Abstract
The growth of leaves is subject to strict time regulation. Several genes influencing leaf growth have been identified, but little is known about how genes regulate the orderly initiation and growth of leaves. Here, we demonstrate that TaKLU/TaCYP78A5 contributes to a time regulation mechanism in leaves from initiation to expansion. TaKLU encodes the cytochrome P450 CYP78A5, and its homolog AtKLU has been described whose deletion is detrimental to organ growth. Our results show that TaKLU overexpression increases leaf size and biomass by altering the time of leaf initiation and expansion. TaKLU-overexpressing plants have larger leaves with more cells. Further dynamic observations indicate that enlarged wheat leaves have experienced a longer expansion time. Different from AtKLU inactivation increases leaf number and initiation rates, TaKLU overexpression only smooths the fluctuations of leaf initiation rates by adjusting the initiation time of local leaves, without affecting the overall leaf number and initiation rates. In addition, complementary analyses suggest TaKLU is functionally conserved with AtKLU in controlling the leaf initiation and size and may involve auxin accumulation. Our results provide a new insight into the time regulation mechanisms of leaf growth in wheat.
1. Introduction
Leaves are one of the most important vegetative organs of vascular plants, and provide organic matter for plants through photosynthesis. The time of leaf initial and growth directly determines the characteristics of leaf size, shape, function, etc., which is the key feature of a plant and the basis for the formation of crop biomass and yield [1,2,3]. The growth of leaves from initiation to growth involves a large number of genes and complex physiological processes. Leaves initiate at the flanks of the shoot apical meristem (SAM) in a species-specific chronological order; subsequently, the leaves grow gradually driven by the process of cell division and expansion, which are controlled by the interaction of multiple genes’ expression in time and space [4,5,6,7,8].
The CYP78A family (CYP78As) is a plant-specific gene family that is highly conserved in land plants [9]. Several members of the CYP78As have been implicated in the control of organ growth by promoting cell division and expansion [10,11,12,13,14]. Among them, AtKLU/AtCYP78A5 regulates the growth of leaves, flowers, grains, siliques, and other organs by regulating cell division [15,16,17,18]. For example, AtKLU is highly expressed in the inner integument of developing ovules, and determines the growth potential of the seed coat and seed by promoting cell division in Arabidopsis [10]. And AtKLU inactivity suppresses megasporocyte cell fate and ultimately determines the size and number of seeds in Arabidopsis [18]. The klu loss-of-function mutants produce smaller leaves and floral organs due to inhibition of cell division [16]. AtKLU and AtCYP78A7 exhibit redundant functions in regulating plastochron by expressing at the edges of meristems in Arabidopsis, and the rosette leaves of cyp78a5 cyp78a7 mutant plants are more and compact than of WT (Wild type) [17,19]. Recently, analyses of multiple mutants revealed that AtKLU, AtCYP78A7 and AMP1 (Altered meristem program1) regulate plastochron length and leaf senescence in the same genetic pathway in Arabidopsis [20]. Thus, it can be seen that KLU may be involved in the entire life cycle of organ growth from initiation, expansion to senescence, and its growth-promoting effect is precisely regulated in time. However, the molecular mechanism of CYP78As controlling organ growth remains elusive.
Recent studies show that AtKLU regulates the plastochron in non-cell-autonomous manners, which is just the classic model of hormone action [20]. Metabolome data indicate that AtKLU positively regulates leaf senescence by activating cytokinin signaling, and also affects the content of auxin [21]. Moreover, our recent research results reveal that TaKLU overexpression promotes auxin accumulation in ovaries [22]. These results confirm the previously hypothesis that the CYP78As may generate a hormone-like growth factor to promote organ growth [11,12,23]. However, there is still a huge knowledge gap in our understanding of the relationship between plant hormones and KLU. Further, given the lack of positive evidence that KLU has a positive effect on leaf and plant growth, it is unclear whether KLU could be used to crop improvement.
Wheat is one of the most important food crops, and its yield is of great value to world food security. Despite the orderly initiation and timely growth of leaves will greatly affect leaf characteristics and crop yields, we still relatively know little about how leaf growth is regulated at the time dimension in wheat. Our previous studies show that localized overexpression of TaKLU in ovary integument is sufficient to increase grain weight and grain yield per plant in wheat, indicating that TaKLU has a great application potential in crop improvement [22]. Here, we demonstrate that TaKLU determines the time of leaf initiation and growth, its overexpression is conducive to the increase of leaf size and biomass in wheat. The overexpression of TaKLU prolongs the time of leaf expansion, which ultimately leads to an increase in leaf size and biomass. The rescue experiment shows that TaKLU and AtKLU have conserved functions in regulating leaf initiation and size, and DR5:GUS marker detection suggests auxin contributes to this regulating growth mechanism. Thus, we suggest a possible role of TaKLU as a time regulator of leaf growth in wheat.
2. Results
2.1. Overexpression of TaKLU Increased Leaf Size and Biomass by Promoting Cell Division
To demonstrate whether TaKLU has a positive effect on promoting leaf growth, two independent transgenic events (UBI::TaKLU, including UBI-1 and UBI-4 events) with a single copy that have been generated previously [22]. Indeed, the overexpression of TaKLU led to a significant increase in the length, width and area of leaves (p < 0.05, n > 7) (Figure 1A–D), suggesting that the overexpression of TaKLU could increase leaf size in wheat. This was complementary to the previous observation that AtKLU loss-of-function mutants form smaller leaves [20]. It is well known that the increase of leaf size may affect biomass, so we further investigated the impact of TaKLU activity on biomass. TaKLU-overexpressing wheat had higher plant height and larger leaves (Figure 1A), which finally led to a significant increase in its biomass (p < 0.05, n > 16), compared with WT (Figure 1E).
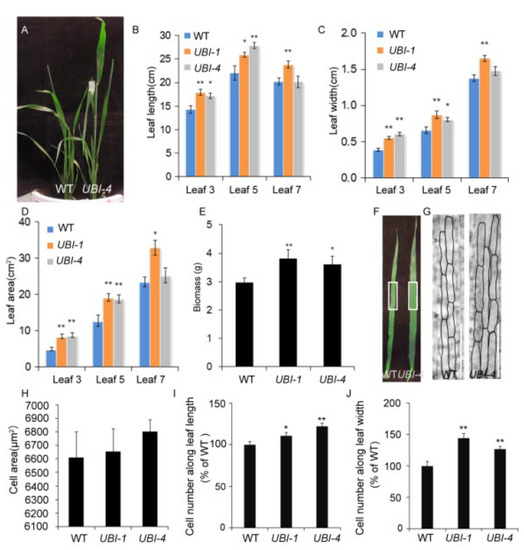
Figure 1.
