Recycled Sericin Hydrolysates Modified by Alcalase® Suppress Melanogenesis in Human Melanin-Producing Cells via Modulating MITF
Abstract
:1. Introduction
2. Results
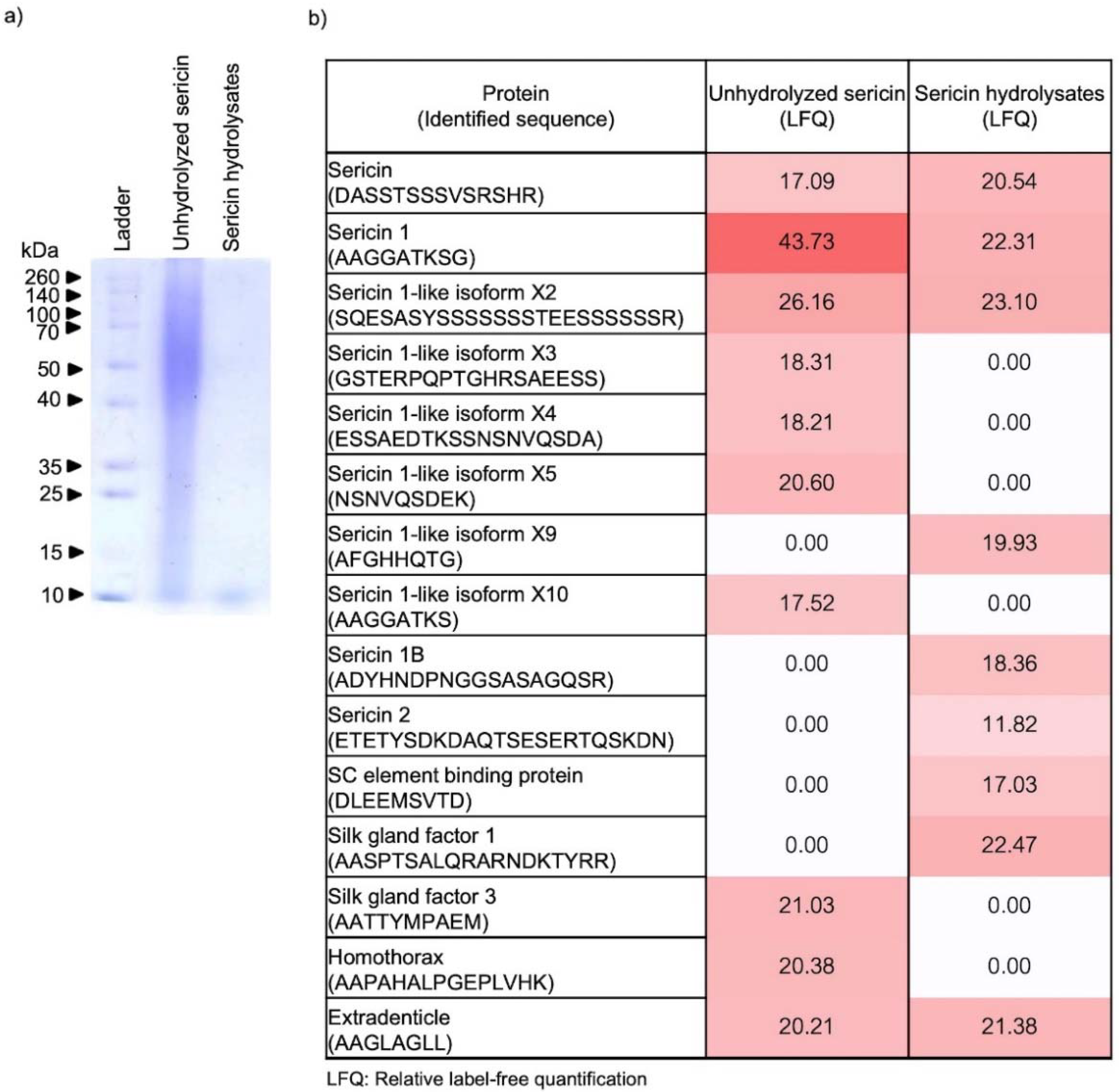
2.1. Peptide Constituents in Sericin Hydrolysates Modified by Alcalase®
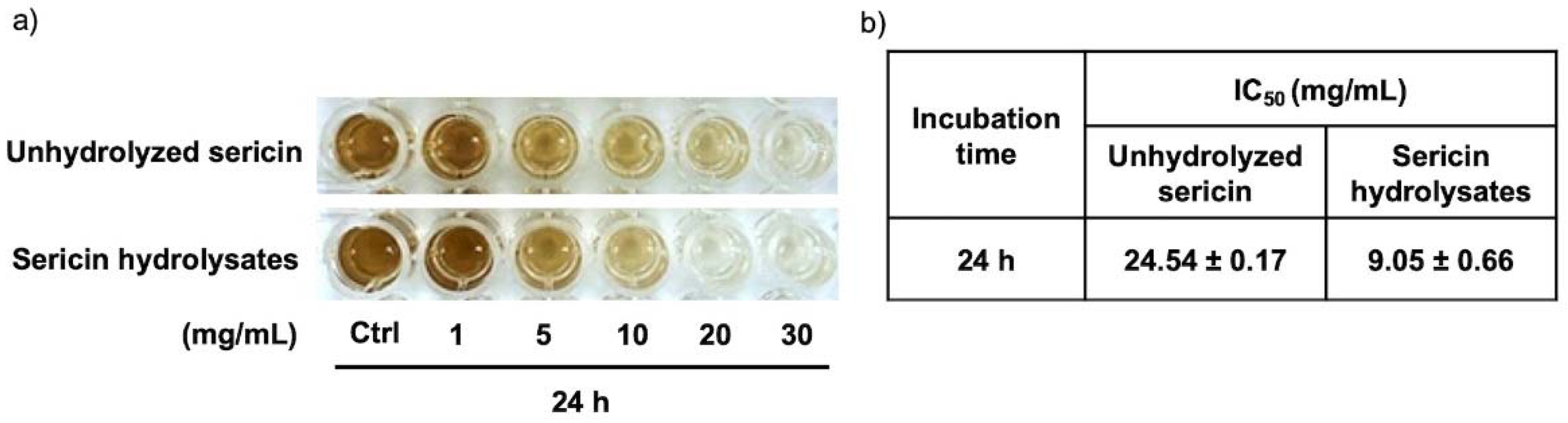
2.2. Suppressive Effect of Sericin Hydrolysates on Melanin Production in Human Melanin-Producing Cells
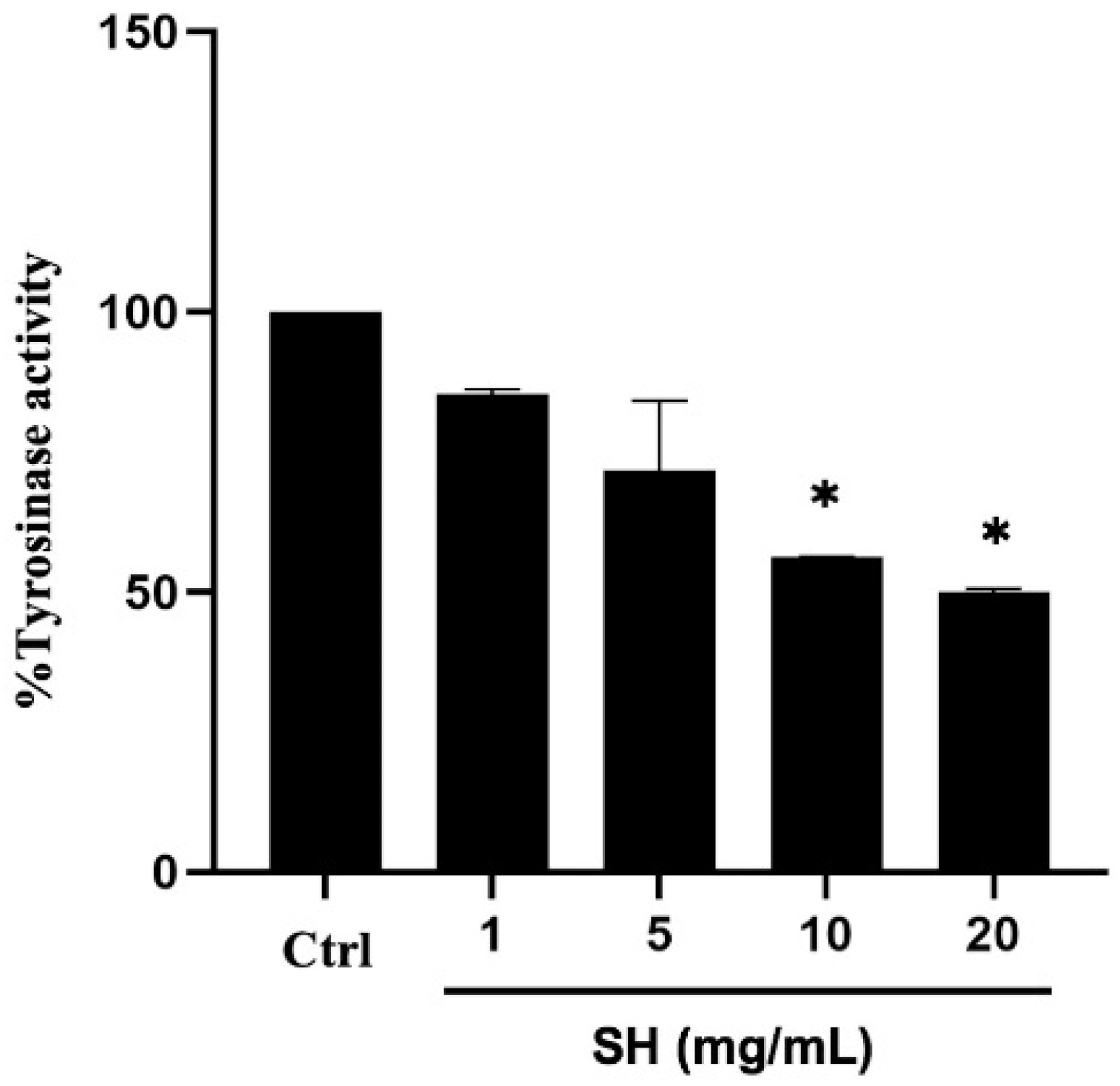
2.3. Alcalase®-Hydrolyzed Sericin as a Human Tyrosinase Inhibitor
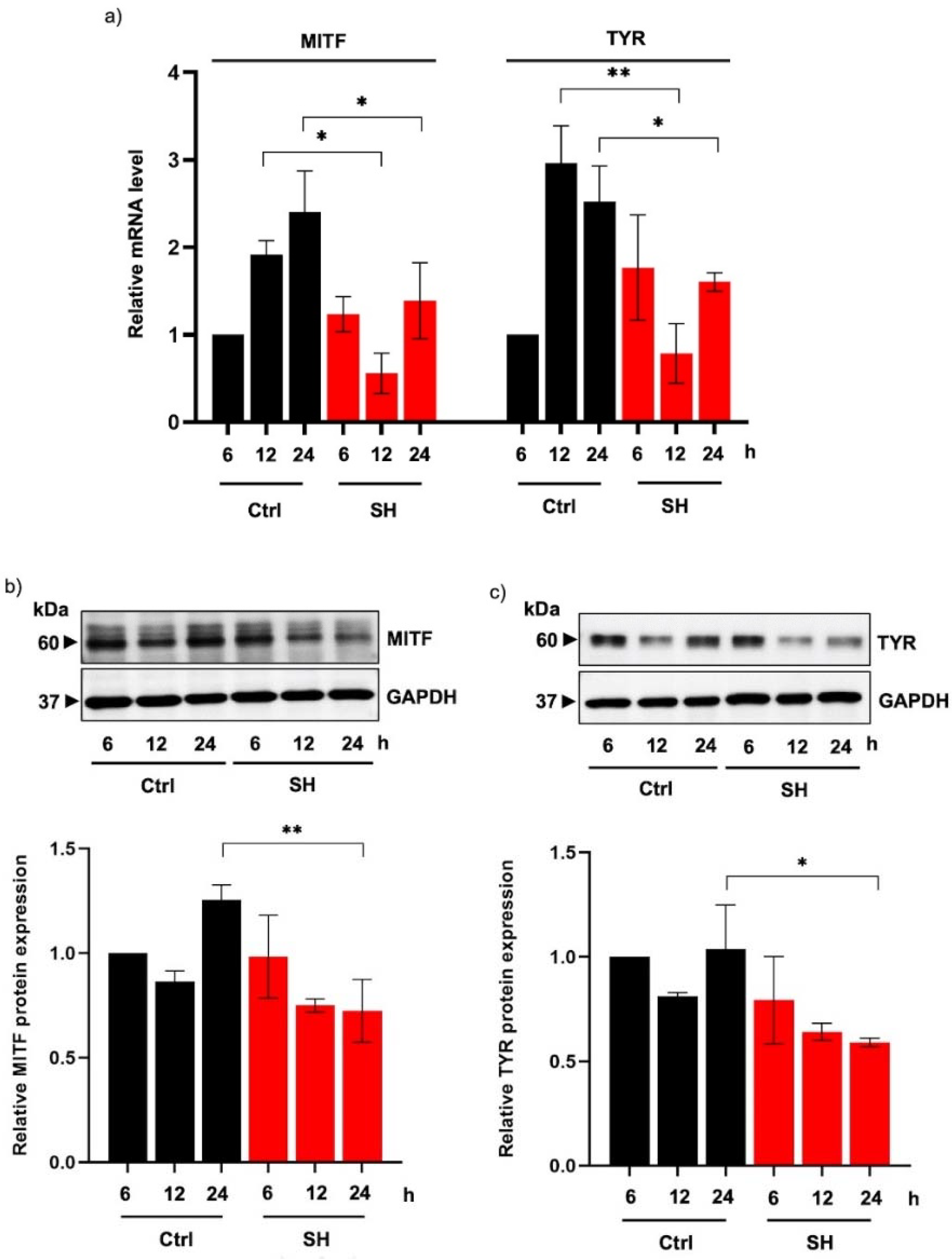
2.4. Sericin Hydrolysates Downregulate Tyrosinase Expression in Human Melanin-Producing Cells
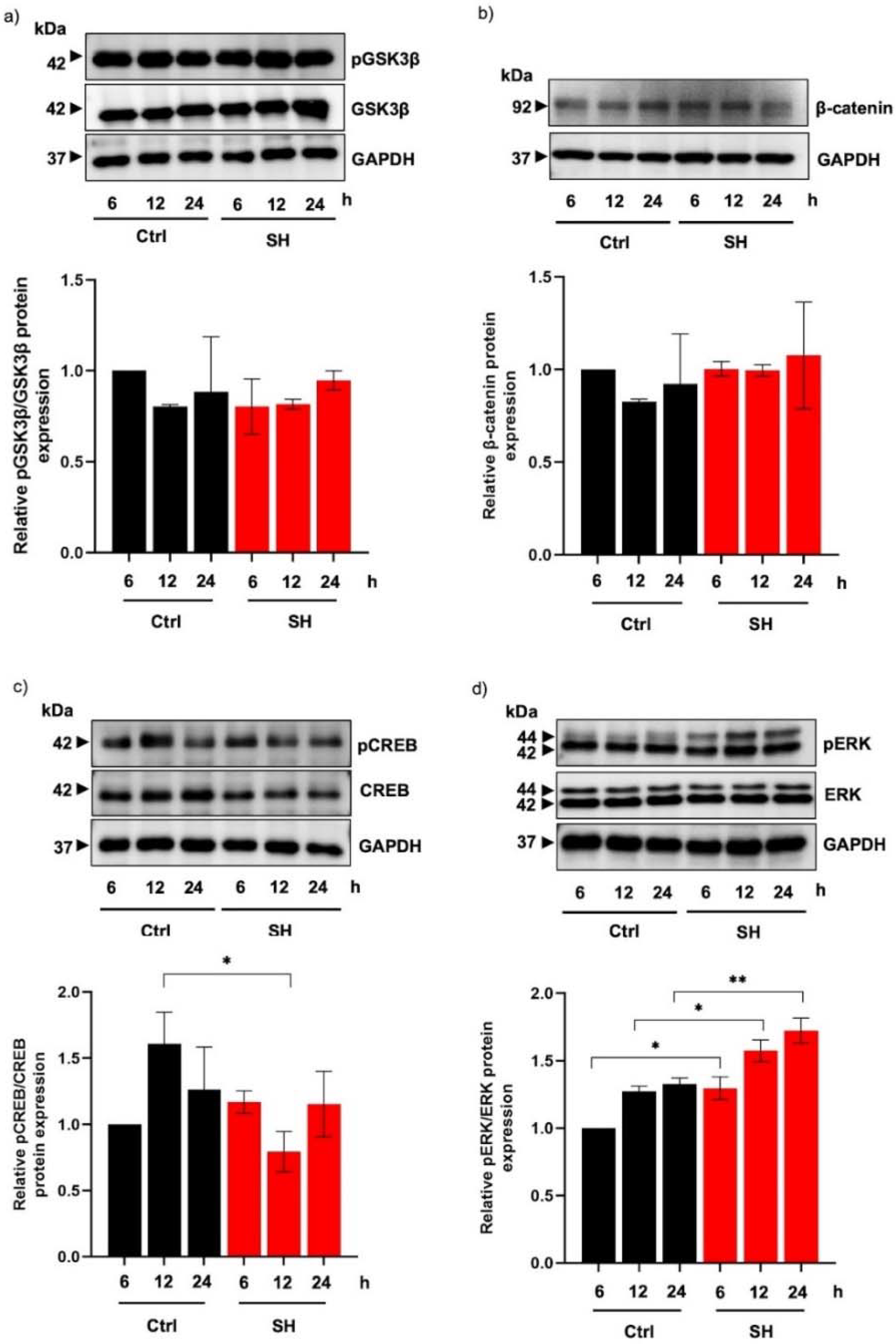
2.5. Alteration of MITF-Regulating Proteins in Human Melanin-Producing Cells Cultured with Sericin Hydrolysates
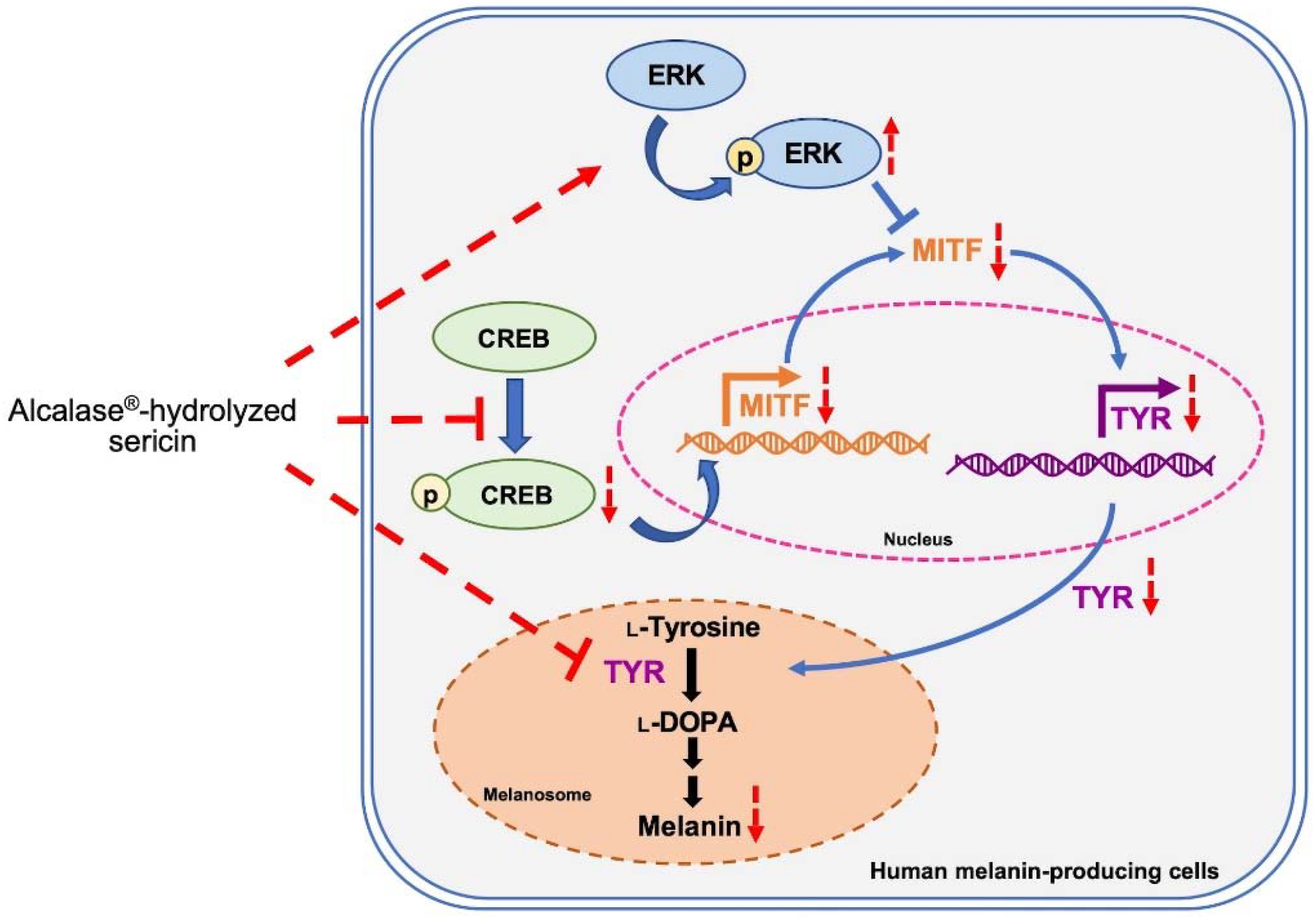
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Preparation of Sericin Hydrolysates
4.3. Molecular Weight Distribution of Sericin Hydrolysates
4.4. Peptidomic Analysis of Alcalase®-Hydrolyzed Sericin
4.4.1. Liquid Chromatography/Electrospray Ionization–Tandem Mass Spectroscopy (LC-ESI-MS/MS)
4.4.2. Bioinformatics and Data Analysis
4.5. Cytotoxicity of Sericin Hydrolysates in Human Melanin-Producing Cells
4.6. Measurement of Cellular Melanin Content
4.7. Determination of Inhibitory Activity of Sericin Hydrolysates against Human Tyrosinase
4.8. Western Blot Analysis
4.9. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
- -Human MITF forward primer: 5′-TCATCCAAAGATCTGGGCTATGACT-3′
- -Human MITF reverse primer: 5′-GTGACGACACAGCAAGCTCAC-3′
- -Human tyrosinase forward primer: 5′-TCATCCAAAGATCTGGGCTATGACT-3′
- -Human tyrosinase reverse primer: 5′-GTGACGACACAGCAAGCTCAC-3′
