1. Introduction
European black elderberries and extracts thereof have been reported to reduce cold and flu-associated symptoms during viral infections and to restrict the time of infections [
1,
2,
3]. Several randomized, placebo-controlled clinical trials, have confirmed such effects during the last decades [
4].
However, the precise underlying mode of action(s) is/are still unclear and both, direct antiviral activities, as well as immune-mediated mechanisms, could play an important role. Regarding the first hypothesis, several authors described a direct antiviral effect showing that the main anthocyanin present in black elderberry, that is, cyanidin-3-sambubiocide, can bind and inhibit the active pocket of the viral enzyme neuraminidase and therefore inhibit the viral replication process in vitro [
5]. In addition, Roschek et al., reported that in vitro flavonoids present in elderberry fruits can directly bind to H1N1 influenza virus particles, thereby inhibiting host cell entry [
6]. This direct antiviral activity of secondary plant components may be very important for the fast and short-term elimination of viral particles; however, for the induction of long-lasting antiviral responses, the immune system must be activated via its innate and adaptive constituents. Therefore, it is very intriguing to speculate that specific components present in elderberry extracts may be able to stimulate/modulate the immune system of the host. Supporting this hypothesis, it has been reported, that in addition to polyphenols,
Sambucus nigra fruits also contain peptic polysaccharides that have been shown to influence the immune system via the activation of macrophages [
7,
8].
While many extracts at an industrial scale are manufactured using alcoholic extraction techniques followed by purification methods to enrich anthocyanins at high levels, it can be assumed that a great part of the polysaccharide fraction is lost during such a process. In this work, we compare the immunomodulatory ability of two different extracts; a water extract produced using ultrafiltration and a conventional ethanolic extract.
For the induction of specific anti-viral immune responses, DCs are absolutely vital since they have the potential to activate also naïve T cells [
9]. DCs connect the innate with the adaptive immune system, and under steady-state conditions, immature DCs reside in peripheral tissues, waiting for the encounter with pathogens including viruses. After the activation via, for example, inflammatory signals, immature DCs (iDCs) change their phenotype and function and become mature DCs (mDCs) [
10]. mDCs increase the antigen processing and presenting capabilities, which goes along with the increased expression of MHC class I and class II, costimulatory molecules such as CD80, CD86 and CD40 as well as CD83, which is an important immune check point molecule expressed by mDCs [
11].
Considering, that DCs are crucial players in the induction of effective antiviral immune responses, and knowing that elderberry-derived substances have been reported to strengthen the protection against viral infections, we investigated if polysaccharide enriched elderberry extract fractions could modulate the phenotype and enhance the immune stimulatory function of DCs. To investigate the effects of elderberry extracts on the DC phenotype, we used FACS analyses. To assess the DC-specific T-cell-stimulatory capacity, the Mixed Leukocyte Reaction (MLR) assay was employed. Furthermore, the expression of inflammatory cytokines present in the cell-free supernatant of DC-T cell co-cultures was analyzed using the Cytokine Bead Assay. Thus, within this study, we investigated elderberry-induced effects with respect to the phenotypic expression of DC-specific cell surface molecules, the T cell stimulatory capacity of mDCs as well as the induced cytokine profile in DC-T cell co-cultures.
3. Discussion
As previously reported, black elderberry has been and is used extensively in a natural way to fight against coughs, colds, and flu [
1]. There is also evidence that black elderberry positively influences the outcome of viral upper respiratory infections; however, the main mode of action is still unclear [
2,
3,
4]. Two scenarios could be responsible for these reported effects: (i) direct antiviral properties, or (ii) immune-enhancing properties of components present in elderberries. Indeed, some studies reported a direct antiviral mechanism, thereby inhibiting viral replication, while others showed that the elderberry extract exhibits strong immunomodulatory properties [
5,
8,
13,
14,
15,
16].
In this study, we analyzed possible immune-modulatory effects in vitro and the results clearly show that an anthocyanin standardized black elderberry extract (ElderCraft®) containing a bioactive polysaccharide fraction, is a potent immune stimulator.
