A.; Lutsey, P.L.; LaMonte, M.J.; Meuer, S.M.; Sahli, M.W.; Andrews, C.A.; Klein, B.E.K.; Klein, R. Serum 25-Hydroxyvitamin D Concentrations and Incidence of Age-Related Macular Degeneration: The Atherosclerosis Risk in Communities Study. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1362–1371. [Google Scholar] [CrossRef]
- Cougnard-Gregoire, A.; Merle, B.M.; Korobelnik, J.F.; Rougier, M.B.; Delyfer, M.N.; Feart, C.; Le Goff, M.; Dartigues, J.F.; Barberger-Gateau, P.; Delcourt, C. Vitamin D Deficiency in Community–Dwelling Elderly Is Not Associated with Age-Related Macular Degeneration. J. Nutr. 2015, 145, 1865–1872. [Google Scholar] [CrossRef]
- Day, S.; Acquah, K.; Platt, A.; Lee, P.P.; Mruthyunjaya, P.; Sloan, F.A. Association of vitamin D deficiency and age-related macular degeneration in medicare beneficiaries. Arch. Ophthalmol. 2012, 130, 1070–1071. [Google Scholar] [CrossRef][Green Version]
- Millen, A.E.; Nie, J.; Sahli, M.W.; Mares, J.A.; Meyers, K.J.; Klein, B.E.K.; LaMonte, M.J.; Lutsey, P.L.; Andrews, C.A.; Klein, R. Vitamin D Status and Prevalent Early Age-Related Macular Degeneration in African Americans and Caucasians: The Atherosclerosis Risk in Communities (ARIC) Study. J. Nutr. Health Aging 2017, 21, 772–780. [Google Scholar] [CrossRef]
- Golan, S.; Shalev, V.; Treister, G.; Chodick, G.; Loewenstein, A. Reconsidering the connection between vitamin D levels and age-related macular degeneration. Eye 2011, 25, 1122–1129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Millen, A.E.; Meyers, K.J.; Liu, Z.; Engelman, C.D.; Wallace, R.B.; LeBlanc, E.S.; Tinker, L.F.; Iyengar, S.K.; Robinson, J.G.; Sarto, G.E.; et al. Association between vitamin D status and age-related macular degeneration by genetic risk. JAMA Ophthalmol. 2015, 133, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.; Chappell, R.J.; Millen, A.E.; Albert, D.M.; Mares, J.A. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch. Ophthalmol. 2007, 125, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Han, K.; Jee, D. Inverse relationship between high blood 25-hydroxyvitamin D and late stage of age-related macular degeneration in a representative Korean population. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4823–4831. [Google Scholar] [CrossRef]
- McKay, G.J.; Young, I.S.; McGinty, A.; Bentham, G.C.; Chakravarthy, U.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; et al. Associations between Serum Vitamin D and Genetic Variants in Vitamin D Pathways and Age-Related Macular Degeneration in the European Eye Study. Ophthalmology 2017, 124, 90–96. [Google Scholar] [CrossRef]
- Itty, S.; Day, S.; Lyles, K.W.; Stinnett, S.S.; Vajzovic, L.M.; Mruthyunjaya, P. Vitamin D deficiency in neovascular versus nonneovascular age-related macular degeneration. Retina 2014, 34, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Voland, R.; Sondel, S.A.; Parekh, N.; Horst, R.L.; Wallace, R.B.; Hageman, G.S.; Chappell, R.; Blodi, B.A.; Klein, M.L.; et al. Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch. Ophthalmol. 2011, 129, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.G.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Chasman, D.I.; Lee, I.M.; Bubes, V.; Li, C.; Haubourg, M.; Schaumberg, D.A.; et al. Effect of Vitamin D and omega–3 Fatty Acid Supplementation on Risk of Age-Related Macular Degeneration: An Ancillary Study of the VITAL Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Silver, R.E.; Rosner, B.; Seddon, J.M. Associations Between Vitamin D Intake and Progression to Incident Advanced Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4569–4578. [Google Scholar] [CrossRef]
- Aoki, A.; Inoue, M.; Nguyen, E.; Obata, R.; Kadonosono, K.; Shinkai, S.; Hashimoto, H.; Sasaki, S.; Yanagi, Y. Dietary n-3 Fatty Acid, alpha-Tocopherol, Zinc, vitamin D, vitamin C, and beta-carotene are Associated with Age-Related Macular Degeneration in Japan. Sci. Rep. 2016, 6, 20723. [Google Scholar] [CrossRef]
- Beauchet, O.; Milea, D.; Graffe, A.; Fantino, B.; Annweiler, C. Association between serum 25-hydroxyvitamin D concentrations and vision: A cross-sectional population-based study of older adults. J. Am. Geriatr. Soc. 2011, 59, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Parravano, M.; Tedeschi, M.; Manca, D.; Costanzo, E.; Di Renzo, A.; Giorno, P.; Barbano, L.; Ziccardi, L.; Varano, M.; Parisi, V. Effects of Macuprev((R)) Supplementation in Age-Related Macular Degeneration: A Double-Blind Randomized Morpho–Functional Study Along 6 Months of Follow-Up. Adv. Ther. 2019, 36, 2493–2505. [Google Scholar] [CrossRef] [PubMed]
- Pérez Serena, A.; Martínez Betancourt, D.P.; González del Valle, F.; Ruiz-Moreno, J.M. Serum 25-hydroxy vitamin D levels in age-related macular degeneration. Int. J. Retin. Vitr. 2022, 8, 17. [Google Scholar] [CrossRef]
- Jacob, J.; Mangelschots, E.; Michez, M.; Sanak, S.N.; Leys, A. Cross-Sectional Study on Vitamin D, Zinc Oxide and Fatty Acid Status in a Population with a Moderate to High Risk of AMD Identified by the STARS((R)) Questionnaire. Ophthalmol. Ther. 2021, 10, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar]
- Mantravadi, A.V.; Vadhar, N. Glaucoma. Prim. Care 2015, 42, 437–449. [Google Scholar] [CrossRef]
- Yoo, T.K.; Oh, E.; Hong, S. Is vitamin D status associated with open–angle glaucoma? A cross-sectional study from South Korea. Public Health Nutr. 2014, 17, 833–843. [Google Scholar] [CrossRef]
- Kim, H.T.; Kim, J.M.; Kim, J.H.; Lee, M.Y.; Won, Y.S.; Lee, J.Y.; Park, K.H. The Relationship between Vitamin D and Glaucoma: A Kangbuk Samsung Health Study. Korean J. Ophthalmol. 2016, 30, 426–433. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, Q.; Ma, W.; Liu, H.; Ji, J.; Li, X. Associations of vitamin D deficiency and vitamin D receptor (Cdx-2, Fok I, Bsm I and Taq I) polymorphisms with the risk of primary open–angle glaucoma. BMC Ophthalmol. 2016, 16, 116. [Google Scholar] [CrossRef]
- Goncalves, A.; Milea, D.; Gohier, P.; Jallet, G.; Leruez, S.; Baskaran, M.; Aung, T.; Annweiler, C. Serum vitamin D status is associated with the presence but not the severity of primary open angle glaucoma. Maturitas 2015, 81, 470–474. [Google Scholar] [CrossRef]
- Vukovic Arar, Z.; Knezevic Pravecek, M.; Miskic, B.; Vatavuk, Z.; Vukovic Rodriguez, J.; Sekelj, S. Association Between Serum Vitamin D Level and Glaucoma in Women. Acta Clin. Croat. 2016, 55, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, R.; Chen, Y.I.; Zangwill, L.M.; Holman, M.; Dirkes, K.; Hai, Y.; Arzumanyan, Z.; Slight, R.; Hammel, N.; Girkin, C.A.; et al. Association of severity of primary open–angle glaucoma with serum vitamin D levels in patients of African descent. Mol. Vis. 2019, 25, 438–445. [Google Scholar] [PubMed]
