Heat-Stress Preconditioning Attenuates Behavioral Responses to Psychological Stress: The Role of HSP-70 in Modulating Stress Responses
Abstract
1. Introduction
2. Results
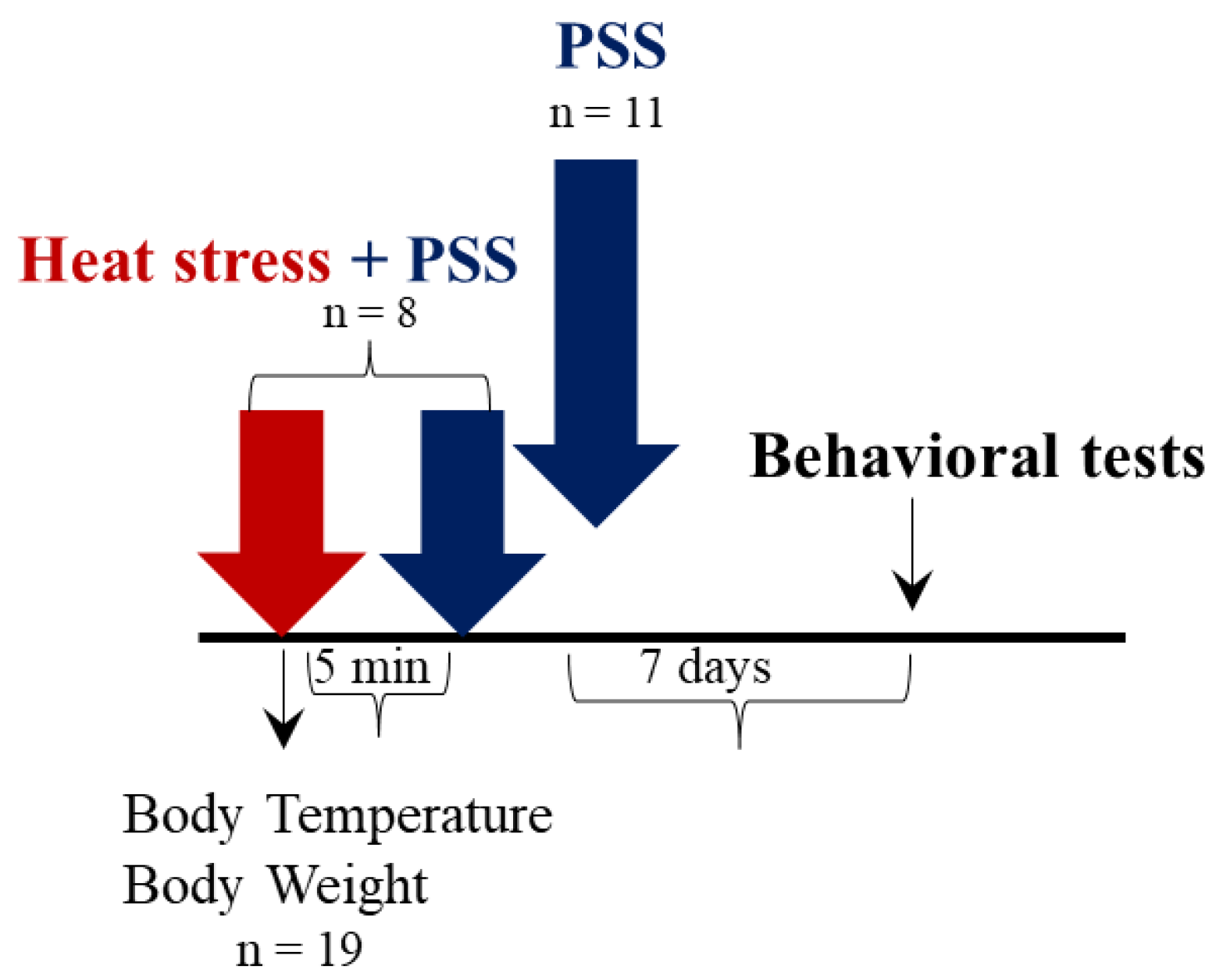
2.1. Experiment 1: The Effect of HS on Physiological, Cellular and Behavioral Responses
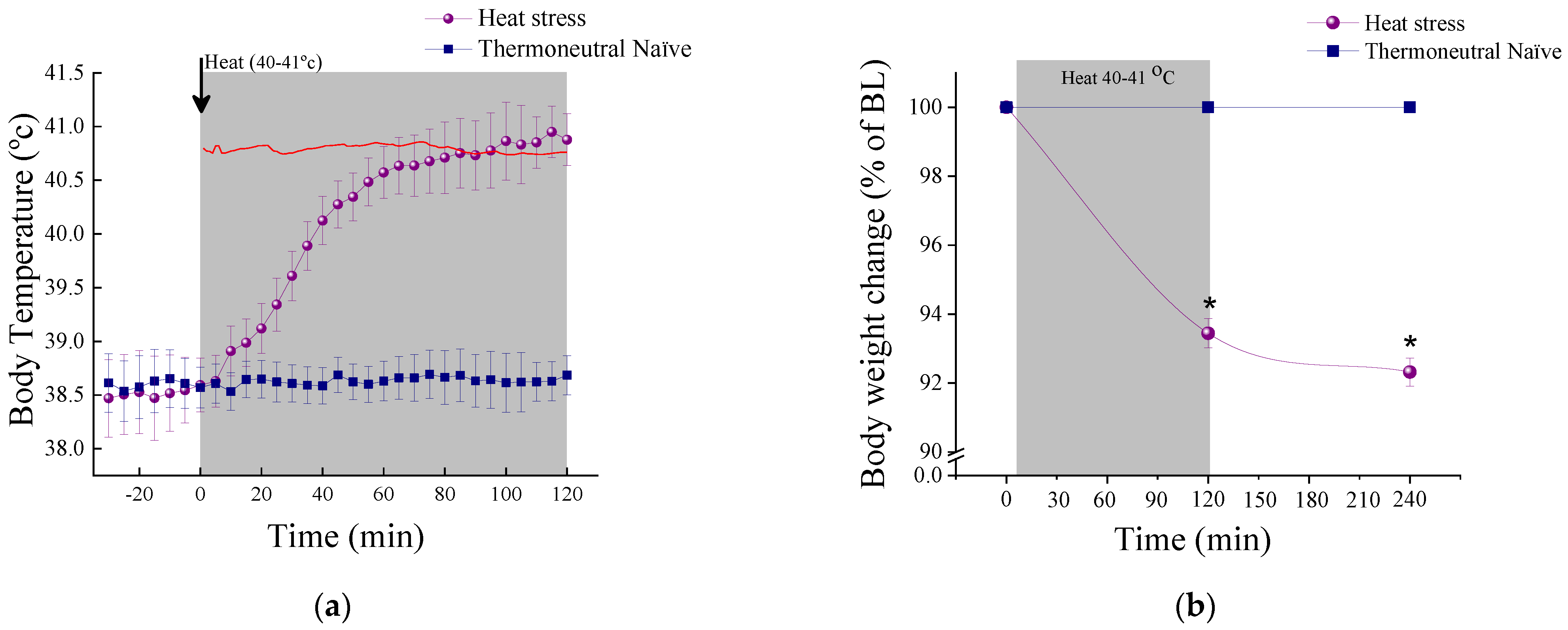
2.1.1. Physiological Measures
2.1.2. Cellular Measures
2.1.3. Acute and Delayed Behavioral Response of HS
2.2. Experiment 2: The Effect of HS-Preconditioning on Resiliency to Psychological Stress
2.2.1. Physiological Measures
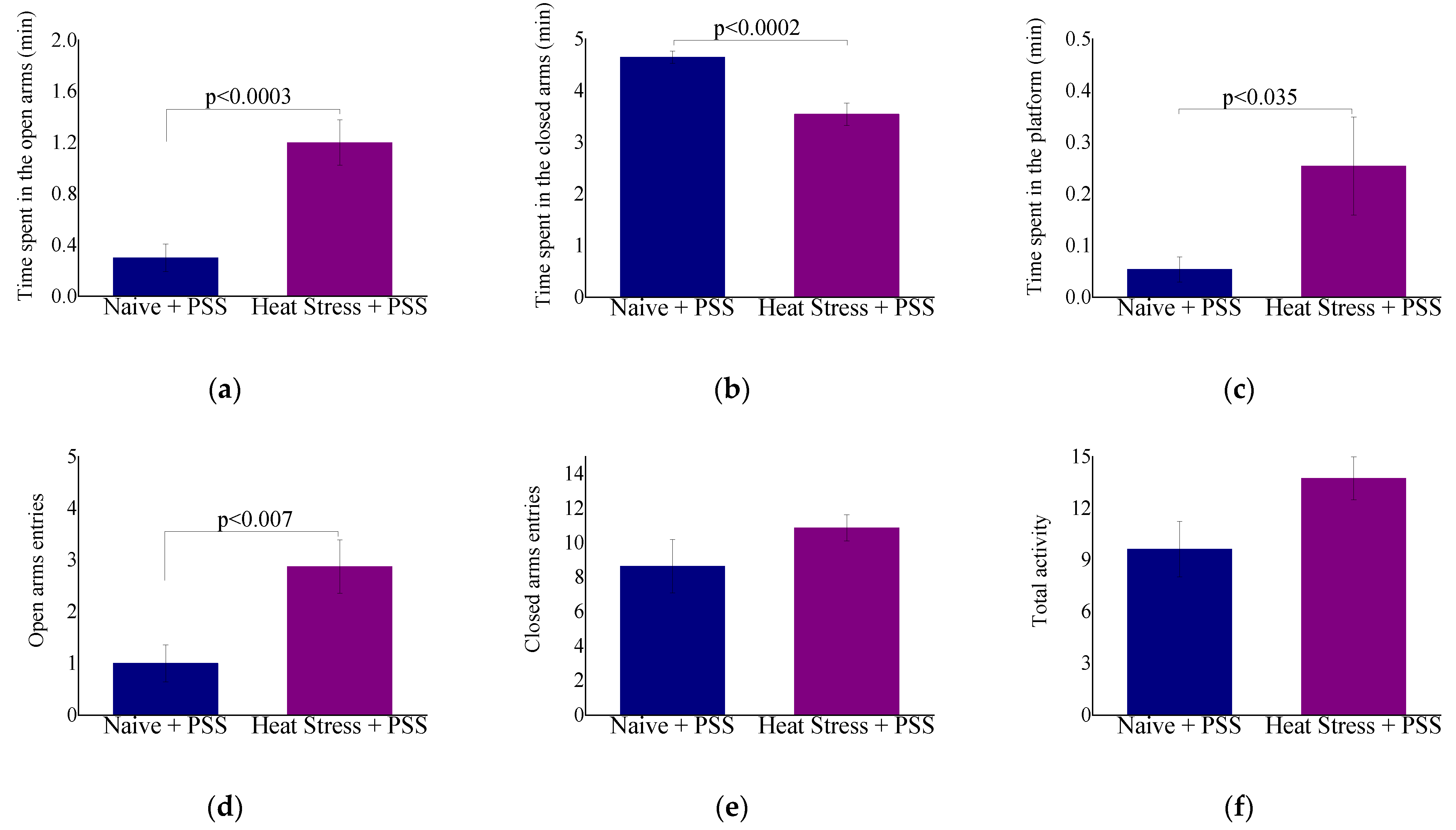
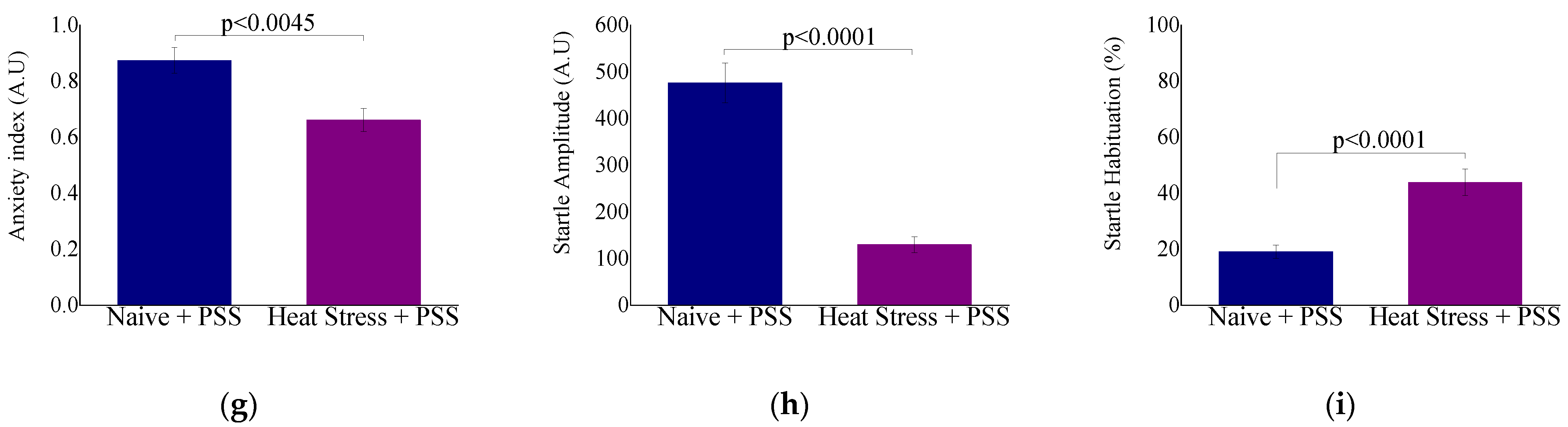
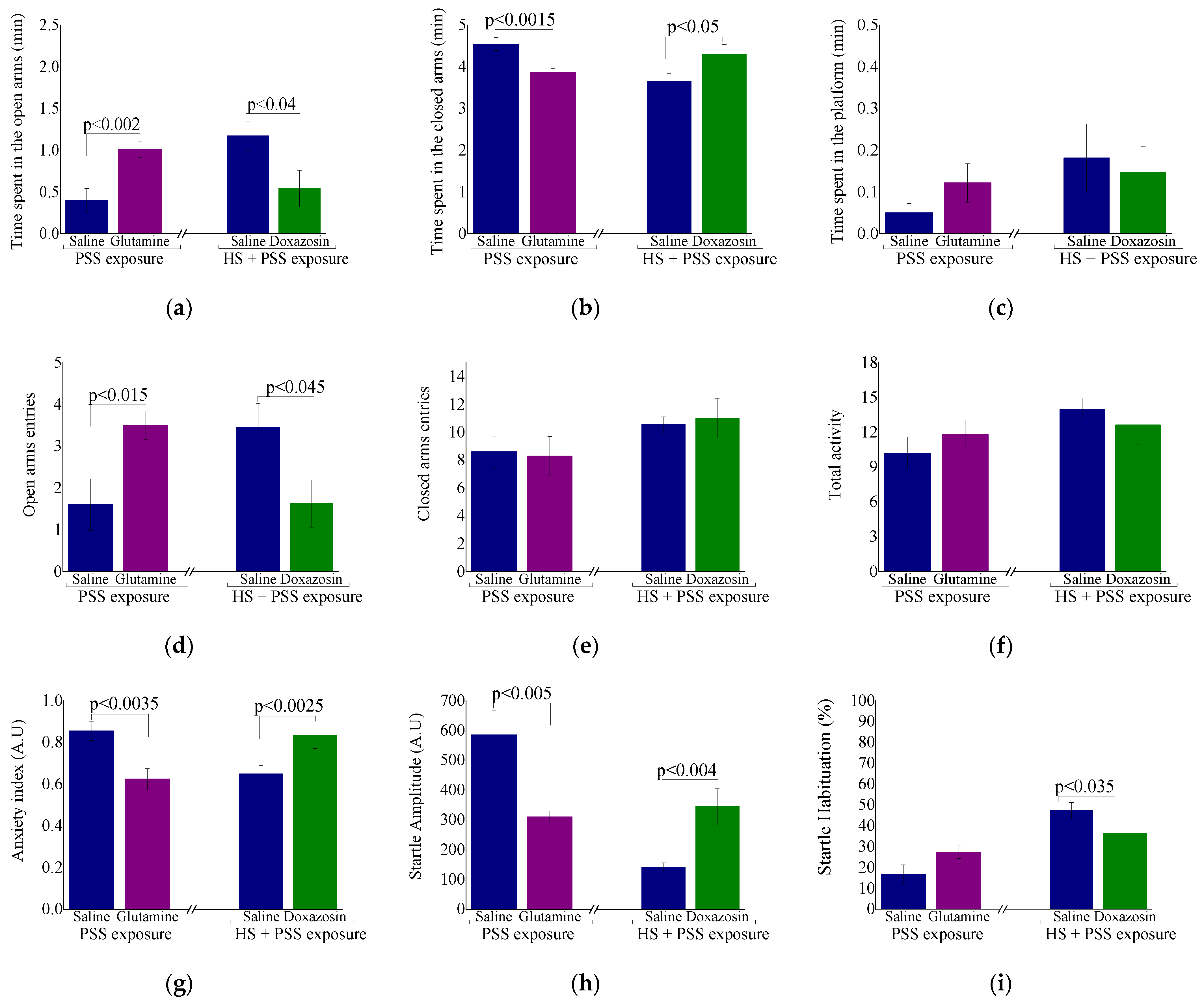
2.2.2. Behavioral Measures: The Effect of HS-Preconditioning on Resiliency to Psychological Stress
2.3. Experiment 3: The Role of Heat-Shock Protein 70 in Modulating the Protective Behavioral Response to Predator-Scent Stress
2.3.1. Physiological Measures
2.3.2. Behavioral Measures: Delayed Behavioral Response to Pharmacological Manipulation of HSP-70 Levels
3. Discussion
3.1. The Effect of HS on Physiological, Cellular and Behavioral Responses
3.2. Stress Associated Response: HS and Pyretic Osmoregulatory Genes
3.3. Neuroinflammation
3.4. Neuroplasticity
3.5. HS-Mediated Behavioral Resiliency
3.6. HS Induced Preconditioning via Cross Tolerance Mechanism
3.7. Preconditioning and HSP-70 Levels
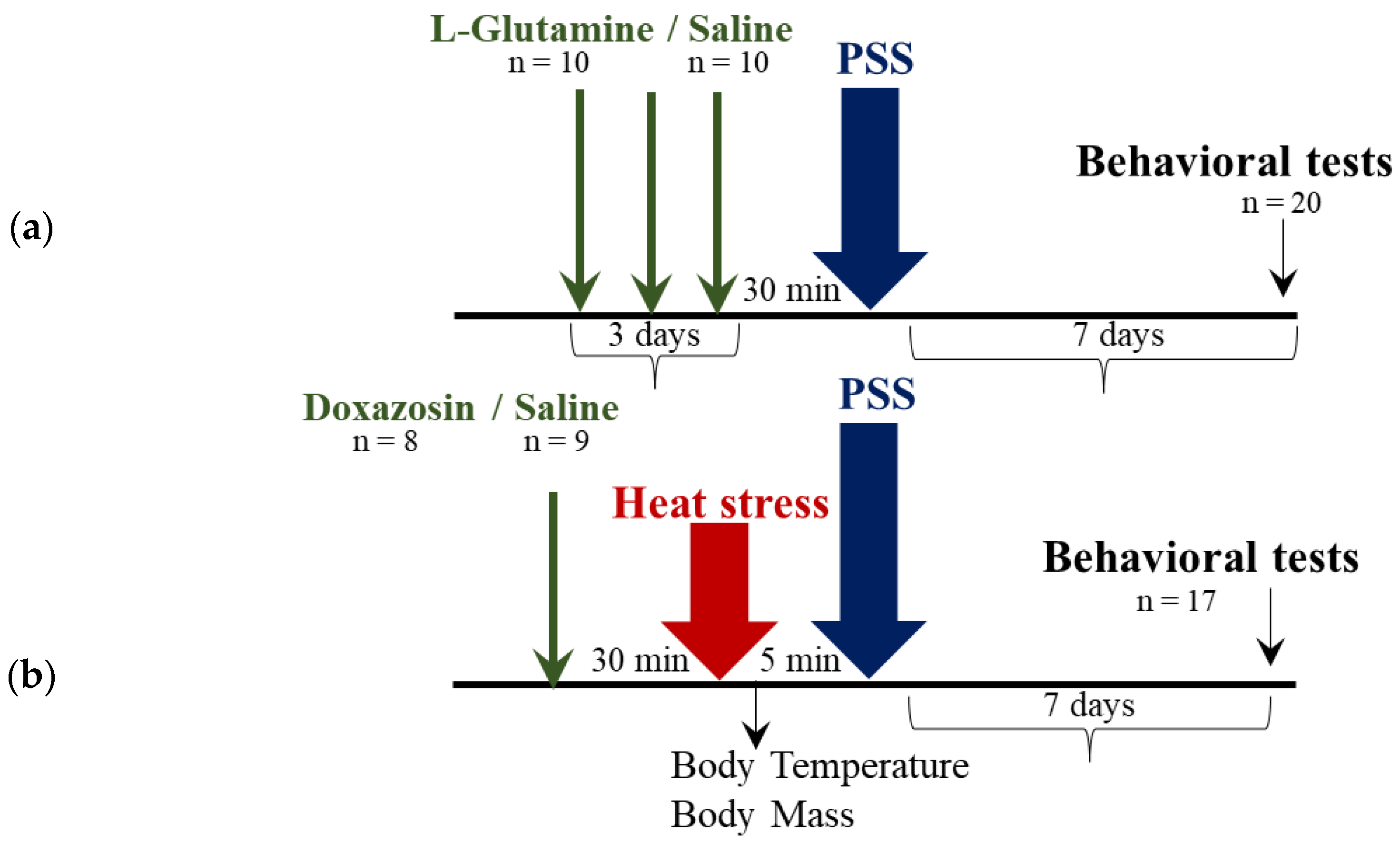
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Surgical Procedure
4.4. Rectal Temperature (Tre) and Body Mass (BM)
4.5. HS Protocol
4.6. Immunohistochemistry
4.7. Quantification
4.8. Regions of Interest
4.9. Genes of Interest
4.10. Predator Scent Stress (PSS)
4.11. Behavioral Assessments
4.12. Measurement of Circulating Corticosterone
4.13. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ASR | Acoustic startle response |
| AVP | Arginine vasopressin |
| BM | Body mass |
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| Tb | Core body temperature |
| CA | Cornu Ammonis |
| COX | Cyclooxygenase |
| DG | Dentate gyrus |
| EPM | Elevated plus-maze |
| GC | Glucocorticoid |
| GR | Glucocorticoid receptor |
| HSP | Heat shock protein |
| HS | Heat-stress |
| HPA | Hypothalamus–pituitary–adrenal |
| Ir | Immunoreactive |
| Iba-1 | Ionized calcium binding adaptor molecule 1 |
| NPY | neuropeptide-Y |
| PSS | Predator scent stress |
| PVN | Paraventricular nucleus of the hypothalamus |
| TN | Thermoneutral naïve |
| Tre | Rectal temperature |
References
- Moran, D.; Shapiro, Y.; Meiri, U.; Laor, A.; Horowitz, M. Heat acclimation: Cardiovascular response to hot/dry and hot/wet heat loads in rats. J. Basic Clin. Physiol. Pharmacol. 1996, 7, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Schochina, M.; Horowitz, M. Central venous pressure, arterial pressure and hypovolemia: Their role in adjustment during heat stress. J. Therm. Biol. 1989, 14, 109–113. [Google Scholar] [CrossRef]
