Cytomolecular Classification of Thyroid Nodules Using Fine-Needle Washes Aspiration Biopsies
Abstract
:1. Introduction
2. Results
2.1. Patient and Nodule Characteristics
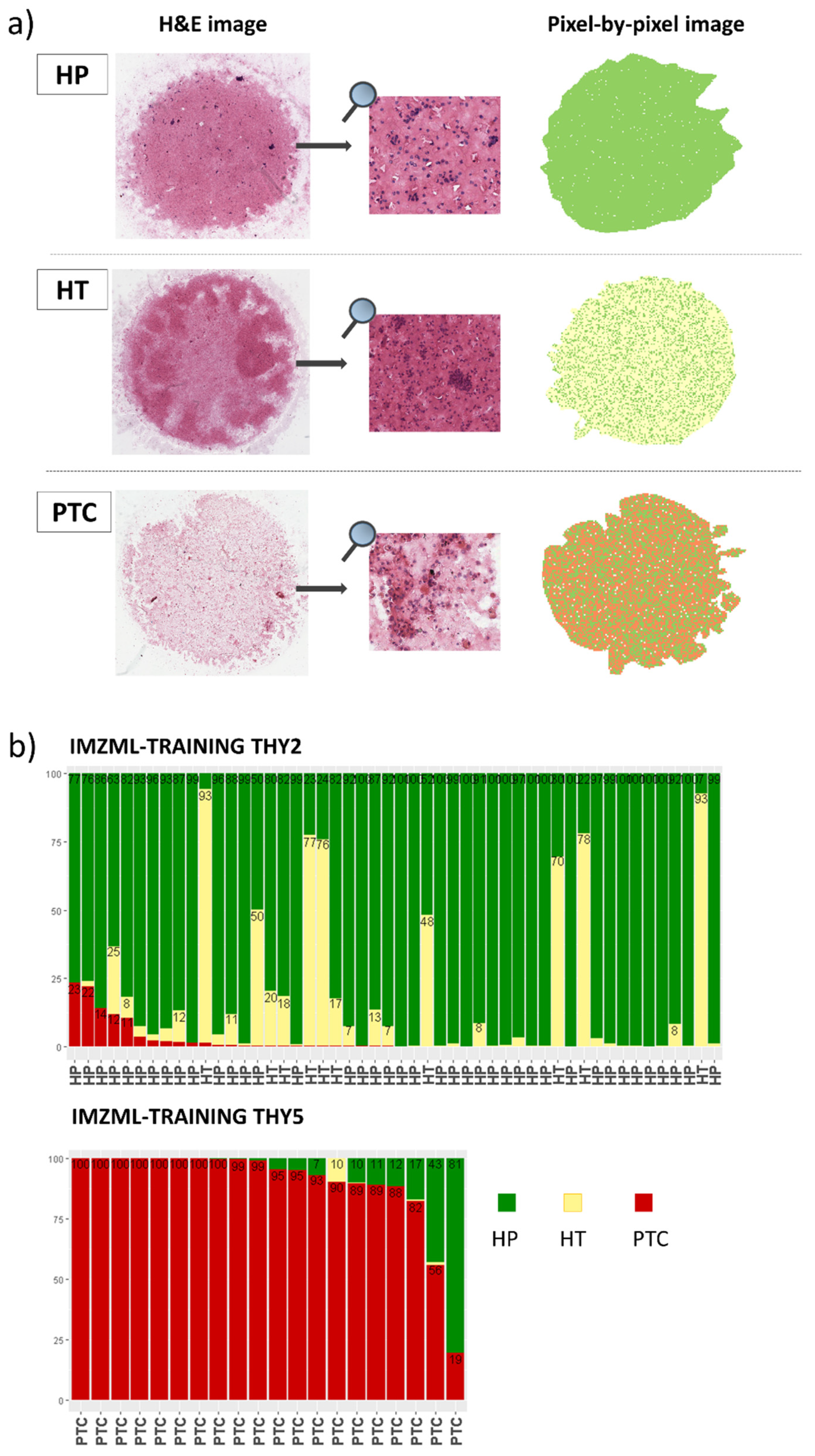
2.2. MALDI-MSI Spectra Characteristics
2.3. MALDI-MSI to Classify Thyroid Nodules: Training Set
2.4. MALDI-MSI to Predict Diagnosis: Validation Set
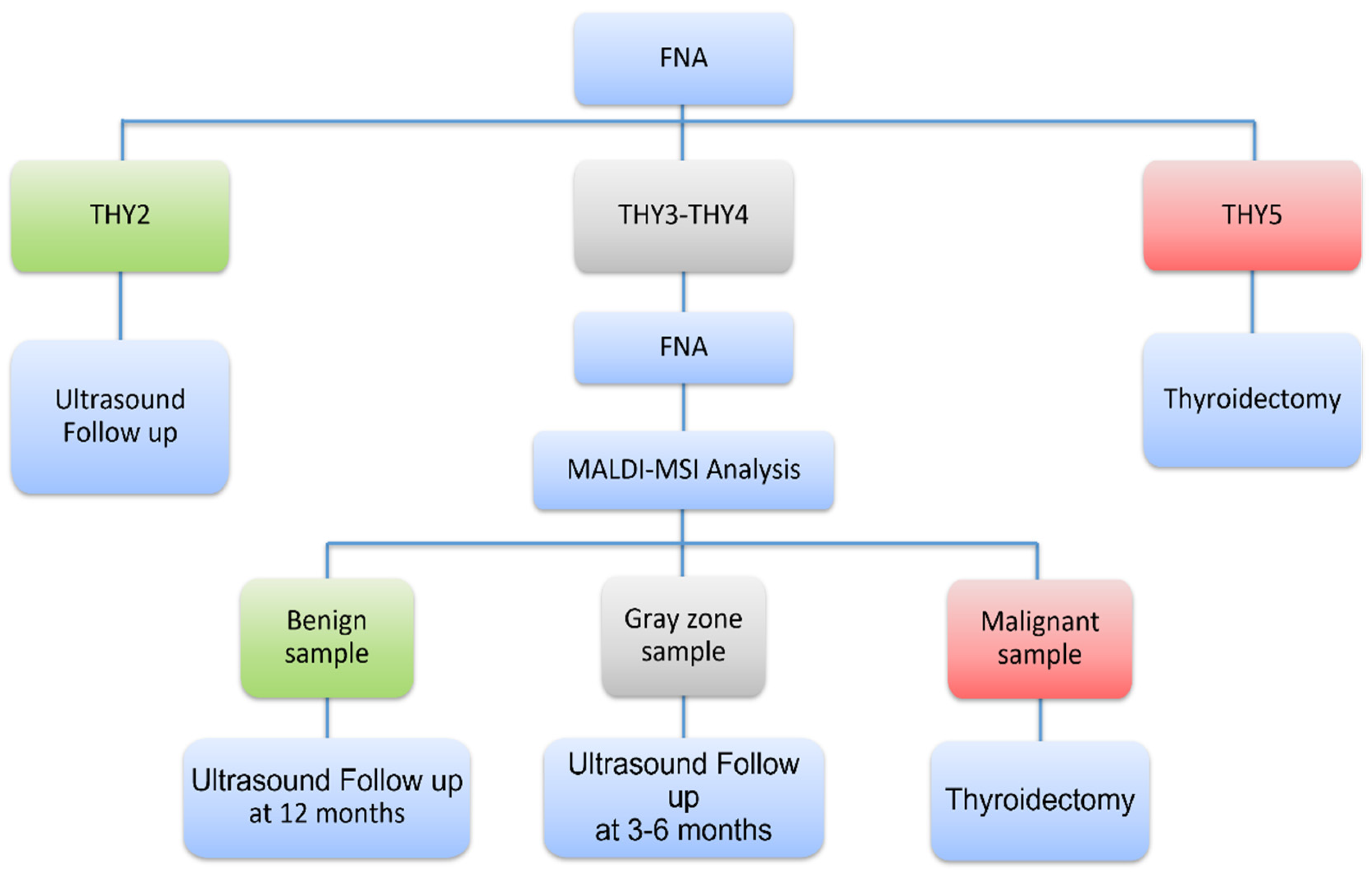
2.5. Novel Diagnostic Workflow Based on MALDI-MSI Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Training and Validation Set
4.3. MALDI-MSI
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Statistical Analysis
References
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian consensus for the classification and reporting of thyroid cytology. J. Endocrinol. Investig. 2014, 37, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Balentine, C.J.; Domingo, R.P.; Patel, R.; Suliburk, J.W. Thyroid Lobectomy for Indeterminate FNA: Not Without Consequence. J. Surg. Res. 2013, 2, 342. [Google Scholar] [CrossRef]
- Nishino, M.; Krane, J.F. Role of Ancillary Techniques in Thyroid Cytology Specimens. Acta Cytol. 2020, 64, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E. Role of Molecular Markers in Thyroid Nodule Management: Then and Now. Endocr. Pr. 2017, 23, 979–989. [Google Scholar] [CrossRef] [PubMed]
- De Sio, G.; Smith, A.J.; Galli, M.; Garancini, M.; Chinello, C.; Bono, F.; Pagni, F.; Magni, F. A MALDI-Mass Spectrometry Imaging method applicable to different formalin-fixed paraffin-embedded human tissues. Mol. Biosyst. 2015, 11, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Pagni, F.; De Sio, G.; Garancini, M.; Scardilli, M.; Chinello, C.; Smith, A.J.; Bono, F.; Leni, D.; Magni, F. Proteomics in thyroid cytopathology: Relevance of MALDI-imaging in distinguishing malignant from benign lesions. Proteomics 2016, 16, 1775–1784. [Google Scholar] [CrossRef]