- Ekiz, T.; Helvaci, S. Bone Mineral Density and 25-Hydroxyvitamin D Levels in Patients with Ocular Pseudoexfoliation Syndrome: A Case-Control Study. J. Clin. Densitom. 2016, 19, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Atalay, K.; Savur, F.G.; Kirgiz, A.; Kaldirim, H.E.; Zengi, O. Serum levels of thyroid hormone, vitamin D, vitamin B12, folic acid, C–reactive protein, and hemoglobin in Pseudoexfoliation and primary open angle Glaucoma. J. Fr. Ophtalmol. 2019, 42, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Dikci, S.; Ozturk, E.; Firat, P.G.; Yilmaz, T.; Taskapan, M.C.; Yologlu, S. The Association of Serum Vitamin D Levels with Pseudoexfoliation Glaucoma/Syndrome. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.D.; Johnson, K.; Larson, J.C.; Thomas, F.; Wactawski-Wende, J.; Bollinger, K.; Chen, Z.; Watsky, M. Association of vitamin D with incident glaucoma: Findings from the Women’s Health Initiative. J. Investig. Med. 2021, 69, 843–850. [Google Scholar] [CrossRef]
- Rudnicka, A.R.; Mt-Isa, S.; Owen, C.G.; Cook, D.G.; Ashby, D. Variations in primary open–angle glaucoma prevalence by age, gender, and race: A Bayesian meta-analysis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4254–4261. [Google Scholar] [CrossRef]
- Krefting, E.A.; Jorde, R.; Christoffersen, T.; Grimnes, G. Vitamin D and intraocular pressure—Results from a case-control and an intervention study. Acta Ophthalmol. 2014, 92, 345–349. [Google Scholar] [CrossRef]
- Cho, Y.; Yun, S.P.; Yoo, W.S.; Kim, R.B.; Cho, M.C.; Kim, S.J. Reduced 25-hydroxyvitamin D concentration in the aqueous humor of cataract patients with open–angle glaucoma. Sci. Rep. 2021, 11, 18785. [Google Scholar] [CrossRef]
- Kocaturk, T.; Bekmez, S.; Unubol, M. Effects of vitamin D deficiency on intraocular pressure values obtained by ocular response analyzer. Int. Ophthalmol. 2020, 40, 697–701. [Google Scholar] [CrossRef]
- Kutuzova, G.D.; Gabelt, B.T.; Kiland, J.A.; Hennes-Beann, E.A.; Kaufman, P.L.; DeLuca, H.F. 1alpha,25-Dihydroxyvitamin D(3) and its analog, 2-methylene-19-nor-(20S)-1alpha,25-dihydroxyvitamin D(3) (2MD), suppress intraocular pressure in non-human primates. Arch. Biochem. Biophys. 2012, 518, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Tohari, A.M.; Alhasani, R.H.; Biswas, L.; Patnaik, S.R.; Reilly, J.; Zeng, Z.; Shu, X. Vitamin D Attenuates Oxidative Damage and Inflammation in Retinal Pigment Epithelial Cells. Antioxidants 2019, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Kutuzova, G.D.; Deluca, H.F. Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D(3) stimulated calcium absorption and clarify its immunomodulatory properties. Arch. Biochem. Biophys. 2004, 432, 152–166. [Google Scholar] [CrossRef]
- Hirooka, K.; Shiraga, F. Potential role for angiotensin–converting enzyme inhibitors in the treatment of glaucoma. Clin. Ophthalmol. 2007, 1, 217–223. [Google Scholar]
- Constad, W.H.; Fiore, P.; Samson, C.; Cinotti, A.A. Use of an angiotensin converting enzyme inhibitor in ocular hypertension and primary open–angle glaucoma. Am. J. Ophthalmol. 1988, 105, 674–677. [Google Scholar] [CrossRef]
- Harris, A.; Arend, O.; Kagemann, L.; Garrett, M.; Chung, H.S.; Martin, B. Dorzolamide, visual function and ocular hemodynamics in normal–tension glaucoma. J. Ocul. Pharmacol. Ther. 1999, 15, 189–197. [Google Scholar] [CrossRef]
- Faralli, J.A.; Schwinn, M.K.; Gonzalez, J.M., Jr.; Filla, M.S.; Peters, D.M. Functional properties of fibronectin in the trabecular meshwork. Exp. Eye Res. 2009, 88, 689–693. [Google Scholar] [CrossRef]
- Tian, B.; Gabelt, B.T.; Geiger, B.; Kaufman, P.L. The role of the actomyosin system in regulating trabecular fluid outflow. Exp. Eye Res. 2009, 88, 713–717. [Google Scholar] [CrossRef]
- Comes, N.; Borrás, T. Individual molecular response to elevated intraocular pressure in perfused postmortem human eyes. Physiol. Genom. 2009, 38, 205–225. [Google Scholar] [CrossRef]
- Aksoy, H.; Akcay, F.; Kurtul, N.; Baykal, O.; Avci, B. Serum 1,25 dihydroxy vitamin D (1,25(OH)2D3), 25 hydroxy vitamin D (25(OH)D) and parathormone levels in diabetic retinopathy. Clin. Biochem. 2000, 33, 47–51. [Google Scholar] [CrossRef]
- Suzuki, A.; Kotake, M.; Ono, Y.; Kato, T.; Oda, N.; Hayakawa, N.; Hashimoto, S.; Itoh, M. Hypovitaminosis D in type 2 diabetes mellitus: Association with microvascular complications and type of treatment. Endocr. J. 2006, 53, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Donaghue, K.C.; Chan, A.K.; Benitez-Aguirre, P.; Hing, S.; Lloyd, M.; Cusumano, J.; Pryke, A.; Craig, M.E. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care 2011, 34, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Patrick, P.A.; Visintainer, P.F.; Shi, Q.; Weiss, I.A.; Brand, D.A. Vitamin D and retinopathy in adults with diabetes mellitus. Arch. Ophthalmol. 2012, 130, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Ahmadieh, H.; Azar, S.T.; Lakkis, N.; Arabi, A. Hypovitaminosis d in patients with type 2 diabetes mellitus: A relation to disease control and complications. ISRN Endocrinol. 2013, 2013, 641098. [Google Scholar] [CrossRef]
- Shimo, N.; Yasuda, T.; Kaneto, H.; Katakami, N.; Kuroda, A.; Sakamoto, F.; Takahara, M.; Irie, Y.; Horikawa, K.; Miyashita, K.; et al. Vitamin D deficiency is significantly associated with retinopathy in young Japanese type 1 diabetic patients. Diabetes Res. Clin. Pract. 2014, 106, e41–e43. [Google Scholar] [CrossRef]
- He, R.; Shen, J.; Liu, F.; Zeng, H.; Li, L.; Yu, H.; Lu, H.; Lu, F.; Wu, Q.; Jia, W. Vitamin D deficiency increases the risk of retinopathy in Chinese patients with type 2 diabetes. Diabet. Med. 2014, 31, 1657–1664. [Google Scholar] [CrossRef]
- Bajaj, S.; Singh, R.P.; Dwivedi, N.C.; Singh, K.; Gupta, A.; Mathur, M. Vitamin D levels and microvascular complications in type 2 diabetes. Indian J. Endocrinol. Metab. 2014, 18, 537–541. [Google Scholar] [CrossRef]
- Zoppini, G.; Galletti, A.; Targher, G.; Brangani, C.; Pichiri, I.; Trombetta, M.; Negri, C.; De Santi, F.; Stoico, V.; Cacciatori, V.; et al. Lower levels of 25-hydroxyvitamin D3 are associated with a higher prevalence of microvascular complications in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2015, 3, e000058. [Google Scholar] [CrossRef]
- Herrmann, M.; Sullivan, D.R.; Veillard, A.S.; McCorquodale, T.; Straub, I.R.; Scott, R.; Laakso, M.; Topliss, D.; Jenkins, A.J.; Blankenberg, S.; et al. Serum 25-hydroxyvitamin D: A predictor of macrovascular and microvascular complications in patients with type 2 diabetes. Diabetes Care 2015, 38, 521–528. [Google Scholar] [CrossRef]
- Usluogullari, C.A.; Balkan, F.; Caner, S.; Ucler, R.; Kaya, C.; Ersoy, R.; Cakir, B. The relationship between microvascular complications and vitamin D deficiency in type 2 diabetes mellitus. BMC Endocr. Disord. 2015, 15, 33. [Google Scholar] [CrossRef]
- Long, M.; Wang, C.; Liu, D. Glycated hemoglobin A1C and vitamin D and their association with diabetic retinopathy severity. Nutr. Diabetes 2017, 7, e281. [Google Scholar] [CrossRef] [PubMed]