- Harikai, N.; Tomogane, K.; Miyamoto, M.; Shimada, K.; Onodera, S.; Tashiro, S.-I. Dynamic responses to acute heat stress between 34 °C and 38.5 °C, and characteristics of heat stress response in mice. Biol. Pharm. Bull. 2003, 26, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Peinnequin, A.; Alonso, A.; Buguet, A.; Cespuglio, R.; Canini, F. Decreased heat tolerance is associated with hypothalamo–pituitary–adrenocortical axis impairment. Neuroscience 2007, 147, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kim, T.M.; Park, G.; Lee, T.H.; Oh, M.S. Repeated heat exposure impairs nigrostriatal dopaminergic neurons in mice. Biol. Pharm. Bull. 2013, b13-00268. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H. Hyperthermia influences excitatory and inhibitory amino acid neurotransmitters in the central nervous system. An experimental study in the rat using behavioural, biochemical, pharmacological, and morphological approaches. J. Neural Transm. 2006, 113, 497–519. [Google Scholar] [CrossRef] [PubMed]
- White, M.G.; Emery, M.; Nonner, D.; Barrett, J.N. Caspase activation contributes to delayed death of heat-stressed striatal neurons. J. Neurochem. 2003, 87, 958–968. [Google Scholar] [CrossRef]
- Mete, F.; Kilic, E.; Somay, A.; Yilmaz, B. Effects of heat stress on endocrine functions & behaviour in the pre-pubertal rat. Indian J. Med. Res. 2012, 135, 233. [Google Scholar]
- Lieberman, H.R.; Georgelis, J.H.; Maher, T.J.; Yeghiayan, S.K. Tyrosine prevents effects of hyperthermia on behavior and increases norepinephrine. Physiol. Behav. 2005, 84, 33–38. [Google Scholar] [CrossRef]
- Bouchama, A.; Knochel, J.P. Heat stroke. New Engl. J. Med. 2002, 346, 1978–1988. [Google Scholar] [CrossRef]
- Lee, W.; Moon, M.; Kim, H.G.; Lee, T.H.; Oh, M.S. Heat stress-induced memory impairment is associated with neuroinflammation in mice. J. Neuroinflamm. 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, A.; Aneja, I.S.; Lexa, J.; Al-Zoabi, B.A. Identification of cerebral neurons and glial cell types inducing heat shock protein Hsp70 following heat stress in the rat. Brain Res 2003, 973, 179–189. [Google Scholar] [CrossRef]
- Blake, M.J.; Nowak, T.S., Jr.; Holbrook, N.J. In vivo hyperthermia induces expression of HSP70 mRNA in brain regions controlling the neuroendocrine response to stress. Brain Res. Mol. Brain Res. 1990, 8, 89–92. [Google Scholar] [CrossRef]
- Feige, U.; Polla, B.S. Hsp70—A multi-gene, multi-structure, multi-function family with potential clinical applications. Experientia 1994, 50, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Robinson, S.D. Heat shock proteins and the heat shock response during hyperthermia and its modulation by altered physiological conditions. Prog. Brain Res. 2007, 162, 433–446. [Google Scholar] [PubMed]
- Moran, D.S.; Horowitz, M.; Meiri, U.; Laor, A.; Pandolf, K.B. The physiological strain index applied to heat-stressed rats. J. Appl. Physiol. 1999, 86, 895–901. [Google Scholar] [CrossRef]
- Horowitz, M. Do cellular heat acclimation responses modulate central thermoregulatory activity? Physiology 1998, 13, 218–225. [Google Scholar] [CrossRef]
