The Role of Hydrogen Sulfide Regulation of Autophagy in Liver Disorders
Abstract
1. Introduction
2. Hydrogen Sulfide Plays a Protective Role by Regulating Autophagy in Nonalcoholic Fatty Liver Disease
3. Hydrogen Sulfide Plays a Protective Role by Regulating Autophagy in Hepatic Ischemia-Reperfusion Injury
4. Hydrogen Sulfide Plays a Protective Role by Regulating Autophagy in Hepatocellular Carcinoma
5. Hydrogen Sulfide Exposure Induces Oxidative Stress and Promotes Hepatocyte Autophagy to Lead Liver Injury
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.; Wu, D.; Wang, H. Hydrogen sulfide plays an important protective role by influencing autophagy in diseases. Physiol. Res. 2019, 68, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.M.; Wang, G.L.; Chen, L.; Xu, Y.Y.; He, S.; Cao, X.L.; Qin, J.; Zhou, J.M.; Zhang, Y.X.; Qun, E. The expression of beclin-1, an autophagic gene, in hepatocellular carcinoma associated with clinical pathological and prognostic significance. BMC Cancer 2014, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Wu, T.; Hu, X.; Su, J.; Chen, X. Autophagy in Skin Diseases. Dermatology 2019, 235, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Regulation of Autophagy by mTOR Signaling Pathway. Adv. Exp. Med. Biol. 2019, 1206, 67–83. [Google Scholar] [CrossRef]
- Ding, Y.; Fu, X.; Wang, Q.; Liu, H.; Wang, H.; Wu, D. The Complex Interplay between Autophagy and NLRP3 Inflammasome in Renal Diseases. Int. J. Mol. Sci. 2021, 22, 12766. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Z.; Wang, J.; Wang, H. Exogenous Hydrogen Sulfide Plays an Important Role Through Regulating Autophagy in Ischemia/Reperfusion Injury. Front. Mol. Biosci. 2021, 8, 681676. [Google Scholar] [CrossRef]
- Lv, S.; Wang, H.; Li, X. The Role of the Interplay Between Autophagy and NLRP3 Inflammasome in Metabolic Disorders. Front. Cell Dev. Biol. 2021, 9, 634118. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Mijaljica, D.; Prescott, M.; Devenish, R.J. Microautophagy in mammalian cells: Revisiting a 40-year-old conundrum. Autophagy 2011, 7, 673–682. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Wada, K.; Kabuta, T. Lysosomal degradation of intracellular nucleic acids-multiple autophagic pathways. J. Biochem. 2017, 161, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Huerta, P.; Troncoso-Escudero, P.; Jerez, C.; Hetz, C.; Vidal, R.L. The intersection between growth factors, autophagy and ER stress: A new target to treat neurodegenerative diseases? Brain Res. 2016, 1649, 173–180. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef]
- Kuma, A.; Komatsu, M.; Mizushima, N. Autophagy-monitoring and autophagy-deficient mice. Autophagy 2017, 13, 1619–1628. [Google Scholar] [CrossRef]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 16, 345–357. [Google Scholar] [CrossRef]