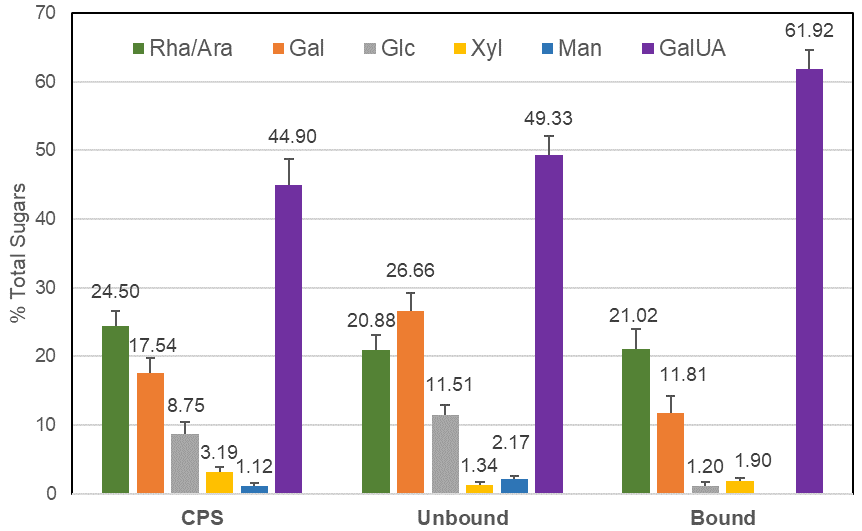
The elderberry extracts we analyzed included a crude polysaccharide enriched fraction (CPS) isolated from the EC15 extract by ethanol precipitation. This CPS fraction was 12-fold enriched in polysaccharides and the anthocyanin content was reduced to >1%, compared to the original EC15 extract. Using anion exchange chromatography, the CPS fraction could be further separated into UNBOUND and BOUND fractions. The monosaccharide composition of the CPS fraction, which was rich in galacturonic acid as well as rhamnose, galactose, and arabinose, suggests the presence of pectic polysaccharides. After ion exchange, the BOUND fraction revealed a composition similar to pectic components as previously reported by [
7]. The UNBOUND fraction contained higher amounts of mannose and galactose, in comparison to the BOUND fraction, which suggests an enrichment of neutral polysaccharides. The substantial amount of galacturonic acid in the unbound fraction strongly indicated that the anion exchange column was overloaded and pectic components were unable to bind. Therefore, the CPS, the BOUND, and the UNBOUND fractions contained pectic polysaccharides.
