Characterization of a Human Platelet Lysate-Loaded Keratin Hydrogel for Wound Healing Applications In Vitro
Abstract
:1. Introduction
2. Results
2.1. Mechanical Properties of Hydrogels
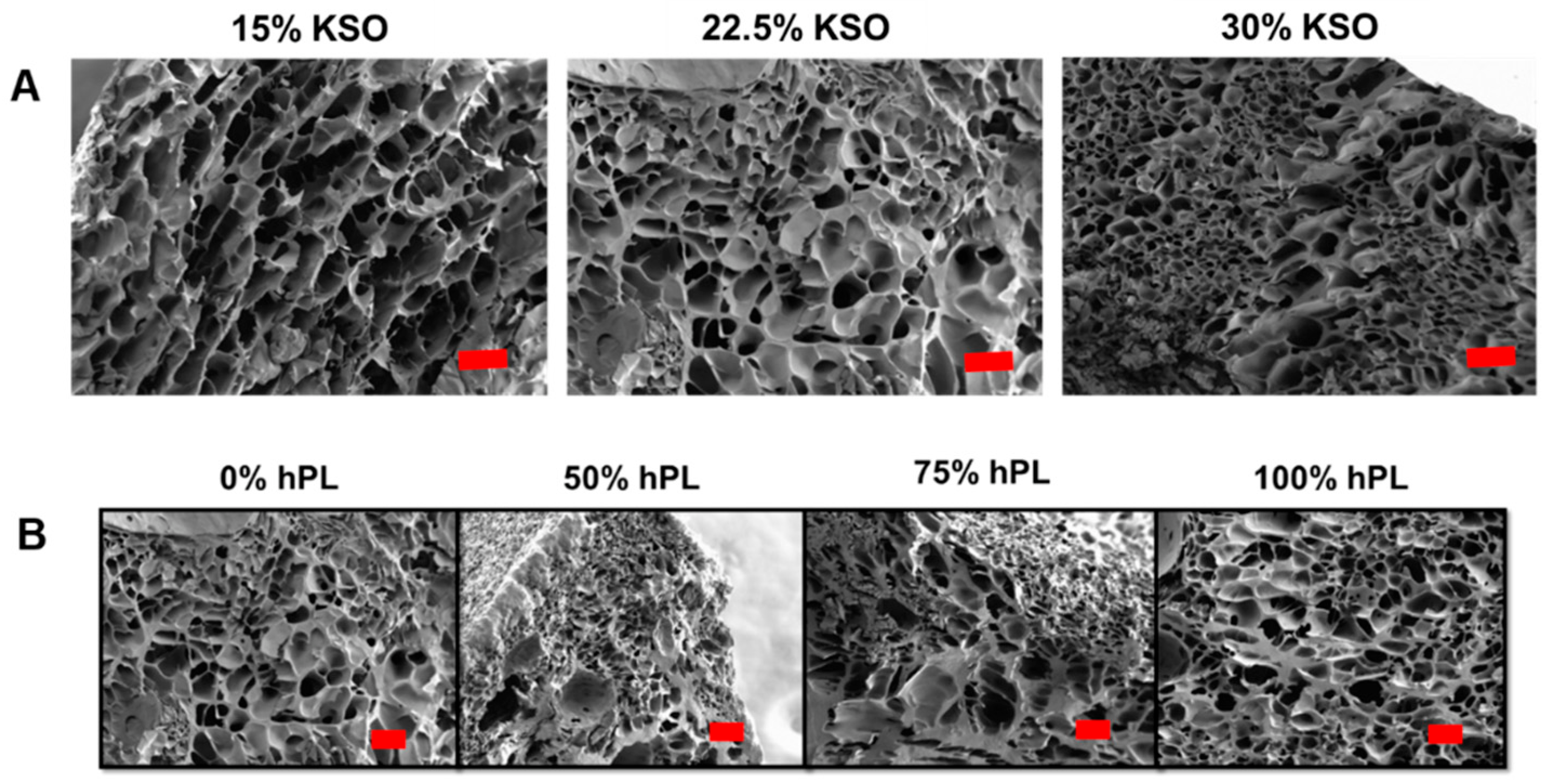
2.2. Hydrogel Structure and Porosity
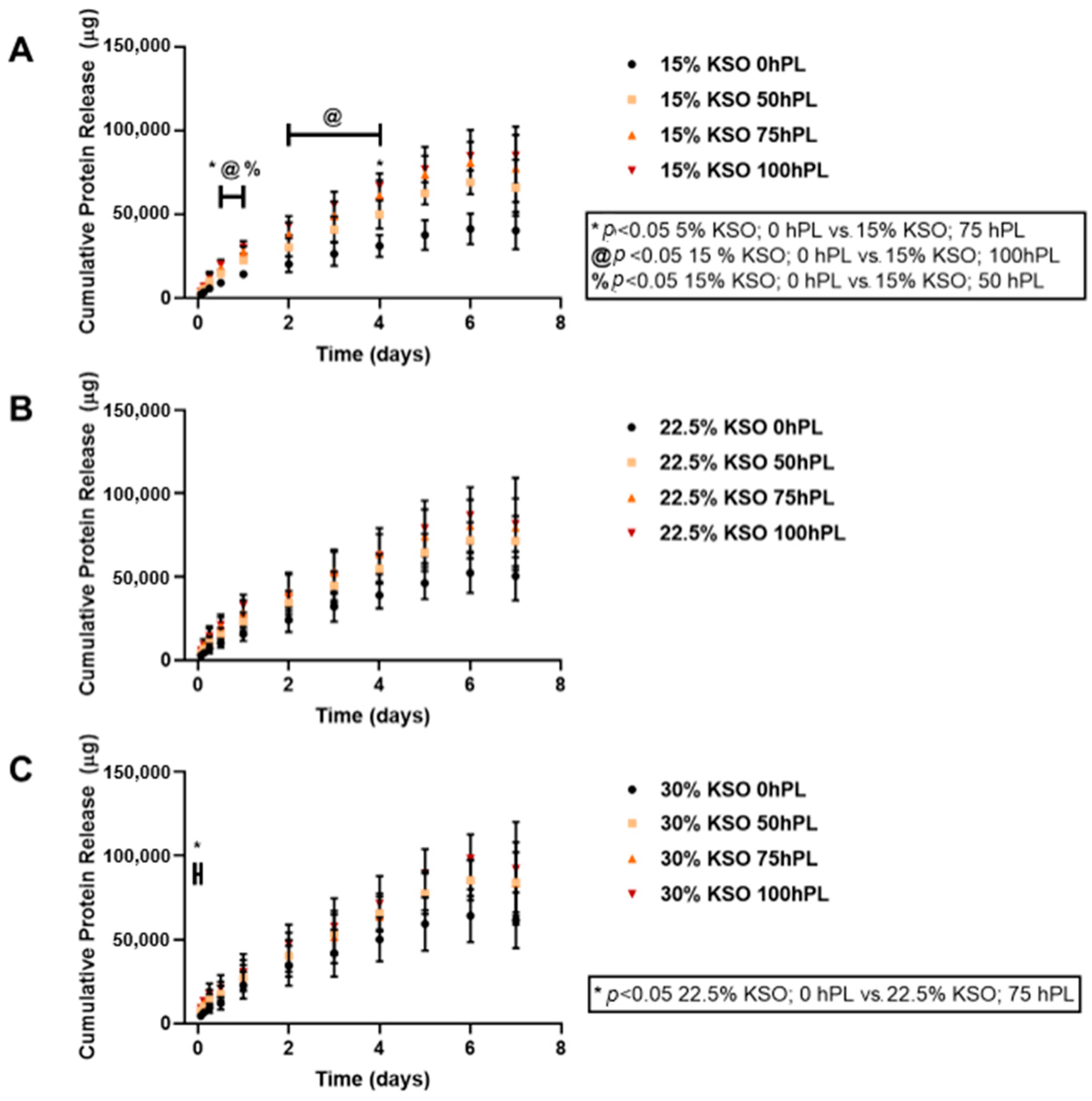
2.3. Cumulative Total Protein Release from Hydrogels
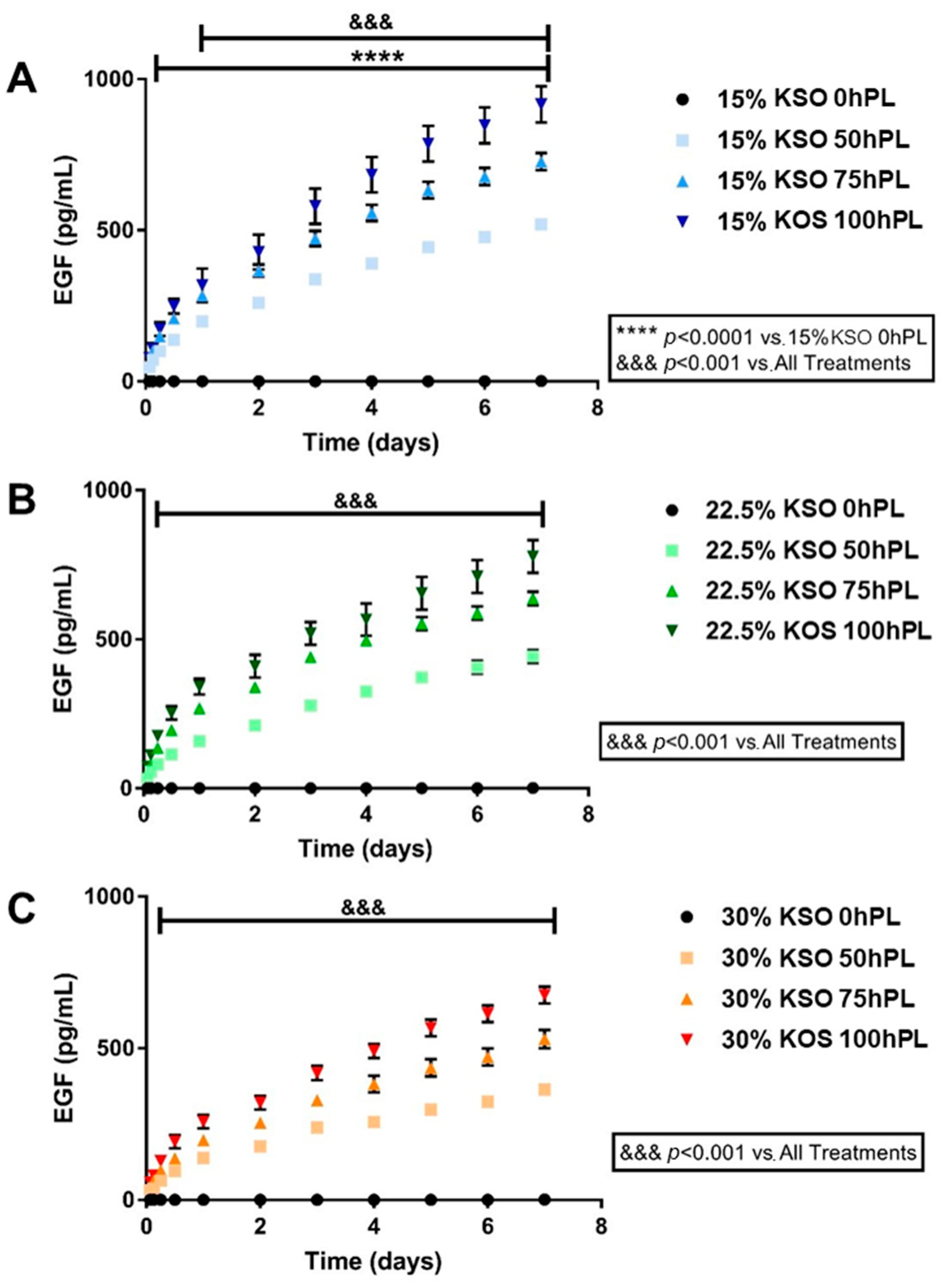
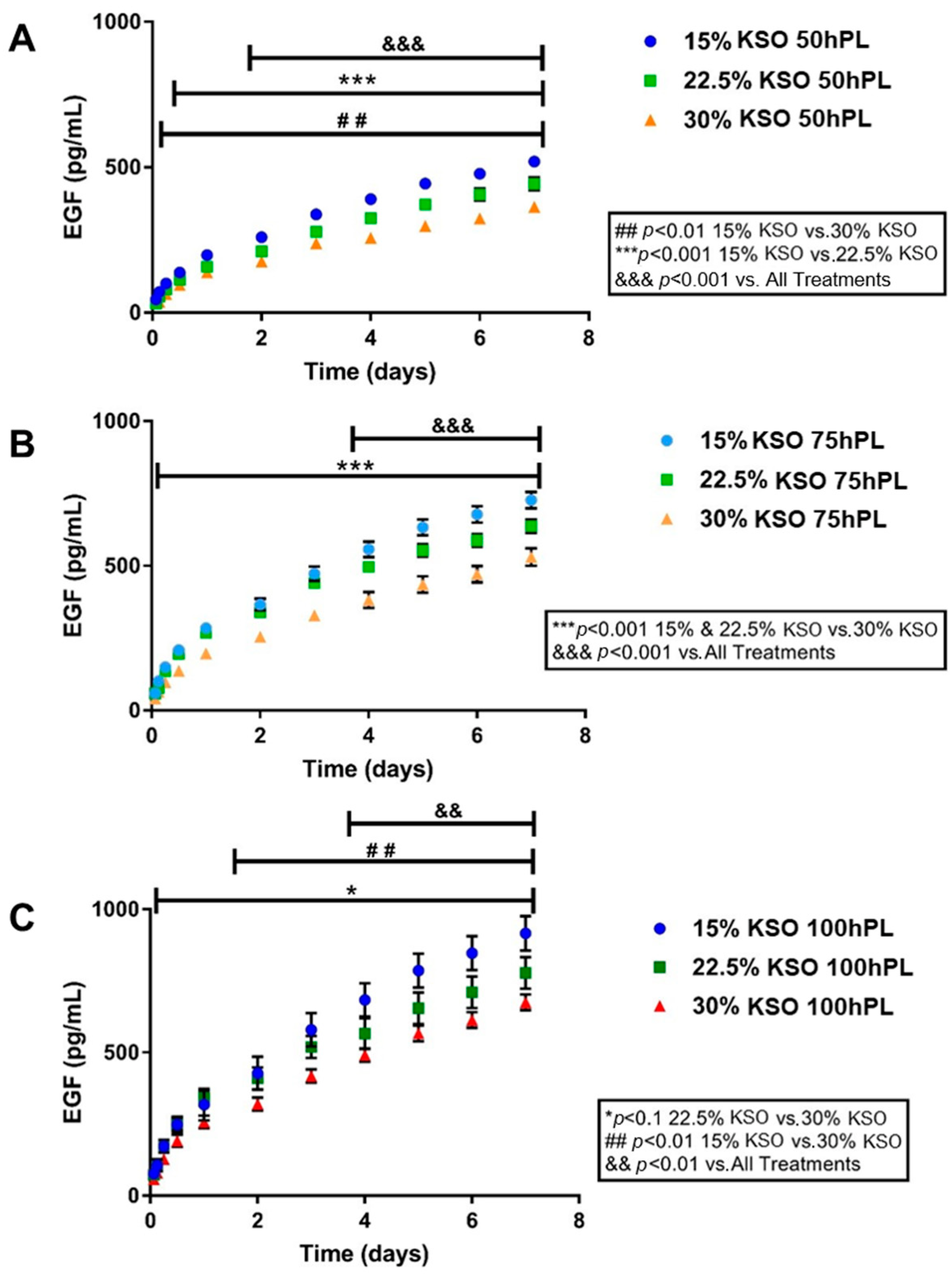
2.4. EGF Release as a Measurement of hPL Release
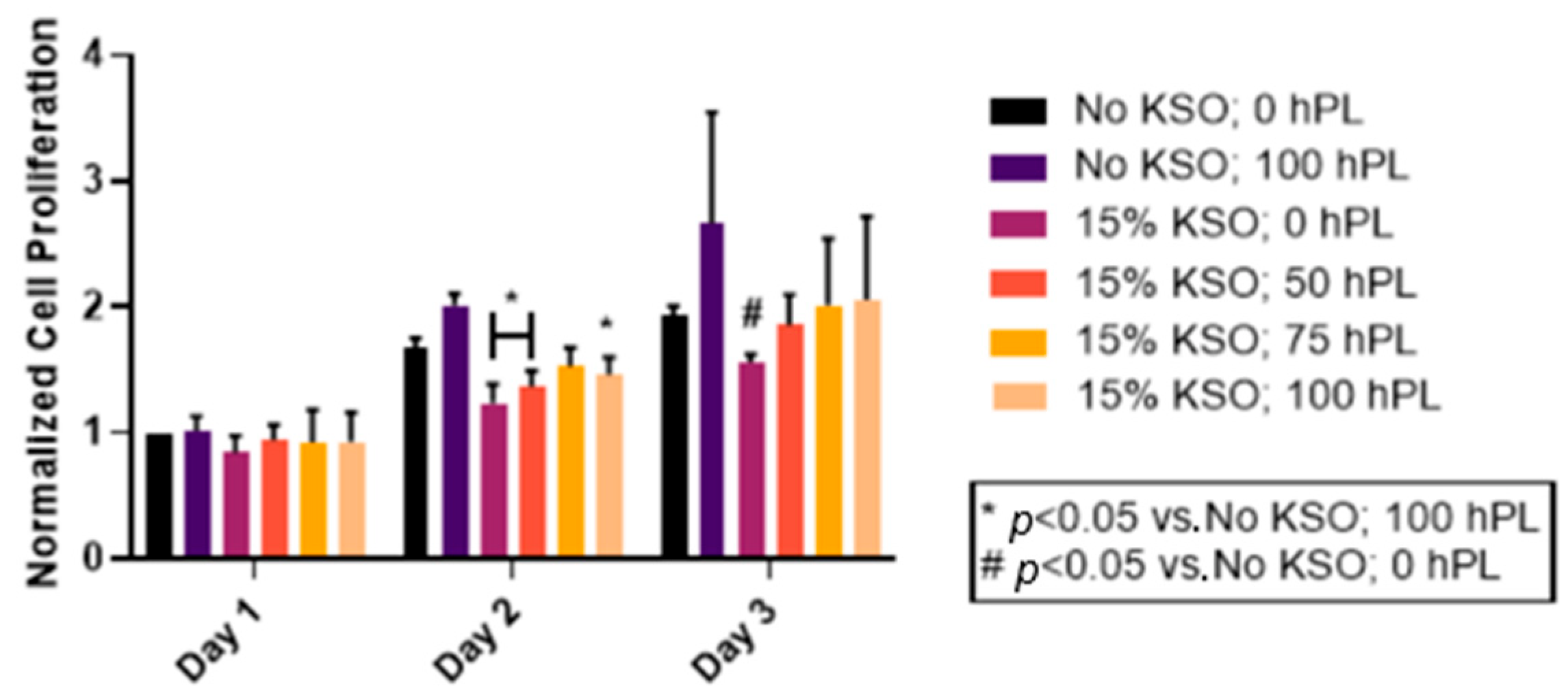
2.5. Cell Proliferation of hDFs
3. Discussion
4. Materials and Methods
4.1. hPL Solution Preparation
4.2. hPL-Loaded Keratin Hydrogel Fabrication
4.3. Rheology
4.4. Scanning Electron Microscopy
4.5. Protein Release
4.6. EGF Quantification
4.7. Cell Culture
4.8. hPL Solution and hPL-Loaded Keratin Hydrogel Treatment
4.9. Cell Proliferation Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnoczky, S.P.; Delos, D.; Rodeo, A.S. What Is Platelet-Rich Plasma? Oper. Tech. Sports Med. 2011, 19, 142–148. [Google Scholar] [CrossRef]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast. Reconstr. Surg. 2006, 118, 147e–159e. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, R.E. Platelet-Rich Plasma: Evidence to Support Its Use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Kazakos, K.; Lyras, D.N.; Verettas, D.; Tilkeridis, K.; Tryfonidis, M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury 2009, 40, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Wrotniak, M.; Bielecki, T.; Gaździk, T.S. Current opinion about using the platelet-rich gel in orthopaedics and trauma surgery. Ortop. Traumatol. Rehabil. 2007, 9, 227–238. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17721419 (accessed on 4 April 2020). [PubMed]
- Mazzucco, L.; Borzini, P.; Gope, R. Platelet-derived factors involved in tissue repair-from signal to function. Transfus. Med. Rev. 2010, 24, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Radziwon-Balicka, A.; de la Rosa, C.M.; Jurasz, P. Platelet-associated angiogenesis regulating factors: A pharmacological perspective. Can. J. Physiol. Pharmacol. 2012, 90, 679–688. [Google Scholar] [CrossRef]