- Lee, W.; Wen, H.; Chang, C.; Chen, M.; Lin, M.-T. Heat shock protein 72 overexpression protects against hyperthermia, circulatory shock, and cerebral ischemia during heatstroke. J. Appl. Physiol. 2006, 100, 2073–2082. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Lin, M.-T. Heat shock protein expression protects against cerebral ischemia and monoamine overload in rat heatstroke. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, H1961–H1967. [Google Scholar] [CrossRef]
- Yellon, D.M.; Pasini, E.; Cargnoni, A.; Marber, M.S.; Latchman, D.S.; Ferrari, R. The protective role of heat stress in the ischaemic and reperfused rabbit myocardium. J. Mol. Cell. Cardiol. 1992, 24, 895–907. [Google Scholar] [CrossRef]
- Chen, D.; Pan, J.; Du, B.; Sun, D. Induction of the heat shock response in vivo inhibits NF-κB activity and protects murine liver from endotoxemia-induced injury. J. Clin. Immunol. 2005, 25, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.R.; Kapoor, M.; Singh, L.P.; Gupta, R.K.; Meena, R.C.; Tulsawani, R.; Nanda, S.; Singh, S.B. Heat stress-induced neuroinflammation and aberration in monoamine levels in hypothalamus are associated with temperature dysregulation. Neuroscience 2017, 358, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Epstein, Y.; Shapiro, Y. Vasopressin in thermoregulation–competitive demands: Experimental evidence and theoretical considerations. Physiol. Res. 1992, 41, 41–48. [Google Scholar] [PubMed]
- Rauch, T.M.; Welch, D.; Gallego, L. Hyperthermia impairs retrieval of an overtrained spatial task in the Morris water maze. Behav. Neural Biol. 1989, 52, 321–330. [Google Scholar] [CrossRef]
- Singleton, K.D.; Wischmeyer, P.E. Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock 2006, 25, 295–299. [Google Scholar] [CrossRef]
- Koko, V.; Djordjeviæ, J.; Cvijiæ, G.; Davidoviæ, V. Effect of acute heat stress on rat adrenal glands: A morphological and stereological study. J. Exp. Biol. 2004, 207, 4225–4230. [Google Scholar] [CrossRef]
- Sinha, R.K. Study of changes in some pathophysiological stress markers in different age groups of an animal model of acute and chronic heat stress. Iran. Biomed. J. 2007, 11, 101–111. [Google Scholar]
- Anderson, R.L.; Kraft, P.E.; Bensaude, O.; Hahn, G.M. Binding activity of glucocorticoid receptors after heat shock. Exp. Cell Res. 1991, 197, 100–106. [Google Scholar] [CrossRef]
- Sanchez, E.R. Heat shock induces translocation to the nucleus of the unliganded glucocorticoid receptor. J. Biol. Chem. 1992, 267, 17–20. [Google Scholar] [CrossRef]
- Matić, G.; Trajković, D.; Susa, M.; Damjanović, S.; Petrović, J. In vitro evidence for modification of rat liver glucocorticoid receptor binding properties and transformation by hyperthermia. J. Steroid Biochem. 1989, 32, 263–270. [Google Scholar] [CrossRef]
- Cvoro, A.; Korać, A.; Matić, G. Immunocytochemical study of the glucocorticoid receptor in rat liver nuclei after hyperthermic stress. Cell Biol. Int. 2003, 27, 403–407. [Google Scholar] [CrossRef]
- Čvoro, A.; Dundjerski, J.; Trajković, D.; Matić, G. Association of the rat liver glucocorticoid receptor with Hsp90 and Hsp70 upon whole body hyperthermic stress. J. Steroid Biochem. Mol. Biol. 1998, 67, 319–325. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Vreugdenhil, E.; Oitzl, M.S.; Joëls, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998, 19, 269–301. [Google Scholar] [PubMed]