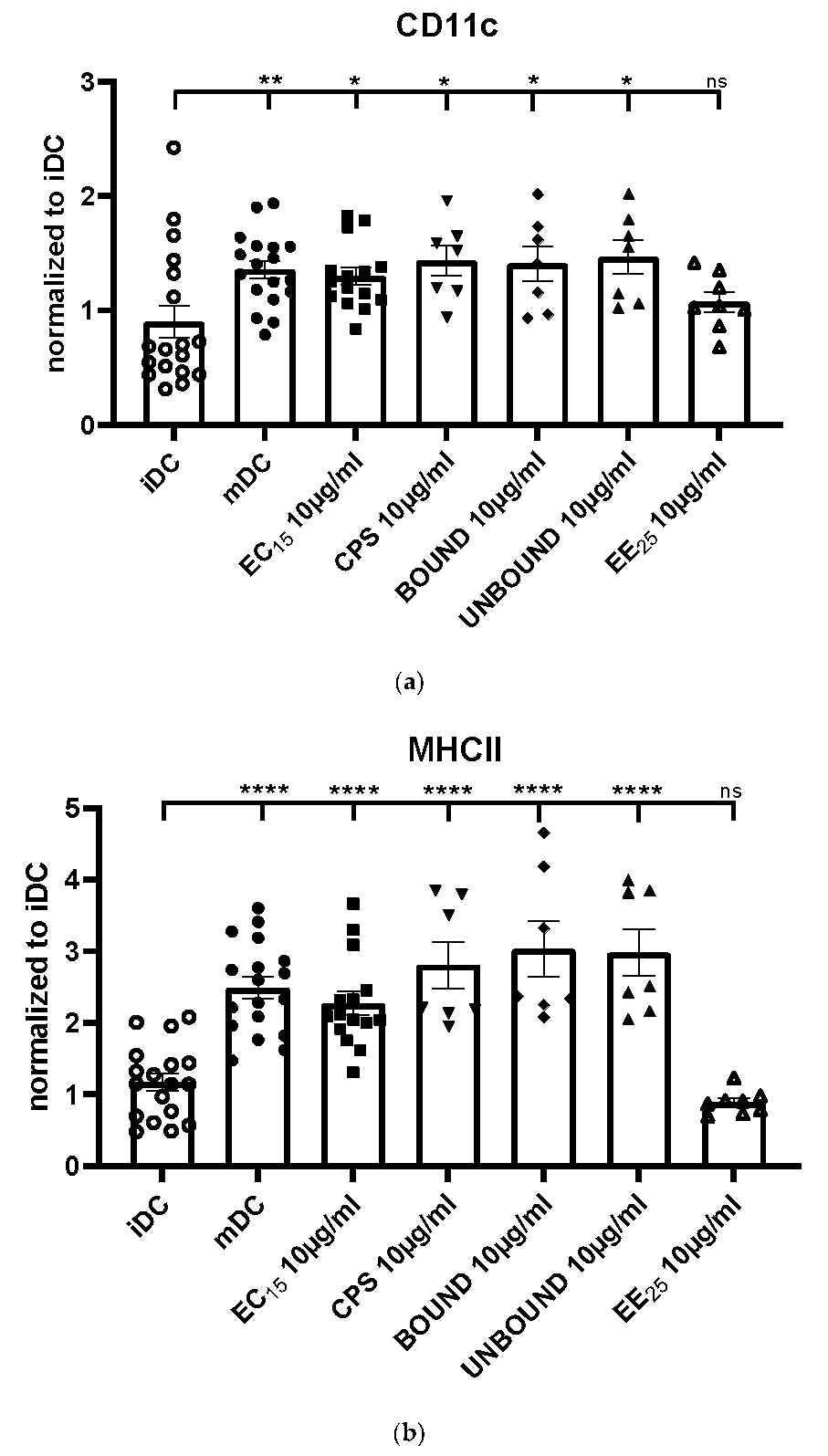
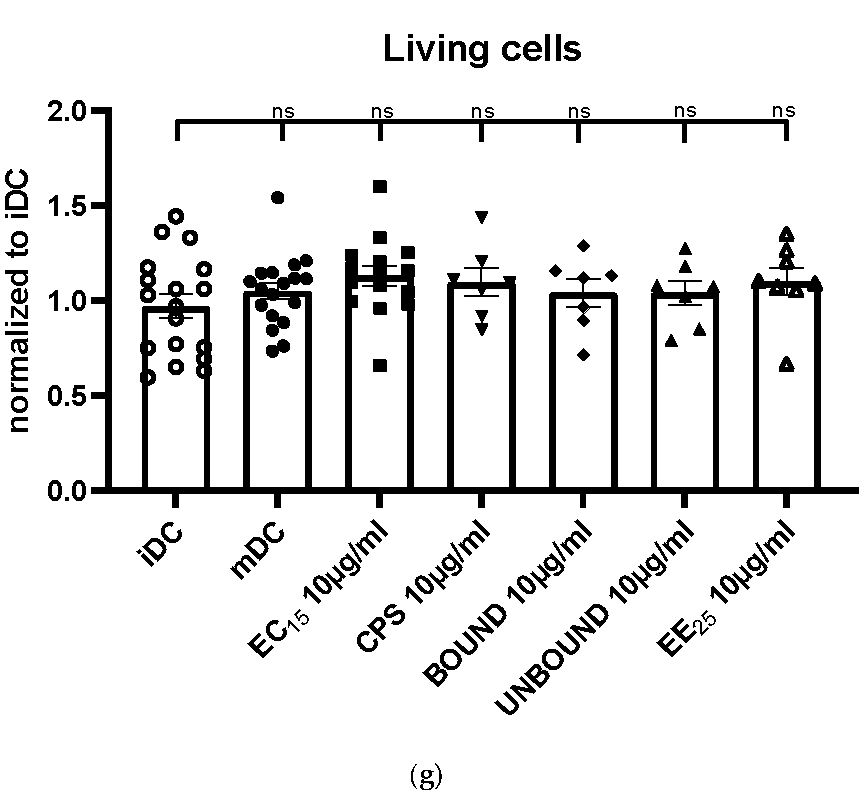
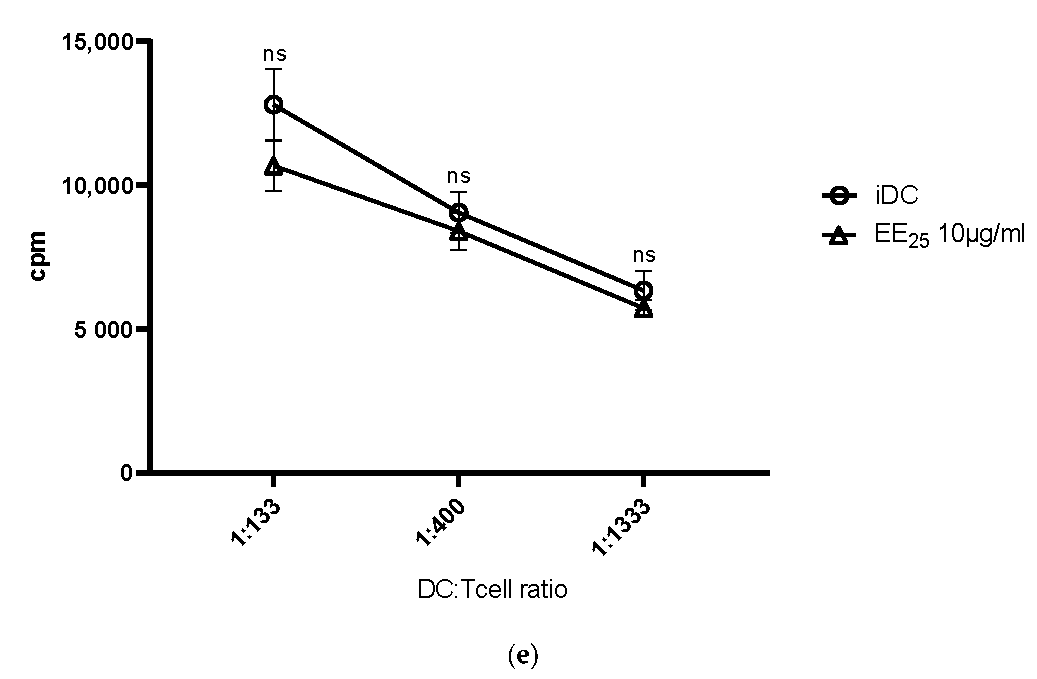
Our results clearly show that all elderberry samples, except for extract EE25, that is, EC15, CPS, BOUND, and UNBOUND, induced a prominent phenotypic maturation of immature DCs. However, extract EE25 had the highest levels of anthocyanins (>2-fold higher than extract EC15) and the CPS fraction had anthocyanin levels <1% of its parent EC15 extract. This strongly suggests that anthocyanins were not correlated with effectiveness.
The maturation of immature DCs is reflected by the upregulation of the surface molecule CD11c, an important integrin to induce adaptive immune responses [
17]. Also, MHC class II molecules were strongly induced by the elderberry extract EC15, and the derived polysaccharide-rich fractions CPS, BOUND, and UNBOUND. Since these molecules present the processed antigenic peptides to the T cell receptor (TCR) expressed by antigen-specific T cells, their presence is an absolute prerequisite to activating virus-specific T cells [
11]. Again, EE25 did not induce upregulation of MHC class II molecules.
To potently activate virus-specific T cells, co-stimulatory molecules such as CD80, CD86 as well as CD40 expressed only by mature DCs are absolutely vital to induce potent immune responses. In contrast, immature DCs do not express co-stimulatory molecules and instead of inducing efficient immune responses, they induce tolerogenic mechanisms [
9]. Noteworthily, all elderberry fractions, except EE25, induced a statistically highly significant upregulation of these costimulatory molecules. Furthermore, also the expression of the CD83 molecule, a very important immune checkpoint only expressed by mature DCs [
11], was highly induced by the elderberry extracts, except by EE25. Thus, these data clearly show that the elderberry extracts EC15, CPS, BOUND and UNBOUND induce the phenotypic maturation of DCs, which is an absolute prerequisite for the induction of potent T cell responses.