- Shirakabe, A.; Ikeda, Y.; Sciarretta, S.; Zablocki, D.K.; Sadoshima, J. Aging and Autophagy in the Heart. Circ. Res. 2016, 118, 1563–1576. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Qiu, M.; Lv, S.; Zheng, H.; Niu, B.; Liu, H. Hydrogen Sulfide Plays an Important Role by Influencing NLRP3 inflammasome. Int. J. Biol. Sci. 2020, 16, 2752–2760. [Google Scholar] [CrossRef]
- Wen, Y.D.; Wang, H.; Zhu, Y.Z. The Drug Developments of Hydrogen Sulfide on Cardiovascular Disease. Oxid. Med. Cell. Longev. 2018, 2018, 4010395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, X.; Li, X.; Wei, X.; Wang, H. Hydrogen Sulfide Plays an Important Role in Diabetic Cardiomyopathy. Front. Cell Dev. Biol. 2021, 9, 627336. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Li, X.; Zhao, S.; Liu, H.; Wang, H. The Role of the Signaling Pathways Involved in the Protective Effect of Exogenous Hydrogen Sulfide on Myocardial Ischemia-Reperfusion Injury. Front. Cell Dev. Biol. 2021, 9, 723569. [Google Scholar] [CrossRef] [PubMed]
- Greaney, J.L.; Kutz, J.L.; Shank, S.W.; Jandu, S.; Santhanam, L.; Alexander, L.M. Impaired Hydrogen Sulfide-Mediated Vasodilation Contributes to Microvascular Endothelial Dysfunction in Hypertensive Adults. Hypertension 2017, 69, 902–909. [Google Scholar] [CrossRef]
- Jin, S.; Teng, X.; Xiao, L.; Xue, H.; Guo, Q.; Duan, X.; Chen, Y.; Wu, Y. Hydrogen sulfide ameliorated L-NAME-induced hypertensive heart disease by the Akt/eNOS/NO pathway. Exp. Biol. Med. 2017, 242, 1831–1841. [Google Scholar] [CrossRef]
- Zhao, A.S.; Zou, D.; Wang, H.H.; Han, X.; Yang, P.; Huang, N. Hydrogen sulphide-releasing aspirin enhances cell capabilities of anti-oxidative lesions and anti-inflammation. Med. Gas. Res. 2019, 9, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, Y.Q.; Zhang, L.; Zhang, H.; Zhang, S.W.; Zhang, Y.; Xuan, X.X.; Wang, M.J.; Zhang, J.Y. Exogenous hydrogen sulfide protects against high glucose-induced apoptosis and oxidative stress by inhibiting the STAT3/HIF-1alpha pathway in H9c2 cardiomyocytes. Exp. Ther. Med. 2019, 18, 3948–3958. [Google Scholar] [CrossRef] [PubMed]
- Tocmo, R.; Parkin, K. S-1-propenylmercaptocysteine protects murine hepatocytes against oxidative stress via persulfidation of Keap1 and activation of Nrf2. Free Radic. Biol. Med. 2019, 143, 164–175. [Google Scholar] [CrossRef]
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H2S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608. [Google Scholar] [CrossRef]
