2-Methoxyestradiol Inhibits Radiation-Induced Skin Injuries
Abstract
:1. Introduction
2. Results
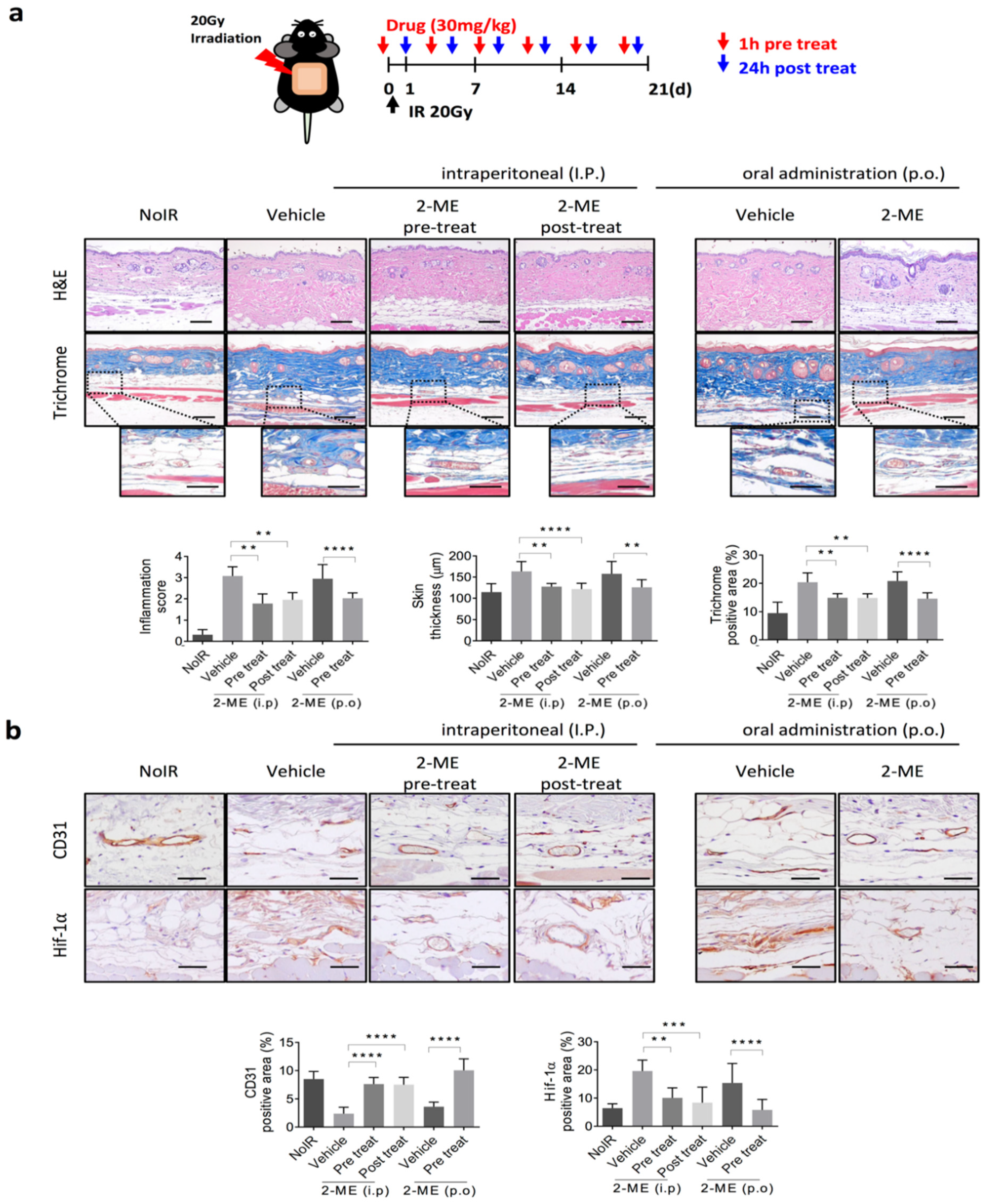
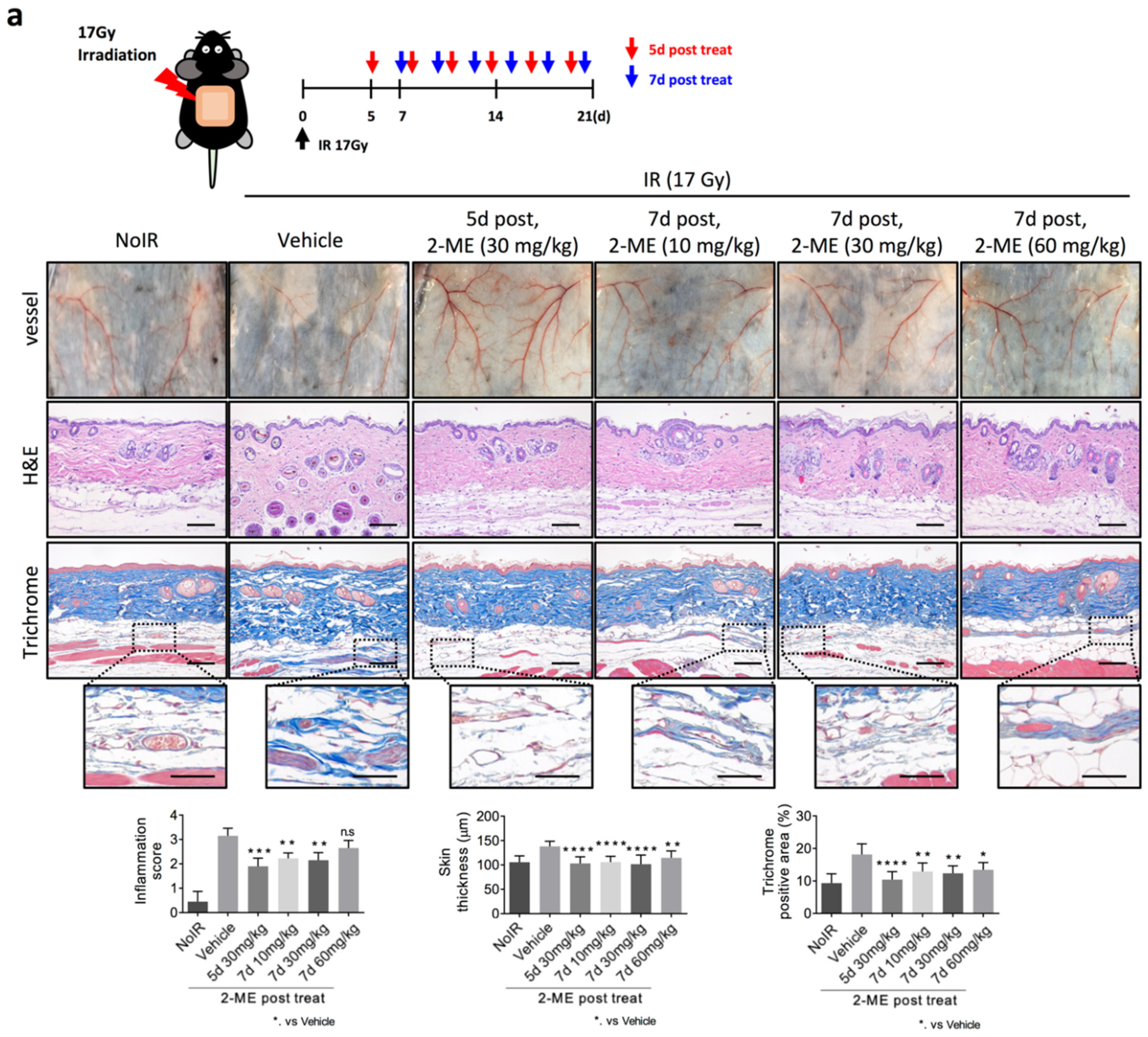
2.1. 2-ME Inhibits RISIs
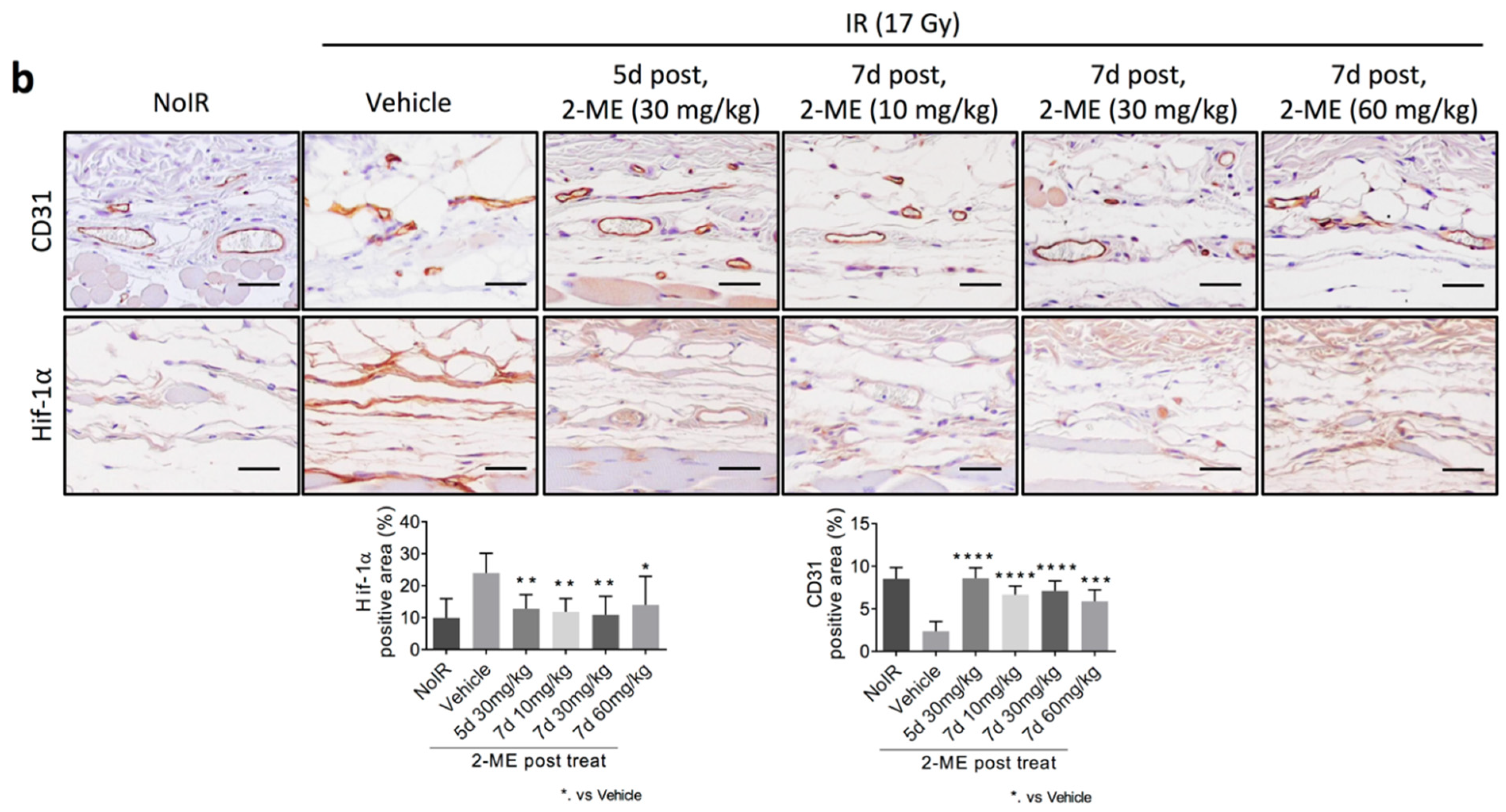
2.2. 2-ME Repairs Radiation-Induced Skin Vascular Damage
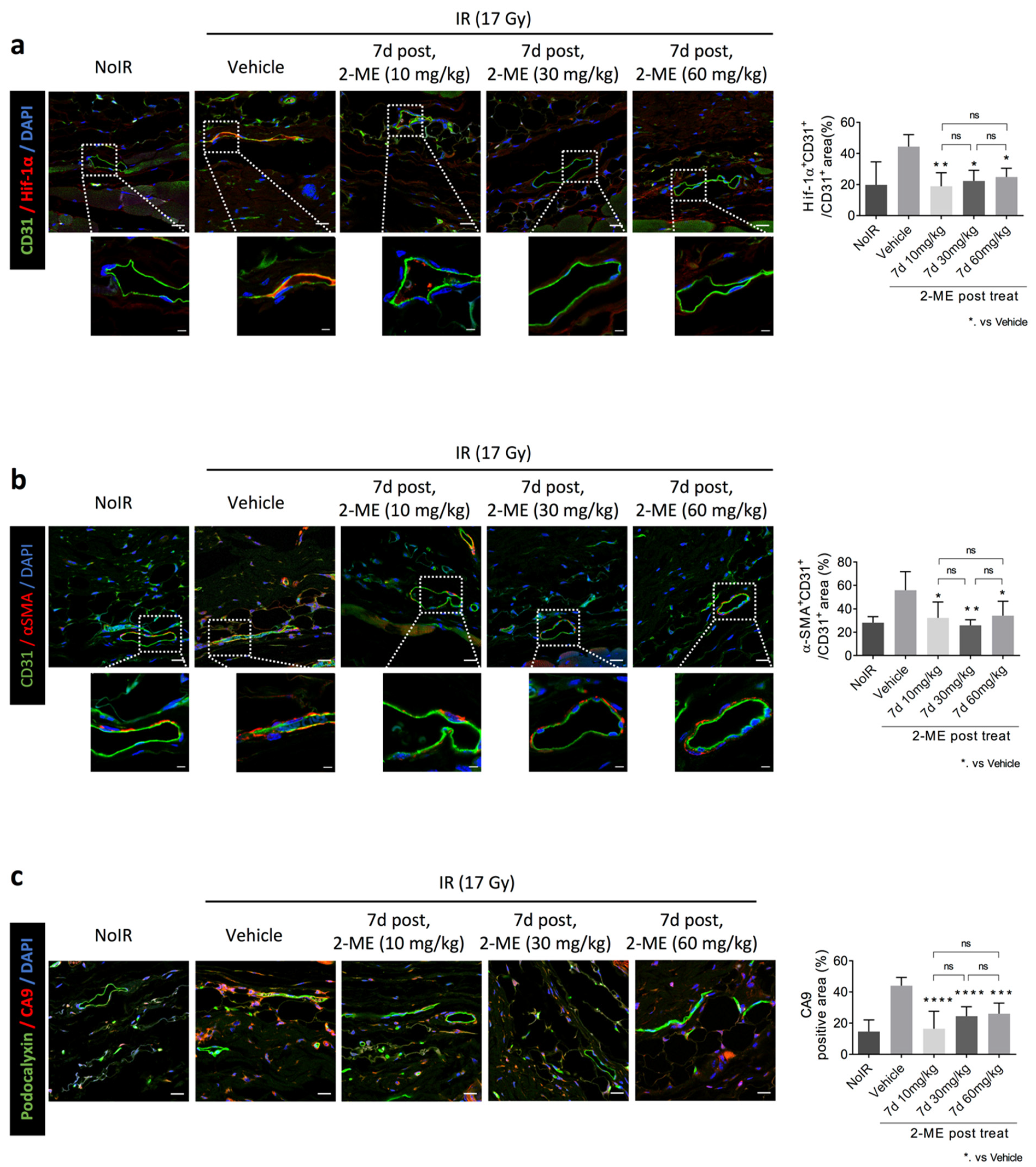
2.3. 2-ME Regulates Vascular EndMT and Hypoxic Status in RISIs
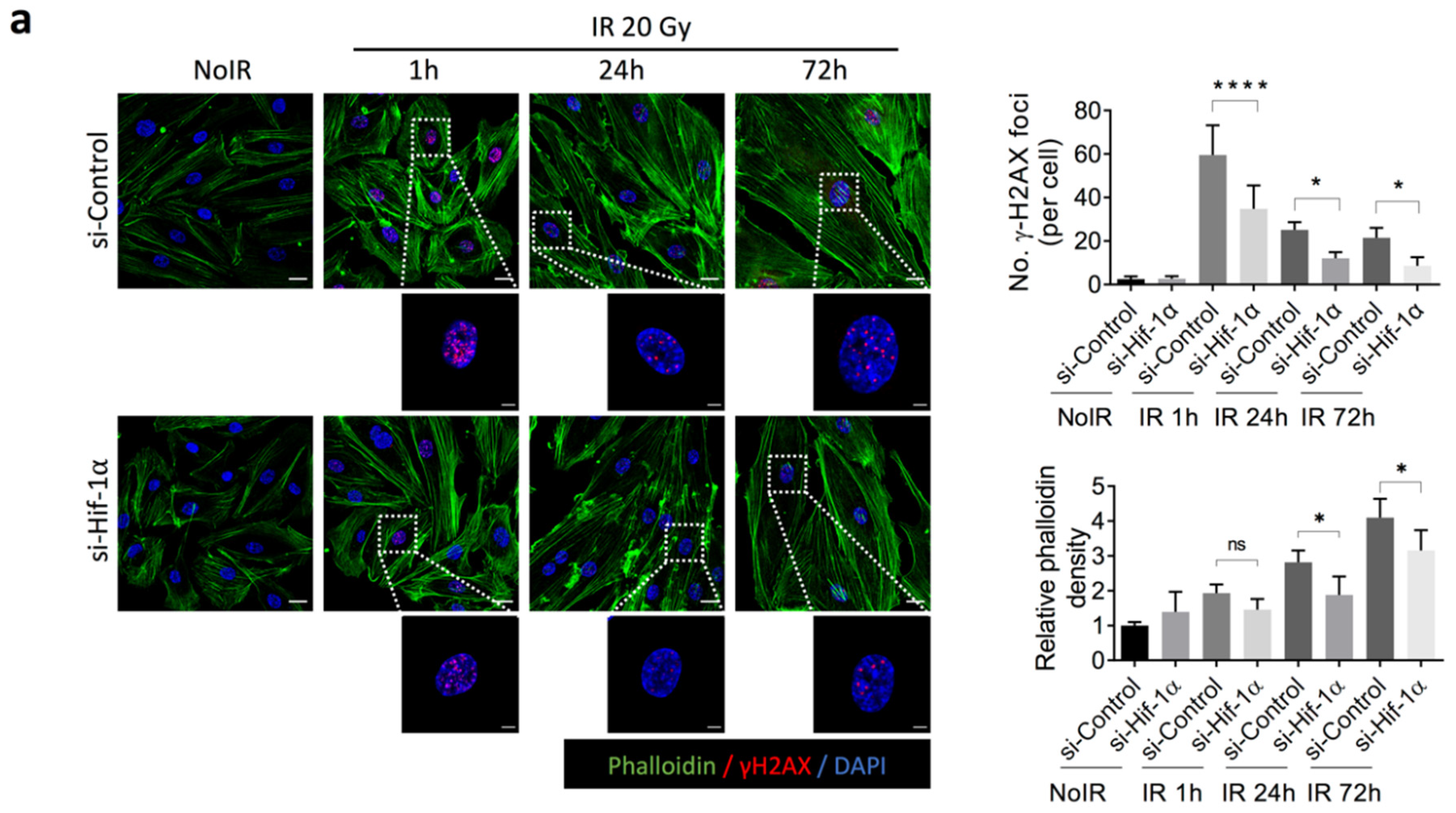
2.4. 2-ME Inhibits Fibrotic Changes in Vascular ECs by Reducing Accumulated DNA Damage in Human Dermal Microvascular ECs (HDMECs)
3. Discussion
4. Materials and Methods
4.1. Radiation-Induced Skin Damage Mice Model
4.2. Histology and Immunohistochemistry Staining
4.3. Cell Culture and Treatment
4.4. Flow Cytometer
4.5. Immunoblotting
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vaz, A.F.; Pinto-Neto, A.M.; Conde, D.M.; Costa-Paiva, L.; Morais, S.S.; Esteves, S.B. Quality of life of women with gynecologic cancer: Associated factors. Arch. Gynecol. Obstet. 2007, 276, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Mol, G.P.S.; Aruldhas, D.; Joe, I.H.; Balachandran, S.; Anuf, A.R.; George, J.; Nadh, A.G. Structural activity, fungicidal activity and molecular dynamics simulation of certain triphenyl methyl imidazole derivatives by experimental and computational spectroscopic techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 212, 105–120. [Google Scholar] [CrossRef]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef]