- Ashinne, B.; Rajalakshmi, R.; Anjana, R.M.; Narayan, K.M.V.; Jayashri, R.; Mohan, V.; Hendrick, A.M. Association of serum vitamin D levels and diabetic retinopathy in Asian Indians with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 139, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Eliacik, M.; Cincik, H.; Ozturk, M.; DeFronzo, R.A.; Abdul-Ghani, M. The Impact of Vitamin D Deficiency on Retinopathy and Hearing Loss among Type 2 Diabetic Patients. Biomed. Res. Int. 2018, 2018, 2714590. [Google Scholar] [CrossRef] [PubMed]
- Nadri, G.; Saxena, S.; Mahdi, A.A.; Kaur, A.; Ahmad, M.K.; Garg, P.; Meyer, C.H. Serum vitamin D is a biomolecular biomarker for proliferative diabetic retinopathy. Int. J. Retin. Vitr. 2019, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhou, J.B.; Zhao, W.; Zhang, R.H.; Cai, Y.H.; Shu, L.P.; Qi, L.; Yang, J.K. Could Vitamin D be Associated with Proliferative Diabetic Retinopathy? Evidence from Pooling Studies. Horm. Metab. Res. 2019, 51, 729–734. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Y.; Zhang, K.; Chen, Y.; Fang, S.; Zhang, W.; Wang, C.; Li, Q.; Xia, F.; Wang, N.; et al. Associations between Vitamin D and Microvascular Complications in Middle-Aged and Elderly Diabetic Patients. Endocr. Pract. 2019, 25, 809–816. [Google Scholar] [CrossRef]
- Butler, A.E.; Dargham, S.R.; Latif, A.; Mokhtar, H.R.; Robay, A.; Chidiac, O.M.; Jayyousi, A.; Al Suwaidi, J.; Crystal, R.G.; Abi Khalil, C.; et al. Association of vitamin D3 and its metabolites in patients with and without type 2 diabetes and their relationship to diabetes complications. Ther. Adv. Chronic. Dis. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Afarid, M.; Ghattavi, N.; Johari, M. Serum Levels of Vitamin D in Diabetic Patients with and Without Retinopathy. J. Ophthalmic Vis. Res. 2020, 15, 172–177. [Google Scholar] [CrossRef]
- Lopes, M.; Laiginhas, R.; Madeira, C.; Neves, J.S.; Barbosa, M.; Rosas, V.; Carvalho, D.; Falcao-Reis, F.; Falcao, M. Association between Serum Vitamin D and Diabetic Retinopathy in Portuguese Patients with Type 1 Diabetes. Acta Med. Port. 2020, 33, 459–465. [Google Scholar] [CrossRef]
- Ahmed, L.H.M.; Butler, A.E.; Dargham, S.R.; Latif, A.; Robay, A.; Chidiac, O.M.; Jayyousi, A.; Al Suwaidi, J.; Crystal, R.G.; Atkin, S.L.; et al. Association of vitamin D2 and D3 with type 2 diabetes complications. BMC Endocr. Disord. 2020, 20, 65. [Google Scholar] [CrossRef]
- Lu, L.; Zou, G.; Chen, L.; Lu, Q.; Wu, M.; Li, C. Elevated NLRP3 Inflammasome Levels Correlate with Vitamin D in the Vitreous of Proliferative Diabetic Retinopathy. Front. Med. 2021, 8, 736316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Xia, X.Y.; Yin, J. Relationship of serum vitamin D levels with diabetic microvascular complications in patients with type 2 diabetes mellitus. Chin. Med. J. 2021, 134, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huo, L.; Yu, X.; Zhang, X. Association of Bone Metabolism Indices and Bone Mineral Density with Diabetic Retinopathy in Elderly Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Inpatient Study in China. J. Diabetes Res. 2021, 2021, 8853622. [Google Scholar] [CrossRef] [PubMed]
- Alcubierre, N.; Valls, J.; Rubinat, E.; Cao, G.; Esquerda, A.; Traveset, A.; Granado-Casas, M.; Jurjo, C.; Mauricio, D. Vitamin D Deficiency Is Associated with the Presence and Severity of Diabetic Retinopathy in Type 2 Diabetes Mellitus. J. Diabetes Res. 2015, 2015, 374178. [Google Scholar] [CrossRef]
- Engelen, L.; Schalkwijk, C.G.; Eussen, S.J.; Scheijen, J.L.; Soedamah-Muthu, S.S.; Chaturvedi, N.; Fuller, J.H.; Stehouwer, C.D. Low 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 levels are independently associated with macroalbuminuria, but not with retinopathy and macrovascular disease in type 1 diabetes: The EURODIAB prospective complications study. Cardiovasc. Diabetol. 2015, 14, 67. [Google Scholar] [CrossRef]
- Gungor, A.; Ates, O.; Bilen, H.; Kocer, I. Retinal Nerve Fiber Layer Thickness in Early-Stage Diabetic Retinopathy with Vitamin D Deficiency. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6433–6437. [Google Scholar] [CrossRef]
- Boyuk, B.; Atalay, H.; Degirmencioglu, S.; Altay, M.; Guzel, S.; Celebi, A.; Ekizoglu, I.; Ayar, Y. Association between Vitamin D Level and Microvascular Complications in Patients with Type 2 Diabetes. Eurasian. J. Med. Oncol. 2017, 1, 190–196. [Google Scholar]
- Chaurasia, A.; Jain, S. Serum Vitamin D Levels in Diabetics and Its Correlation with Diabetic Retinopathy. J. Evol. Med. Dent. Sci. 2017, 6, 2735–2740. [Google Scholar] [CrossRef]
- Richter, J.; Zavorkova, M.; Vetvicka, V.; Liehneova, I.; Kral, V.; Rajnohova Dobiasova, L. Effects of beta-glucan and Vitamin D Supplementation on Inflammatory Parameters in Patients with Diabetic Retinopathy. J. Diet. Suppl. 2019, 16, 369–378. [Google Scholar] [CrossRef]
- Senyigit, A. The association between 25-hydroxy vitamin D deficiency and diabetic complications in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 1381–1386. [Google Scholar] [CrossRef]
- Almoosa, A.; Ayachit, S.; Aldoseri, A.; Wagih, W. Incidence of Vitamin D Deficiency in Patients with Type II Diabetes Mellitus and its Relation to the Severity of Retinopathy. Bahrain Med. Bull. 2019, 41, 238–240. [Google Scholar]
- Ahmed, L.H.M.; Butler, A.E.; Dargham, S.R.; Latif, A.; Ahmed, E.A.; Hassan, A.; Atkin, S.L. Relationship between total vitamin D metabolites and complications in patients with type 2 diabetes. Biomed. Rep. 2021, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Nadri, G.; Saxena, S.; Kaur, A.; Ahmad, K.; Garg, P.; Mahdi, A.A.; Akduman, L.; Gazdikova, K.; Caprnda, M.; Vesely, P.; et al. Correlation between vitamin D serum levels and severity of diabetic retinopathy in patients with type 2 diabetes mellitus. J. Endocrinol. Metab. Diabetes South Afr. 2021, 26, 82–88. [Google Scholar] [CrossRef]
- Castillo-Oti, J.M.; Galvan-Manso, A.I.; Callejas-Herrero, M.R.; Vara-Gonzalez, L.A.; Salas-Herrera, F.; Munoz-Cacho, P. Vitamin D Deficiency Is Significantly Associated with Retinopathy in Type 2 Diabetes Mellitus: A Case-Control Study. Nutrients 2021, 14, 84. [Google Scholar] [CrossRef]
- Tomic, M.; Vrabec, R.; Ljubic, S.; Bulum, T.; Rahelic, D. Plasma homocysteine is associated with nonproliferative retinopathy in patients with type 2 diabetes without renal disease. Diabetes Metab. Syndr. 2022, 16, 102355. [Google Scholar] [CrossRef]
- Xiao, Y.; Wei, L.; Xiong, X.; Yang, M.; Sun, L. Association Between Vitamin D Status and Diabetic Complications in Patients With Type 2 Diabetes Mellitus: A Cross-Sectional Study in Hunan China. Front. Endocrinol. 2020, 11, 564738. [Google Scholar] [CrossRef]
- Reddy, G.B.; Sivaprasad, M.; Shalini, T.; Satyanarayana, A.; Seshacharyulu, M.; Balakrishna, N.; Viswanath, K.; Sahay, M. Plasma vitamin D status in patients with type 2 diabetes with and without retinopathy. Nutrition 2015, 31, 959–963. [Google Scholar] [CrossRef]
- Reheem, R.; Fattah, M. Serum vitamin D and parathormone (PTH) concentrations as predictors of the development and severity of diabetic retinopathy. Alex. J. Med. 2013, 49, 119–123. [Google Scholar] [CrossRef]