- Mehta, S.; Watson, J.T. Platelet rich concentrate: Basic science and current clinical applications. J. Orthop. Trauma 2008, 22, 432–438. [Google Scholar] [CrossRef]
- Hom, D.B.; Linzie, B.M.; Huang, T.C. The healing effects of autologous platelet gel on acute human skin wounds. Arch. Facial Plast. Surg. 2007, 9, 174–183. [Google Scholar] [CrossRef] [Green Version]
- De Leon, J.M.; Driver, V.R.; Fylling, C.P.; Carter, M.J.; Anderson, C.; Wilson, J.; Dougherty, R.M.; Fuston, D.; Trigilia, D.; Valenski, V.; et al. The clinical relevance of treating chronic wounds with an enhanced near-physiological concentration of platelet-rich plasma gel. Adv. Ski. Wound Care 2011, 24, 357–368. [Google Scholar] [CrossRef]
- Marck, R.E.; Middelkoop, E.; Breederveld, R.S. Considerations on the use of platelet-rich plasma, specifically for burn treatment. J. Burn. Care Res. 2014, 35, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, N.; Kushida, S.; Minakata, T.; Suzuki, K.; Kusumoto, K. Platelet-rich plasma promotes epithelialization and angiogenesis in a split-thickness skin graft donor site. Med. Mol. Morphol. 2011, 44, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Battinelli, E.M.; Markens, B.A.; Italiano, J.E. Release of angiogenesis regulatory proteins from platelet alpha granules: Modulation of physiologic and pathologic angiogenesis. Blood 2011, 118, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Achora, S.; Muliira, J.K.; Thanka, A.N. Strategies to promote healing of split thickness skin grafts: An integrative review. J. Wound Ostomy Cont. Nurs. 2014, 41, 335–339. [Google Scholar] [CrossRef]
- Burnouf, T.; Goubran, H.A.; Chen, T.M.; Ou, K.L.; El-Ekiaby, M.; Radosevic, M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013, 27, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Assoian, R.K.; Sporn, M.B. Type β transforming growth factor in human platelets: Release during platelet degranulation and action on vascular smooth muscle cells. J. Cell Biol. 1986, 102, 1217–1223. [Google Scholar] [CrossRef] [Green Version]