- Hymes, S.R.; Strom, E.A.; Fife, C. Radiation dermatitis: Clinical presentation, pathophysiology, and treatment 2006. J. Am. Acad. Dermatol. 2006, 54, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, L.; Passante, M.; Cameli, N.; Cristaudo, A.; Patruno, C.; Nistico, S.P.; Silvestri, M. Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review. Bioengineering 2021, 8, 153. [Google Scholar] [CrossRef]
- Venkatesulu, B.P.; Mahadevan, L.S.; Aliru, M.L.; Yang, X.; Bodd, M.H.; Singh, P.K.; Yusuf, S.W.; Abe, J.-I.; Krishnan, S. Radiation-Induced Endothelial Vascular Injury. JACC Basic Transl. Sci. 2018, 3, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Holler, V.; Buard, V.; Gaugler, M.H.; Guipaud, O.; Baudelin, C.; Sache, A.; Perez Mdel, R.; Squiban, C.; Tamarat, R.; Milliat, F.; et al. Pravastatin limits radiation-induced vascular dysfunction in the skin. J. Investig. Dermatol. 2009, 129, 1280–1291. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Meng, L.; Hou, X.; Qu, C.; Wang, B.; Xin, Y.; Jiang, X. Radiation-induced skin reactions: Mechanism and treatment. Cancer Manag. Res. 2019, 11, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Alavi, A.; Wong, R.; Akita, S. Radiodermatitis: A Review of Our Current Understanding. Am. J. Clin. Dermatol. 2016, 17, 277–292. [Google Scholar] [CrossRef]