- -Human GAPDH forward primer: 5′-GAGTCCACTGGCGTCTTCA-3′
- -Human GAPDH reverses primer: 5′-TTCAGCTCAGGGATGACCTT-3′
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandya, A.G.; Guevara, I.L. Disorders of hyperpigmentation. Dermatol. Clin. 2000, 18, 91–98. [Google Scholar] [CrossRef]
- D’Mello, A.N.S.; Finlay, J.G.; Baguley, C.B.; Askarian-Amiri, E.M. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Baek, N.; Nam, T.G. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzyme. Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- García-Gavín, J.; González-Vilas, D.; Fernández-Redondo, V.; Toribio, J. Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact. Dermatitis. 2010, 62, 63–64. [Google Scholar] [CrossRef]
- Nisticò, S.P.; Tolone, M.; Zingoni, T.; Tamburi, F.; Scali, E.; Bennardo, L.; Cannarozzo, G. A New 675 nm laser device in the treatment of melasma: Results of a prospective observational study. Photobiomodul. Photomed. Laser. Surg. 2020, 38, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, M.; Bennardo, L.; Zappia, E.; Tamburi, F.; Cameli, N.; Cannarozzo, G.; Nisticò, S.P. Q-switched 1064/532 nm laser with picosecond pulse to treat benign hyperpigmentations: A single-center retrospective study. Appl. Sci. 2021, 11, 7478. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Sarkar, R.; Arora, P.; Garg, K.V. Cosmeceuticals for hyperpigmentation: What is available? J. Cutan. Aesthet. Surg. 2013, 6, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Kamiya, T.; Kawakami, A.; Ogino, J.; Sohma, H.; Uhara, H.; Jimbow, K. Elucidation of melanogenesis cascade for identifying pathophysiology and therapeutic approach of pigmentary disorders and melanoma. Int. J. Mol. Sci. 2020, 21, 6129. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.I.; Setaluri, V. Cyclic AMP (cAMP) signaling in melanocytes and melanoma. Arch. Biochem. Biophys. 2014, 563, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Schepsky, A.; Bruser, K.; Gunnarsson, G.J.; Goodall, J.; Hallsson, J.H.; Goding, C.R.; Steingrimsson, E.; Hecht, A. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol. Cell. Biol. 2006, 26, 8914–8927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Baek, S.H.; Kim, D.H.; Choi, T.Y.; Yoon, T.J.; Hwang, J.S.; Kim, M.R.; Kwon, H.J.; Lee, C.H. Downregulation of melanin synthesis by haginin A and its application to in vivo lightening model. J. Investig. Dermatol. 2008, 128, 1227–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.S.; Park, M.; Han, J.; Lee, J.H.; Bae, I.H.; Choi, H.; Son, E.D.; Park, Y.H.; Lim, K.M. Liver X Receptor activation inhibits melanogenesis through the acceleration of ERK-mediated MITF degradation. J. Investig. Dermatol. 2013, 133, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Zhao, G.; Zhang, H.; Guan, D.; Meng, R.; Zhang, Y.; Yang, Q.; Jia, H.; Dou, K.; Liu, C.; et al. MITF-siRNA formulation is a safe and effective therapy for human melasma. Mol. Ther. 2011, 19, 362–371. [Google Scholar] [CrossRef]
- Ersel, M.; Uyanikgil, Y.; Karbek Akarca, F.; Ozcete, E.; Altunci, Y.A.; Karabey, F.; Cavusoglu, T.; Meral, A.; Yigitturk, G.; Oyku Cetin, E. Effects of silk sericin on incision wound healing in a dorsal skin flap wound healing rat model. Med. Sci. Monit. 2016, 22, 1064–1078. [Google Scholar] [CrossRef] [Green Version]
- Ampawong, S.; Isarangkul, D.; Aramwit, P. Sericin ameliorated dysmorphic mitochondria in high-cholesterol di-et/streptozotocin rat by antioxidative property. Exp. Biol. Med. 2017, 242, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Manesa, K.C.; Kebede, T.G.; Dube, S.; Nindi, M.M. Profiling of silk sericin from cocoons of three southern African wild silk moths with a focus on their antimicrobial and antioxidant properties. Materials 2020, 13, 5706. [Google Scholar] [CrossRef]