TaKLU regulated leaf size and biomass by affecting cell division in wheat. (A) WT (Wild type) and UBI::TaKLU transgenic wheat lines (UBI-4) plants were shown at 80 days after sowing. (B–D) Statistics on the length (B), width (C) and area (D) of the leaves when they stopped elongating (n > 7). (E) Statistical analysis of biomass in different genotypes wheat (n > 16). (F–J) Cytological analysis of leaf 5 from WT and UBI::TaKLU plants in wheat. (F) Leaf 5 of the indicated genotypes at 16 days after its appearance. (G) Cell morphology characteristics in the middle of the leaf 5 in panel (F) (the position of white rectangle). (H) Statistics on the cell area (n > 68) of leaf 5 at 16 days after its appearance. (I,J) Statistics on the cell number along leaf length and leaf width of leaf 5 at 16 days after its appearance (n > 9). Bars indicate SE. Size bars represent 200 μm (G). Asterisks (*) and (**) indicate significant differences from their WT at p < 0.05 and p < 0.01 (t-test), respectively.
CYP78As has been shown previously to regulate flower and grain growth by promoting the proliferation of maternal integument cells [10,11]. However, similar evidence for leaf size is lacking. To determine whether TaKLU acts as this maternal factor to promote leaf growth, we investigated the characteristics of leaf epidermal cells of different genotypes of wheat. The results showed that the elongated leaves of TaKLU-overexpressing plants had more epidermal cells than those of WT (Figure 1G–J), and there was no significant difference in cell size (n > 68) (Figure 1H), indicating that the change in leaf size is due to the increase in cell number. To gain insight into the mechanism of TaKLU regulating cell division, we investigated the expression level of several marker genes related to cell wall and cell cycle. Consistent with the overexpression of TaKLU leading to a change in the number of leaf epidermal cells in wheat, all of the three cell cycle-related marker genes [24,25] (CDKA;1 (CYCLIN-DEPENDENT KINASE 1), CYCT1;1 (CYCLIN T1;1) and CDC20 (CELL DIVISION CYCLE 20)) in TaKLU-overexpressing wheat showed consistent and significant up-regulation (Figure S1A), two of the three cell wall metabolism-related marker genes [5,26,27] (XTH8 (XYLOGLUCAN ENDOTRANSGLUCOSYLASE/HYDROLASE 8), EXPA4 (EXPANSIN A4) and PAE3 (PECTIN ACETYLESTERASE 3)) showed differential expression compared with WT in wheat leaves (Figure S1B). Thus, the overexpression of TaKLU contributed to increasing leaf size by promoting cell division.
2.2. TaKLU Overexpression Extended the Time of Leaf Elongation
Our previous study showed that TaKLU promotes grain enlargement by prolonging the time of cell division [22]. To explore whether the promoting effect of TaKLU on leaf growth is also regulated by time, we conducted a dynamic investigation on the characteristics of the leaf of WT and TaKLU-overexpressing plants in wheat. Dynamic morphological observations showed that although the elongation rate of the leaf 5 of TaKLU-overexpressing plants was always higher than that of WT, only in the later stages of leaf development (from 8 to 10 days after leaf 5 appearance) was a statistically significant difference (Figure 2A). Correspondingly, the expansion of leaf 5 of WT plants almost stopped at the 8 days after its appearance, while the expansion of the leaf 5 of TaKLU-overexpressing plants could be maintained after 10 days after its appearance (Figure 2B,C). Further leaf elongation was prolonged by about two days due to TaKLU- overexpression (Figure 2D). Thus, the increase in leaf size of TaKLU-overexpressing plants was attributed to a longer leaf expansion time more than a faster growth rate (Figure 2A–D and Figure S1A).
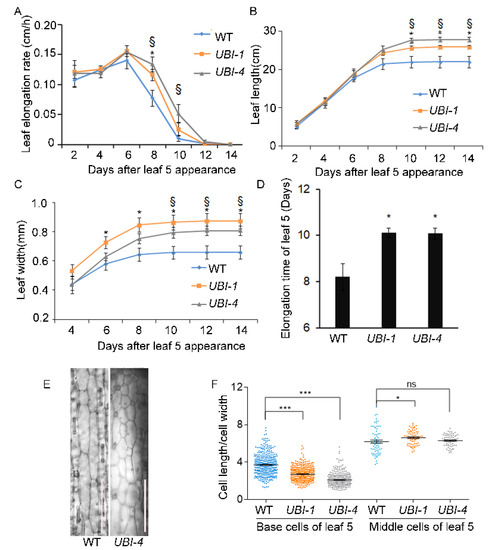
Figure 2.
TaKLU regulated the time of leaf elongation and cell division. (A–C) Dynamic analysis of the elongation rate (A), length (B) and width (C) of leaf 5 from 2 to 14 days after its appearance in WT and UBI::TaKLU plants (n > 7). (D) Elongation time of leaf 5 (n > 7). Leaf elongation time represents the minimum time required for leaf growth to enter the plateau stage (no significant change in leaf length). (E) Characteristics of base cells of leaf 5 were shown at 16 days after its appearance in WT (left) and UBI::TaKLU (right, UBI-4) plants. (F) The aspect ratio of the leaf base cells in panel (E) (n > 68). Bars indicate SE. Size bars represent 200 μm (E). * indicates significant differences between UBI-1 and WT plants at p < 0.05 (t-test), § indicates significant differences between UBI-4 and WT plants at p < 0.05 (t-test) in panel (A–C). Asterisks (*) and (***) indicate significant differences from their WT at p < 0.05 and p < 0.001 (t-test) in panel (D) and (F), respectively. “ns” means no significant difference.
Moreover, in view of the fact that leaf cell division mainly occurs in the division zone at the base of leaf, and TaKLU was highly expressed in meristems (Figure S2), we investigated the cell characteristices of division zone to analyze the promoting effect of TaKLU on cell division time. Interestingly, cytological observations revealed that the cell division of TaKLU-overexpressing leaves may last a longer time than that of WT, even the division zone of the leaf 5 of WT plants basically disappeared on the 16 days after leaf 5 appearance, the division zone of TaKLU-overexpressing leaves still existed (Figure 2E). Statistics data showed that the base cells of TaKLU-overexpressing leaves were smaller and more round that had a smaller aspect ratio than those of WT at 16 days after its appearance in wheat, and different from the cell morphology in the middle of their leaves (Figure 2E,F). These results implied that the overexpression of TaKLU extended the time of cell division in wheat leaves. In summary, these results indicated that TaKLU promoted leaf growth by extending the duration of leaf expansion in wheat.