- Srinivasan, G.; Patel, N.T.; Thompson, E.B. Heat shock protein is tightly associated with the recombinant human glucocorticoid receptor: Glucocorticoid response element complex. Mol. Endocrinol. 1994, 8, 189–196. [Google Scholar]
- Djordjevic, A.; Adzic, M.; Djordjevic, J.; Radojcic, M.B. Stress type dependence of expression and cytoplasmic-nuclear partitioning of glucocorticoid receptor, hsp90 and hsp70 in Wistar rat brain. Neuropsychobiology 2009, 59, 213–221. [Google Scholar] [CrossRef]
- Stavreva, D.A.; Müller, W.G.; Hager, G.L.; Smith, C.L.; McNally, J.G. Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Mol. Cell. Biol. 2004, 24, 2682–2697. [Google Scholar] [CrossRef]
- Elbi, C.; Walker, D.A.; Romero, G.; Sullivan, W.P.; Toft, D.O.; Hager, G.L.; DeFranco, D.B. Molecular chaperones function as steroid receptor nuclear mobility factors. Proc. Natl. Acad. Sci. USA 2004, 101, 2876–2881. [Google Scholar] [CrossRef]
- Liu, J.; DeFranco, D.B. Chromatin recycling of glucocorticoid receptors: Implications for multiple roles of heat shock protein 90. Mol. Endocrinol. 1999, 13, 355–365. [Google Scholar] [CrossRef]
- Jasnic, N.; Dakic, T.; Bataveljic, D.; Vujovic, P.; Lakic, I.; Jevdjovic, T.; Djurasevic, S.; Djordjevic, J. Distinct vasopressin content in the hypothalamic supraoptic and paraventricular nucleus of rats exposed to low and high ambient temperature. J. Therm. Biol. 2015, 52, 1–7. [Google Scholar] [CrossRef]
- Jasnic, N.; Djordjevic, J.; Vujovic, P.; Lakic, I.; Djurasevic, S.; Cvijic, G. The effect of vasopressin 1b receptor (V1bR) blockade on HPA axis activity in rats exposed to acute heat stress. J. Exp. Biol. 2013, 216, 2302–2307. [Google Scholar] [CrossRef]
- Volpi, S.; Rabadan-Diehl, C.; Aguilera, G. Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress 2004, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G.; Rabadan-Diehl, C. Vasopressinergic regulation of the hypothalamic–pituitary–adrenal axis: Implications for stress adaptation. Regul. Pept. 2000, 96, 23–29. [Google Scholar] [CrossRef]
- Koshimizu, T.A.; Nakamura, K.; Egashira, N.; Hiroyama, M.; Nonoguchi, H.; Tanoue, A. Vasopressin V1a and V1b receptors: From molecules to physiological systems. Physiol. Rev. 2012, 92, 1813–1864. [Google Scholar] [CrossRef] [PubMed]
- Szmydynger-Chodobska, J.; Fox, L.M.; Lynch, K.M.; Zink, B.J.; Chodobski, A. Vasopressin amplifies the production of proinflammatory mediators in traumatic brain injury. J. Neurotrauma 2010, 27, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Bhosle, M.R.; Lu, S.F.; Simon, N.S.; Iriah, S.; Brownstein, M.J.; Ferris, C.F. Evidence of early vasogenic edema following minor head impact that can be reduced with a vasopressin V1a receptor antagonist. Brain Res. Bull. 2020, 165, 218–227. [Google Scholar] [CrossRef]
- Shuaib, A.; Xu Wang, C.; Yang, T.; Noor, R. Effects of nonpeptide V1 vasopressin receptor antagonist SR-49059 on infarction volume and recovery of function in a focal embolic stroke model. Stroke 2002, 33, 3033–3037. [Google Scholar] [CrossRef]