- Nie, L.; Liu, M.; Chen, J.; Wu, Q.; Li, Y.; Yi, J.; Zheng, X.; Zhang, J.; Chu, C.; Yang, J. Hydrogen sulfide ameliorates doxorubicininduced myocardial fibrosis in rats via the PI3K/AKT/mTOR pathway. Mol. Med. Rep. 2021, 23, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.K.; Bajeli, S.; Sahu, S.; Bhat, S.A.; Kumar, A. Hydrogen sulfide-induced GAPDH sulfhydration disrupts the CCAR2-SIRT1 interaction to initiate autophagy. Autophagy 2021, 17, 3511–3529. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Santhekadur, P.K.; Kumar, D.P.; Sanyal, A.J. Preclinical models of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Simha, V. Management of hypertriglyceridemia. BMJ 2020, 371, m3109. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.A.; Raposo, H.F.; Wanschel, A.C.; Nardelli, T.R.; Oliveira, H.C. Apolipoprotein CIII Overexpression-Induced Hypertriglyceridemia Increases Nonalcoholic Fatty Liver Disease in Association with Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2017, 2017, 1838679. [Google Scholar] [CrossRef]
- Esteve-Luque, V.; Padro-Miquel, A.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Pinto, X.; Candas-Estebanez, B. Implication between Genetic Variants from APOA5 and ZPR1 and NAFLD Severity in Patients with Hypertriglyceridemia. Nutrients 2021, 13, 552. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, S.; Yu, C.; Pan, Z.; Liu, Y.; Zhao, J.; Wang, X.; Yun, F.; Zhao, H.; Yan, S.; et al. Hydrogen sulfide reduces serum triglyceride by activating liver autophagy via the AMPK-mTOR pathway. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E925–E935. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.M.; Xie, J.; Hu, Z.W. Mycelium of Hirsutella hepiali Chen et Shen activates autophagy and protects against metabolic syndrome in mice fed with high fat diet. Yao Xue Xue Bao 2014, 49, 615–621. [Google Scholar] [PubMed]
- Shin, D.W. Lipophagy: Molecular Mechanisms and Implications in Metabolic Disorders. Mol. Cells 2020, 43, 686–693. [Google Scholar] [CrossRef]
- Wang, H.-G.; Zhang, Y.-J.; Huang, C.-S.; Chen, L.M.; Wang, J.; Wu, D.D.; Xie, X.Z.; Li, Z.I.; Ji, L.A. Effect of exogenous hydrogen sulfide on lipophagy in mouse primary hepatocytes. Chin. J. Pathophysiol. 2017, 33, 1901–1905. [Google Scholar]
- Wu, D.; Zhong, P.; Wang, J.; Wang, H. Exogenous hydrogen sulfide mitigates LPS + ATP-induced inflammation by inhibiting NLRP3 inflammasome activation and promoting autophagy in L02 cells. Mol. Cell. Biochem. 2019, 457, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhong, P.; Sun, L. Exogenous hydrogen sulfide mitigates NLRP3 inflammasome-mediated inflammation through promoting autophagy via the AMPK-mTOR pathway. Biol. Open 2019, 8, bio043653. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhong, P.; Wang, Y.; Zhang, Q.; Li, J.; Liu, Z.; Ji, A.; Li, Y. Hydrogen Sulfide Attenuates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease by Inhibiting Apoptosis and Promoting Autophagy via Reactive Oxygen Species/Phosphatidylinositol 3-Kinase/AKT/Mammalian Target of Rapamycin Signaling Pathway. Front. Pharmacol. 2020, 11, 585860. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update1. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef]