- Jee, D.; Han, K.; Kim, E.C. Inverse association between high blood 25-hydroxyvitamin D levels and diabetic retinopathy in a representative Korean population. PLoS ONE 2014, 9, e115199. [Google Scholar] [CrossRef]
- Alele, J.D.; Luttrell, L.M.; Hollis, B.W.; Luttrell, D.K.; Hunt, K.J.; Group, V.S. Relationship between vitamin D status and incidence of vascular events in the Veterans Affairs Diabetes Trial. Atherosclerosis 2013, 228, 502–507. [Google Scholar] [CrossRef]
- Alam, U.; Amjad, Y.; Chan, A.W.; Asghar, O.; Petropoulos, I.N.; Malik, R.A. Vitamin D Deficiency Is Not Associated with Diabetic Retinopathy or Maculopathy. J. Diabetes Res. 2016, 2016, 6156217. [Google Scholar] [CrossRef] [PubMed]
- Balbaba, M.; Ulas, F.; Erdag, M.; Yildirim, H.; Celiker, U.; Aydin, S. Evaluation of aqueous humor and serum cortistatin levels in diabetic patients with and without diabetic retinopathy. Eur. J. Ophthalmol. 2021, 31, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Girard, E.; Nacher, M.; Bukasa-Kakamba, J.; Fahrasmane, A.; Adenis, A.; Massicard, M.; Drak Alsibai, K.; De Toffol, B.; Bekima, R.; Thelusme, L.; et al. Vitamin D Deficiency in Patients with Diabetes in French Guiana: Epidemiology and Relation with Microvascular and Macrovascular Complications. Nutrients 2021, 13, 4302. [Google Scholar] [CrossRef] [PubMed]
- Bonakdaran, S.; Shoeibi, N. Is there any correlation between vitamin D insufficiency and diabetic retinopathy? Int. J. Ophthalmol. 2015, 8, 326–331. [Google Scholar] [PubMed]
- Joergensen, C.; Hovind, P.; Schmedes, A.; Parving, H.H.; Rossing, P. Vitamin D levels, microvascular complications, and mortality in type 1 diabetes. Diabetes Care 2011, 34, 1081–1085. [Google Scholar] [CrossRef]
- Payne, J.F.; Ray, R.; Watson, D.G.; Delille, C.; Rimler, E.; Cleveland, J.; Lynn, M.J.; Tangpricha, V.; Srivastava, S.K. Vitamin D insufficiency in diabetic retinopathy. Endocr. Pract. 2012, 18, 185–193. [Google Scholar] [CrossRef]
- Poon, M.; Craig, M.E.; Kaur, H.; Cusumano, J.; Sasongko, M.B.; Wong, T.Y.; Donaghue, K.C. Vitamin D deficiency is not associated with changes in retinal geometric parameters in young people with type 1 diabetes. J. Diabetes Res. 2013, 2013, 280691. [Google Scholar] [CrossRef]
- Millen, A.E.; Sahli, M.W.; Nie, J.; LaMonte, M.J.; Lutsey, P.L.; Klein, B.E.; Mares, J.A.; Meyers, K.J.; Andrews, C.A.; Klein, R. Adequate vitamin D status is associated with the reduced odds of prevalent diabetic retinopathy in African Americans and Caucasians. Cardiovasc. Diabetol. 2016, 15, 128. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, K.J.; Kim, B.Y.; Kim, C.H.; Kang, S.K.; Mok, J.O. Relationship between vitamin D status and vascular complications in patients with type 2 diabetes mellitus. Nutr. Res. 2016, 36, 117–124. [Google Scholar] [CrossRef]
- Yi, X.; Sun, J.; Li, L.; Wei, Q.; Qian, Y.; Chen, X.; Ma, L. 1,25-Dihydroxyvitamin D3 Deficiency is Involved in the Pathogenesis of Diabetic Retinopathy in the Uygur Population of China. IUBMB Life 2016, 68, 445–451. [Google Scholar] [CrossRef]
- Ezhilarasi, K.; Dhamodharan, U.; Vijay, V. BSMI single nucleotide polymorphism in vitamin D receptor gene is associated with decreased circulatory levels of serum 25-hydroxyvitamin D among micro and macrovascular complications of type 2 diabetes mellitus. Int. J. Biol. Macromol. 2018, 116, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Zavorkova, M.; Vetvicka, V.; Richter, J.; Kral, V.; Liehnova, I.; Rajnohova, D.L. Effects of Glucan and Vitamin D Supplementation on Obesity and Lipid Metabolism in Diabetic Retinopathy. Open Biochem. J. 2018, 12, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Movafaghi, V.; Arabi, A.; Shahraki, T.; Safi, S. Effects of Oral Vitamin D Supplement Therapy on Clinical Outcomes of Intravitreal Bevacizumab in Diabetic Macular Edema. J. Ophthalmic. Vis. Res. 2021, 16, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.T.; Li, X.F.; Sun, Y.M.; Li, Y.B.; Su, Y. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Biomed. Pharmacother. 2015, 74, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.; Brownstein, S. Advanced glycation end products and diabetic retinopathy. Amino Acids 2013, 44, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W.; Frankel, J.B.; Heldt, A.M.; Grodsky, G.M. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Cade, C.; Norman, A.W. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 1986, 119, 84–90. [Google Scholar] [CrossRef]
- Ren, Z.; Li, W.; Zhao, Q.; Ma, L.; Zhu, J. The impact of 1,25-dihydroxy vitamin D3 on the expressions of vascular endothelial growth factor and transforming growth factor–beta(1) in the retinas of rats with diabetes. Diabetes Res. Clin. Pract. 2012, 98, 474–480. [Google Scholar] [CrossRef]
- Zhang, J.; Upala, S.; Sanguankeo, A. Relationship between vitamin D deficiency and diabetic retinopathy: A meta-analysis. Can. J. Ophthalmol. 2017, 52, 219–224. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, W.; Lu, P.; Yuan, H. The Association between VDR Gene Polymorphisms and Diabetic Retinopathy Susceptibility: A Systematic Review and Meta–Analysis. Biomed. Res. Int. 2016, 2016, 5305282. [Google Scholar] [CrossRef]
- Sugden, J.A.; Davies, J.I.; Witham, M.D.; Morris, A.D.; Struthers, A.D. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 2008, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Dolan, L.M.; Khoury, P.R.; Urbina, E.M.; Kimball, T.R.; Shah, A.S. Low Serum Vitamin D Levels Are Associated with Increased Arterial Stiffness in Youth With Type 2 Diabetes. Diabetes Care 2015, 38, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Ertek, S.; Akgül, E.; Cicero, A.F.; Kütük, U.; Demirtaş, S.; Cehreli, S.; Erdoğan, G. 25-Hydroxy vitamin D levels and endothelial vasodilator function in normotensive women. Arch. Med. Sci. 2012, 8, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tohari, A.M.; Almarhoun, M.; Alhasani, R.H.; Biswas, L.; Zhou, X.; Reilly, J.; Zeng, Z.; Shu, X. Protection by vitamin D against high–glucose–induced damage in retinal pigment epithelial cells. Exp. Cell Res. 2020, 392, 112023. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zuo, D.; Yu, L.; Zhang, L.; Tang, J.; Cui, C.; Bao, L.; Zan, K.; Zhang, Z.; Yang, X.; et al. ROS/TXNIP pathway contributes to thrombin induced NLRP3 inflammasome activation and cell apoptosis in microglia. Biochem. Biophys. Res. Commun. 2017, 485, 499–505. [Google Scholar] [CrossRef]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D(3) Protects against Diabetic Retinopathy by Inhibiting High–Glucose–Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef]
- Lee, V.; Rekhi, E.; Hoh Kam, J.; Jeffery, G. Vitamin D rejuvenates aging eyes by reducing inflammation, clearing amyloid beta and improving visual function. Neurobiol. Aging 2012, 33, 2382–2389. [Google Scholar] [CrossRef]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin–angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Burgess, E.D.; Hawkins, R.G.; Watanabe, M. Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. Am. J. Hypertens. 1990, 3, 903–905. [Google Scholar] [CrossRef]
