Co-Application with Tannic Acid Prevents Transdermal Sensitization to Ovalbumin in Mice
Abstract
:1. Introduction
2. Results
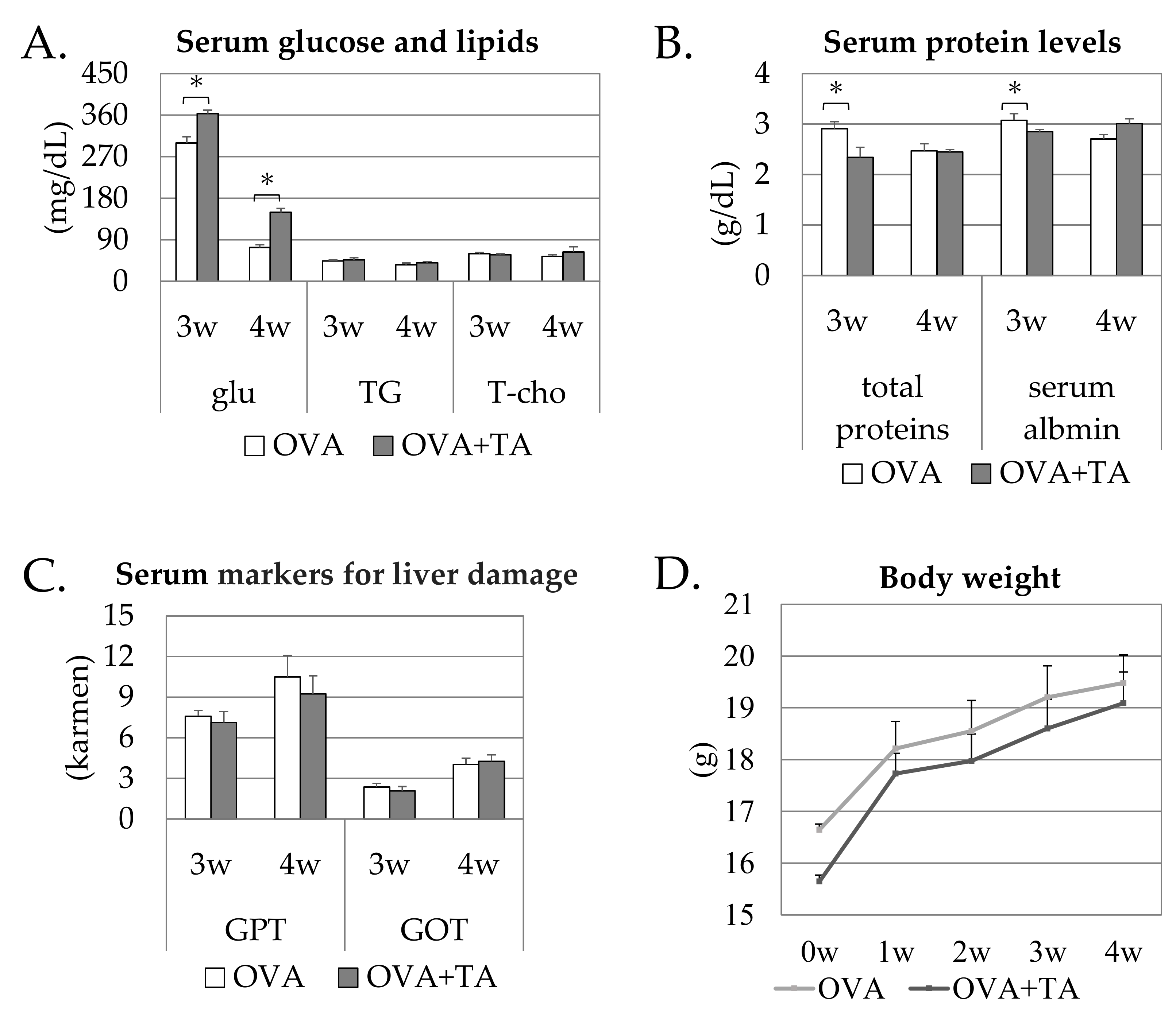
2.1. Effects of the Topical Application of OVA and TA on Growth and Serum Parameters
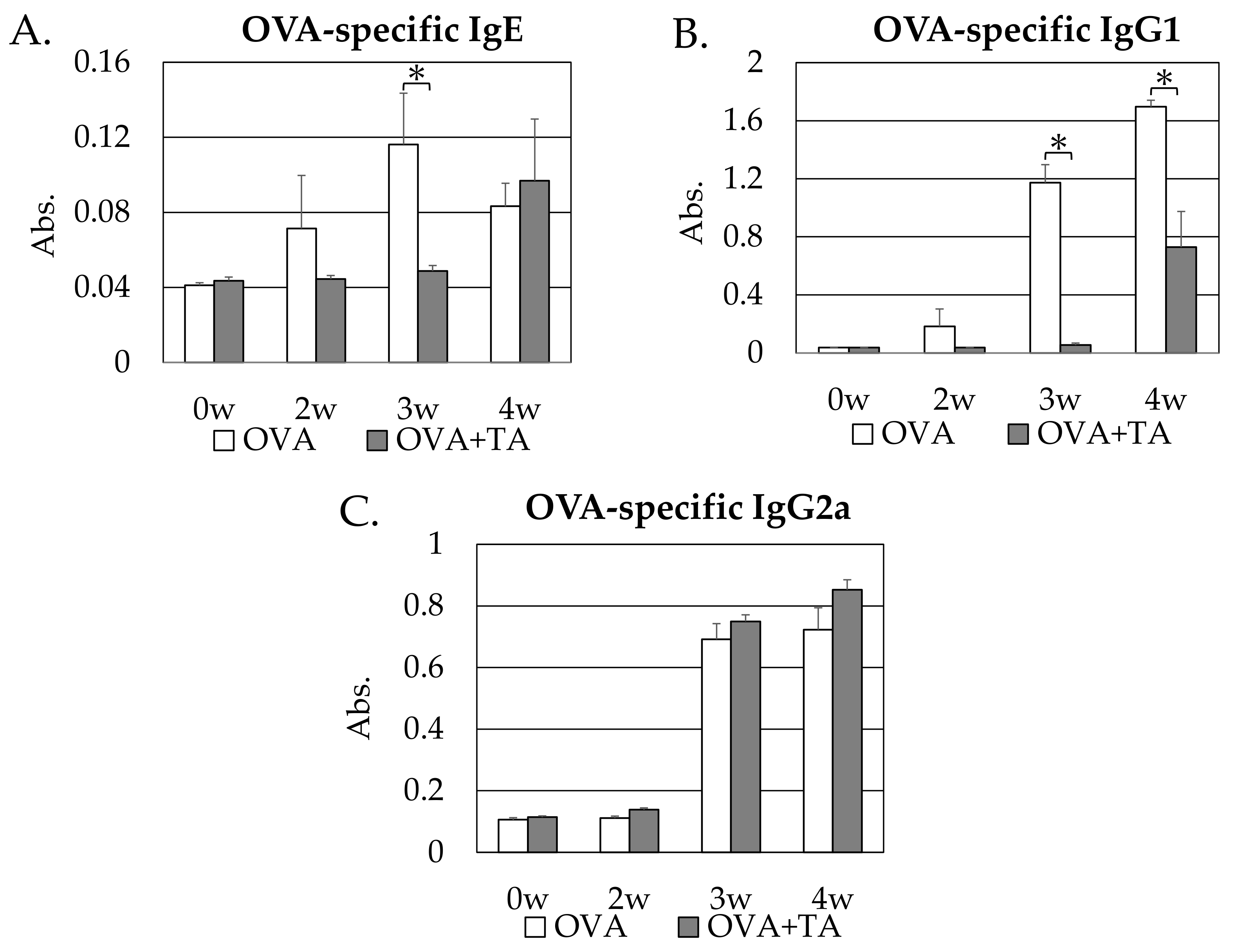
2.2. Effect of Co-Application of TA and OVA on OVA-Specific IgE, IgG1 and IgG2a Production
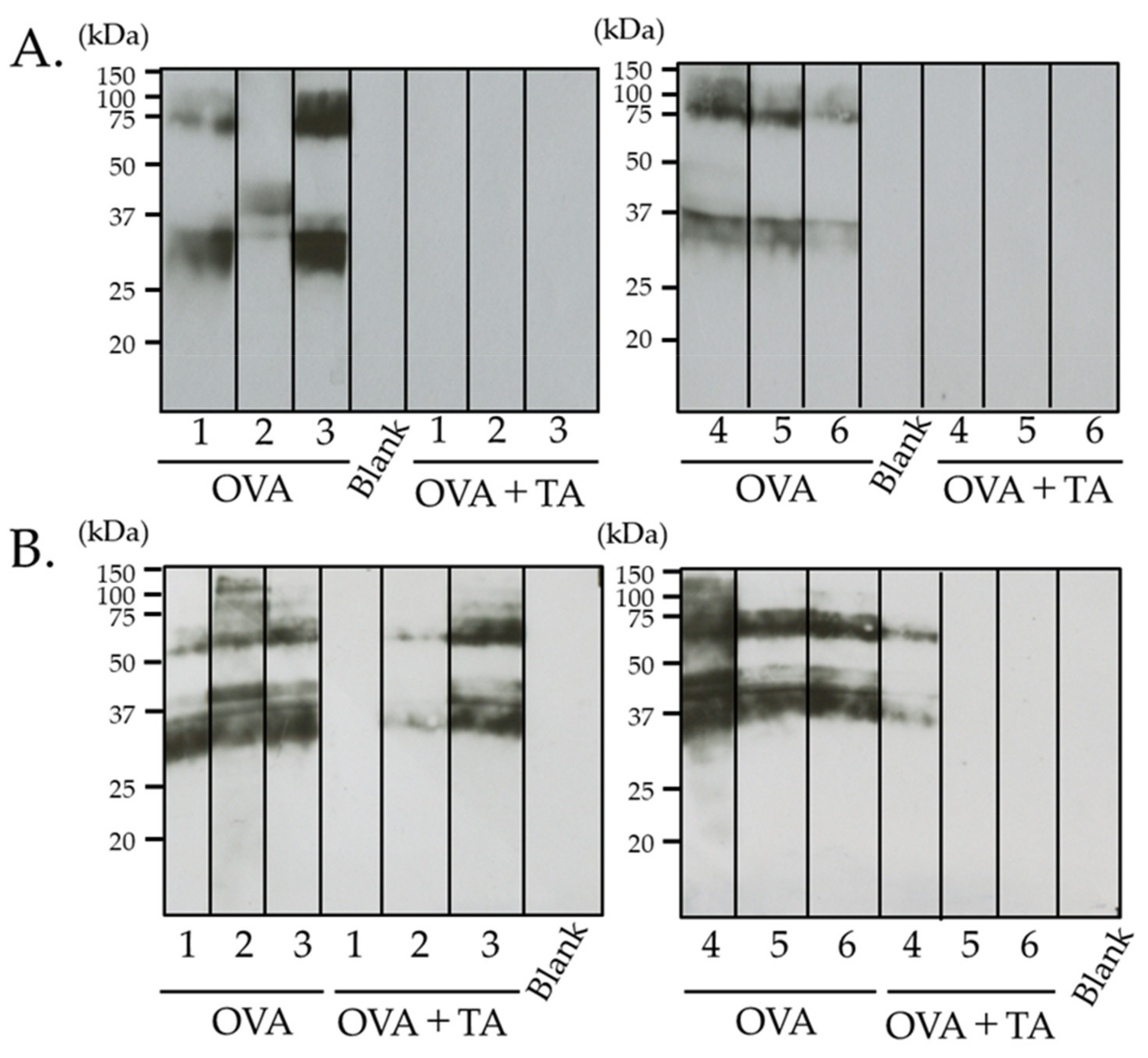
2.3. Effect of Co-Application of TA and OVA on OVA-Specific IgG1 Production
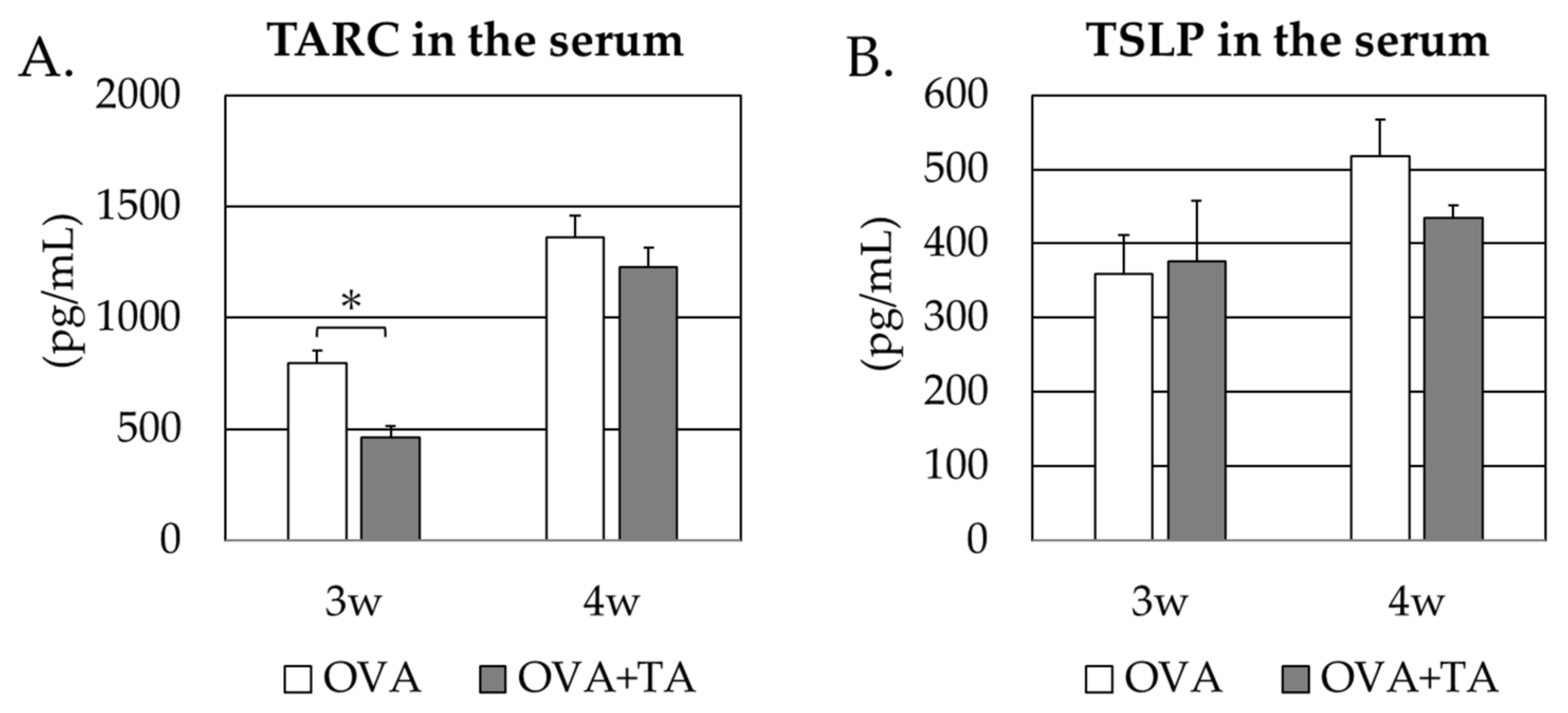
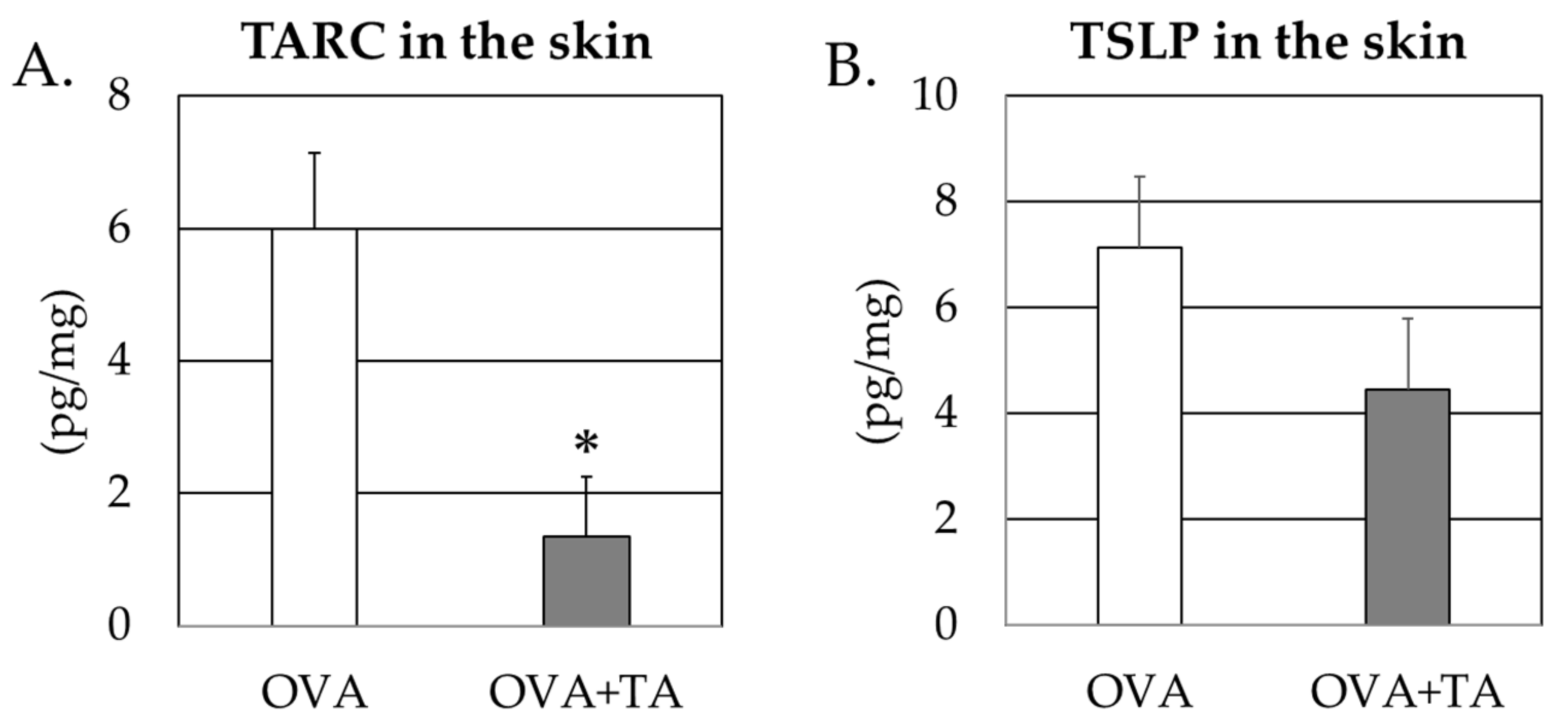