- Fan, J.B.; Zheng, L.H.; Wang, F.; Guo, H.Y.; Jiang, L.; Ren, F.Z. Enzymatic hydrolysis of silk sericin by proteases and antioxidant activities of the hydrolysates. J. Food. Biochem. 2010, 34, 382–398. [Google Scholar] [CrossRef]
- Aramwit, P.; Damrongsakkul, S.; Kanokpanont, S.; Srichana, T. Properties and antityrosinase activity of sericin from various extraction methods. Biotechnol. Appl. Biochem. 2010, 55, 91–98. [Google Scholar] [CrossRef]
- Manosroi, A.; Boonpisuttinant, K.; Winitchai, S.; Manosroi, W.; Manosroi, J. Free radical scavenging and tyrosinase inhibition activity of oils and sericin extracted from Thai native silkworms (Bombyx mori). Pharm. Biol. 2010, 48, 855–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNaughton, B.R.; Gareiss, P.C.; Jacobs, S.E.; Fricke, A.F.; Scott, G.A.; Miller, B.L. A potent activator of melanogenesis identified from small-molecule screening. Chem. Med. Chem. 2009, 4, 1583–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolbe, L.; Mann, T.; Gerwat, W.; Batzer, J.; Ahlheit, S.; Scherner, C.; Wenck, H.; Stäb, F. 4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2013, 27 (Suppl. S1), 19–23. [Google Scholar] [CrossRef] [PubMed]
- Alexis, A.F. New and emerging treatments for hyperpigmentation. J. Drugs. Dermatol. 2014, 13, 382–385. [Google Scholar] [PubMed]