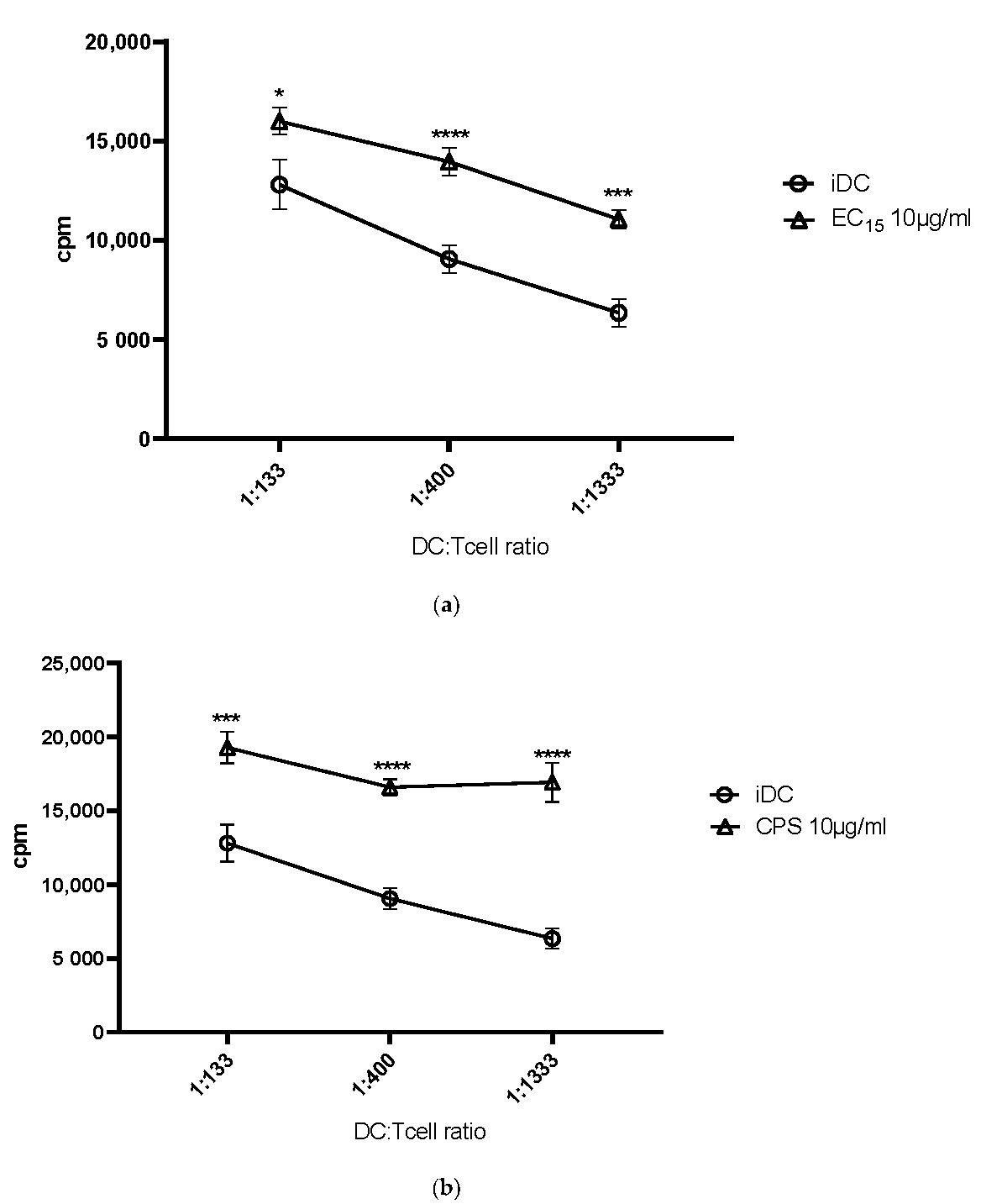
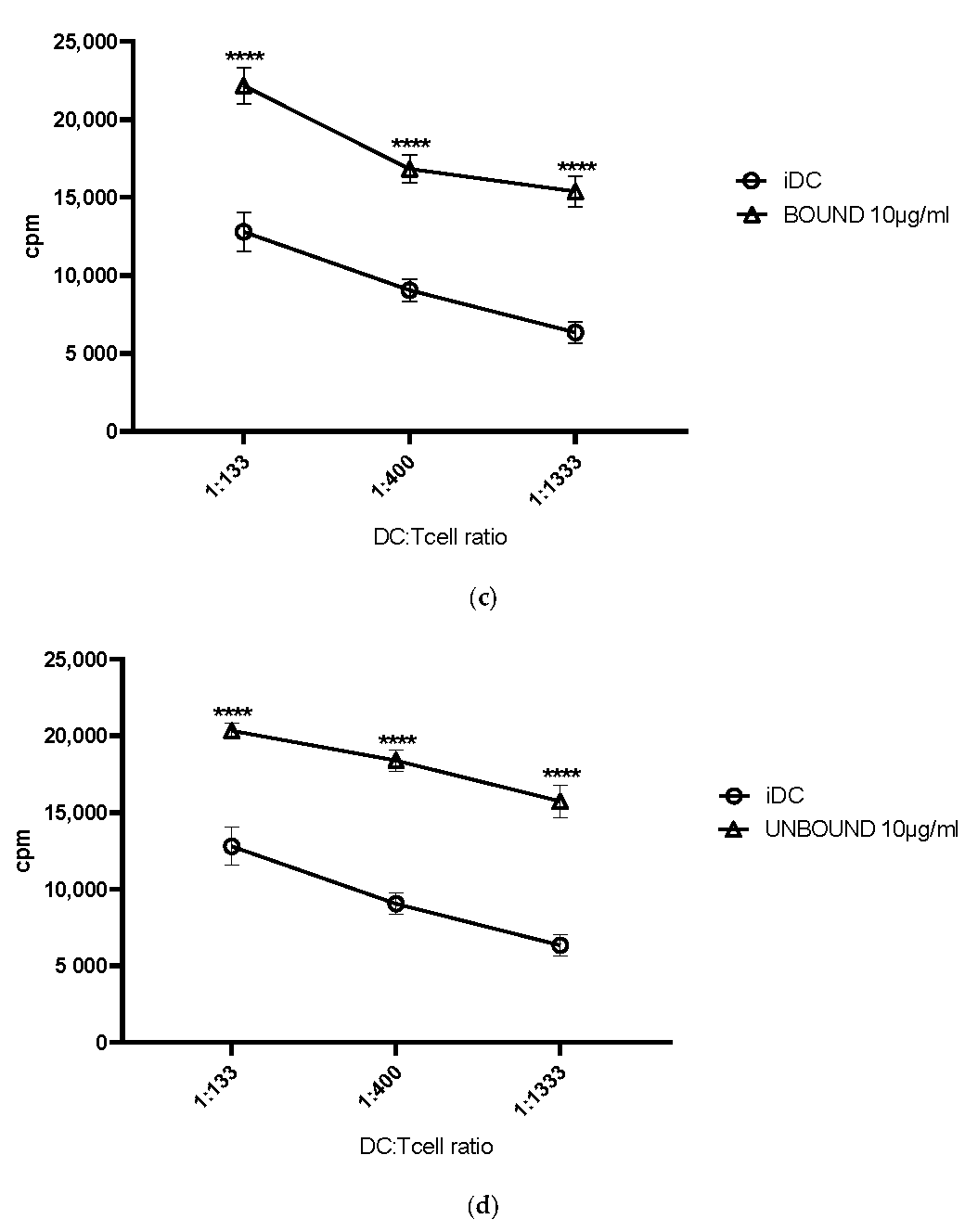
The next obvious question was, if these observed phenotypic changes, leading to a strong DC maturation, would also translate into a better T cell stimulation. To assess this, we used the MLR assay. Indeed, all elderberry extracts, with the exception of EE25, induced a statistically highly significant DC-mediated T cell stimulation when compared to immature DCs. The stimulation index was comparable to, for example, LPS or cocktail matured DCs, which we and others use to mature DCs [
11,
18].
A typical functional property of mature DC-T cells interactions, and characteristic of MLR assays, is the production of pro-inflammatory cytokines. Thus, using CBA we assessed the cytokine secretion into the supernatant, induced by different elderberry extract matured DCs in the co-culture with T cells. Interestingly, especially the extracts CPS, BOUND, and UNBOUND induced a statistically significant expression of the cytokines IL-6, TNF-α as well as IFN-γ. These inflammatory cytokines are mechanistically very important for the induction of potent T-cell-mediated immune responses [
19]. Therefore, these data support the hypothesis that the elderberry derived extracts EC15, CPS, BOUND and UNBOUND modulate the immune system, in order to fight against viral infections, as reported, for example, by Ho et al. before [
7,
8].
These authors described that elderberry-derived pectic polymers not only increase the stimulatory activity of macrophages but also induce a strong and dose-dependent complement fixating activity of these cells [
7]. In subsequent work, they suggested that anthocyanins and procyanidins induce complement activation and modulate NO production in macrophages [
8]. Based on these data, we hypothesize that polysaccharides, as well as anthocyanins, may contribute to the potent immune-modulatory properties of the elderberry extracts. In addition, Kinoshita et al. reported murine data showing that the oral uptake of elderberry juice induced a dose-dependent stimulatory effect, leading to increased titers of virus-neutralizing antibodies in bronchoalveolar lavage fluids as well as in the serum of the treated mice [
15]. Since DCs are also important for the activation of B cells and thus for the production of antibodies [
20], it is conceivable to speculate that the here reported elderberry extract (EC15), and its fractions, that is, CPS, BOUND and UNBOUND, could also enhance antibody production against, for example, different viruses, in vivo.