- Talmor, Y.; Golan, E.; Benchetrit, S.; Bernheim, J.; Klein, O.; Green, J.; Rashid, G. Calcitriol blunts the deleterious impact of advanced glycation end products on endothelial cells. Am. J. Physiol. Renal. Physiol. 2008, 294, F1059–F1064. [Google Scholar] [CrossRef] [PubMed]
- Omidian, M.; Djalali, M.; Javanbakht, M.H.; Eshraghian, M.R.; Abshirini, M.; Omidian, P.; Alvandi, E.; Mahmoudi, M. Effects of vitamin D supplementation on advanced glycation end products signaling pathway in T2DM patients: A randomized, placebo-controlled, double blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.F.; Das, S.K.; Li, M.Q. Vitamin D Ameliorates Impaired Wound Healing in Streptozotocin–Induced Diabetic Mice by Suppressing Endoplasmic Reticulum Stress. J. Diabetes Res. 2018, 2018, 1757925. [Google Scholar] [CrossRef] [PubMed]
- Riek, A.E.; Oh, J.; Sprague, J.E.; Timpson, A.; de las Fuentes, L.; Bernal-Mizrachi, L.; Schechtman, K.B.; Bernal-Mizrachi, C. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J. Biol. Chem. 2012, 287, 38482–38494. [Google Scholar] [CrossRef]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K. Vitamin D3 treatment regulates apoptosis, antioxidant defense system, and DNA integrity in the epididymal sperm of an aged rat model. Mol. Reprod. Dev. 2019, 86, 1951–1962. [Google Scholar] [CrossRef]
- Van der Wijk, A.E.; Hughes, J.M.; Klaassen, I.; Van Noorden, C.J.F.; Schlingemann, R.O. Is leukostasis a crucial step or epiphenomenon in the pathogenesis of diabetic retinopathy? J. Leukoc. Biol. 2017, 102, 993–1001. [Google Scholar] [CrossRef]
- Sabbaghi, H.; Daftarian, N.; Suri, F.; Mirrahimi, M.; Madani, S.; Sheikhtaheri, A.; Khorrami, F.; Saviz, P.; Nejad, M.Z.; Tivay, A.; et al. The First Inherited Retinal Disease Registry in Iran: Research Protocol and Results of a Pilot Study. Arch. Iran. Med. 2020, 23, 445–454. [Google Scholar] [CrossRef]
- Papas, E.B. The global prevalence of dry eye disease: A Bayesian view. Ophthalmic Physiol. Opt. 2021, 41, 1254–1266. [Google Scholar] [CrossRef]
- Kanellopoulos, A.J.; Asimellis, G. In pursuit of objective dry eye screening clinical techniques. Eye Vis. 2016, 3, 1. [Google Scholar] [CrossRef]
- Kastelan, S.; Tomic, M.; Salopek-Rabatic, J.; Novak, B. Diagnostic procedures and management of dry eye. Biomed. Res. Int. 2013, 2013, 309723. [Google Scholar] [CrossRef]
- Bang, B.; Asmussen, K.; Sorensen, O.H.; Oxholm, P. Reduced 25-hydroxyvitamin D levels in primary Sjogren’s syndrome. Correlations to disease manifestations. Scand. J. Rheumatol. 1999, 28, 180–183. [Google Scholar] [PubMed]
- Galor, A.; Gardener, H.; Pouyeh, B.; Feuer, W.; Florez, H. Effect of a Mediterranean dietary pattern and vitamin D levels on Dry Eye syndrome. Cornea 2014, 33, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kurtul, B.E.; Ozer, P.A.; Aydinli, M.S. The association of vitamin D deficiency with tear break-up time and Schirmer testing in non-Sjogren dry eye. Eye 2015, 29, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, P.; Garip, Y.; Karci, A.A.; Guler, T. Dry eye in vitamin D deficiency: More than an incidental association. Int. J. Rheum. Dis. 2016, 19, 49–54. [Google Scholar] [CrossRef]
- Jin, K.W.; Ro, J.W.; Shin, Y.J.; Hyon, J.Y.; Wee, W.R.; Park, S.G. Correlation of vitamin D levels with tear film stability and secretion in patients with dry eye syndrome. Acta Ophthalmol. 2017, 95, e230–e235. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Bae, S.H.; Shin, Y.J.; Park, S.G.; Hwang, S.H.; Hyon, J.Y.; Wee, W.R. Low Serum 25-Hydroxyvitamin D Levels Are Associated with Dry Eye Syndrome. PLoS ONE 2016, 11, e0147847. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Sethu, S.; Deshmukh, R.; Deshpande, K.; Ghosh, A.; Agrawal, A.; Shroff, R. Corneal Dendritic Cell Density Is Associated with Subbasal Nerve Plexus Features, Ocular Surface Disease Index, and Serum Vitamin D in Evaporative Dry Eye Disease. Biomed. Res. Int. 2016, 2016, 4369750. [Google Scholar] [CrossRef]
- Shetty, R.; Sethu, S.; Chevour, P.; Deshpande, K.; Pahuja, N.; Nagaraja, H.; Pindipapanahalli, N.; Ghosh, A. Lower Vitamin D Level and Distinct Tear Cytokine Profile Were Observed in Patients with Mild Dry Eye Signs but Exaggerated Symptoms. Transl. Vis. Sci. Technol. 2016, 5, 16. [Google Scholar] [CrossRef]
- Bae, S.H.; Shin, Y.J.; Kim, H.K.; Hyon, J.Y.; Wee, W.R.; Park, S.G. Vitamin D Supplementation for Patients with Dry Eye Syndrome Refractory to Conventional Treatment. Sci. Rep. 2016, 6, 33083. [Google Scholar] [CrossRef]
- Meng, Y.F.; Lu, J.; Xing, Q.; Tao, J.J.; Xiao, P. Lower Serum Vitamin D Level Was Associated with Risk of Dry Eye Syndrome. Med. Sci. Monit. 2017, 23, 2211–2216. [Google Scholar] [CrossRef]
- Demirci, G.; Karaman Erdur, S.; Ozsutcu, M.; Eliacik, M.; Olmuscelik, O.; Aydin, R.; Kocabora, M.S. Dry Eye Assessment in Patients With Vitamin D Deficiency. Eye Contact Lens 2018, 44 (Suppl. S1), S62–S65. [Google Scholar] [CrossRef] [PubMed]
- Khamar, P.; Nair, A.P.; Shetty, R.; Vaidya, T.; Subramani, M.; Ponnalagu, M.; Dhamodaran, K.; D’Souza, S.; Ghosh, A.; Pahuja, N.; et al. Dysregulated Tear Fluid Nociception–Associated Factors, Corneal Dendritic Cell Density, and Vitamin D Levels in Evaporative Dry Eye. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.J.; Byun, Y.S.; Lee, J.; Park, S.H.; Chung, S.H. The Association of Serum Vitamin D Level With the Severity of Dry Eye Parameters in Primary Sjogren Syndrome. Cornea 2020, 39, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Arita, R.; Mizoguchi, T.; Kawashima, M.; Koh, S.; Shirakawa, R.; Suzuki, T.; Sasaki, S.; Morishige, N. Relation of Dietary Fatty Acids and Vitamin D to the Prevalence of Meibomian Gland Dysfunction in Japanese Adults: The Hirado–Takushima Study. J. Clin. Med. 2021, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Aksoy Aydemir, G.; Aydemir, E.; Asik, A. Changes in Tear Meniscus Analysis of Children Who Have Type 1 Diabetes Mellitus, With and Without Vitamin D Deficiency. Cornea 2021. [Google Scholar] [CrossRef]
- Dikci, S.; Akatli, A.N.; Yildirim, T. Conjunctival impression cytology and tear–film changes in cases with vitamin D deficiency. Int. Ophthalmol. 2020, 40, 1687–1694. [Google Scholar] [CrossRef]
- Jee, D.; Kang, S.; Yuan, C.; Cho, E.; Arroyo, J.G.; The Epidemiologic Survey Committee of the Korean Ophthalmologic Society. Serum 25-Hydroxyvitamin D Levels and Dry Eye Syndrome: Differential Effects of Vitamin D on Ocular Diseases. PLoS ONE 2016, 11, e0149294. [Google Scholar]
- Kim, M.J.; Hwang, H.R.; Kim, Y.J.; Lee, S.Y.; Lee, J.G.; Jeong, D.W.; Kim, Y.H. Association Between Serum 25-Hydroxyvitamin D Levels and Dry Eye in Korean Adults: A Study Based on Korean National Health and Nutrition Examination Survey, 2010–2011. Korean J. Fam. Med. 2017, 38, 81–85. [Google Scholar] [CrossRef]