2.3. Overexpression of TaKLU Smoothed the Fluctuations of Leaf Initiation Time in Wheat
Given that loss of AtKLU function led to an increase in leaf initiation rates due to accelerating cell division around the shoot apical meristems [16,17]. Combining that both AtKLU and TaKLU were highly expressed in the shoot apical meristem and affected cell division [15] (Figure S2), we speculated that TaKLU might also be involved in the regulation of leaf initiation and leaf number in wheat. Thus, to further understand the regulation of the effects of TaKLU on leaf development by time, we investigated the initiation times of all leaves at seeding stage. Before this, we first analyzed the grain germination rate of WT and transgenic plants at 24 h after sowing to exclude the effect of germination factors on leaf initiation time. The results showed that the germination time and characteristics of their grains cannot be distinguished throughout the germination stage (Figure 3A,B).
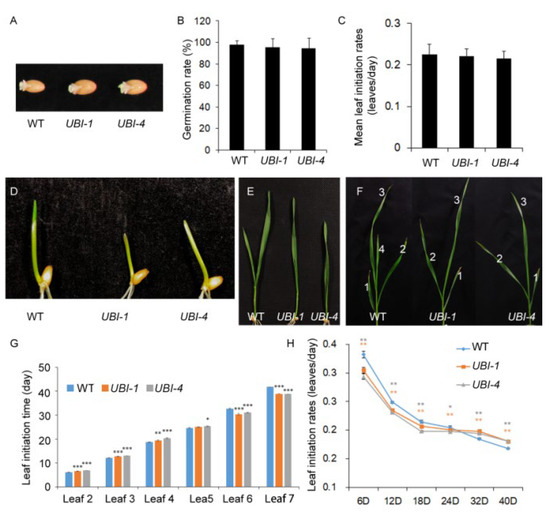
Figure 3.
Overexpression of TaKLU in wheat influenced leaf initiation time of local leaves. (A) Comparison of grain germination between WT and UBI::TaKLU plants at 24 h after sowing. (B) Statistics of germination rates of WT and UBI::TaKLU plants (n > 20) at 24 h after sowing. (C) Statistics of mean leaf initiation rates of WT and UBI::TaKLU plants (n > 10). (D–F) Comparison of growth status between WT and UBI::TaKLU plants at the seedling stage (The numbers in subfigure (F) indicate the order in which the leaves appeared). (G) Dynamic analysis of the leaf initiation time of WT and UBI::TaKLU plants (n > 10). (H) Statistics of leaf initiation rates of WT and UBI::TaKLU plants after sowing (n > 10). Bars indicate SE. Asterisks (*), (**), and (***) indicate significant differences from their WT at p < 0.05, p < 0.01, and p < 0.001 (t-test), respectively.
Unlike klu mutant plants, which had faster leaf initiation rates and more leaves [17] (Figure 4), both WT and TaKLU-overexpressing plants had only 7 leaves and there were no difference in their mean leaf initiation rates of whole growth stages (Figure 3C). However, TaKLU overexpressing plants grew slower than WT at the early stages of the seedling (Figure 3D–F), and then the leaf initiation time of the TaKLU-overexpressing plants was lower than that of WT (Figure 3G). Interestingly, with the increase in the number of leaves, the leaf initiation rates of all genotype plants was declining, but the decay of the leaf initiation rates of TaKLU overexpressing plants was slower than that of WT, even the initial leaf rates of TaKLU overexpressing plants was higher than that of WT at the later stage of seedling. (Figure 3G,H). During the whole vegetative stage, TaKLU-overexpressing plants appeared to weaken the fluctuation of leaf initiation time, leading to the leaf initiation being gentler (Figure 3C). These results indicated that TaKLU overexpression smoothed the fluctuations in leaf initiation by adjusting the initiation time of local leaves, rather than affecting the overall level of the leaf number and initiation rates at the seedling stage.
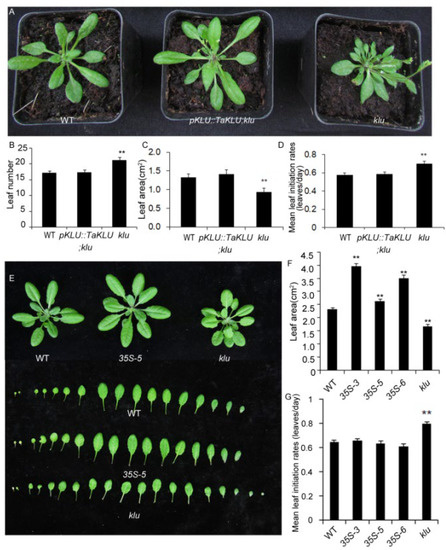
Figure 4.
Conserved function of TaKLU and AtKLU in regulating organ growth. (A) TaKLU rescued the multi-leaf phenotype of klu mutants at 30 days after germination. (B–D) Quantification of rosette leaf number (B), n > 10), area (C), n > 5) and leaf initiation rates (D), n > 22) in the indicated genotypes at 30 days after germination. (E–G) Comparison of the leaf morphological characteristics between WT, 35S::TaKLU (35S-5) and klu mutant plants at 30 days after germination in Arabidopsis. (E) The seedling leaves of the indicated genotypes. (F–G) Statistics on the leaf area (F), (n > 21) and leaf initiation rate (G), (n > 13) of WT, 35S::TaKLU and klu mutant plants. Bars indicate SE. Asterisks (**) indicate significant differences from their WT at p < 0.01 (t-test).
2.4. TaKLU Was Functionally Conserved in Both Monocots and Dicots
To verify whether TaKLU and AtKLU are functionally conserved in regulating leaf growth, we constructed a vector pAtKLU::TaKLU that expressed TaKLU driven by the promoter of AtKLU (pAtKLU) in Arabidopsis, and transformed pAtKLU::TaKLU vector into klu mutants that have been generated previously [28]. Exactly, the phenotype of the increased leaf number, accelerated leaf initiation rates, and small leaves of the klu mutants were rescued to normal levels by transferring the pAtKLU::TaKLU construct, compared with WT (Figure 4A–D). Moreover, the leaf phenotypes of pAtKLU::TaKLU;klu and wild-type plants were almost indistinguishable at the seedling stage (Figure 4A), suggesting that wheat TaKLU could rescue the phenotype of klu mutants well.