Of note, the elderberry extract EC15 was obtained using a water extraction protocol followed by an ultrafiltration process to increase the anthocyanin and polyphenol concentration. We compared this water extract and its derived polysaccharide-rich fractions with the commercially purchased ethanol extract EE25. However, the ethanol-derived EE25 fraction had no potential to modulate and increase the investigated immune responses. This observation is in line with a recent report investigating the effect of an ethanolic extract on cytokine production [
21]. Interestingly, the authors actually observed a reduced expression of TNF-α and IFN-γ, which fits with our results, as we did not see a significant increase in TNF-α and IFN-γ production when cells were incubated with the ethanol extract EE25 compared to the water extract EC15, and derived fractions CPS, BOUND and UNBOUND. Thus, we conclude that the polysaccharide content plays an important role in induced immune-stimulatory activities.
In summary, the elderberry extract EC15, and its fractions CPS, BOUND, and UNBOUND, but not EE25, induced (i) a strong DC maturation, (ii) a potent DC-mediated T cell stimulation, and (iii) a significant induction of inflammatory cytokines. Thus, they have an interesting potential to stimulate, for example, antiviral immune responses also in vivo.
4. Materials and Methods
4.1. Plant Material
European black elderberry (Sambucus nigra L.) extract (ElderCraft®) was provided by IPRONA AG/SPA. ElderCraft® is a full spectrum water extract, standardized to 15% anthocyanins (EE15) and containing up to 10% soluble fiber from the elderberry fruit. The ethanolic black elderberry extract (EE25) was provided by Medtrue Enterprise Co., Ltd., Nanjing, China, and is standardized to 25% anthocyanins.
4.2. HPLC Analysis of Elderberry Extracts
The HPLC was performed as published by IFU No. 71 as established by International Fruit and Vegetable Juice Association with modifications [
22]. Anthocyanin concentration was Cyanidin-chloride was used as standard and a conversion factor of 1.393 was used to convert the result from cyanidin-chloride into cyanidin-3-glucoside.
4.3. Fractionation and Purification Methods
To isolate polysaccharide fractions, 100 g of dried elderberry extract EC15 was dissolved in 2.5 L of ultra-pure water (UPW) with stirring, then chilled ethanol was added to 75% (
v/
v). The solution was stirred vigorously, covered with cling film, and left overnight at 5 °C in a cold room. The precipitate was collected by successive centrifugations (20 min at 10,000×
g at 5 °C) in a Sorvall RC5C Plus ultrafuge (Fisher Scientific Ltd., Loughborough, UK). The pellets were resuspended in UPW, and samples were taken for total sugar and total anthocyanin tests. The supernatants were combined and retained. The pelleted material was then re-precipitated at 75% (
v/
v) ethanol using the same procedure. The second precipitate was resuspended in UPW then dialyzed overnight against an excess of UPW. The crude polysaccharide sample (CPS) was frozen and then freeze-dried. Samples were assessed for anthocyanin content [
23] and polysaccharide content using the phenol sulfuric acid method [
24].
For ion exchange, 500 mg of the sample was resuspended in 10 mL of 100 mM Tris HCl pH 8.0 and applied to a 100 mL column of DEAE-Sepharose equilibrated in the same buffer. The column was eluted with 2 × 50 mL volumes of Tris buffer to give the unbound fraction then sequentially with 50 mL of 0.25 M NaCl, 0.5 M NaCl then 3 × 50 mL of 1 M NaCl in Tris buffer.
The procedure was repeated eight times and the fractions were precipitated with 75% (v/v) ethanol, resuspended in UPW, and freeze-dried. The bulk of the polysaccharides were recovered in the UNBOUND fraction and in the BOUND fraction 1 (0.25 M NaCl).
4.4. Determination of Carbohydrate Composition
The CPS, UNBOUND fraction 1, and 0.25 M BOUND fractions were acid hydrolyzed by heating in 2 M trifluoracetic acid at 120 °C for 1 h. After drying in a speed-vac, their monosaccharide composition was determined using high-performance anion-exchange chromatography (HPAEC) coupled with pulsed electrochemical detection (ECD), as described previously [
25]. Quantification was carried out by comparing the peak areas against standard curves for specific monosaccharides.
4.5. Sample Preparations for the Treatment of DCs and MLR Assays
The spray-dried elderberry extract, standardized to 15% anthocyanins (EC15), extract EE25 and the CPS, BOUND, UNBOUND fractions were reconstituted in 1 × PBS, sterile filtered using a Millex-GP 0.22 µm PES Membrane (Merck Millipore Ltd., Dublin, Ireland) and stored at +4 °C.
4.6. Generation of Human Monocyte-Derived Dendritic Cells
To generate immature DCs (iDCs) from human monocytic precursor cells leukoreduction system chambers (LRSCs) were used as reported [
26]. In brief, peripheral blood mononuclear cells (PBMCs), donated by healthy volunteers were enriched by gradient centrifugation using Lymphoprep (Axis-Shield/Alere Technologies AS, Oslo, Norway). In the next step, PBMCs were allowed to adhere to plastic tissue culture dishes (Falcon, Fisher Scientific, Erlangen, Germany) for 90 min at 37 °C.