- Jeon, D.H.; Yeom, H.; Yang, J.; Song, J.S.; Lee, H.K.; Kim, H.C. Are Serum Vitamin D Levels Associated With Dry Eye Disease? Results From the Study Group for Environmental Eye Disease. J. Prev. Med. Public Health 2017, 50, 369–376. [Google Scholar] [CrossRef]
- Arman, A.; Petricli, I.S.; Kara, C.; Koksal, Z.; Gucel, F. The relationship between serum vitamin D levels and dry eye syndrome in postmenopausal women. Ann. Clin. Anal. Med. 2020, 11, 91–94. [Google Scholar]
- Arita, R.; Kawashima, M.; Ito, M.; Tsubota, K. Clinical safety and efficacy of vitamin D3 analog ointment for treatment of obstructive meibomian gland dysfunction. BMC Ophthalmol. 2017, 17, 84. [Google Scholar] [CrossRef]
- Kizilgul, M.; Kan, S.; Ozcelik, O.; Beysel, S.; Apaydin, M.; Ucan, B.; Cakal, E. Vitamin D Replacement Improves Tear Osmolarity in Patients with Vitamin D Deficiency. Semin. Ophthalmol. 2018, 33, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Albietz, J.; Harkin, D.G.; Kimlin, M.G.; Schmid, K.L. Impact of oral vitamin D supplementation on the ocular surface in people with dry eye and/or low serum vitamin D. Cont. Lens Anterior Eye 2018, 41, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Lee, Y.P.; Shin, Y.J. Vitamin D Enhances the Efficacy of Topical Artificial Tears in Patients With Dry Eye Disease. Cornea 2019, 38, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.; Sahai, A.; Kumar, P.R.; Shamshad, M.A.; Trivedi, G.K.; Tyagi, L. A prospective study to assess the role of vitamin D individually and in combination with cyclosporine in the treatment of dry eye in patients with deficient serum 25(OH)D levels. Indian J. Ophthalmol. 2020, 68, 1020–1026. [Google Scholar] [CrossRef]
- Karaca, E.E.; Kemer, O.E.; Ozek, D.; Berker, D.; Imga, N.N. Clinical outcomes of ocular surface in patients treated with vitamin D oral replacement. Arq. Bras. Oftalmol. 2020, 83, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Eslampoor, A.; Najjaran, M.; Arjmand Askari, E.; Zarei-Ghanavati, S.; Ziaei, M. Effect of oral vitamin D supplementation on dry eye disease patients with vitamin D deficiency. Clin. Exp. Optom. 2022, 1–6. [Google Scholar] [CrossRef]
- Jain, N.; Sharma, P.; Chouhan, J.K. A study of the association between Vitamin D deficiency and Dry Eye Syndrome (DES) in the Indian population. Indian J. Ophthalmol. 2022, 70, 500–504. [Google Scholar]
- Shab-Bidar, S.; Neyestani, T.R.; Djazayery, A.; Eshraghian, M.R.; Houshiarrad, A.; Gharavi, A.; Kalayi, A.; Shariatzadeh, N.; Zahedirad, M.; Khalaji, N.; et al. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: A randomized double-blind clinical trial. BMC Med. 2011, 9, 125. [Google Scholar] [CrossRef]
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 2005, 26, 662–687. [Google Scholar] [CrossRef]
- Froicu, M.; Weaver, V.; Wynn, T.A.; McDowell, M.A.; Welsh, J.E.; Cantorna, M.T. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol. Endocrinol. 2003, 17, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Fattori, E.; Cappelletti, M.; Costa, P.; Sellitto, C.; Cantoni, L.; Carelli, M.; Faggioni, R.; Fantuzzi, G.; Ghezzi, P.; Poli, V. Defective inflammatory response in interleukin 6-deficient mice. J. Exp. Med. 1994, 180, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Bascom, A.; Redfern, R.L.; Reins, R.Y. Characterization of Vitamin D Levels in Ocular Surface Tissues and their Association with Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1414. [Google Scholar]
- Meng, Y.F.; Xin, Q.; Lu, J.; Xiao, P.; Li, J. Association Between Single Nucleotide Polymorphisms in the Vitamin D Receptor and Incidence of Dry Eye Disease in Chinese Han Population. Med. Sci. Monit. 2019, 25, 4759–4765. [Google Scholar] [CrossRef]
- Sadaka, A.; Nguyen, K.; Malik, A.; Brito, R.; Berry, S.; Lee, A.G. Vitamin D and Selenium in a Thyroid Eye Disease Population in Texas. Neuroophthalmology 2019, 43, 291–294. [Google Scholar] [CrossRef]
- Heisel, C.J.; Riddering, A.L.; Andrews, C.A.; Kahana, A. Serum Vitamin D Deficiency Is an Independent Risk Factor for Thyroid Eye Disease. Ophthalmic Plast Reconstr. Surg. 2020, 36, 17–20. [Google Scholar] [CrossRef]
- Planck, T.; Shahida, B.; Malm, J.; Manjer, J. Vitamin D in Graves Disease: Levels, Correlation with Laboratory and Clinical Parameters, and Genetics. Eur. Thyroid J. 2018, 7, 27–33. [Google Scholar] [CrossRef]
- Nian, H.; Liang, D.; Zuo, A.; Wei, R.; Shao, H.; Born, W.K.; Kaplan, H.J.; Sun, D. Characterization of autoreactive and bystander IL-17+ T cells induced in immunized C57BL/6 mice. Investig. Ophthalmol. Vis. Sci. 2012, 53, 897–905. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, R.; Luger, D.; Zhu, W.; Silver, P.B.; Grajewski, R.S.; Su, S.B.; Chan, C.-C.; Adorini, L.; Caspi, R.R. Calcitriol Suppresses Antiretinal Autoimmunity through Inhibitory Effects on the Th17 Effector Response. J. Immunol. 2009, 182, 4624–4632. [Google Scholar] [CrossRef]
- González, M.M.; Solano, M.M.; Porco, T.C.; Oldenburg, C.E.; Acharya, N.R.; Lin, S.C.; Chan, M.F. Epidemiology of uveitis in a US population-based study. J. Ophthalmic Inflamm. Infect. 2018, 8, 6. [Google Scholar] [CrossRef]
- Llop, S.M.; Davoudi, S.; Stanwyck, L.K.; Sathe, S.; Tom, L.; Ahmadi, T.; Grotting, L.; Papaliodis, G.N.; Sobrin, L. Association of Low Vitamin D Levels with Noninfectious Uveitis and Scleritis. Ocul. Immunol. Inflamm. 2019, 27, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Sobrin, L.; Stanwyck, L.K.; Pan, W.; Hubbard, R.A.; Kempen, J.H.; VanderBeek, B.L. Association of Hypovitaminosis D With Increased Risk of Uveitis in a Large Health Care Claims Database. JAMA Ophthalmol. 2018, 136, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Mitulescu, T.C.; Stavaru, C.; Voinea, L.M.; Banica, L.M.; Matache, C.; Predeteanu, D. The role of Vitamin D in immuno–inflammatory responses in Ankylosing Spondylitis patients with and without Acute Anterior Uveitis. J. Med. Life 2016, 9, 26–33. [Google Scholar]
- Dadaci, Z.; Cetinkaya, S.; Oncel Acir, N.; Oncel, M.; Borazan, M. Serum Vitamin D Levels in Patients with Acute Anterior Uveitis. Ocul. Immunol. Inflamm. 2017, 25, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Grotting, L.A.; Davoudi, S.; Palenzuela, D.; Papaliodis, G.N.; Sobrin, L. Association of Low Vitamin D Levels With Noninfectious Anterior Uveitis. JAMA Ophthalmol. 2017, 135, 150–153. [Google Scholar] [CrossRef]
- Yi, X.; Yang, P.; Sun, M.; Yang, Y.; Li, F. Decreased 1,25-Dihydroxyvitamin D3 level is involved in the pathogenesis of Vogt-Koyanagi-Harada (VKH) disease. Mol. Vis. 2011, 17, 673–679. [Google Scholar] [PubMed]
- Rohmer, J.; Hadjadj, J.; Bouzerara, A.; Salah, S.; Paule, R.; Groh, M.; Blanche, P.; Mouthon, L.; Monnet, D.; Le Jeunne, C.; et al. Serum 1,25(OH)2 Vitamin D and 25(OH) Vitamin D Ratio for the Diagnosis of Sarcoidosis–Related Uveitis. Ocul. Immunol. Inflamm. 2020, 28, 341–347. [Google Scholar] [CrossRef]