- Horton, J.A.; Li, F.; Chung, E.J.; Hudak, K.; White, A.; Krausz, K.; Gonzalez, F.; Citrin, D. Quercetin inhibits radiation-induced skin fibrosis. Radiat. Res. 2013, 180, 205–215. [Google Scholar] [CrossRef]
- Kim, J.M.; Yoo, H.; Kim, J.Y.; Oh, S.H.; Kang, J.W.; Yoo, B.R.; Han, S.Y.; Kim, C.S.; Choi, W.H.; Lee, E.J.; et al. Metformin Alleviates Radiation-Induced Skin Fibrosis via the Downregulation of FOXO3. Cell Physiol. Biochem. 2018, 48, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.A.; Chung, E.J.; Hudak, K.E.; Sowers, A.; Thetford, A.; White, A.O.; Mitchell, J.B.; Citrin, D.E. Inhibition of radiation-induced skin fibrosis with imatinib. Int. J. Radiat. Biol. 2013, 89, 162–170. [Google Scholar] [CrossRef]
- Nam, J.K.; Kim, A.R.; Choi, S.H.; Kim, J.H.; Choi, K.J.; Cho, S.; Lee, J.W.; Cho, H.J.; Kwon, Y.W.; Cho, J.; et al. An antibody against L1 cell adhesion molecule inhibits cardiotoxicity by regulating persistent DNA damage. Nat. Commun. 2021, 12, 3279. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.K.; Kim, A.R.; Choi, S.H.; Kim, J.H.; Han, S.C.; Park, S.; Lee, Y.J.; Kim, J.; Cho, J.; Lee, H.J.; et al. Pharmacologic Inhibition of HIF-1alpha Attenuates Radiation-Induced Pulmonary Fibrosis in a Preclinical Image Guided Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, N.J.; Sarkar, M.A.; Venitz, J.; Figg, W.D. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy 2003, 23, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Ricker, J.L.; Chen, Z.; Yang, X.P.; Pribluda, V.S.; Swartz, G.M.; Van Waes, C. 2-Methoxyestradiol inhibits hypoxia-inducible factor 1alpha, tumor growth, and angiogenesis and augments paclitaxel efficacy in head and neck squamous cell carcinoma. Clin. Cancer Res. 2004, 10, 8665–8673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tevaarwerk, A.J.; Holen, K.D.; Alberti, D.B.; Sidor, C.; Arnott, J.; Quon, C.; Wilding, G.; Liu, G. Phase I trial of 2-Methoxyestradiol NanoCrystal dispersion in advanced solid malignancies. Clin. Cancer Res. 2009, 15, 1460–1465. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Lee, D.W.; Choi, W.H.; Jeon, Y.R.; Kim, S.H.; Cho, H.; Lee, E.J.; Hong, Z.Y.; Lee, W.J.; Cho, J. Development of a porcine skin injury model and characterization of the dose-dependent response to high-dose radiation. J. Radiat. Res. 2013, 54, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.K.; Kim, J.H.; Park, M.S.; Kim, E.H.; Kim, J.; Lee, Y.J. Radiation-Induced Fibrotic Tumor Microenvironment Regulates Anti-Tumor Immune Response. Cancers 2021, 13, 5232. [Google Scholar] [CrossRef]