Afterward, the non-adherent fraction (NAF), containing T cells was rinsed from the plates, centrifuged, and frozen at −80 °C in 11% human serum albumin (HSA; CSL Behring, King of Prussia, PA, USA), 10% glucose (Merck, Darmstadt, Germany) and 20% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA), for subsequent co-culture experiments.
The mononuclear cells that had adhered to the plastic were inoculated for six days in a medium consisting of RPMI 1640 (Lonza, Basel, Switzerland), supplemented with 1% (v/v) heat-inactivated human serum type AB (Sigma-Aldrich), 1% (v/v) penicillin streptomycin L-glutamine (Sigma-Aldrich) and 10 mM HEPES (Lonza), in the presence of 800 IU/mL (day 0) or 400 IU/mL (day 3 and day 5) recombinant human GM-CSF and 250 IU/mL (day 0, 3 and 5) recombinant human IL 4 (both from Miltenyi Biotec, Bergisch Gladbach, Germany).
Subsequently, cells were treated with 10 µg/mL of spray-dried ultrafiltrated dry elderberry extract EC15, CPS, BOUND, UNBOUND, and EE25, respectively, for 24 h. As maturation control iDCs were treated with 100 ng/mL LPS (Sigma Aldrich, Darmstadt Germany).
4.7. Mixed Lymphocytes Reaction (MLR)
On day 7, the above described and pre-treated human iDCs were analyzed regarding their phenotype and function. On day 7, pre-treated iDCs were inoculated at different ratios with 4 × 10
5 allogeneic NAF cells, and ultrafiltrated elderberry extract EC15, CPS, BOUND, UNBOUND, and EE25 were added freshly, at the same concentration as used on day 6. As control, untreated iDC were also co-cultured with 4 × 10
5 allogeneic NAF cells, without the addition of elderberry-derived substances. Subsequently, cells were cultured further for 72 h in 96-well cell culture plates (Falcon, Fisher Scientific), in a total volume of 200 µL/well and 10 µg/mL of elderberry-derived substances. Three days later, cell-free culture supernatants were collected and analyzed for their cytokine content [
26]. To investigate the T cell stimulatory potential of elderberry treated DCs, cells were pulsed with 3H-thymidine (1 μC/well; PerkinElmer, Rodgau, Germany) for 16−20 h, and the allogeneic T cell proliferation was assessed using a Wallac 1420 Victor2 Microplate Reader (PerkinElmer). As a control, NAF cells were treated with DynabeadsTM Human T-Activator CD3/CD28 (Thermo Fisher Scientific, Waltham, MA, USA).
4.8. Cytometric Bead Array (CBA)
To analyze the expression of the pro-inflammatory cytokines IL-6, TNF-a, and IFN-g within the supernatant of DC-T cell co-cultures, the cytometric beads array (CBA) LEGENDplex™ HU Inflammation Cytokine Panel (BioLegend, San Diego, CA, USA), was used according to the manufacturer’s instructions. Briefly, the HU Inflammation Cytokine Panel is a bead-based multiplex assay panel, using fluorescence-encoded beads. Each bead has specific antibodies on the surface, which binds the specific cytokine. The amount of cytokines was assessed by FACS.
4.9. Flow Cytometric Analyses
To characterize human DCs on a phenotypic level, we used the subsequent monoclonal antibodies (all purchased from BioLegend, unless stated otherwise): CD11c-APC (clone 3.9), CD14-BV510 (clone M5E2), CD40-PECy7 (clone 5C3), CD80-BV421 (clone 2D10) CD83-PE (clone HB15e), CD86-FITC (clone FUN-1; BD) and MHCII-APCCy7 (clone L243). For dead cell discrimination, 7-AAD viability staining solution (Thermo Fisher Scientific) was added directly to the surface staining. The cell surface staining was performed for 30 min in the dark at 4°C using Dulbecco’s phosphate-buffered saline (DPBS).
4.10. Statistical Analyses
For statistical analyses, we worked with GraphPad Prism 8.3, using the ordinary one-way ANOVA (
Figure 2 and
Figure 4), and the 2 way ANOVA
Figure 3. Data presented mean values including the SEM. *
p < 0.05; **
p < 0.01; ***
p < 0.001; and ****
p < 0.0001 were considered statistically significant.