- Sengler, C.; Zink, J.; Klotsche, J.; Niewerth, M.; Liedmann, I.; Horneff, G.; Kessel, C.; Ganser, G.; Thon, A.; Haas, J.P.; et al. Vitamin D deficiency is associated with higher disease activity and the risk for uveitis in juvenile idiopathic arthritis—Data from a German inception cohort. Arthritis Res. Ther. 2018, 20, 276. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Z.K.; Lim, L.L.; Rogers, S.L.; Hall, A.J. Patterns of Vitamin D Levels and Exposures in Active and Inactive Noninfectious Uveitis Patients. Ophthalmology 2020, 127, 230–237. [Google Scholar] [CrossRef]
- Cooper, J.D.; Smyth, D.J.; Walker, N.M.; Stevens, H.; Burren, O.S.; Wallace, C.; Greissl, C.; Ramos-Lopez, E.; Hypponen, E.; Dunger, D.B.; et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes 2011, 60, 1624–1631. [Google Scholar] [CrossRef]
- Sawcer, S.; Hellenthal, G.; Pirinen, M.; Spencer, C.C.; Patsopoulos, N.A.; Moutsianas, L.; Dilthey, A.; Su, Z.; Freeman, C.; Hunt, S.E.; et al. Genetic risk and a primary role for cell–mediated immune mechanisms in multiple sclerosis. Nature 2011, 476, 214–219. [Google Scholar] [PubMed]
- Pillar, S.; Amer, R. The association between vitamin D and uveitis: A comprehensive review. Surv. Ophthalmol. 2021, 67, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Hou, S.; Xiang, Q.; Qi, J.; Yu, H.; Shi, Y.; Zhou, Y.; Kijlstra, A.; Yang, P. Polymorphisms in genetics of vitamin D metabolism confer susceptibility to ocular Behcet disease in a Chinese Han population. Am. J. Ophthalmol. 2014, 157, 488–494.e6. [Google Scholar] [CrossRef] [PubMed]
- Audo, I.; Darjatmoko, S.R.; Schlamp, C.L.; Lokken, J.M.; Lindstrom, M.J.; Albert, D.M.; Nickells, R.W. Vitamin D analogues increase p53, p21, and apoptosis in a xenograft model of human retinoblastoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4192–4199. [Google Scholar] [CrossRef]
- Albert, D.M.; Kumar, A.; Strugnell, S.A.; Darjatmoko, S.R.; Lokken, J.M.; Lindstrom, M.J.; Patel, S. Effectiveness of vitamin D analogues in treating large tumors and during prolonged use in murine retinoblastoma models. Arch. Ophthalmol. 2004, 122, 1357–1362. [Google Scholar]
- Mejia-Rodriguez, F.; Flores-Aldana, M.E.; Quezada-Sanchez, A.D.; Shamah-Levy, T.; Villalpando, S.; Contreras-Manzano, A.; Bhatt-Carreno, S.; Orjuela-Grimm, M. Association between Predictors of Vitamin D Serum Levels and Risk of Retinoblastoma in Children: A Case-Control Study. Nutrients 2021, 13, 2510. [Google Scholar] [CrossRef]
- Orjuela-Grimm, M.; Carreno, S.B.; Liu, X.; Ruiz, A.; Medina, P.; Ramirez Ortiz, M.A.; Rendon, J.R.; Molina, N.C.L.; Pinilla, H.; Hinojosa, D.; et al. Sunlight exposure in infancy decreases risk of sporadic retinoblastoma, extent of intraocular disease. Cancer Rep. 2021, 4, e1409. [Google Scholar] [CrossRef]
- Schündeln, M.M.; Hauffa, P.K.; Bauer, J.J.; Temming, P.; Sauerwein, W.; Biewald, E.; Bornfeld, N.; Hauffa, B.P.; Grasemann, C. Pediatric Survivors of Retinoblastoma Are at Risk for Altered Bone Metabolism After Chemotherapy Treatment Early in Life. Pediatr. Hematol. Oncol. 2015, 32, 455–466. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Delcourt, C.; Cougnard-Gregoire, A.; Boniol, M.; Carriere, I.; Dore, J.F.; Delyfer, M.N.; Rougier, M.B.; Le Goff, M.; Dartigues, J.F.; Barberger-Gateau, P.; et al. Lifetime exposure to ambient ultraviolet radiation and the risk for cataract extraction and age-related macular degeneration: The Alienor Study. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7619–7627. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J.; Gao, Q.; Wang, Y.; Hu, L.; Zheng, Y.; Wang, F.; Liu, Y. The relationship between disability–adjusted life years of cataracts and ambient erythemal ultraviolet radiation in China. J. Epidemiol. 2015, 25, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.M.; Yang, D.Q.; Wang, H.; Xu, J.; Gao, Q.; Hu, L.W.; Wang, F.; Wang, Y.; Yan, Q.C.; Zhang, J.S.; et al. Prevalence and risk factors of lens opacities in rural populations living at two different altitudes in China. Int. J. Ophthalmol. 2016, 9, 610–616. [Google Scholar] [PubMed]
- Vinson, J.A. Oxidative stress in cataracts. Pathophysiology 2006, 13, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, N.K. Serum 25-hydroxyvitamin D and Age-Related Cataract. Ophthalmic Epidemiol. 2017, 24, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Jee, D.; Kim, E.C. Association between serum 25-hydroxyvitamin D levels and age-related cataracts. J. Cataract Refract. Surg. 2015, 41, 1705–1715. [Google Scholar] [CrossRef]
- Abdellah, M.M.; Mohamed Mostafa, E.; Salama, E.H.; Roshdy Mohamed, E. Association of Serum 25-Hydroxyl Vitamin D Deficiency and Age-Related Cataract: A Case-Control Study. J. Ophthalmol. 2019, 2019, 9312929. [Google Scholar] [CrossRef]
- Atalay, K.; Gezer Savur, F.; Kirgiz, A.; Erdogan Kaldirim, H.; Zengi, O. Serum Vitamin D Levels in Different Morphologic Forms of Age Related Cataract. Acta Endocrinol. 2020, 16, 178–182. [Google Scholar] [CrossRef]
- Oktem, C.; Aslan, F. Vitamin D Levels in Young Adult Cataract Patients: A Case-Control Study. Ophthalmic Res. 2021, 64, 116–120. [Google Scholar]
- Brown, C.J.; Akaichi, F. Vitamin D deficiency and posterior subcapsular cataract. Clin. Ophthalmol. 2015, 9, 1093–1098. [Google Scholar] [CrossRef]
- Rao, P.; Millen, A.E.; Meyers, K.J.; Liu, Z.; Voland, R.; Sondel, S.; Tinker, L.; Wallace, R.B.; Blodi, B.A.; Binkley, N.; et al. The Relationship Between Serum 25-Hydroxyvitamin D Levels and Nuclear Cataract in the Carotenoid Age-Related Eye Study (CAREDS), an Ancillary Study of the Women’s Health Initiative. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4221–4230. [Google Scholar] [CrossRef]
- Jee, D.; Kim, E.C.; Cho, E.; Arroyo, J.G. Positive Association between Blood 25-Hydroxyvitamin D Levels and Pterygium after Control for Sunlight Exposure. PLoS ONE 2016, 11, e0157501. [Google Scholar] [CrossRef] [PubMed]
- Samadi Aidenloo, N.; Motarjemizadeh, Q.; Moradi Nahriq, S. Serum concentrations of 25-hydroxyvitamin D and different subtypes of age-related cataract; a case-control study. Immunopathol. Persa 2022, 8, e16. [Google Scholar] [CrossRef]
- Cho, M.C.; Kim, R.B.; Ahn, J.Y.; Yoo, W.S.; Kim, S.J. Aqueous humor and serum 25-Hydroxyvitamin D levels in patients with cataracts. BMC Ophthalmol. 2020, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Karatas Durusoy, G.; Karakucuk, Y.; Tulu Aygun, B.; Solmaz, B. Serum Vitamin D Level and Body Mass Index in Children with Vernal Keratoconjunctivitis. Beyoglu Eye J. 2020, 5, 102–107. [Google Scholar] [CrossRef]
- Bozkurt, B.; Artac, H.; Ozdemir, H.; Ünlü, A.; Bozkurt, M.K.; Irkec, M. Serum Vitamin D Levels in Children with Vernal Keratoconjunctivitis. Ocul. Immunol. Inflamm. 2018, 26, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Zicari, A.M.; Cafarotti, A.; Occasi, F.; Lollobrigida, V.; Nebbioso, M.; Pecorella, I.; De Castro, G.; Spalice, A.; Loffredo, L.; Villa, M.P.; et al. Vitamin D levels in children affected by vernal keratoconjunctivitis. Curr. Med. Res. Opin. 2017, 33, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Sorkhabi, R.; Ahoor, M.H.; Ghorbanihaghjo, A.; Jafari, S. Serum vitamin D levels in patients with vernal keratoconjunctivitis and its relationship with disease severity. Eur. J. Ophthalmol. 2021, 31, 3259–3264. [Google Scholar] [CrossRef]