- Borzini, P.; Mazzucco, L. Platelet gels and releasates. Curr. Opin. Hematol. 2005, 12, 473–479. [Google Scholar] [CrossRef]
- Rothan, H.A.; Djordjevic, I.; Bahrani, H.; Paydar, M.; Ibrahim, F.; Rahmanh, N.A.; Yusof, R. Three-dimensional culture environment increases the efficacy of platelet rich plasma releasate in prompting skin fibroblast differentiation and extracellular matrix formation. Int. J. Med. Sci. 2014, 11, 1029–1038. [Google Scholar] [CrossRef] [Green Version]
- Kubotal, S.; Kawatal, K.; Yanagita1, T.; Doi, H.; Kitohl, T.; Takigawal, M. Abundant Retention and Release of Connective Tissue Growth Factor (CTGF/CCN2) by Platelets. J. Biochem. 2004, 136, 279–282. [Google Scholar] [CrossRef]
- Iudicone, P.; Fioravanti, D.; Bonanno, G.; Miceli, M.; Lavorino, C.; Totta, P.; Frati, L.; Nuti, M.; Pierelli, L. Pathogen-free, plasma-poor platelet lysate and expansion of human mesenchymal stem cells. J. Transl. Med. 2014, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Santo, V.E.; Babo, P.; Amador, M.; Correia, C.; Cunha, B.; Coutinho, D.F.; Neves, N.M.; Mano, F.; Reis, R.L.; Gomes, M.E. Engineering Enriched Microenvironments with Gradients of Platelet Lysate in Hydrogel Fibers. Biomacromolecules 2016, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Tunable Collagen I Hydrogels for Engineered Physiological Tissue Micro-Environments. PLoS ONE 2015, 10, e0122500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.R.; Mooney, D.J. Polymeric growth factor delivery strategies for tissue engineering. Pharm. Res. 2003, 20, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Pérez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef]
- Rosellini, E.; Cristallini, C.; Guerra, G.D.; Barbani, N. Surface chemical immobilization of bioactive peptides on synthetic polymers for cardiac tissue engineering. J. Biomater. Sci. Polym. Ed. 2015, 26, 515–533. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Jones, R.R.; Hamley, I.W.; Connon, C.J. The bioactivity of composite Fmoc-RGDS-collagen gels. Biomater. Sci. 2014, 2, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Hosoyama, K.; Lazurko, C.; Muñoz, M.; McTiernan, C.D.; Alarcon, E.I. Peptide-based functional biomaterials for soft-tissue repair. Front. Bioeng. Biotechnol. 2019, 7, 205. [Google Scholar] [CrossRef]