In addition, to further demonstrate whether TaKLU has a conserved function in regulating leaf size in both monocots and dicots, we constructed multiple transgenic plants with constitutive promoters (35S: CaMV35S promoter) driving TaKLU expression in Arabidopsis (35S::TaKLU, including 35S-3, 35S-5 and 35S-6) to investigate leaf phenotypes. QRT-PCR detected a significant increase in the expression level of TaKLU in all of the transgenic lines (Figure S3). Similar to the phenotype of TaKLU-overexpressing wheat, TaKLU-overexpressing Arabidopsis had the same leaf number and initiation rates, and formed larger leaves, compared with WT (Figure 4E–G). These results suggested that TaKLU had a conserved function in regulating leaf growth in both monocots and dicots.
2.5. TaKLU Promoted Leaf Growth via Auxin Signaling
Many studies suggest that KLU depends on a mobile growth factor downstream to promote organ growth, but there has been a lack of strong molecular evidence linking it to known growth-promoting factors [10,23]. Considering the klu mutants showed reduced apical dominance and shortened longevity (Figure S4) [16], and the overexpression of TaKLU in wheat led to enhanced apical dominance [22], these phenotypes are classic phenotypes related to auxin [29,30]. We speculated that the regulation effect of TaKLU on leaf growth might involve auxin signaling. To test this, we analyzed the accumulation of auxin by using the auxin response reporter DR5:GUS in Arabidopsis. The strength of the GUS signals suggested that the auxin accumulation in TaKLU-overexpressing leaves was increased, while the auxin accumulation of klu mutant leaves was decreased than that of WT (Figure 5A–C). Moreover, the constitutive expression of TaKLU changed the original accumulation pattern of auxin. Auxin, originally mainly concentrated in the local area as shown in WT, was now distributed accumulated throughout the leaves by constitutively expressing TaKLU (Figure 5A–C). To further confirm whether ectopic TaKLU expression affects the auxin pathway in wheat and Arabidopsis, we tested the expression level of three marker genes related to auxin metabolism and response [31,32]. Compared with WT plants, the expression levels of YUC 1 (YUCCA 1), ARF 13 (AUXIN RESPONSE FACTOR 13), and PIN1 (PINFORMED 1) genes in TaKLU-overexpressing plants all changed significantly (Figure S1C,D). These results indicated that TaKLU activity affected the accumulation of auxin.
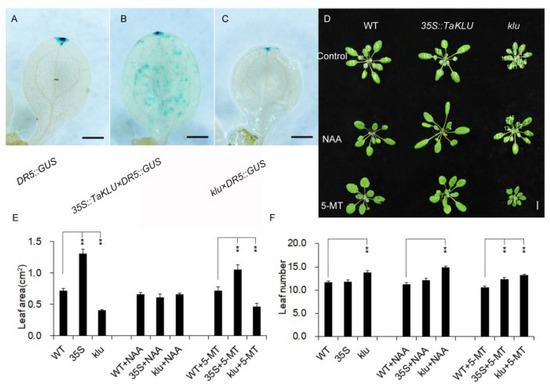
Figure 5.
TaKLU regulated the accumulation and response of auxin in leaves. (A–C) DR5:GUS marker was used to detect the auxin accumulation in DR5:GUS (A), 35S::TaKLU × DR5:GUS hybrids (B) and klu × DR5:GUS hybrids (C). After resistance screening, the positive hybrids were planted in the same environment with the control groups. Cotyledons of different genotypes Arabidopsis were taken for GUS staining at two weeks post-germination. (D) Responses of WT, 35S::TaKLU and klu mutant plants to 1-Naphthaleneacetic acid (NAA) and the auxin synthesis inhibitor 5-methyl-tryptophan (5-MT) treatment. Individual plants (n > 24) at the same developmental stage were selected for treatment according to previous reports [33]. (E,F) Statistics on the leaf area (E), n > 10) and leaf number (F), (n > 9) of WT, 35S::TaKLU and klu mutant plants. Size bars represent 200 μm (A–C) and 1 cm (D). Bars indicate SE. Asterisks (**) indicate significant differences from their WT at p < 0.01 (t-test).
To further understand the relationship between auxin accumulation and the leaf phenotype of different genotypes, we used the auxin analog 1-Naphthaleneacetic acid (NAA) and the auxin synthesis inhibitor 5-methyl-tryptophan (5-MT) to treat different genotypes of Arabidopsis. The results showed that the TaKLU-overexpressing Arabidopsis was more sensitive to NAA treatment, and its leaves were slenderer and even more curled than that of WT, which led to the disappearance of the significant difference between the leaf size of TaKLU-overexpressing plants and that of WT (Figure 5D,E). Moreover, although NAA treatment could not completely recover the phenotype of the increased leaf number of the klu mutants (Figure 5F), it could improve the phenotype of klu mutants, which showed that the petiole length and leaf area of klu mutants increased after NAA treatment (Figure 5D,E). In contrast, the leaves of klu mutant were smaller and more compact than that of WT after 5-MT treatment, (Figure 5D,E), which was very similar to the leaf phenotype of cyp78a5 cyp78a7 double-mutants [17], suggesting that the klu mutants were more sensitive to 5-MT treatment. These observations indicated that both TaKLU and AtKLU regulating leaf growth depended on the auxin signaling.
3. Discussion
3.1. KLU Plays as a Time Regulator of Leaf Growth
Although the leaves of monocot and dicot plants are very different in morphology and structure, the growth of their leaves is both driven by cell division and cell expansion regulating by space and time [34]. AtKLU promotes organ growth by stimulating cell division, and the altered AtKLU activity can change the time of leaf initiation and senescence [17,21]. Interestingly, our results further revealed that enhanced expression of TaKLU prolonged the time of leaf elongation in dynamic observation, which is caused by prolonging the time of cell division (Figure 2). These indicated that TaKLU had a time effect on promoting leaf growth.
CYP78As have been shown to control organ size by promoting cell division and cell expansion [11,16]. Among them, OsPLA1, AtKLU, and AtCYP78A7 are likely homologous genes and reported to act on the initial and subsequent growth of leaves. Their common features include expressing at the periphery of the shoot apical meristem and affecting the development and fate of shoot apical meristem [17,19,35]. For example, pla1 plants had enlarged shoot apical meristem due to activated cell divisions, which gave rise to a faster leaf initiation rate, and finally formed the doubled number of leaves compared with WT [35]. Based on the expression pattern and its mutant phenotype, PLA1 was considered to be a timekeeper of plant development [35]. Similarly, inactivation of AtKLU accelerated the cell division in the shoot apical meristems in klu mutants, which formed a bigger shoot apical meristem to compensate for an increase in leaf initiation rate compared with WT, even cyp78a5 cyp78a7 double-mutants often die as embryos with supernumerary cotyledon primordial [17]. Combined with the expression of TaKLU in young tissues and shoot apical meristem (Figure S2), our work further supports such function of leaf growth time regulation by showing that TaKLU overexpression leads to smooth fluctuations in leaf initiation rates and prolonged growth of leaves (Figure 2 and Figure 3).