- Foretz, M.; Pacot, C.; Dugail, I.; Lemarchand, P.; Guichard, C.; Le Liepvre, X.; Berthelier-Lubrano, C.; Spiegelman, B.; Kim, J.B.; Ferre, P.; et al. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol. Cell. Biol. 1999, 19, 3760–3768. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Bashmakov, Y.; Ikemoto, S.; Horton, J.D.; Brown, M.S.; Goldstein, J.L. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA 1999, 96, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Bian, H.; Wang, L.; Sun, X.; Xu, X.; Yan, H.; Xia, M.; Chang, X.; Lu, Y.; Li, Y.; et al. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic. Biol. Med. 2019, 141, 192–204. [Google Scholar] [CrossRef]
- Ziamajidi, N.; Khaghani, S.; Hassanzadeh, G.; Vardasbi, S.; Ahmadian, S.; Nowrouzi, A.; Ghaffari, S.M.; Abdirad, A. Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPARalpha and SREBP-1. Food Chem. Toxicol. 2013, 58, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Saleh Al-Maamari, J.N.; Rahmadi, M.; Panggono, S.M.; Prameswari, D.A.; Pratiwi, E.D.; Ardianto, C.; Balan, S.S.; Suprapti, B. The effects of quercetin on the expression of SREBP-1c mRNA in high-fat diet-induced NAFLD in mice. J. Basic. Clin. Physiol. Pharmacol. 2021, 32, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.P.; Kim, D.Y.; Lee, Y.G.; Lee, Y.S.; Truong, X.T.; Lee, J.H.; Song, D.K.; Kwon, T.K.; Park, S.H.; Jung, C.H.; et al. SREBP-1c impairs ULK1 sulfhydration-mediated autophagic flux to promote hepatic steatosis in high-fat-diet-fed mice. Mol. Cell 2021, 81, 3820–3832.e7. [Google Scholar] [CrossRef] [PubMed]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Van der Windt, D.J.; Sud, V.; Zhang, H.; Tsung, A.; Huang, H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018, 18, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gomez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Cuthbertson, D.J.; Shojaee-Moradie, F.; Sprung, V.S.; Jones, H.; Pugh, C.J.; Richardson, P.; Kemp, G.J.; Barrett, M.; Jackson, N.C.; Thomas, E.L.; et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin. Sci. 2016, 130, 93–104. [Google Scholar] [CrossRef]
- Shojaee-Moradie, F.; Cuthbertson, D.J.; Barrett, M.; Jackson, N.C.; Herring, R.; Thomas, E.L.; Bell, J.; Kemp, G.J.; Wright, J.; Umpleby, A.M. Exercise Training Reduces Liver Fat and Increases Rates of VLDL Clearance But Not VLDL Production in NAFLD. J. Clin. Endocrinol. Metab. 2016, 101, 4219–4228. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Morgan, L.; Truby, H. A review of the effects of exercise on appetite regulation: An obesity perspective. Int. J. Obes. 2008, 32, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jung, K.S.; Kim, S.U.; Yoon, H.J.; Yun, Y.J.; Lee, B.W.; Kang, E.S.; Han, K.H.; Lee, H.C.; Cha, B.S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, W.; Zeng, L.Q.; Bai, H.; Li, J.; Zhou, J.; Zhou, G.Y.; Fang, C.W.; Wang, F.; Qin, X.J. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020, 36, 101635. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Liu, M.; Xi, Y.; Chen, M.; Tian, L.; Xie, J.; Chen, M.; Wang, Z.; Yang, M.; Yu, Z.; et al. Long-term exercise prevents hepatic steatosis: A novel role of FABP1 in regulation of autophagy-lysosomal machinery. FASEB J. 2019, 33, 11870–11883. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zeng, J.; Gu, Q. Exercise restores bioavailability of hydrogen sulfide and promotes autophagy influx in livers of mice fed with high-fat diet. Can. J. Physiol. Pharmacol. 2017, 95, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Temiz, C.; Solmaz, I.; Tehli, O.; Kaya, S.; Onguru, O.; Arslan, E.; Izci, Y. The effects of splenectomy on lipid peroxidation and neuronal loss in experimental spinal cord ischemia/reperfusion injury. Turk. Neurosurg. 2013, 23, 67–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Usul, H.; Cakir, E.; Cobanoglu, U.; Alver, A.; Peksoylu, B.; Topbas, M.; Baykal, S. The effects of tyrphostine Ag 556 on experimental spinal cord ischemia reperfusion injury. Surg. Neurol. 2004, 61, 45–54; discussion 54. [Google Scholar] [CrossRef]
- Musayeva, A.; Unkrig, J.C.; Zhutdieva, M.B.; Manicam, C.; Ruan, Y.; Laspas, P.; Chronopoulos, P.; Gobel, M.L.; Pfeiffer, N.; Brochhausen, C.; et al. Betulinic Acid Protects from Ischemia-Reperfusion Injury in the Mouse Retina. Cells 2021, 10, 2240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, M.; He, M.; Wang, X.; Sun, D.; Huang, Y.; Cheng, X.; Fu, J.; Cai, J.; Ma, T.; et al. TNFAIP3 Interacting Protein 3 Is an Activator of Hippo-YAP Signaling Protecting Against Hepatic Ischemia/Reperfusion Injury. Hepatology 2021, 74, 2133–2153. [Google Scholar] [CrossRef] [PubMed]
- Christou, C.D.; Tsoulfas, G. The Role of microRNA in Hepatic Ischemia/Reperfusion Injury. Microrna 2020, 9, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Dusabimana, T.; Kim, S.R.; Kim, H.J.; Park, S.W.; Kim, H. Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, Y.; Lu, J.; Zhang, W.; Zhu, Y.; Zhang, S.; Ma, G.; Jiang, P.; Zhang, W. PINK1-mediated mitophagy protects against hepatic ischemia/reperfusion injury by restraining NLRP3 inflammasome activation. Free Radic. Biol. Med. 2020, 160, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Rezq, S.; Hassan, R.; Mahmoud, M.F. Rimonabant ameliorates hepatic ischemia/reperfusion injury in rats: Involvement of autophagy via modulating ERK- and PI3K/AKT-mTOR pathways. Int. Immunopharmacol. 2021, 100, 108140. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Du, Y.; Qui, M.; Wang, M.; Chen, K.; Huang, Z.; Jiang, M.; Xiong, F.; Chen, J.; Zhou, J.; et al. Ophiopogonin B-induced autophagy in non-small cell lung cancer cells via inhibition of the PI3K/Akt signaling pathway. Oncol. Rep. 2013, 29, 430–436. [Google Scholar] [CrossRef]