2.4. Effect of Co-Application of TA and OVA on the Thymic Stromal Lymphopoietin and TARC Levels in the Skin
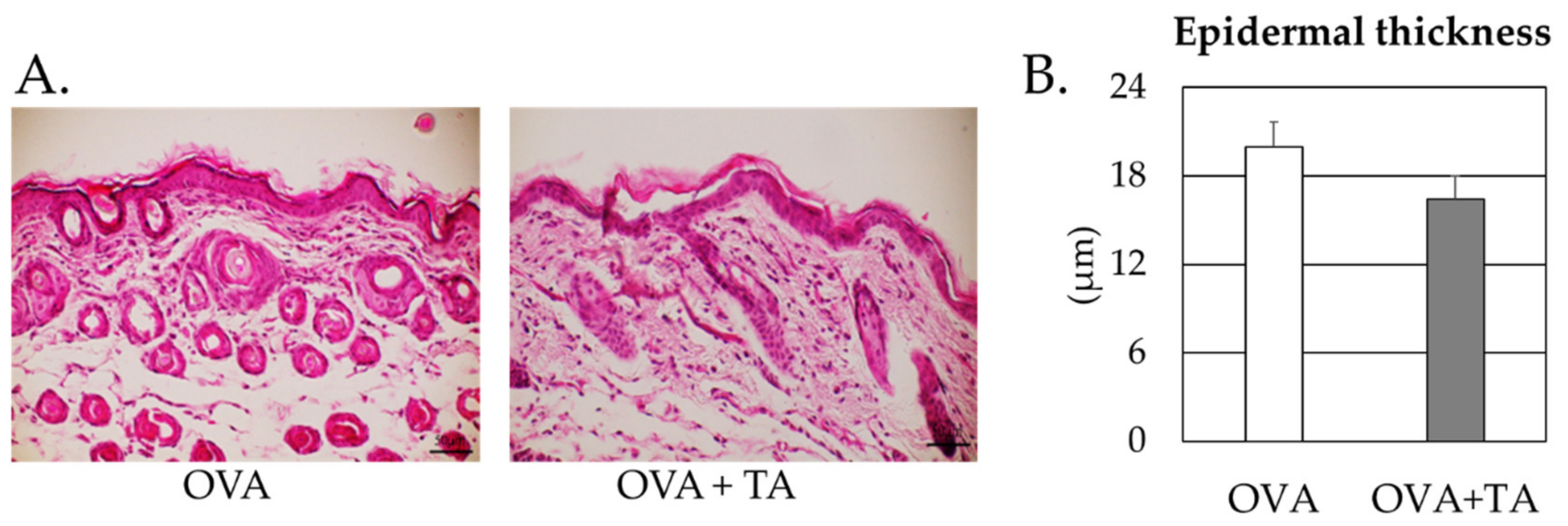
2.5. Effect of Co-Application of TA and OVA on the Skin
3. Discussion
4. Materials and Methods
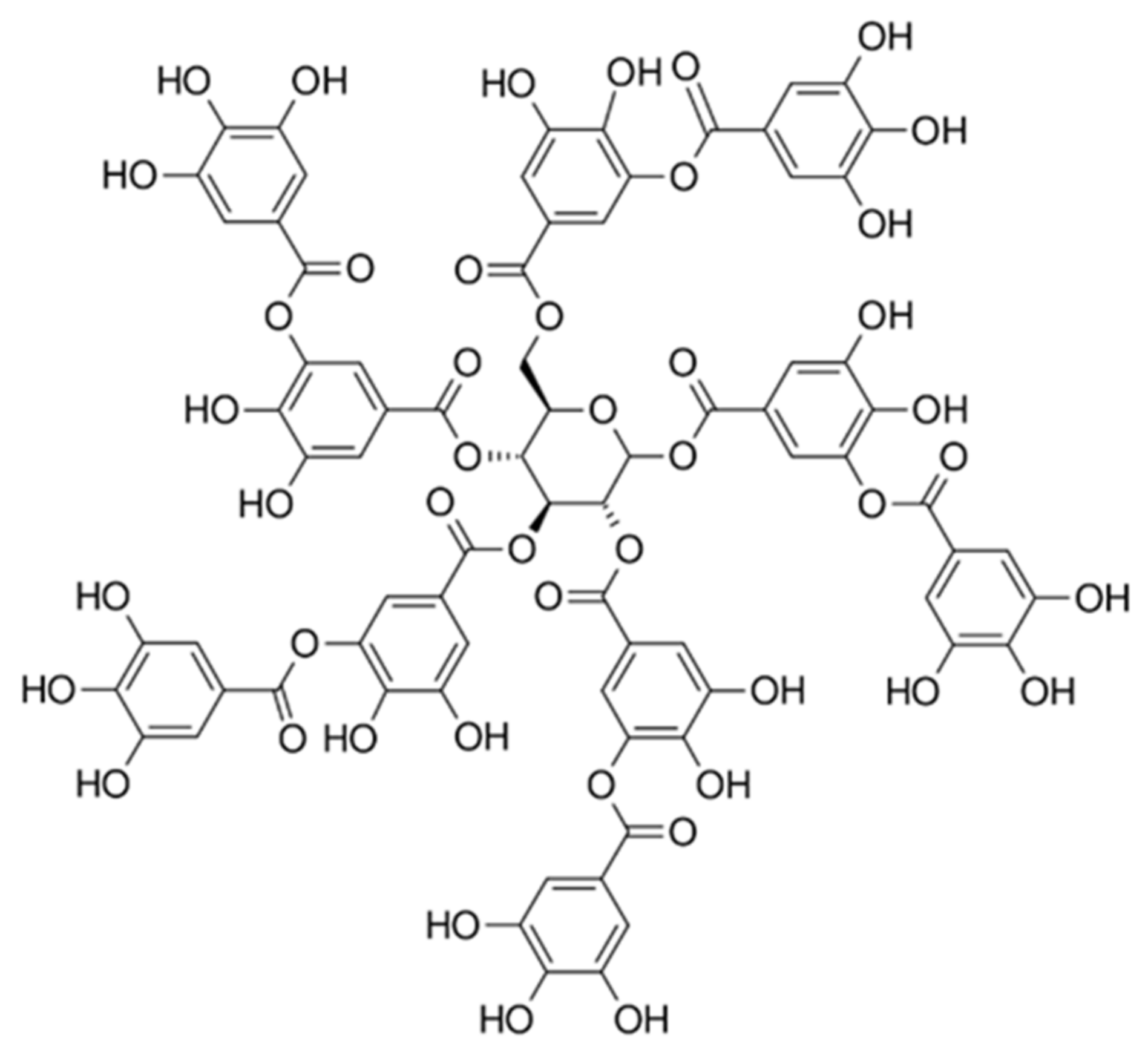
4.1. Materials
4.2. Percutaneous Sensitization Treatment
4.3. Enzyme-Linked Immunosorbent Assay
4.4. Electrophoresis and Immunoblotting
4.5. Determination of Serum or Skin TSLP and TARC Concentrations in Mice
4.6. H&E Stain
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef]