3.2. Relationship between KLU and Plant Hormones
As a monooxygenase, CYP78As may play an important role in biosynthesis, especially the synthesis of secondary metabolites such as fatty acids [10,16]. For example, the overexpression of AtKLU led to an increase in Arabidopsis seed oil content. Transcriptome analysis found that AtKLU regulated the expression of several cytochrome P450 genes involved in fatty acid modification [10,16]. Similarly, biochemical analysis showed that CYP78A1 can catalyze the monooxygenase reaction of certain fatty acids [36], and PLA1 may be involved in the fatty acid synthesis and metabolism pathways required for leaf development [35]. Therefore, it was proposed previously that CYP78As were involved in generating a novel signaling factor derived from fatty acids, which can move and promote the growth of cells and organs [10,37]. For AtKLU, it has been shown that it promoted growth depending on a mobile growth factor as a traditional hormone in a non-cell autonomous manner [10,16].
Phytohormones are proven to be the determinants of controlling non-cell autonomous growth [38]. However, there has been a controversy about the relationship between KLU and hormones. Some researchers believe that AtKLU does not directly contribute to biosynthesis or degradation of one of the classical hormones, because of the lack of consistent overlap between the transcriptional responses to AtKLU and to phytohormones, and the klu mutant’s phenotype cannot be rescued by treatment with exogenous hormones [16]. However, a more recent study showed that AtKLU contributed to leaf longevity by activation of cytokinin signaling, because metabolome results showed that the overexpression of AtKLU led to increased cytokinin accumulation in leaves [21]. However, changes in auxin accumulation were also detected in their metabolome studies [21]. Here, the expression of the auxin-responsive DR5:GUS marker showed TaKLU-overexpressing plants and klu mutant plants had significant changes in the auxin accumulation in their leaves, and these changes were consistent with TaKLU/KLU activity (Figure 5A–C). And our recent research also found that TaKLU regulates grain size by affecting the accumulation of auxin [22]. Similarly, in two independent experiments, it was detected that PLA1 acts on organ development and involved the metabolism of auxin and gibberellin, respectively [39,40]. It is well known that the metabolism and function of different hormones have synergistic and antagonistic effects. Therefore, the changes in the accumulation of multiple hormones have been detected in independent tests related to AtKLU and PLA1.
Finally, AtKLU was known to regulate plant growth by promoting cell division, determining cell fate, and delaying leaf senescence in non-cell autonomous manner [15,16,17,18,21], which were all classic functions of both auxin and cytokinin. In previous experiments, it seemed difficult to determine whether the main factor that AtKLU depends on to promote organ development is auxin, cytokinin, or neither. Here, we found that the activity of KLU positively regulated the accumulation of auxin in the leaves (Figure 5A–C), and TaKLU-overexpressing plants were more sensitive to NAA treatment (Figure 5D), while klu mutant plants were more sensitive to the auxin synthesis inhibitor 5-MT treatment (Figure 5D). Thus, we are inclined to support the view that KLU regulates leaf development via auxin signaling, but more strong evidence is still needed to prove that KLU plays a role in the auxin pathway.
3.3. Strategies of TaKLU Applied in Wheat Breeding
Although TaKLU appeared to be a potent positive regulator of vegetative organ size (Figure 1), unfortunately, constitutive overexpression did not result in a significant increase in yield due to increased apical dominance of wheat [22]. Combined with the fact that constitutive overexpression of TaKLU could change the accumulation and distribution of auxin in leaves (Figure 5A,B), it was reasonable to speculate that constitutive overexpression of TaKLU could also alter the accumulation and distribution of auxin in whole plant, thereby changing the plant type of wheat. How to apply TaKLU to high-yield crop breeding seemed to be a problem that needed to be constantly explored. Our previous study proposed an effective strategy to locally overexpress TaKLU only in the integument of wheat ovary, which not only overcomes the apparent apical dominance of the TaKLU overexpressing-plants, but also improved grain weight and grain yield per plant. Similarly, local overexpression of an expansin/TaExpA6 in developing grain resulted in grain enlargement without affecting the number of grains in wheat, which ultimately overcame the opposing relationship between grain size and grain number, resulting in an increase in wheat yield [41]. However, a moderate increase in the size of the vegetative organ was also beneficial to the increase in yield, so we proposed that by overexpressing TaKLU in expanding leaves specifically, an increase in leaf size could be achieved without affecting the whole plant type, which may be a possible strategy for TaKLU application in wheat improvement.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
The Arabidopsis materials used in this study were all based on the genetic background of Arabidopsis thaliana ecotype Columbia. The cyp78a5/klu mutants (Salk_024697C) was obtained from Arabidopsis Biological Resources (https://abrc.osu.edu/ accessed on 1 April 2022) and had been identified previously [28]. The transgenic Arabidopsis lines and the control materials were grown under the same conditions, which grew under a 16-h/8-h light/dark cycle at 22 °C in the greenhouse, until all of the silique is completely ripe.
The wheat materials used in this study all took JW1 as the genetic background. All of the wheat materials used in this study were grown in the greenhouse, which grew under 24 °C day temperature, 18 °C night temperature and 50% humidity until all of the ears and the leaves were completely yellow.
4.2. Vector Construction and Transformation
The UBI::TaKLU:GFP/GUS transgenic wheat used in this study was obtained in the early stage of the laboratory [22]. The bar gene was used as a selection marker. The positive transgenic lines were identified based on 0.2% glufosinate (BASTA) through leaf daubing.
To generate overexpression Arabidopsis plants, two vectors contained TaKLU with different promoters (CaMV 35S promoter and the promoter of AtCYP78A5/AtKLU) were constructed to transform Arabidopsis. The coding region of TaKLU-A without stop codon was amplified and cloned into pCAMBIA 3301 vector at Xba I/Bgl II site under the control of the CaMV 35S promoter to generate expression vector p3301-p35S::TaKLU. Based on this vector, the promoter of AtCYP78A5/AtKLU (AT1G13710) (from –1bp to –2229bp) was amplified from A. thaliana genome and replaced the CaMV 35S promoter with EcoR I/Xba I restriction site to construct expression vector p3301-pKLU::TaKLU. The primer sequences were listed in Table S1. These two vectors were introduced into Arabidopsis (Columbia-0) by Agrobacterium tumefaciens transformation method as previously described [42]. The transgenic plants were selected by spraying 0.1% glufosinate (BASTA) on seedling leaves. At least three independent transgenic homozygous lines were obtained for each construct.