- Shen, M.; Lu, J.; Dai, W.; Wang, F.; Xu, L.; Chen, K.; He, L.; Cheng, P.; Zhang, Y.; Wang, C.; et al. Ethyl pyruvate ameliorates hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway of apoptosis and autophagy. Mediat. Inflamm. 2013, 2013, 461536. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, F.; Chen, K.; Shen, M.; Dai, W.; Xu, L.; Zhang, Y.; Wang, C.; Li, J.; Yang, J.; et al. Hydrogen sulfide ameliorates ischemia/reperfusion-induced hepatitis by inhibiting apoptosis and autophagy pathways. Mediat. Inflamm. 2014, 2014, 935251. [Google Scholar] [CrossRef]
- Ohnishi, K.; Komohara, Y.; Fujiwara, Y.; Takemura, K.; Lei, X.; Nakagawa, T.; Sakashita, N.; Takeya, M. Suppression of TLR4-mediated inflammatory response by macrophage class A scavenger receptor (CD204). Biochem. Biophys. Res. Commun. 2011, 411, 516–522. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, L.; Zong, G.; Ma, K.; Zhu, X.; Zhang, H.; Li, N.; Yang, Q.; Bai, H.; Ben, J.; et al. Class A scavenger receptor promotes cerebral ischemic injury by pivoting microglia/macrophage polarization. Neuroscience 2012, 218, 35–48. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Zhuang, Y.; Li, N.; Zhu, X.; Hu, J.; Ben, J.; Yang, Q.; Bai, H.; Chen, Q. Class A scavenger receptor activation inhibits endoplasmic reticulum stress-induced autophagy in macrophage. J. Biomed. Res. 2014, 28, 213–221. [Google Scholar] [CrossRef]
- Ruan, Z.; Liang, M.; Deng, X.; Lai, M.; Shang, L.; Su, X. Exogenous hydrogen sulfide protects fatty liver against ischemia-reperfusion injury by regulating endoplasmic reticulum stress-induced autophagy in macrophage through mediating the class A scavenger receptor pathway in rats. Cell Biol. Int. 2019, 44, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Hartke, J.; Johnson, M.; Ghabril, M. The diagnosis and treatment of hepatocellular carcinoma. Semin. Diagn. Pathol. 2017, 34, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.W.; Knox, J. Hepatocellular carcinoma in the era of immunotherapy. Curr. Probl. Cancer 2018, 42, 40–48. [Google Scholar] [CrossRef]
- Clark, T.; Maximin, S.; Meier, J.; Pokharel, S.; Bhargava, P. Hepatocellular Carcinoma: Review of Epidemiology, Screening, Imaging Diagnosis, Response Assessment, and Treatment. Curr. Probl. Diagn. Radiol. 2015, 44, 479–486. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; El-Serag, H.B. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin. Gastroenterol. Hepatol. 2015, 13, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Garshell, J.; Miller, D.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2010; National Cancer Institute: Bethesda, MD, USA, 2013. [Google Scholar]