- Burmeister, D.M.; Roy, D.C.; Becerra, S.C.; Natesan, S.; Christy, R.J. In Situ Delivery of Fibrin-Based Hydrogels Prevents Contraction and Reduces Inflammation. J. Burn Care Res. 2017, 39, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K. Hybrid hydrogels based on keratin and alginate for tissue engineering. J. Mater. Chem. B 2014, 2, 5441–5451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouse, J.G.; van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, A.; Cavaco-Paulo, A. The Use of Keratin in Biomedical Applications. Curr. Drug Targets 2013, 14, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Tomblyn, S.; Kneller, E.L.P.; Walker, S.J.; Ellenburg, M.D.; Kowalczewski, C.J.; van Dyke, M.; Burnett, L.; Saul, J.M. Keratin hydrogel carrier system for simultaneous delivery of exogenous growth factors and muscle progenitor cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 864–879. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Noh, K.; Lee, S.C.; Kwon, I.K.; Han, D.W.; Lee, I.S.; Hwang, Y.S. Human hair keratin and its-based biomaterials for biomedical applications. Tissue Eng. Regen. Med. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Roy, D.C.; Tomblyn, S.; Isaac, K.M.; Kowalczewski, C.J.; Burmeister, D.M.; Burnett, L.R.; Christy, R.J. Ciprofloxacin-loaded keratin hydrogels reduce infection and support healing in a porcine partial-thickness thermal burn. Wound Repair Regen. 2016, 24, 657–668. [Google Scholar] [CrossRef]
- Ham, T.R.; Lee, R.T.; Han, S.; Haque, S.; Vodovotz, Y.; Gu, J.; Burnett, L.R.; Tomblyn, S.; Saul, J.M. Tunable keratin hydrogels for controlled erosion and growth factor delivery. Biomacromolecules 2016, 17, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, Z.; Foo, S.E.M.; Tan, N.S.; Yuan, Y.; Lin, W.; Zhang, Z.; Ng, K.W. Culturing fibroblasts in 3D human hair keratin hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 5187–5198. [Google Scholar] [CrossRef]