4.3. RNA Isolation and QRT-PCR Analysis
To detect the expression level of TaKLU, lateral inflorescences of different genotype Arabidopsis plants were collected at 45 days after germination for reverse transcriptase-polymerase chain reaction (RT-PCR) analysis. To detect the expression level of cell cycle (CDKA;1, CYCT;1, CDC20), cell wall (XTH8, EXPA4, PAE3), and auxin-related genes (YUCCA1, PIN1, ARF13), young leaves in the same position from different genotype plants were collected at 10 days after its appearance in wheat and Arabidopsis. All samples were frozen in liquid nitrogen and stored at −80°C until RNA extraction.
Total RNA was isolated using the SteadyPure Plant RNA Extraction Kit (Accurate Biotechnology, Changsha, China), and the first-strand cDNA was synthesized via reverse transcription reaction with the Evo M-MLVRT Premix Kit (Accurate Biotechnology, Changsha, China). The expression levels of target genes were detected by Quantitative Real-time PCR using the SYBR Green premix Pro Taq HS qPCR Kit (Accurate Biotechnology, Changsha, China). Each test has three biologically independent repeats, and the experiment was repeated twice. The results were normalized by the expression levels of internal reference genes UBC (for Arabidopsis) or ACTIN (for wheat). The test results were analyzed with reference to the method of Pfaffl et al. [43]. The primer information used for QRT-PCR detection is shown in Table S2.
4.4. Germination Experiment
We selected transgenic wheat and the control harvested in the same period for germination experiments. The grains of the above genotypes were kept in a moist state and the same environment, and the number of grains germinated which endosperm broken through the seed coat was counted every 12 h. Until all of the grains germinated.
4.5. Morphological Analysis
To detect the leaf initiation time of transgenic wheat and the control, we observed the above genotype wheat every day after its germination, and we regard that day when the leaf appeared and the new leaf was visible to the naked eye and appeared about 1–2 cm, as the second day of the appearance of the leaf. The wheat above mentioned was grown in the Hogland nutrient solution according to the instructions.
To dynamically investigate the phenotypic changes of leaves, from the emergence of the fifth wheat leaf (about 50 days after sowing), the length and width of the fifth leaf were measured every other day until the elongation stopped. The length and width of the third and seventh leaves were respectively measured when they were no longer elongated. The leaf area of wheat was calculated by leaf length and width as previously described [44].
In order to explore the conservative function of TaKLU and AtKLU, we constructed a vector pAtKLU::TaKLU and transformed it into homozygous klu mutants [28]. The number of leaves of the above genotypes Arabidopsis and the control plants was counted at 30 days after its germination and at least 10 plants were measured per line. For accurate measurement, the eighth rosette leaves of different genotypes were photographed with the digital camera, and then their areas were measured with Image J software (https://imagej.en.softonic.com/, accessed on 10 March 2022), at least 5 plants were measured per line. And the images were collected 30 days after germination.
To investigate the phenotypic of leaves of TaKLU-overexpressing Arabidopsis, the length, width, and area of the eighth rosettes leaves of Arabidopsis were measured at 30 days after germination, at least 21 plants were measured per line. The leaf initiation rates in this study was calculated from the ratio of the number of leaves to the growth time (days).
4.6. Chemical Treatments
To investigate the relationship between KLU and auxin, the 4-week-old 35S::TaKLU Arabidopsis, klu mutants and wild type plants were sprayed with the auxin analogue 1-Naphthaleneacetic acid (NAA) and the auxin synthesis inhibitor 5-methyl-tryptophan (5-MT) (in water), respectively. The spraying concentration of NAA and 5-MT was selected as 0.1 and 500 μM, respectively, according to relevant literature [30,33]. Individual plants at approximately the same developmental stage were selected for treatment. Evenly sprayed IAA, 5-MT, or control solution every 4 days (28, 32, and 36 days after germination) to treat the plants, with at least twenty-four individual plants per line and three lines totally. The observation was made every other day from the first exogenous spray, and final phenotypes were imaged 4 days after the last treatment (40 days after germination). And the images were used to measure the number and area of leaves with Image J software.
4.7. Cytological Analysis
In order to determine the sizes of epidermal cells of the leaves, we collected leaf 5 of wheat which appears on 16 days. All of the samples were immersed in carnoy-fixation (Methanol:Glacial acetic acid = 3:1) at 4 °C for 20 min, and then immersed in alcohol at 30%, 50%, 75%, 95%, and 100% in concentration gradient successively until the leaves were decolorized, all of the samples were immersed in each gradient for 30 min. Observed and collected the image of the middle and the bottom part of the leaves through an optical microscope (Olympus, System Microscope BX51, Tokyo, Japan), and finally used the Image J software to measure the cell size. At least 300 cells were measured per line. The total number of epidermal cells per leaf was calculated by dividing the total leaf area by the average cell area.
4.8. Histochemical Staining
The DR5:GUS line was used as the carrier for in situ analysis of auxins. DR5:GUS homozygous was used as the female parent, and the TaKLU-overexpressing Arabidopsis and klu mutant plants were used as the male parent, respectively. Cross and propagate the above parental plants to obtain F1 hybrids. The GUS staining assay was performed on the leaves of the F1 hybrids at two weeks post-germination, referring to the method reported by Disch et al. [45].
4.9. Statistical Analysis
All data obtained in this study were processed in Excel 2013, and an unpaired Student’s t-test was used for p-values. Significant differences were considered if p-values < 0.05, very significant differences were considered if p-values < 0.01.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23084219/s1.