- Yagami, A.; Suzuki, K.; Nakamura, M.; Sano, A.; Iwata, Y.; Kobayashi, T.; Suzuki, M.; Hara, K.; Teshima, R.; Matsunaga, K. Case of anaphylactic reaction to soy following percutaneous sensitization by soy-based ingredients in cosmetic products. J. Dermatol. 2015, 42, 917–918. [Google Scholar] [CrossRef]
- Murakami, H.; Ogawa, T.; Takafuta, A.; Yano, E.; Zaima, N.; Moriyama, T. Identification of the 7S and 11S globulins as percutaneously sensitizing soybean allergens as demonstrated through epidermal application of crude soybean extract. Biosci. Biotechnol. Biochem. 2018, 82, 1408–1416. [Google Scholar] [CrossRef] [Green Version]
- Murakami, H.; Ogawa, T.; Takafuta, A.; Yano, E.; Zaima, N.; Moriyama, T. Percutaneous sensitization to soybean proteins is attenuated by oral tolerance. J. Nutr. Sci. Vitaminol. 2018, 64, 483–486. [Google Scholar] [CrossRef]
- Izumi, E. Thaumatin-like protein (Pru av 2) is a cherry allergen that triggers percutaneous sensitization in mice. Foods 2021, 10, 134. [Google Scholar] [CrossRef]
- Kinugasa, S. Kiwifruit defense protein, kiwellin (Act d 5) percutaneously sensitizes mouse models through the epidermal application of crude kiwifruit extract. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Wavrin, S.; Bernard, H.; Wal, J.M.; Adel-Patient, K. Influence of the route of exposure and the matrix on the sensitisation potency of a major cows’ milk allergen. Clin. Transl. Allergy 2015, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Spergel, J.M.; Mizoguchi, E.; Brewer, J.P.; Martin, T.R.; Bhan, A.K.; Geha, R.S. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J. Clin. Investig. 1998, 101, 1614–1622. [Google Scholar] [CrossRef]
- Takafuta, A.; Department of Applied Biological Chemistry, Graduate School of Agriculture, Kindai University, Naka-machi, Nara, Japan; Kakuki, A.; Department of Applied Biological Chemistry, Graduate School of Agriculture, Kindai University, Naka-machi, Nara, Japan; Hidaka, S.; Department of Applied Biological Chemistry, Graduate School of Agriculture, Kindai University, Naka-machi, Nara, Japan; Zaima, N.; Department of Applied Biological Chemistry, Graduate School of Agriculture, Kindai University, Naka-machi, Nara, Japan; Moriyama, T.; Department of Applied Biological Chemistry, Graduate School of Agriculture, Kindai University, Naka-machi, Nara, Japan. Unpublished work. 2021.