- Wang, S.; Taraballi, F.; Tan, L.P.; Ng, K.W. Human keratin hydrogels support fibroblast attachment and proliferation In Vitro. Cell Tissue Res. 2012, 347, 795–802. [Google Scholar] [CrossRef]
- Kowalczewski, C.J.; Tombyln, S.; Wasnick, D.C.; Hughes, M.R.; Ellenburg, M.D.; Callahan, M.F.; Smith, T.L.; van Dyke, M.E.; Burnett, L.R.; Saul, J.M. Reduction of ectopic bone growth in critically-sized rat mandible defects by delivery of rhBMP-2 from kerateine biomaterials. Biomaterials 2014, 35, 3220–3228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierpinski, P.; Garrett, J.; Ma, J.; Apel, P.; Klorig, D.; Smith, T.; Koman, L.A.; Atala, A.; van Dyke, M. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 2008, 29, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Saul, J.M.; Ellenburg, M.D.; de Guzman, R.C.; van Dyke, M. Keratin hydrogels support the sustained release of bioactive ciprofloxacin. J. Biomed. Mater. Res. Part A 2011, 98, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.A.; Xu, Q. Biocompatibility and degradation of tendon-derived scaffolds. Regen. Biomater. 2016, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, L.; Hu, M.; Wen, W.; Xiao, H.; Niu, Y. Effect of chondroitin sulfate modification on rhBMP-2 release kinetics from collagen delivery system. J. Biomed. Mater. Res. Part A 2010, 92, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, R.; Dijkstra, P.J.; van Wachem, P.B.; van Luyn, M.J.A.; Hendriks, M.; Cahalan, P.T.; Feijen, J. Successive epoxy and carbodiimide cross-linking of dermal sheep collagen. Biomaterials 1999, 20, 921–931. [Google Scholar] [CrossRef]
- Mangum, L.H.; Natesan, S.; Ii, R.S.; Wrice, N.L.; Larson, D.A.; Florell, K.F.; Christy, B.A.; Herzig, M.C.; Cap, A.P.; Christy, R.J. Tissue Source and Cell Expansion Condition Influence Phenotypic Changes of Adipose-Derived Stem Cells. Stem Cells Int. 2017, 2017, 7108458. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.-Y.; Wu, J.; Zhu, J.-J.; Xiao, Z.-C.; He, C.-C.; Shi, H.-X.; Li, X.-K.; Yang, S.-L.; Xiao, J. Reserach advances in tissue engineering materials for sustained release of growth factors. Biomed. Res. Int. 2015, 2015, 808202. [Google Scholar] [CrossRef] [Green Version]