- Galappatthy, P.; Rathnayake, D. Depigmenting Agents. In Pigmentary Skin Disorders. Updates in Clinical Dermatology; Kumarasinghe, P., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.L.; Junior, A.D.S.; Ribani, M.; Vieira, M.G.A.; Gimenes, M.L.; da Silva, M.G. Evaluation of molecular weight distribution of sericin in solutions concentrated via precipitation by ethanol and precipitation by freezing/thawing. Chem. Eng. Trans. 2014, 38, 103–108. [Google Scholar]
- Kunz, R.I.; Brancalhão, R.M.; Ribeiro, L.F.; Natali, M.R. Silkworm sericin: Properties and biomedical applications. Biomed. Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, A.; Gao, Y.; Wubulikasimu, A.; Arken, A.; Aisa, H.A.; Yili, A. Effects of trypsin-induced limited hydrolysis on the structural, functional, and bioactive properties of sericin. RSC. Adv. 2021, 11, 25431–25440. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska-Cagnazzo, A.; Kulczyk, A. Use of different proteases to obtain flaxseed protein hydrolysates with antioxidant activity. Int. J. Mol. Sci. 2016, 17, 1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panyam, D.; Kilara, A. Enhancing the functionality of food proteins by enzymatic modification. Trends. Food. Sci. Technol. 1996, 7, 120–125. [Google Scholar] [CrossRef]
- Tavano, O.L. Protein hydrolysis using proteases: An important tool for food biotechnology. J. Mol. Catal. B Enzym. 2013, 90, 1–11. [Google Scholar] [CrossRef]
- Puangphet, A.; Tiyaboonchai, W.; Thongsook, T. Inhibitory effect of sericin hydrolysate on polyphenol oxidase and browning of fresh-cut products. Int. Food. Res. J. 2015, 22, 1623–1630. [Google Scholar]
- Da Silva, J.D.F.; Correa, A.P.F.; Kechinski, C.P.; Brandelli, A. Buffalo cheese whey hydrolyzed with Alcalase as an antibrowning agent in minimally processed apple. J. Food. Sci. Technol. 2018, 55, 3731–37388. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods. Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurink, M.; van Berkel, W.J.; Wichers, H.J.; Boeriu, C.G. Novel peptides with tyrosinase inhibitory activity. Peptides. 2007, 28, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.G.; Gan, C.Y. Multifunctional tyrosinase inhibitor peptides with copper chelating, UV-absorption and antioxidant activities: Kinetic and docking studies. Foods. 2021, 10, 675. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, Z.; Xu, S.Y. Enzymatic production of bioactive peptides from sericin recovered from silk industry wastewater. Process. Biochem. 2008, 43, 480–487. [Google Scholar] [CrossRef]
- Zhou, S.; Sakamoto, K. Citric acid promoted melanin synthesis in B16F10 mouse melanoma cells, but inhibited it in human epidermal melanocytes and HMV-II melanoma cells via the GSK3β/β-catenin signaling pathway. PLoS ONE 2020, 15, e0243565. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, K.T.; Lee, H.S.; Kim, M.; Lim, Y.H. Evaluation of the inhibition of mushroom tyrosinase and cellular tyrosinase activities of oxyresveratrol: Comparison with mulberroside A. J. Enzym. Inhib. Med. Chem. 2021, 27, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Soler-Lopez, M.; Wichers, H.J.; Dijkstra, B.W. Large-scale recombinant expression and purification of human tyrosinase suitable for structural studies. PLoS ONE 2016, 11, e0161697. [Google Scholar] [CrossRef]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef] [Green Version]
- Goding, C.; Meyskens, F.L., Jr. Microphthalmic-associated transcription factor integrates melanocyte biology and melanoma progression. Clin. Cancer Res. 2006, 12, 1069–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, W.; Choi, D.; Park, S.; Park, T. Carvone decreases melanin content by inhibiting melanoma cell proliferation via the cyclic adenosine monophosphate (cAMP) pathway. Molecules 2020, 25, 5191. [Google Scholar] [CrossRef] [PubMed]