Author Contributions
M.M., H.P. and W.L. conceived and designed the experiments. M.Z. and L.W. conducted the experiments and analyzed the data. L.W., L.G. and H.C. conducted genotype analysis of wheat accessions. M.L. and B.W. helped to construct expression vector. M.M. and M.Z. wrote the draft of the manuscript. X.L. and H.Z. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Nature Science Foundation of China (32072003 and 32072059) and the Key Research and Development Program of Shaanxi Province (2021NY-079).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data can be found in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xiong, Y.; Jiao, Y. The Diverse Roles of Auxin in Regulating Leaf Development. Plants 2019, 8, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Fang, Y.; Hu, P.; Tan, Y.; Wang, Y.; Hou, L.; Deng, X.; Wu, H.; Zhu, L.; Zhu, L.; et al. Construction of a High-Density Genetic Map Based on Slaf Markers and Qtl Analysis of Leaf Size in Rice. Front. Plant Sci. 2020, 11, 1143. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tu, Y.; Zhu, J.; Luo, W.; Liu, H.; Li, C.; Li, S.; Liu, J.; Ding, P.; Habib, A.; et al. Flag Leaf Size and Posture of Bread Wheat: Genetic Dissection, Qtl Validation and Their Relationships with Yield-Related Traits. Theor. Appl. Genet. 2020, 133, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Sizani, B.L.; Kalve, S.; Markakis, M.N.; Domagalska, M.A.; Stelmaszewska, J.; AbdElgawad, H.; Zhao, X.; De Veylder, L.; De Vos, D.; Broeckhove, J.; et al. Multiple Mechanisms Explain How Reduced Krp Expression Increases Leaf Size of Arabidopsis Thaliana. New Phytol. 2019, 221, 1345–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Rhee, J.Y.; Lee, S.H.; Chung, G.C.; Park, S.J.; Segami, S.; Maeshima, M.; Choi, G. Functionally Redundant Lng3 and Lng4 Genes Regulate Turgor-Driven Polar Cell Elongation through Activation of Xth17 and Xth24. Plant Mol. Biol. 2018, 97, 23–36. [Google Scholar] [CrossRef]
- Vanhaeren, H.; Nam, Y.J.; De Milde, L.; Chae, E.; Storme, V.; Weigel, D.; Gonzalez, N.; Inzé, D. Forever Young: The Role of Ubiquitin Receptor Da1 and E3 Ligase Big Brother in Controlling Leaf Growth and Development. Plant Physiol. 2017, 173, 1269–1282. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.; Lowndes, L.; Regan, S.; Beardmore, T. Overexpression of Cycd1;2 in Activation-Tagged Populus Tremula X Populus Alba Results in Decreased Cell Size and Altered Leaf Morphology. Tree Genet. Genomes 2015, 11, 66. [Google Scholar] [CrossRef]
- Donnelly, P.M.; Bonetta, D.; Tsukaya, H.; Dengler, R.E.; Dengler, N.G. Cell Cycling and Cell Enlargement in Developing Leaves of Arabidopsis. Dev. Biol. 1999, 215, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, M.; Ohta, D. Diversification of P450 Genes During Land Plant Evolution. Annu. Rev. Plant Biol. 2010, 61, 291–315. [Google Scholar] [CrossRef]
- Adamski, N.M.; Anastasiou, E.; Eriksson, S.; O’Neill, C.M.; Lenhard, M. Local Maternal Control of Seed Size by Kluh/Cyp78a5-Dependent Growth Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20115–20120. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Wang, Z.; Cui, R.; Li, J.; Li, Y. Maternal Control of Seed Size by Eod3/Cyp78a6 in Arabidopsis Thaliana. Plant J. Cell Mol. Biol. 2012, 70, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Silveira, M.; Cucinotta, M.; Chauvin, A.L.; Chávez Montes, R.A.; Colombo, L.; Marsch-Martínez, N.; de Folter, S. Cytochrome P450 Cyp78a9 Is Involved in Arabidopsis Reproductive Development. Plant Physiol. 2013, 162, 779–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Fang, J.; Ou, S.; Gao, S.; Zhang, F.; Du, L.; Xiao, Y.; Wang, H.; Sun, X.; Chu, J.; et al. Variations in Cyp78a13 Coding Region Influence Grain Size and Yield in Rice. Plant Cell Environ. 2015, 38, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, C.; Song, L.; Li, Y.; Li, M. Pacyp78a9, a Cytochrome P450, Regulates Fruit Size in Sweet Cherry (Prunus Avium L.). Front. Plant Sci. 2017, 8, 2076. [Google Scholar] [CrossRef] [Green Version]
- Zondlo, S.C.; Irish, V.F. Cyp78a5 Encodes a Cytochrome P450 That Marks the Shoot Apical Meristem Boundary in Arabidopsis. Plant J. 1999, 19, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Anastasiou, E.; Kenz, S.; Gerstung, M.; MacLean, D.; Timmer, J.; Fleck, C.; Lenhard, M. Control of Plant Organ Size by Kluh/Cyp78a5-Dependent Intercellular Signaling. Dev. Cell 2007, 13, 843–856. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.W.; Schwab, R.; Czech, B.; Mica, E.; Weigel, D. Dual Effects of Mir156-Targeted Spl Genes and Cyp78a5/Kluh on Plastochron Length and Organ Size in Arabidopsis Thaliana. Plant Cell 2008, 20, 1231–1243. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Cai, H.; Su, Z.; Wang, L.; Huang, X.; Zhang, M.; Chen, P.; Dai, X.; Zhao, H.; Palanivelu, R.; et al. Klu Suppresses Megasporocyte Cell Fate through Swr1-Mediated Activation of Wrky28 Expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E526–E535. [Google Scholar] [CrossRef] [Green Version]
- Poretska, O.; Yang, S.; Pitorre, D.; Poppenberger, B.; Sieberer, T. Amp1 and Cyp78a5/7 Act through a Common Pathway to Govern Cell Fate Maintenance Inarabidopsis Thaliana. PLoS Genet. 2020, 16, e1009043. [Google Scholar] [CrossRef]
- Nobusawa, T.; Kamei, M.; Ueda, H.; Matsushima, N.; Yamatani, H.; Kusaba, M. Highly Pleiotropic Functions of Cyp78as and Amp1 Are Regulated in Non-Cell-Autonomous/Organ-Specific Manners. Plant Physiol. 2021, 186, 767–781. [Google Scholar] [CrossRef]