- Robles, H. Tannic Acid. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 474–475. [Google Scholar] [CrossRef]
- Wongsamitkul, N.; Sirianant, L.; Muanprasat, C.; Chatsudthipong, V. A plant-derived hydrolysable tannin inhibits CFTR chloride channel: A potential treatment of diarrhea. Pharm. Res. 2010, 27, 490–497. [Google Scholar] [CrossRef]
- Plein, K.; Burkard, G.; Hotz, J. Treatment of chronic diarrhea in Crohn disease. A pilot study of the clinical effect of tannin albuminate and ethacridine lactate. Fortschr. Med. 1993, 111, 114–118. [Google Scholar]
- Matsumoto, K.; Yokoyama, S. Induction of uncoupling protein-1 and -3 in brown adipose tissue by kaki-tannin in type 2 diabetic NSY/Hos mice. Food Chem. Toxicol. 2012, 50, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Tebib, K.; Besancon, P.; Rouanet, J.M. Dietary grape seed tannins affect lipoproteins, lipoprotein lipases and tissue lipids in rats fed hypercholesterolemic diets. J. Nutr. 1994, 124, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Touriño, S.; Lizárraga, D.; Carreras, A.; Lorenzo, S.; Ugartondo, V.; Mitjans, M.; Vinardell, M.P.; Juliá, L.; Cascante, M.; Torres, J.L. Highly galloylated tannin fractions from witch hazel (Hamamelis virginiana) bark: Electron transfer capacity, in vitro antioxidant activity, and effects on skin-related cells. Chem. Res. Toxicol. 2008, 21, 696–704. [Google Scholar] [CrossRef]
- Noti, M.; Kim, B.S.; Siracusa, M.C.; Rak, G.D.; Kubo, M.; Moghaddam, E.A.; Comeau, M.R.; Spergel, J.M.; Artis, D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin–basophil axis. J. Allergy Clin. Immunol. 2014, 133, 1390–1399. [Google Scholar] [CrossRef] [Green Version]
- Saeki, H.; Tamaki, K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J. Dermatol. Sci. 2006, 43, 75–84. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Baer-Dubowska, W. The effects of tannic acid on cytochrome P450 and phase II enzymes in mouse liver and kidney. Toxicol. Lett. 2003, 143, 209–216. [Google Scholar] [CrossRef]
- Wu, S.; Pang, Y.; He, Y.; Zhang, X.; Peng, L.; Guo, J.; Zeng, J. A comprehensive review of natural products against atopic dermatitis: Flavonoids, alkaloids, terpenes, glycosides and other compounds. Biomed. Pharmacother. 2021, 140, 111741. [Google Scholar] [CrossRef]
- Jung, M.; Hur, D.; Song, S.; Park, Y.; Kim, T.; Bang, S.; Kim, S.; Song, H.; Park, H.; Cho, D. Tannic Acid and Quercetin Display a Therapeutic Effect in Atopic Dermatitis via Suppression of Angiogenesis and TARC Expression in Nc/Nga Mice. J. Investig. Dermatol. 2010, 130, 1459–1463. [Google Scholar] [CrossRef] [Green Version]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchamani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Tannic acid modulates NFκB signaling pathway and skin inflammation in NC/Nga mice through PPARγ expression. Cytokine 2015, 76, 206–213. [Google Scholar] [CrossRef]
- Takahagi, S.; Hatada, N.; Kamegashira, A.; Suzuki, S.; Shindo, H.; Kanatani, H.; Tanaka, A.; Mizuno, H.; Hide, M. Randomized double-blind cross-over trial of bath additive containing tannic acid in patients with atopic dermatitis. J. Cutan. Immunol. Allergy 2020, 3, 56–61. [Google Scholar] [CrossRef]
- Kim, Y.; Yoshimoto, M.; Nakayama, K.; Tanino, S.; Fujimura, Y.; Yamada, K.; Tachibana, H. Tannic acid, a higher galloylated pentagalloylglucose, suppresses antigen-specific IgE production by inhibiting ɛ germline transcription induced by STAT6 activation. FEBS Open Bio. 2013, 3, 341–345. [Google Scholar] [CrossRef] [Green Version]