- Trabold, R.; Krieg, S.; Schöller, K.; Plesnila, N. Role of vasopressin V1a and V2 receptors for the development of secondary brain damage after traumatic brain injury in mice. J. Neurotrauma 2008, 25, 1459–1465. [Google Scholar] [CrossRef]
- Vakili, A.; Kataoka, H.; Plesnila, N. Role of arginine vasopressin V1 and V2 receptors for brain damage after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2005, 25, 1012–1019. [Google Scholar] [CrossRef]
- Dickinson, L.D.; Betz, A.L. Attenuated development of ischemic brain edema in vasopressin-deficient rats. J. Cereb. Blood Flow Metab. 1992, 12, 681–690. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. IJPR 2011, 10, 655. [Google Scholar]
- Nakayama, M.; Uchimura, K.; Zhu, R.L.; Nagayama, T.; Rose, M.E.; Stetler, R.A.; Isakson, P.C.; Chen, J.; Graham, S.H. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc. Natl. Acad. Sci. USA 1998, 95, 10954–10959. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, S.; Zhang, F.; Ross, M.E.; Iadecola, C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 1997, 17, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Baik, E.J.; Kim, E.J.; Lee, S.H.; Moon, C.-H. Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neuronal cell death in the hippocampus. Brain Res. 1999, 843, 118–129. [Google Scholar] [CrossRef]
- Akaike, A.; Kaneko, S.; Tamura, Y.; Nakata, N.; Shiomi, H.; Ushikubi, F.; Narumiya, S. Prostaglandin E2 protects cultured cortical neurons against N-methyl-D-aspartate receptor-mediated glutamate cytotoxicity. Brain Res. 1994, 663, 237–243. [Google Scholar] [CrossRef]
- Dymond, J.B.; Kalmus, G.W. The cytoprotective properties of prostaglandin E2 against the toxic effects of actinomycin C on embryonic neural retina cells. Prostaglandins 1992, 44, 129–134. [Google Scholar] [CrossRef]
- Ehrlich, K.; Plate, S.; Stroff, T.; Gretzer, B.; Respondek, M.; Peskar, B.M. Peptidergic and cholinergic neurons and mediators in peptone-induced gastroprotection: Role of cyclooxygenase-2. Am. J. Physiol. 1998, 274, G955–G964. [Google Scholar] [CrossRef]
- Gądek-Michalska, A.; Tadeusz, J.; Rachwalska, P.; Bugajski, J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol. Rep. 2013, 65, 1655–1662. [Google Scholar] [CrossRef]
- Fujii, N.; Pastore, O.L.; McGarr, G.W.; Meade, R.D.; McNeely, B.D.; Nishiyasu, T.; Kenny, G.P. Cyclooxygenase-1 and -2 modulate sweating but not cutaneous vasodilation during exercise in the heat in young men. Physiol. Rep. 2018, 6, e13844. [Google Scholar] [CrossRef]
- Delpech, J.-C.; Madore, C.; Nadjar, A.; Joffre, C.; Wohleb, E.S.; Layé, S. Microglia in neuronal plasticity: Influence of stress. Neuropharmacology 2015, 96, 19–28. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Thoenen, H. Neurotrophins and activity-dependent plasticity. Prog. Brain Res. 2000, 128, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lombès, M.; Le Menuet, D. Glucocorticoid receptor represses brain-derived neurotrophic factor expression in neuron-like cells. Mol. Brain 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Bramham, C.R.; Duarte, C.B. BDNF and Hippocampal Synaptic Plasticity. Vitam. Horm. 2017, 104, 153–195. [Google Scholar] [CrossRef] [PubMed]