- Conroy, L.R.; Stanback, A.E.; Young, L.E.A.; Clarke, H.A.; Austin, G.L.; Liu, J.; Allison, D.B.; Sun, R.C. In Situ Analysis of N-Linked Glycans as Potential Biomarkers of Clinical Course in Human Prostate Cancer. Mol. Cancer Res. 2021, 19, 1727–1738. [Google Scholar] [CrossRef]
- Calligaris, D.; Feldman, D.R.; Norton, I.; Olubiyi, O.; Changelian, A.N.; Machaidze, R.; Vestal, M.L.; Laws, E.R.; Dunn, I.F.; Santagata, S.; et al. MALDI mass spectrometry imaging analysis of pituitary adenomas for near-real-time tumor delineation. Proc. Natl. Acad. Sci. 2015, 112, 9978–9983. [Google Scholar] [CrossRef] [Green Version]
- Piga, I.; Capitoli, G.; Tettamanti, S.; Denti, V.; Smith, A.; Chinello, C.; Stella, M.; Leni, D.; Garancini, M.; Galimberti, S.; et al. Feasibility Study for the MALDI-MSI Analysis of Thyroid Fine Needle Aspiration Biopsies: Evaluating the Morphological and Proteomic Stability Over Time. PROTEOMICS–Clin. Appl. 2019, 13, 1700170. [Google Scholar] [CrossRef] [Green Version]
- Piga, I.; Capitoli, G.; Denti, V.; Tettamanti, S.; Smith, A.; Stella, M.; Chinello, C.; Leni, D.; Garancini, M.; Galimberti, S.; et al. The management of haemoglobin interference for the MALDI-MSI proteomics analysis of thyroid fine needle aspiration biopsies. Anal. Bioanal. Chem. 2019, 411, 5007–5012. [Google Scholar] [CrossRef] [PubMed]
- DeHoog, R.J.; Zhang, J.; Alore, E.; Lin, J.Q.; Yu, W.; Woody, S.; Almendariz, C.; Lin, M.; Engelsman, A.F.; Sidhu, S.B.; et al. Preoperative metabolic classification of thyroid nodules using mass spectrometry imaging of fine-needle aspiration biopsies. Proc. Natl. Acad. Sci. 2019, 116, 21401–21408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurczyk, A.; Gawin, M.; Chekan, M.; Wilk, A.; Łakomiec, K.; Mrukwa, G.; Frątczak, K.; Polanska, J.; Fujarewicz, K.; Pietrowska, M.; et al. Classification of Thyroid Tumors Based on Mass Spectrometry Imaging of Tissue Microarrays; a Single-Pixel Approach. Int. J. Mol. Sci. 2020, 21, 6289. [Google Scholar] [CrossRef] [PubMed]
- Mascini, N.E.; Teunissen, J.; Noorlag, R.; Willems, S.M.; Heeren, R.M. Tumor classification with MALDI-MSI data of tissue microarrays: A case study. Methods 2018, 151, 21–27. [Google Scholar] [CrossRef]