- Li, Q.; Ni, Y.; Zhang, L.; Jiang, R.; Xu, J.; Yang, H.; Hu, Y.; Qiu, J.; Pu, L.; Tang, J.; et al. HIF-1alpha-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct. Target. Ther. 2021, 6, 76. [Google Scholar] [CrossRef]
- Sun, R.; Zhai, R.; Ma, C.; Miao, W. Combination of aloin and metformin enhances the antitumor effect by inhibiting the growth and invasion and inducing apoptosis and autophagy in hepatocellular carcinoma through PI3K/AKT/mTOR pathway. Cancer Med. 2020, 9, 1141–1151. [Google Scholar] [CrossRef]
- Wang, S.S.; Chen, Y.H.; Chen, N.; Wang, L.J.; Chen, D.X.; Weng, H.L.; Dooley, S.; Ding, H.G. Hydrogen sulfide promotes autophagy of hepatocellular carcinoma cells through the PI3K/Akt/mTOR signaling pathway. Cell Death Dis. 2017, 8, e2688. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, B.R.; Wang, Y.G. Role of autophagy in tumorigenesis, metastasis, targeted therapy and drug resistance of hepatocellular carcinoma. World. J. Gastroenterol. 2018, 24, 4643–4651. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, M.R.; Szabo, C. Hydrogen Sulfide and Cancer. Handb. Exp. Pharmacol. 2015, 230, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Si, W.; Wang, M.; Lv, S.; Ji, A.; Li, Y. Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide 2015, 50, 38–45. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, Z.; Chen, L.; Huang, W.; Zhang, F.; Wang, L.; Wang, Y.; Cao, P.; Zheng, S. Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy. Redox Biol. 2020, 36, 101600. [Google Scholar] [CrossRef]
- Xie, L.; Yu, S.; Yang, K.; Li, C.; Liang, Y. Hydrogen Sulfide Inhibits Autophagic Neuronal Cell Death by Reducing Oxidative Stress in Spinal Cord Ischemia Reperfusion Injury. Oxid. Med. Cell. Longev. 2017, 2017, 8640284. [Google Scholar] [CrossRef]
- Honma, Y.; Sato-Morita, M.; Katsuki, Y.; Mihara, H.; Baba, R.; Hino, K.; Kawashima, A.; Ariyasu, T.; Harada, M. Trehalose alleviates oxidative stress-mediated liver injury and Mallor-Denk body formation via activating autophagy in mice. Med. Mol. Morphol. 2021, 54, 41–51. [Google Scholar] [CrossRef]
- Greabu, M.; Totan, A.; Miricescu, D.; Radulescu, R.; Virlan, J.; Calenic, B. Hydrogen Sulfide, Oxidative Stress and Periodontal Diseases: A Concise Review. Antioxidants 2016, 5, 3. [Google Scholar] [CrossRef]
- Guo, J.M.; Xing, H.J.; Cai, J.Z.; Zhang, H.F.; Xu, S.W. H2S exposure-induced oxidative stress promotes LPS-mediated hepatocyte autophagy through the PI3K/AKT/TOR pathway. Ecotoxicol. Environ. Saf. 2021, 209, 111801. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Sun, J.; Fei, A.; Gao, C.; Pan, S.; Wu, Z. Hydrogen sulfide treatment alleviated ventilator-induced lung injury through regulation of autophagy and endoplasmic reticulum stress. Int. J. Biol. Sci. 2019, 15, 2872–2884. [Google Scholar] [CrossRef] [PubMed]