- Poo, M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001, 2, 24–32. [Google Scholar] [CrossRef]
- Smith, M.A.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995, 15, 1768–1777. [Google Scholar] [CrossRef]
- Shi, S.-S.; Shao, S.-H.; Yuan, B.-P.; Pan, F.; Li, Z.-L. Acute stress and chronic stress change brain-derived neurotrophic factor (BDNF) and tyrosine kinase-coupled receptor (TrkB) expression in both young and aged rat hippocampus. Yonsei Med. J. 2010, 51, 661–671. [Google Scholar] [CrossRef]
- Molteni, R.; Calabrese, F.; Cattaneo, A.; Mancini, M.; Gennarelli, M.; Racagni, G.; Riva, M.A. Acute stress responsiveness of the neurotrophin BDNF in the rat hippocampus is modulated by chronic treatment with the antidepressant duloxetine. Neuropsychopharmacology 2009, 34, 1523–1532. [Google Scholar] [CrossRef]
- Smith, M.A.; Makino, S.; Kim, S.; Kvetnansky, R. Stress increases brain-derived neurotropic factor messenger ribonucleic acid in the hypothalamus and pituitary. Endocrinology 1995, 136, 3743–3750. [Google Scholar] [CrossRef]
- Lambert, W.M.; Xu, C.F.; Neubert, T.A.; Chao, M.V.; Garabedian, M.J.; Jeanneteau, F.D. Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation. Mol. Cell. Biol. 2013, 33, 3700–3714. [Google Scholar] [CrossRef]
- Gray, J.D.; Milner, T.A.; McEwen, B.S. Dynamic plasticity: The role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience 2013, 239, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lievano, M.; Lambert, W.M.; Bath, K.G.; Garabedian, M.J.; Chao, M.V.; Jeanneteau, F. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc. Natl. Acad. Sci. USA 2015, 112, 15737–15742. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, F.; Holzer, P. Neuropeptide Y: A stressful review. Neuropeptides 2016, 55, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bahry, M.A.; Chowdhury, V.S.; Yang, H.; Tran, P.V.; Do, P.H.; Han, G.; Ikeda, H.; Cockrem, J.F.; Furuse, M. Central administration of neuropeptide Y differentially regulates monoamines and corticosterone in heat-exposed fed and fasted chicks. Neuropeptides 2017, 62, 93–100. [Google Scholar] [CrossRef]
- Cohen, H.; Liu, T.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Mathé, A.A. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 2012, 37, 350–363. [Google Scholar] [CrossRef]
- Eltahan, H.M.; Bahry, M.A.; Yang, H.; Han, G.; Nguyen, L.T.N.; Ikeda, H.; Ali, M.N.; Amber, K.A.; Furuse, M.; Chowdhury, V.S. Central NPY-Y5 sub-receptor partially functions as a mediator of NPY-induced hypothermia and affords thermotolerance in heat-exposed fasted chicks. Physiol. Rep. 2017, 5, e13511. [Google Scholar] [CrossRef]
- Ito, K.; Bahry, M.A.; Hui, Y.; Furuse, M.; Chowdhury, V.S. Acute heat stress up-regulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acids in chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 13–19. [Google Scholar] [CrossRef]
- Conrad, C.; McEwen, B. Acute stress increases neuropeptide Y mRNA within the arcuate nucleus and hilus of the dentate gyrus. Mol. Brain Res. 2000, 79, 102–109. [Google Scholar] [CrossRef]