- Pagni, F.; Mainini, V.; Garancini, M.; Bono, F.; Vanzati, A.; Giardini, V.; Scardilli, M.; Goffredo, P.; Smith, A.J.; Galli, M.; et al. Proteomics for the diagnosis of thyroid lesions: Preliminary report. Cytopathology 2015, 26, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Piga, I.; Capitoli, G.; Clerici, F.; Mahajneh, A.; Brambilla, V.; Smith, A.; Leni, D.; L’Imperio, V.; Galimberti, S.; Pagni, F.; et al. Ex vivo thyroid fine needle aspirations as an alternative for MALDI-MSI proteomic investigation: Intra-patient comparison. Anal. Bioanal. Chem. 2021, 413, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Capitoli, G.; Piga, I.; Clerici, F.; Brambilla, V.; Mahajneh, A.; Leni, D.; Garancini, M.; Pincelli, A.I.; L’Imperio, V.; Galimberti, S.; et al. Analysis of Hashimoto’s thyroiditis on fine needle aspiration samples by MALDI-Imaging. Biochim. Et Biophys. Acta-Proteins Proteom. 2020, 1868, 140481. [Google Scholar] [CrossRef]
- Capitoli, G.; Piga, I.; Galimberti, S.; Leni, D.; Pincelli, A.I.; Garancini, M.; Clerici, F.; Mahajneh, A.; Brambilla, V.; Smith, A.; et al. MALDI-MSI as a Complementary Diagnostic Tool in Cytopathology: A Pilot Study for the Characterization of Thyroid Nodules. Cancers 2019, 11, 1377. [Google Scholar] [CrossRef] [Green Version]
- Piga, I.; Capitoli, G.; Clerici, F.; Brambilla, V.; Leni, D.; Scardilli, M.; Canini, V.; Cipriani, N.; Bono, F.; Valsecchi, M.G.; et al. Molecular trait of follicular-patterned thyroid neoplasms defined by MALDI-imaging. Biochim. Et Biophys. Acta-Proteins Proteom. 2020, 1868, 140511. [Google Scholar] [CrossRef]
- Alexander, E.K.; Kennedy, G.C.; Baloch, Z.W.; Cibas, E.S.; Chudova, D.; Diggans, J.; Friedman, L.; Kloos, R.T.; LiVolsi, V.A.; Mandel, S.J.; et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. New Engl. J. Med. 2012, 367, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, S.; Nakayama, T. MALDI-Based Mass Spectrometry in Clinical Testing: Focus on Bacterial Identification. Appl. Sci. 2022, 12, 2814. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Seminati, D.; Capitoli, G.; Leni, D.; Fior, D.; Vacirca, F.; Di Bella, C.; Galimberti, S.; L’Imperio, V.; Pagni, F. Use of Diagnostic Criteria from ACR and EU-TIRADS Systems to Improve the Performance of Cytology in Thyroid Nodule Triage. Cancers 2021, 13, 5439. [Google Scholar] [CrossRef] [PubMed]
- Leni, D.; Seminati, D.; Fior, D.; Vacirca, F.; Capitoli, G.; Cazzaniga, L.; Di Bella, C.; L’Imperio, V.; Galimberti, S.; Pagni, F. Diagnostic Performances of the ACR-TIRADS System in Thyroid Nodules Triage: A Prospective Single Center Study. Cancers 2021, 13, 2230. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs; International Agency for Research on Cancer (IARC): Lyon, France, 2017. [Google Scholar]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.R.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
| TP | FP | TN | FN | % Sensitivity (95% CI) | % Specificity (95% CI) | % PPV (95% CI) | % NPV (95% CI) | % Accuracy | |
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 170) | |||||||||
| Pixel-by-pixel | 28 | 18 | 87 | 37 | 43.1 (30.9–56.0) | 82.9 (74.3–89.5) | 60.9 (45.4–74.9) | 70.2 (61.3–78.0) | 67.7 |
| ROIs | 26 | 18 | 87 | 39 | 40.0 (28.0–52.9) | 82.9 (74.3–89.5) | 59.1 (43.3–73.7) | 69.1 (43.3–73.7) | 66.5 |
| Average spectra | 20 | 15 | 90 | 45 | 30.8 (19.9–43.5) | 85.7 (77.5–91.8) | 57.1 (39.4–73.7) | 66.7 (58.0–74.5) | 64.7 |
| Borderline diagnosis ° excluded (n = 141) | |||||||||
| Pixel-by-pixel | 28 | 9 | 67 | 37 | 43.1 (30.9–56.0) | 88.2 (78.7–94.4) | 75.7 (58.8–88.2) | 64.4 (54.4–73.6) | 67.4 |
| ROIs | 26 | 10 | 66 | 39 | 40.0 (28.0–52.9) | 86.8 (77.1–93.5) | 72.2 (54.8–85.8) | 62.9 (52.9–72.1) | 65.3 |
| Average spectra | 20 | 7 | 69 | 45 | 30.8 (19.9–43.5) | 90.8 (81.9–96.2) | 74.1 (53.7–88.9) | 60.5 (50.9–69.6) | 63.1 |
| Samples with adequate cellularity (n = 72) * | |||||||||
| Pixel-by-pixel | 26 | 7 | 31 | 8 | 76.5 (58.8–89.3) | 81.6 (65.7–92.3) | 78.8 (61.1–91.0) | 79.5 (63.5–90.7) | 79.2 |