- Rajasekar, N.; Sivanantham, A.; Kar, A.; Mukhopadhyay, S.; Mahapatra, S.K.; Paramasivam, S.G.; Rajasekaran, S. Anti-asthmatic effects of tannic acid from Chinese natural gall nuts in a mouse model of allergic asthma. Int. Immunopharmacol. 2021, 98, 107847. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.; Jeong, H.; Kim, H. Potential anti-inflammatory effect of Madi-Ryuk and its active ingredient tannic acid on allergic rhinitis. Mol. Immunol. 2019, 114, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Maibach, H.I. Squamometry: An evaluation method for a barrier protectant (tannic acid). Contact Dermat. 2007, 40, 189–191. [Google Scholar] [CrossRef]
- Chung, S.; Reed, S. Removing peanut allergens by tannic acid. Food Chem. 2012, 134, 1268–1473. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wehling, R.L.; Ciftci, O.; Zhang, Y. Formation of complexes between tannic acid with bovine serum albumin, egg ovalbumin and bovine beta-lactoglobulin. Food Res. Int. 2017, 102, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, J.; Yi, X.; Ding, B.; Sun, W.; Yan, F.; Wei, S.; Li, Z. Interactions and emulsifying properties of ovalbumin with tannic acid. LWT 2018, 95, 282–288. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef]
- Alhamwe, B.A.; Khalaila, R.; Wolf, J.; von Bülow, V.; Harb, H.; Alhamdan, F.; Hii, C.S.; Prescott, S.L.; Ferrante, A.; Renz, H.; et al. Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin. Immunol. 2018, 14, 39. [Google Scholar] [CrossRef] [Green Version]
- Čelakovská, J.; Bukač, J.; Vaňková, R.; Krejsek, J.; Krcmova, I.; Andrýs, C. Egg allergy in atopic dermatitis patients-analysis of results of specific extract IgE and specific IgE to molecular components. Bird-egg syndrome. Food Agric. Immunol. 2021, 32, 265–282. [Google Scholar] [CrossRef]
- Čelakovská, J.; Bukač, J.; Vaňková, R.; Krcmova, I.; Krejsek, J.; Andrýs, C. ISAC multiplex testing-results of examination in 100 patients suffering from atopic dermatitis. Food Agric Immunol. 2020, 31, 1014–1035. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kyhse-Andersen, J. Electroblotting of multiple gels: A simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Methods 1984, 10, 203–209. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumi, E.; Tanahashi, N.; Kinugasa, S.; Hidaka, S.; Zaima, N.; Moriyama, T. Co-Application with Tannic Acid Prevents Transdermal Sensitization to Ovalbumin in Mice. Int. J. Mol. Sci. 2022, 23, 3933. https://doi.org/10.3390/ijms23073933
Izumi E, Tanahashi N, Kinugasa S, Hidaka S, Zaima N, Moriyama T. Co-Application with Tannic Acid Prevents Transdermal Sensitization to Ovalbumin in Mice. International Journal of Molecular Sciences. 2022; 23(7):3933. https://doi.org/10.3390/ijms23073933
Chicago/Turabian StyleIzumi, Eri, Nana Tanahashi, Serina Kinugasa, Shota Hidaka, Nobuhiro Zaima, and Tatsuya Moriyama. 2022. "Co-Application with Tannic Acid Prevents Transdermal Sensitization to Ovalbumin in Mice" International Journal of Molecular Sciences 23, no. 7: 3933. https://doi.org/10.3390/ijms23073933
APA StyleIzumi, E., Tanahashi, N., Kinugasa, S., Hidaka, S., Zaima, N., & Moriyama, T. (2022). Co-Application with Tannic Acid Prevents Transdermal Sensitization to Ovalbumin in Mice. International Journal of Molecular Sciences, 23(7), 3933. https://doi.org/10.3390/ijms23073933