- Croce, N.; Gelfo, F.; Ciotti, M.T.; Federici, G.; Caltagirone, C.; Bernardini, S.; Angelucci, F. NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: A possible role in neuroprotection? Mol. Cell. Biochem. 2013, 376, 189–195. [Google Scholar] [CrossRef]
- Barnea, A.; Roberts, J. Induction of functional and morphological expression of neuropeptide Y (NPY) in cortical cultures by brain-derived neurotrophic factor (BDNF): Evidence for a requirement for extracellular-regulated kinase (ERK)-dependent and ERK-independent mechanisms. Brain Res. 2001, 919, 57–69. [Google Scholar] [CrossRef]
- Cohen, H.; Kozlovsky, N.; Matar, M.A.; Zohar, J.; Kaplan, Z. Distinctive hippocampal and amygdalar cytoarchitectural changes underlie specific patterns of behavioral disruption following stress exposure in an animal model of PTSD. Eur. Neuropsychopharmacol. 2014, 24, 1925–1944. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Cohen, H.; Cai, G.; Buxbaum, J.D.; Yehuda, R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proc. Natl. Acad. Sci. USA 2014, 111, 13529–13534. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, N.; Matar, M.A.; Kaplan, Z.; Kotler, M.; Zohar, J.; Cohen, H. Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. Int. J. Neuropsychopharmacol. 2007, 10, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, N.; Matar, M.A.; Kaplan, Z.; Zohar, J.; Cohen, H. A distinct pattern of intracellular glucocorticoid-related responses is associated with extreme behavioral response to stress in an animal model of post-traumatic stress disorder. Eur. Neuropsychopharmacol. 2009, 19, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Latchman, D.S. Heat shock proteins and cardiac protection. Cardiovasc. Res. 2001, 51, 637–646. [Google Scholar] [CrossRef]
- Brown, I.R. Heat shock proteins and protection of the nervous system. Ann. N. Y. Acad. Sci. 2007, 1113, 147–158. [Google Scholar] [CrossRef]
- Latchman, D.S. Protective effect of heat shock proteins in the nervous system. Curr. Neurovasc. Res. 2004, 1, 21–27. [Google Scholar] [CrossRef]
- Udelsman, R.; Li, D.G.; Stagg, C.A.; Gordon, C.B.; Kvetnansky, R. Adrenergic regulation of adrenal and aortic heat shock protein. Surgery 1994, 116, 177–182. [Google Scholar]
- Inaguma, Y.; Hasegawa, K.; Goto, S.; Ito, H.; Kato, K. Induction of the synthesis of hsp27 and alpha B crystallin in tissues of heat-stressed rats and its suppression by ethanol or an alpha 1-adrenergic antagonist. J. Biochem. 1995, 117, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Peng, M.; Cheng, M.; Xiao, X.; Yi, J.; Yao, S.; Zhang, X. Hyperthermia protects mice against chronic unpredictable stress-induced anxiety-like behaviour and hippocampal CA3 cell apoptosis. Int. J. Hyperth. 2011, 27, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Matar, M.A.; Buskila, D.; Kaplan, Z.; Zohar, J. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol. Psychiatry 2008, 64, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Zohar, J.; Matar, M. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol. Psychiatry 2003, 53, 463–473. [Google Scholar] [CrossRef]