| ROIs | 21 | 8 | 30 | 13 | 61.8 (43.6–77.8) | 79.0 (62.7–90.5) | 72.4 (52.8–87.3) | 69.8 (53.9–82.8) | 70.8 |
| Average spectra | 19 | 5 | 33 | 15 | 55.9 (37.9–72.8) | 86.8 (71.9–95.6) | 79.2 (57.9–92.9) | 68.8 (53.8–81.3) | 72.2 |
| Histopathology/Follow-Up | Total | Predicted Diagnosis | ||
|---|---|---|---|---|
| Benign | Gray Zone | Malignant | ||
| Benign (n = 34) | ||||
| HP | 21 | 17 * | 0 | 4 ° |
| HT | 0 | 0 | 0 | 0 |
| FA | 9 | 3 | 0 | 6 |
| NIFTP | 3 | 2 | 0 | 1 |
| WDT-UMP | 1 | 1 | 0 | 0 |
| Malignant (n = 11) | ||||
| PTC | 8 | 1 | 1 | 6 # |
| MTC | 3 | 0 | 0 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capitoli, G.; Piga, I.; L’Imperio, V.; Clerici, F.; Leni, D.; Garancini, M.; Casati, G.; Galimberti, S.; Magni, F.; Pagni, F. Cytomolecular Classification of Thyroid Nodules Using Fine-Needle Washes Aspiration Biopsies. Int. J. Mol. Sci. 2022, 23, 4156. https://doi.org/10.3390/ijms23084156
Capitoli G, Piga I, L’Imperio V, Clerici F, Leni D, Garancini M, Casati G, Galimberti S, Magni F, Pagni F. Cytomolecular Classification of Thyroid Nodules Using Fine-Needle Washes Aspiration Biopsies. International Journal of Molecular Sciences. 2022; 23(8):4156. https://doi.org/10.3390/ijms23084156
Chicago/Turabian StyleCapitoli, Giulia, Isabella Piga, Vincenzo L’Imperio, Francesca Clerici, Davide Leni, Mattia Garancini, Gabriele Casati, Stefania Galimberti, Fulvio Magni, and Fabio Pagni. 2022. "Cytomolecular Classification of Thyroid Nodules Using Fine-Needle Washes Aspiration Biopsies" International Journal of Molecular Sciences 23, no. 8: 4156. https://doi.org/10.3390/ijms23084156
APA StyleCapitoli, G., Piga, I., L’Imperio, V., Clerici, F., Leni, D., Garancini, M., Casati, G., Galimberti, S., Magni, F., & Pagni, F. (2022). Cytomolecular Classification of Thyroid Nodules Using Fine-Needle Washes Aspiration Biopsies. International Journal of Molecular Sciences, 23(8), 4156. https://doi.org/10.3390/ijms23084156