- Dumitriu, S. Polymeric Biomaterials, Revised and Expanded, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001; Available online: https://books.google.com/books?hl=en&lr=&id=iCm1SJBDZwkC&oi=fnd&pg=PR5&ots=0-r_bEKqxC&sig=MVOlv9Z1_nKsxT9hdGNVF5t9x54 (accessed on 19 April 2020).
- Strelkov, S.V.; Schumacher, J.; Burkhard, P.; Aebi, U.; Herrmann, H. Crystal structure of the human lamin a coil 2B dimer: Implications for the head-to-tail association of nuclear lamins. J. Mol. Biol. 2004, 343, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, R.C.; Merrill, M.R.; Richter, J.R.; Hamzi, R.I.; Greengauz-Roberts, O.K.; van Dyke, M.E. Mechanical and biological properties of keratose biomaterials. Biomaterials 2011, 32, 8205–8217. [Google Scholar] [CrossRef]
- Popescu, C.; Höcker, H. Hair—The most sophisticated biological composite material. Chem. Soc. Rev. 2007, 36, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Jooybar, E.; Abdekhodaie, M.J.; Alvi, M.; Mousavi, A.; Karperien, M.; Dijkstra, P.J. An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomater. 2019, 83, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, L.S.M.; Leijten, J.C.H.; Wennink, J.W.H.; Chatterjea, A.G.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. The effect of platelet lysate supplementation of a dextran-based hydrogel on cartilage formation. Biomaterials 2012, 33, 3651–3661. [Google Scholar] [CrossRef] [PubMed]
- Gerecht, S.; Townsend, S.A.; Pressler, H.; Zhu, H.; Nijst, C.L.E.; Bruggeman, J.P.; Nichol, J.W.; Langer, R. A porous photocurable elastomer for cell encapsulation and culture. Biomaterials 2007, 28, 4826–4835. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.; Erwin, M.; Atigh, M.; Christians, U.; Saul, J.M.; Yazdani, S.K. In Vitro and In Vivo Assessment of Keratose as a Novel Excipient of Paclitaxel Coated Balloons. Front. Pharmacol. 2018, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Conrads, A.; Phan, K.H.; van de Löcht, M.; Zahn, H. In Vitro reconstitution of wool intermediate filaments. Int. J. Biol. Macromol. 1986, 8, 258–264. [Google Scholar] [CrossRef]