- Aslan, M.G.; Findik, H.; Okutucu, M.; Aydin, E.; Oruç, Y.; Arpa, M.; Uzun, F. Serum 25-Hydroxy Vitamin D, Vitamin B12, and Folic Acid Levels in Progressive and Nonprogressive Keratoconus. Cornea 2021, 40, 334–341. [Google Scholar] [CrossRef]
- Akkaya, S.; Ulusoy, D.M. Serum Vitamin D Levels in Patients with Keratoconus. Ocul. Immunol. Inflamm. 2020, 28, 348–353. [Google Scholar] [CrossRef]
- Zarei-Ghanavati, S.; Yahaghi, B.; Hassanzadeh, S.; Mobarhan, M.G.; Hakimi, H.R.; Eghbali, P. Serum 25-Hydroxyvitamin D, Selenium, Zinc and Copper in Patients with Keratoconus. J. Curr. Ophthalmol. 2020, 32, 26–31. [Google Scholar] [CrossRef]
- Epstein, D.; Kvanta, A.; Lindqvist, P.G. Vitamin D Deficiency in Patients with Central Retinal Vein Occlusion: A Case Control Study. Curr. Eye Res. 2017, 42, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Oli, A.; Joshi, D. Serum vitamin D levels in Indian patients with retinal venous occlusions. Saudi J. Ophthalmol. 2017, 31, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yao, X.; Ding, J.; Hong, R.; Wu, Y.; Huang, H.; Zhuang, L.; Li, Z.; Wang, Y.; Zhang, Y.; et al. Low levels of vitamin D and the relationship between vitamin D and Th2 axis–related cytokines in neuromyelitis optica spectrum disorders. J. Clin. Neurosci. 2019, 61, 22–27. [Google Scholar] [CrossRef]
- Jitprapaikulsan, J.; Siritho, S.; Prayoonwiwat, N. Vitamin D level status in Thai neuromyelitis optica patients. J. Neuroimmunol. 2016, 295–296, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kusumadewi, W.; Imran, D.; Witjaksono, F.; Pakasi, T.A.; Rusmana, A.I.; Pangeran, D.; Marwadhani, S.S.; Maharani, K.; Estiasari, R. Low vitamin D-25(OH) level in Indonesian multiple sclerosis and neuromyelitis optic patients. Mult. Scler. Relat. Disord. 2018, 25, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Goksugur, S.B.; Erdurmus, M.; Bekdas, M.; Erkocoglu, M.; Agca, S.; Tosun, M.; Goksugur, N.; Demircioglu, F. Tear and serum vitamin D levels in children with allergic rhinoconjunctivitis. Allergol. Immunopathol. 2015, 43, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Al-wailmi, F.K.; Shah, S.I.A.; Al-Mazaideh, G.M.; Sikandar, M.Z. Serum Vitamin D and Immunoglobulin E Levels in Patients With Seasonal Allergic Conjunctivitis. IIUM Med. J. Malays. 2021, 20. [Google Scholar] [CrossRef]
- Yenigun, A.; Dadaci, Z.; Oncel, M. Plasma vitamin D levels of patients with allergic rhino–conjunctivitis with positive skin prick test. Am. J. Rhinol. Allergy 2015, 29, e46–e49. [Google Scholar] [CrossRef]
- Dadaci, Z.; Borazan, M.; Kiyici, A.; Oncel Acir, N. Plasma vitamin D and serum total immunoglobulin E levels in patients with seasonal allergic conjunctivitis. Acta Ophthalmol. 2014, 92, e443–e446. [Google Scholar] [CrossRef]
- Arikan, S.; Kamis, F. Effect of vitamin D deficiency on spatial contrast sensitivity function. Clin. Exp. Optom. 2021, 1–7. [Google Scholar] [CrossRef]
- Ozturk, E.; Cankaya, C. Effect of Vitamin D Deficiency on Contrast Sensitivity Function. Curr. Eye Res. 2020, 45, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Aksoy Aydemir, G.; Yetkin, E.; Aydemir, E.; Bolu, S.; Asik, A. Changes in the macular choroidal thickness of children who have type–1 diabetes mellitus, with and without vitamin D deficiency. Int. Ophthalmol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Vural, E.; Hazar, L.; Çağlayan, M.; Şeker, Ö.; Çelebi, A.R.C. Peripapillary choroidal thickness in patients with vitamin D deficiency. Eur. J. Ophthalmol. 2021, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Öncül, H.; Alakus, M.F.; Çağlayan, M.; Öncül, F.Y.; Dag, U.; Arac, E. Changes in choroidal thickness after vitamin D supplementation in patients with vitamin D deficiency. Can. J. Ophthalmol. 2020, 55, 486–491. [Google Scholar] [CrossRef]
- Cankaya, C.; Cumurcu, T.; Gunduz, A. Corneal endothelial changes in patients with vitamin D deficiency. Indian J. Ophthalmol. 2018, 66, 1256. [Google Scholar] [CrossRef]
- Graffe, A.; Beauchet, O.; Fantino, B.; Milea, D.; Annweiler, C. Vitamin D and Macular Thickness in the Elderly: An Optical Coherence Tomography Study. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5298–5303. [Google Scholar] [CrossRef]
- Kara, N.; Ceri, S. Vitamin D level in patients with pterygium. Arq. Bras. Oftalmol. 2017, 80, 229–233. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kim, S.Y.; Park, Y.G.; Han, K.D.; Kim, J.W.; Chae, H.S.; Lee, Y.C. Evaluation of socioeconomic status as a risk factor of pterygium using the Korean National Health and Nutrition Examination Survey 2010 to 2011: A STROBE–compliant article. Medicine 2017, 96, e6343. [Google Scholar] [CrossRef]
- Maxia, C.; Murtas, D.; Corrias, M.; Zucca, I.; Minerba, L.; Piras, F.; Marinelli, C.; Perra, M.T. Vitamin D and vitamin D receptor in patients with ophthalmic pterygium. Eur. J. Histochem. 2017, 61, 2837. [Google Scholar] [CrossRef]
- Ornek, N.; Ogurel, T.; Kisa, U. Tear Fluid and Serum Vitamin D Concentrations in Unilateral Pterygium. Optom. Vis. Sci. 2021, 98, 170–174. [Google Scholar] [CrossRef]
- Droutsas, K.; Sekundo, W. Epidemiology of pterygium. A review. Ophthalmologe 2010, 107, 511–512, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K. Pterygium: Epidemiology prevention and treatment. Community Eye Health 2017, 30, S5–S6. [Google Scholar] [PubMed]
- Ghiglioni, D.G.; Bruschi, G.; Gandini, S.; Osnaghi, S.; Peroni, D.; Marchisio, P. Vitamin D serum levels in children with vernal keratoconjunctivitis and disease control. Int. J. Immunopathol. Pharmacol. 2019, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, X.; Xiang, Q.; Zheng, Y.; Pi, L.; Liu, Q.; Xiao, J.; Ke, N.; Fang, J. Prevalence of Low Serum Vitamin A Levels in Young Children with Chalazia in Southwest China. Am. J. Ophthalmol. 2014, 157, 1103–1108.e2. [Google Scholar] [CrossRef] [PubMed]
- Kabataş, E.U.; Dinlen, N.F.; Zenciroğlu, A.; Dilli, D.; Beken, S.; Okumuş, N. Relationship between serum 25-hydroxy vitamin D levels and retinopathy of prematurity. Scott. Med. J. 2017, 62, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, U.; Ikram, M.A.; Hofman, A.; de Jong, P.; Uitterlinden, A.G.; Klaver, C.C.W.; Ikram, M.K. Vitamin D and retinal microvascular damage: The Rotterdam Study. Medicine 2016, 95, e5477. [Google Scholar] [CrossRef] [PubMed]
- Daldal, H.; Gokmen Salici, A. Ocular Findings Among Patients with Vitamin D Deficiency. Cureus 2021, 13, e15159. [Google Scholar] [CrossRef]
- Karabulut, M.; Karabulut, S.; Canbek, T.D.; Sül, S.; Karalezli, A. The effect of vitamin D deficiency on the retinal microvasculature: An observational case-control study. Arq. Bras. Oftalmol. 2022. [Google Scholar] [CrossRef]
- Malabanan, A.; Veronikis, I.E.; Holick, M.F. Redefining vitamin D insufficiency. Lancet 1998, 351, 805–806. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).