- Cohen, H.; Zohar, J. An animal model of posttraumatic stress disorder: The use of cut-off behavioral criteria. Ann. N. Y. Acad. Sci. 2004, 1032, 167–178. [Google Scholar] [CrossRef]
- Fleischmann, C.; Bar-Ilan, N.; Horowitz, M.; Bruchim, Y.; Deuster, P.; Heled, Y. Astaxanthin supplementation impacts the cellular HSP expression profile during passive heating. Cell Stress Chaperones 2020, 25, 549–558. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. A Stereotaxic Atlas of the Rat Brain; Academic Press: New York, NY, USA, 1988. [Google Scholar]
- Sapolsky, R.M. A mechanism for glucocorticoid toxicity in the hippocampus: Increased neuronal vulnerability to metabolic insults. J. Neurosci. 1985, 5, 1228–1232. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Schwimmer, H.; Gerstberger, R.; Horowitz, M. Nitric oxide and angiotensin II: Neuromodulation of thermoregulation during combined heat and hypohydration stress. Brain Res. 2004, 1006, 177–189. [Google Scholar] [CrossRef]
- Badoer, E. Role of the hypothalamic PVN in the regulation of renal sympathetic nerve activity and blood flow during hyperthermia and in heart failure. Am. J. Physiol. Ren. Physiol. 2010, 298, F839–F846. [Google Scholar] [CrossRef]
- Cham, J.L.; Badoer, E. Hypothalamic paraventricular nucleus is critical for renal vasoconstriction elicited by elevations in body temperature. Am. J. Physiol. Ren. Physiol. 2008, 294, F309–F315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cohen, S.; Vainer, E.; Matar, M.A.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Mathé, A.A.; Cohen, H. Diurnal fluctuations in HPA and neuropeptide Y-ergic systems underlie differences in vulnerability to traumatic stress responses at different zeitgeber times. Neuropsychopharmacology 2015, 40, 774–790. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belity, T.; Horowitz, M.; Hoffman, J.R.; Epstein, Y.; Bruchim, Y.; Todder, D.; Cohen, H. Heat-Stress Preconditioning Attenuates Behavioral Responses to Psychological Stress: The Role of HSP-70 in Modulating Stress Responses. Int. J. Mol. Sci. 2022, 23, 4129. https://doi.org/10.3390/ijms23084129
Belity T, Horowitz M, Hoffman JR, Epstein Y, Bruchim Y, Todder D, Cohen H. Heat-Stress Preconditioning Attenuates Behavioral Responses to Psychological Stress: The Role of HSP-70 in Modulating Stress Responses. International Journal of Molecular Sciences. 2022; 23(8):4129. https://doi.org/10.3390/ijms23084129
Chicago/Turabian StyleBelity, Tal, Michal Horowitz, Jay R. Hoffman, Yoram Epstein, Yaron Bruchim, Doron Todder, and Hagit Cohen. 2022. "Heat-Stress Preconditioning Attenuates Behavioral Responses to Psychological Stress: The Role of HSP-70 in Modulating Stress Responses" International Journal of Molecular Sciences 23, no. 8: 4129. https://doi.org/10.3390/ijms23084129
APA StyleBelity, T., Horowitz, M., Hoffman, J. R., Epstein, Y., Bruchim, Y., Todder, D., & Cohen, H. (2022). Heat-Stress Preconditioning Attenuates Behavioral Responses to Psychological Stress: The Role of HSP-70 in Modulating Stress Responses. International Journal of Molecular Sciences, 23(8), 4129. https://doi.org/10.3390/ijms23084129