- Jiang, L.; Yoshida, T.; Stiegert, S.; Jing, Y.; Alseekh, S.; Lenhard, M.; Pérez-Alfocea, F.; Fernie, A.R. Multi-Omics Approach Reveals the Contribution of Klu to Leaf Longevity and Drought Tolerance. Plant Physiol. 2021, 185, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ma, M.; Wu, L.; Zhou, M.; Li, M.; Wu, B.; Li, L.; Liu, X.; Jing, R.; Chen, W.; et al. Modified Expression of Tacyp78a5 Enhances Grain Weight with Yield Potential by Accumulating Auxin in Wheat (Triticum Aestivum L.). Plant Biotechnol. J. 2021, 20, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.; Stransfeld, L.; Adamski, N.M.; Breuninger, H.; Lenhard, M. Kluh/Cyp78a5-Dependent Growth Signaling Coordinates Floral Organ Growth in Arabidopsis. Curr. Biol. 2010, 20, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalve, S.; De Vos, D.; Beemster, G.T. Leaf Development: A Cellular Perspective. Front. Plant Sci. 2014, 5, 362. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Kong, H.; Sun, Y.; Zhang, X.; Zhang, W.; Altman, N.; De Pamphilis, C.W.; Ma, H. Genome-Wide Analysis of the Cyclin Family in Arabidopsis and Comparative Phylogenetic Analysis of Plant Cyclin-Like Proteins. Plant Physiol. 2004, 135, 1084–1099. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.J.; Zou, W.S.; Wu, G.; Lin, H.H.; Xi, D.H. Tobacco Alpha-Expansin Expa4 Plays a Role in Nicotiana Benthamiana Defence against Tobacco Mosaic Virus. Planta 2018, 247, 355–368. [Google Scholar] [CrossRef]
- De Souza, A.; Hull, P.A.; Gille, S.; Pauly, M. Identification and Functional Characterization of the Distinct Plant Pectin Esterases Pae8 and Pae9 and Their Deletion Mutants. Planta 2014, 240, 1123–1138. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Wang, Q.; Li, Z.; Cheng, H.; Li, Z.; Liu, X.; Song, W.; Appels, R.; Zhao, H. Expression of Tacyp78a3, a Gene Encoding Cytochrome P450 Cyp78a3 Protein in Wheat (Triticum Aestivum L.), Affects Seed Size. Plant J. 2015, 83, 312–325. [Google Scholar]
- Kim, J.I.; Murphy, A.S.; Baek, D.; Lee, S.W.; Yun, D.J.; Bressan, R.A.; Narasimhan, M.L. Yucca6 over-expression Demonstrates Auxin Function in Delaying Leaf Senescence in Arabidopsis Thaliana. J. Exp. Bot. 2011, 62, 3981–3992. [Google Scholar] [CrossRef]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A Role for Flavin Monooxygenase-Like Enzymes in Auxin Biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef]
- Chandler, J.W. The Hormonal Regulation of Flower Development. J. Plant Growth Regul. 2010, 30, 242–254. [Google Scholar] [CrossRef]
- Vernoux, T.; Brunoud, G.; Farcot, E.; Morin, V.; Van den Daele, H.; Legrand, J.; Oliva, M.; Das, P.; Larrieu, A.; Wells, D.; et al. The Auxin Signalling Network Translates Dynamic Input into Robust Patterning at the Shoot Apex. Mol. Syst. Biol. 2011, 7, 508. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Du, Q.; Zhuo, C.; Qi, L.; Wang, H. Lbd29-Involved Auxin Signaling Represses Nac Master Regulators and Fiber Wall Biosynthesis. Plant Physiol. 2019, 181, 595–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelissen, H.; Gonzalez, N.; Inze, D. Leaf Growth in Dicots and Monocots: So Different yet So Alike. Curr. Opin. Plant Biol. 2016, 33, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, K.; Ahn, B.O.; Kawakatsu, T.; Ito, Y.; Itoh, J.; Nagato, Y.; Kurata, N. Plastochron1, a Timekeeper of Leaf Initiation in Rice, Encodes Cytochrome P450. Proc. Natl. Acad. Sci. USA 2004, 101, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Imaishi, H.; Matsuo, S.; Swai, E.; Ohkawa, H. Cyp78a1 Preferentially Expressed in Developing Inflorescences of Zea Mays Encoded a Cytochrome P450-Dependent Lauric Acid 12-Monooxygenase. Biosci. Biotechnol. Biochem. 2000, 64, 1696–1701. [Google Scholar] [CrossRef]
- Yang, W.; Gao, M.; Yin, X.; Liu, J.; Xu, Y.; Zeng, L.; Li, Q.; Zhang, S.; Wang, J.; Zhang, X.; et al. Control of Rice Embryo Development, Shoot Apical Meristem Maintenance, and Grain Yield by a Novel Cytochrome P450. Mol. Plant 2013, 6, 1945–1960. [Google Scholar] [CrossRef] [Green Version]
- Santner, A.; Calderon-Villalobos, L.I.; Estelle, M. Plant Hormones Are Versatile Chemical Regulators of Plant Growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- Mimura, M.; Nagato, Y.; Itoh, J. Rice Plastochron Genes Regulate Leaf Maturation Downstream of the Gibberellin Signal Transduction Pathway. Planta 2012, 235, 1081–1089. [Google Scholar] [CrossRef]
- Sun, X.; Cahill, J.; Van Hautegem, T.; Feys, K.; Whipple, C.; Novák, O.; Delbare, S.; Versteele, C.; Demuynck, K.; De Block, J.; et al. Altered Expression of Maize Plastochron1 Enhances Biomass and Seed Yield by Extending Cell Division Duration. Nat. Commun. 2017, 8, 14752. [Google Scholar] [CrossRef] [Green Version]
- Calderini, D.F.; Castillo, F.M.; Arenas-M, A.; Molero, G.; Reynolds, M.P.; Craze, M.; Bowden, S.; Milner, M.J.; Wallington, E.J.; Dowle, A.; et al. Overcoming the Trade-Off between Grain Weight and Number in Wheat by the Ectopic Expression of Expansin in Developing Seeds Leads to Increased Yield Potential. New Phytol. 2021, 230, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-Mediated Transformation of Arabidopsis Thaliana Using the Floral Dip Method. Nat. Protocols 2006, 1, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative Expression Software Tool (Rest) for Group-Wise Comparison and Statistical Analysis of Relative Expression Results in Real-Time Pcr. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Cui, F.; Zhao, C.; Zhang, W.; Yang, L.; Zhao, X.; Han, J.; Su, Q.; Ji, J.; Zhao, Z.; et al. Qtls for Flag Leaf Size and Their Influence on Yield-Related Traits in Wheat (Triticum Aestivum L.). Mol. Breed. 2015, 35, 24. [Google Scholar] [CrossRef]
- Disch, S.; Anastasiou, E.; Sharma, V.K.; Laux, T.; Fletcher, J.C.; Lenhard, M. The E3 Ubiquitin Ligase Big Brother Controls Arabidopsis Organ Size in a Dosage-Dependent Manner. Curr. Biol. 2006, 16, 272–279. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).