- Choi, S.H.; Hong, Z.Y.; Nam, J.K.; Lee, H.J.; Jang, J.; Yoo, R.J.; Lee, Y.J.; Lee, C.Y.; Kim, K.H.; Park, S.; et al. A Hypoxia-Induced Vascular Endothelial-to-Mesenchymal Transition in Development of Radiation-Induced Pulmonary Fibrosis. Clin. Cancer Res. 2015, 21, 3716–3726. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.R.; Rzucidlo, E. Acute and chronic radiation injury. J. Vasc. Surg. 2011, 53, 15S–21S. [Google Scholar] [CrossRef] [PubMed]
- Demidov, V.; Zhao, X.; Demidova, O.; Pang, H.Y.M.; Flueraru, C.; Liu, F.F.; Vitkin, I.A. Preclinical quantitative in-vivo assessment of skin tissue vascularity in radiation-induced fibrosis with optical coherence tomography. J. Biomed. Opt. 2018, 23, 106003. [Google Scholar] [CrossRef] [PubMed]
- Korpela, E.; Liu, S.K. Endothelial perturbations and therapeutic strategies in normal tissue radiation damage. Radiat. Oncol. 2014, 9, 266. [Google Scholar] [CrossRef] [Green Version]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis--a common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Glasser, S.W.; Hagood, J.S.; Wong, S.; Taype, C.A.; Madala, S.K.; Hardie, W.D. Mechanisms of Lung Fibrosis Resolution. Am. J. Pathol. 2016, 186, 1066–1077. [Google Scholar] [CrossRef] [Green Version]