- Steinert, P.M.; Gullino, M.I. Bovine epidermal keratin filament assembly In Vitro. Biochem. Biophys. Res. Commun. 1976, 70, 221–227. [Google Scholar] [CrossRef]
- Verma, V.; Verma, P.; Ray, P.; Ray, A.R. Preparation of scaffolds from human hair proteins for tissue-engineering applications. Biomed. Mater. 2008, 3, 025007. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, A.; Furuta, Y.; Takeshima, H.; Tanabe, T.; Yamauchi, K. Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J. Biotechnol. 2002, 93, 165–170. [Google Scholar] [CrossRef]
- Roy, D.C.; Tomblyn, S.; Burmeister, D.M.; Wrice, N.L.; Becerra, S.C.; Burnett, L.R.; Saul, J.M.; Christy, R.J. Ciprofloxacin-Loaded Keratin Hydrogels Prevent Pseudomonas aeruginosa Infection and Support Healing in a Porcine Full-Thickness Excisional Wound. Adv. Wound Care 2015, 4, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Konop, M.; Sulejczak, D.; Czuwara, J.; Kosson, P.; Misicka, A.; Lipkowski, A.W.; Rudnicka, L. The role of allogenic keratin-derived dressing in wound healing in a mouse model. Wound Repair Regen. 2017, 25, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, A.; Kowalkowski, T.; et al. Evaluation of keratin biomaterial containing silver nanoparticles as a potential wound dressing in full-thickness skin wound model in diabetic mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Vakilian, S.; Jamshidi-Adegani, F.; Al-Shidhani, S.; Anwar, M.U.; Al-Harrasi, R.; Al-Wahaibi, N.; Qureshi, A.; Alyaqoobi, S.; Al-Amri, I.; Al-Harrasi, A.; et al. Keratin-based biomaterial as a promising dresser for skin wound healing. Wound Med. 2019, 25, 100155. [Google Scholar] [CrossRef]

| Hydrogel Rheological Properties at 1 Hz within the LVER (n = 3) | |||||
|---|---|---|---|---|---|