- Kleszczyński, K.; Kim, T.K.; Bilska, B.; Sarna, M.; Mokrzynski, K.; Stegemann, A.; Pyza, E.; Reiter, R.J.; Steinbrink, K.; Böhm, M.; et al. Melatonin exerts oncostatic capacity and decreases melanogenesis in human MNT-1 melanoma cells. J. Pineal. Res. 2019, 67, e12610. [Google Scholar] [CrossRef] [PubMed]
- Netcharoensirisuk, P.; Umehara, K.; De-Eknamkul, W.; Chaotham, C. Cajanin suppresses melanin synthesis through modulating MITF in human melanin-producing cells. Molecules 2021, 26, 6040. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.B.; Babiarz, L.; Liebel, F.; Roydon Price, E.; Kizoulis, M.; Gendimenico, G.J.; Fisher, D.E.; Seiberg, M. Modulation of microphthalmia-associated transcription factor gene expression alters skin pigmentation. J. Investig. Dermatol. 2002, 119, 1330–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishio, T.; Usami, M.; Awaji, M.; Shinohara, S.; Sato, K. Dual effects of acetylsalicylic acid on ERK signaling and Mitf transcription lead to inhibition of melanogenesis. Mol. Cell. Biochem. 2016, 412, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K.; Favre, M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 1973, 80, 575–599. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods. 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and its metabolites ameliorate UVR-induced mitochondrial oxidative stress in human MNT-1 melanoma cells. Int. J. Mol. Sci. 2018, 19, 3786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, T.I.; Yun, J.M.; Park, E.J.; Kim, Y.S.; Lee, Y.M.; Lim, J.H. Plumbagin suppresses α-MSH-induced melanogenesis in B16F10 mouse melanoma cells by inhibiting