- Dubey, R.K.; Jackson, E.K. Estrogen-induced cardiorenal protection: Potential cellular, biochemical, and molecular mechanisms. Am. J. Physiol. Renal. Physiol. 2001, 280, F365–F388. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Hart, J.C.; Norton, L.; Dannenberg, A.J. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. Evidence for involvement of ERK1/2 AND p38 mitogen-activated protein kinase pathways. J. Biol. Chem. 2000, 275, 14838–14845. [Google Scholar] [CrossRef] [Green Version]
- Mabjeesh, N.J.; Escuin, D.; LaVallee, T.M.; Pribluda, V.S.; Swartz, G.M.; Johnson, M.S.; Willard, M.T.; Zhong, H.; Simons, J.W.; Giannakakou, P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 2003, 3, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef] [Green Version]
- Petrek, M.; Lenhart, K. Occurrence of griseofulvin-resistant mutants of trichophyton mentagrophytes. Acta Univ. Palacki. Olomuc. Fac. Med. 1986, 113, 53–60. [Google Scholar]
- Zhao, H.; Jiang, H.; Li, Z.; Zhuang, Y.; Liu, Y.; Zhou, S.; Xiao, Y.; Xie, C.; Zhou, F.; Zhou, Y. 2-Methoxyestradiol enhances radiosensitivity in radioresistant melanoma MDA-MB-435R cells by regulating glycolysis via HIF-1alpha/PDK1 axis. Int. J. Oncol. 