| Keratin (KSO) % (w/v) | hPL % | G’ (Pa) | G” (Pa) | G* (Pa) | Tan δ |
| 15 | 0 | 533.7 ± 40.9 | 63.48 ± 4.1 | 537.5 ± 41.0 | 0.11 ± 0.004 |
| 50 | 338.5 ± 67.1 | 44.1 ± 6.2 | 341.4 ± 67.3 | 0.13 ± 0.009 | |
| 100 | 240.2 ± 33.3 | 34.6 ± 5.0 | 242.7 ± 33.7 | 0.14 ± 0.000 | |
| 22.5 | 0 | 2079.0 ± 95.6 | 266.7 ± 32.5 | 2096.1 ± 99.0 | 0.13 ± 0.01 |
| 50 | 1191.1 ± 93.3 | 150.3 ± 14.6 | 1200.7 ± 91.9 | 0.13 ± 0.02 | |
| 100 | 1085.0 ± 108.8 | 142.0 ± 13.9 | 1094.3 ± 109.6 | 0.13 ± 0.000 | |
| 30 | 0 | 6826.0 ± 271.4 | 999.3 ± 64.0 | 6898.8 ± 277.4 | 0.15 ± 0.004 |
| 50 | 6028.8 ± 709.9 | 936.1 ± 120.6 | 6101.1 ± 719.6 | 0.16 ± 0.004 | |
| 100 | 5926.7 ± 962.0 | 978.0 ± 191.5 | 6006.9 ± 980.3 | 0.16 ± 0.005 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuniga, K.; Isaac, A.; Christy, S.; Wrice, N.; Mangum, L.; Natesan, S.; Burnett, L.; Christy, R.; Kowalczewski, C. Characterization of a Human Platelet Lysate-Loaded Keratin Hydrogel for Wound Healing Applications In Vitro. Int. J. Mol. Sci. 2022, 23, 4100. https://doi.org/10.3390/ijms23084100
Zuniga K, Isaac A, Christy S, Wrice N, Mangum L, Natesan S, Burnett L, Christy R, Kowalczewski C. Characterization of a Human Platelet Lysate-Loaded Keratin Hydrogel for Wound Healing Applications In Vitro. International Journal of Molecular Sciences. 2022; 23(8):4100. https://doi.org/10.3390/ijms23084100
Chicago/Turabian StyleZuniga, Kameel, Alisa Isaac, Sean Christy, Nicole Wrice, Lauren Mangum, Shanmugasundaram Natesan, Luke Burnett, Robert Christy, and Christine Kowalczewski. 2022. "Characterization of a Human Platelet Lysate-Loaded Keratin Hydrogel for Wound Healing Applications In Vitro" International Journal of Molecular Sciences 23, no. 8: 4100. https://doi.org/10.3390/ijms23084100
APA StyleZuniga, K., Isaac, A., Christy, S., Wrice, N., Mangum, L., Natesan, S., Burnett, L., Christy, R., & Kowalczewski, C. (2022). Characterization of a Human Platelet Lysate-Loaded Keratin Hydrogel for Wound Healing Applications In Vitro. International Journal of Molecular Sciences, 23(8), 4100. https://doi.org/10.3390/ijms23084100