tyrosinase activity. Int. J. Mol. Sci. 2017, 18, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netcharoensirisuk, P.; Abrahamian, C.; Tang, R.; Chen, C.C.; Rosato, A.S.; Beyers, W.; Chao, Y.K.; Filippini, A.; Di Pietro, S.; Bartel, K.; et al. Flavonoids increase melanin production and reduce proliferation, migration and invasion of melanoma cells by blocking endolysosomal/melanosomal TPC2. Sci. Rep. 2021, 11, 8515. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joyjamras, K.; Netcharoensirisuk, P.; Roytrakul, S.; Chanvorachote, P.; Chaotham, C. Recycled Sericin Hydrolysates Modified by Alcalase® Suppress Melanogenesis in Human Melanin-Producing Cells via Modulating MITF. Int. J. Mol. Sci. 2022, 23, 3925. https://doi.org/10.3390/ijms23073925
Joyjamras K, Netcharoensirisuk P, Roytrakul S, Chanvorachote P, Chaotham C. Recycled Sericin Hydrolysates Modified by Alcalase® Suppress Melanogenesis in Human Melanin-Producing Cells via Modulating MITF. International Journal of Molecular Sciences. 2022; 23(7):3925. https://doi.org/10.3390/ijms23073925
Chicago/Turabian StyleJoyjamras, Keerati, Ponsawan Netcharoensirisuk, Sittiruk Roytrakul, Pithi Chanvorachote, and Chatchai Chaotham. 2022. "Recycled Sericin Hydrolysates Modified by Alcalase® Suppress Melanogenesis in Human Melanin-Producing Cells via Modulating MITF" International Journal of Molecular Sciences 23, no. 7: 3925. https://doi.org/10.3390/ijms23073925
APA StyleJoyjamras, K., Netcharoensirisuk, P., Roytrakul, S., Chanvorachote, P., & Chaotham, C. (2022). Recycled Sericin Hydrolysates Modified by Alcalase® Suppress Melanogenesis in Human Melanin-Producing Cells via Modulating MITF. International Journal of Molecular Sciences, 23(7), 3925. https://doi.org/10.3390/ijms23073925