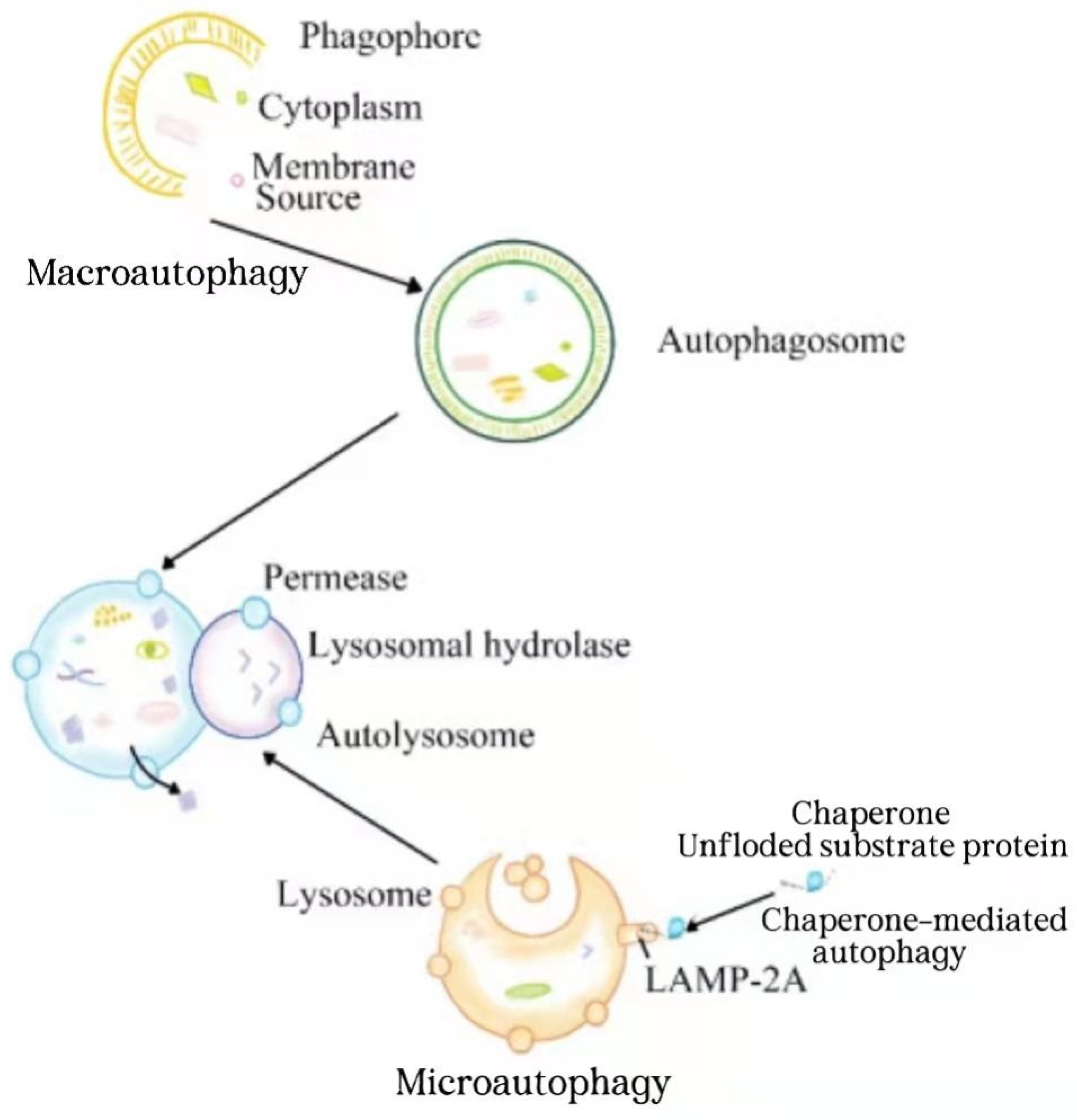
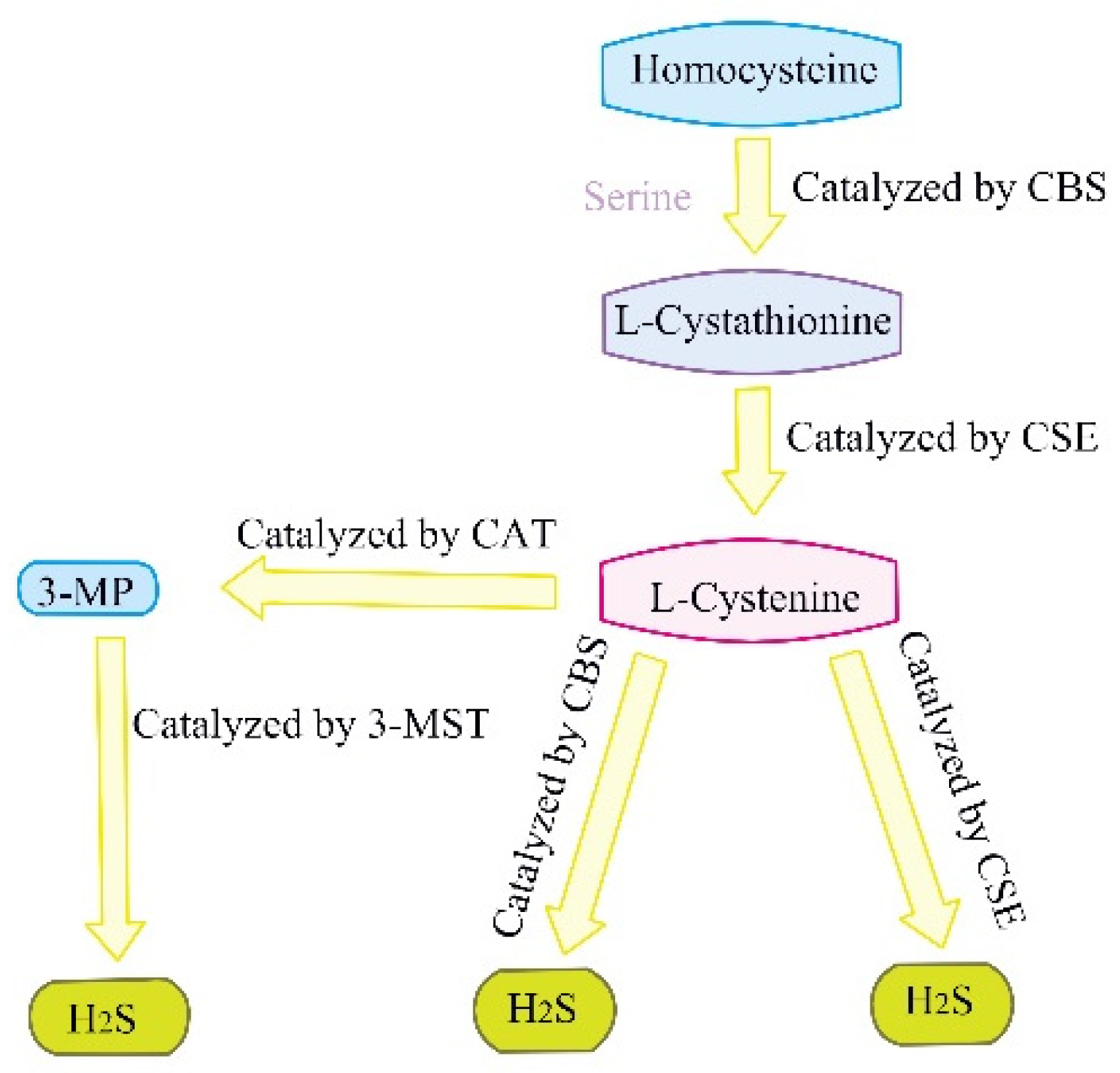

| The Type of Liver Disorder | The Role Hydrogen Sulfide Regulation of Autophagy | Experimental Model | References |
|---|---|---|---|
| Nonalcoholic fatty liver disease (NAFLD) | H2S improved NAFLD through autophagy promotion by activating AMPK/mTOR pathway | male C57BL/6 mice model of NAFLD | [43] |
| NAFLD | H2S promoted autophagy through the inhibition of reactive oxygen species (ROS)-mediated phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) | Mouse/mouse hepatocytes model of NAFLD | [49] |
| hepatic steatosis | H2S improved hepatic steatosis through promotion of ULK1 sulfhydration-mediated autophagy | Mouse model of NAFLD | [56] |
| NAFLD | Exercise promoted H2S bioavailability and autophagy to improve HFD-induced NAFLD | male C57BL/6J mice model of NAFLD | [67] |
| Hepatic ischemia-reperfusion injury(HIRI) | H2S improved hepatic I/R injury through autophagy reduction by inhibiting JNK pathway, which needs to be further confirmed | Mouse/mouse hepatocytes model of HIRI | [78] |
| HIRI | H2S improved fatty liver I/R injury through autophagy promotion via the inhibition of SRA pathway, which requires to be further confirmed | Sprague Dawley rats model of HIRI | [82] |
| hepatocellular carcinoma | H2S improved hepatocellular carcinoma through autophagy promotion via the inhibition of PI3K/AKT/mTOR pathway | hepatocellular carcinoma (HCC): HepG2 and HLE | [91] |
| liver injury | high concentrations of H2S induced liver injury through autophagy promotionby inhibiting PI3K/Akt/TOR pathway | chicken hepatocytess | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Ding, Y.; Liu, H.; Sun, M.; Chen, C.; Yang, Y.; Wang, H. The Role of Hydrogen Sulfide Regulation of Autophagy in Liver Disorders. Int. J. Mol. Sci. 2022, 23, 4035. https://doi.org/10.3390/ijms23074035
Lu X, Ding Y, Liu H, Sun M, Chen C, Yang Y, Wang H. The Role of Hydrogen Sulfide Regulation of Autophagy in Liver Disorders. International Journal of Molecular Sciences. 2022; 23(7):4035. https://doi.org/10.3390/ijms23074035
Chicago/Turabian StyleLu, Xueqin, Yueming Ding, Huiyang Liu, Mengyao Sun, Chaoran Chen, Yihan Yang, and Honggang Wang. 2022. "The Role of Hydrogen Sulfide Regulation of Autophagy in Liver Disorders" International Journal of Molecular Sciences 23, no. 7: 4035. https://doi.org/10.3390/ijms23074035
APA StyleLu, X., Ding, Y., Liu, H., Sun, M., Chen, C., Yang, Y., & Wang, H. (2022). The Role of Hydrogen Sulfide Regulation of Autophagy in Liver Disorders. International Journal of Molecular Sciences, 23(7), 4035. https://doi.org/10.3390/ijms23074035