2017, 50, 1531–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, H.; Adachi, M.; Imai, K.; Hareyama, M.; Yoshioka, K.; Zhao, S.; Shinomura, Y. 2-Methoxyestradiol, an endogenous mammalian metabolite, radiosensitizes colon carcinoma cells through c-Jun NH2-terminal kinase activation. Clin. Cancer Res. 2006, 12, 6532–6539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Nam, J.-K.; Kim, A.-R.; Park, M.-S.; Lee, H.-J.; Park, J.; Kim, J.; Lee, Y.-J. 2-Methoxyestradiol Inhibits Radiation-Induced Skin Injuries. Int. J. Mol. Sci. 2022, 23, 4171. https://doi.org/10.3390/ijms23084171
Kim J-H, Nam J-K, Kim A-R, Park M-S, Lee H-J, Park J, Kim J, Lee Y-J. 2-Methoxyestradiol Inhibits Radiation-Induced Skin Injuries. International Journal of Molecular Sciences. 2022; 23(8):4171. https://doi.org/10.3390/ijms23084171
Chicago/Turabian StyleKim, Ji-Hee, Jae-Kyung Nam, A-Ram Kim, Min-Sik Park, Hae-June Lee, Joonho Park, Joon Kim, and Yoon-Jin Lee. 2022. "2-Methoxyestradiol Inhibits Radiation-Induced Skin Injuries" International Journal of Molecular Sciences 23, no. 8: 4171. https://doi.org/10.3390/ijms23084171
APA StyleKim, J.-H., Nam, J.-K., Kim, A.-R., Park, M.-S., Lee, H.-J., Park, J., Kim, J., & Lee, Y.-J. (2022). 2-Methoxyestradiol Inhibits Radiation-Induced Skin Injuries. International Journal of Molecular Sciences, 23(8), 4171. https://doi.org/10.3390/ijms23084171





