Impact of Rutin and Other Phenolic Substances on the Digestibility of Buckwheat Grain Metabolites
Abstract
:1. Introduction
2. Flavonoids in Tartary Buckwheat Grain and Herb
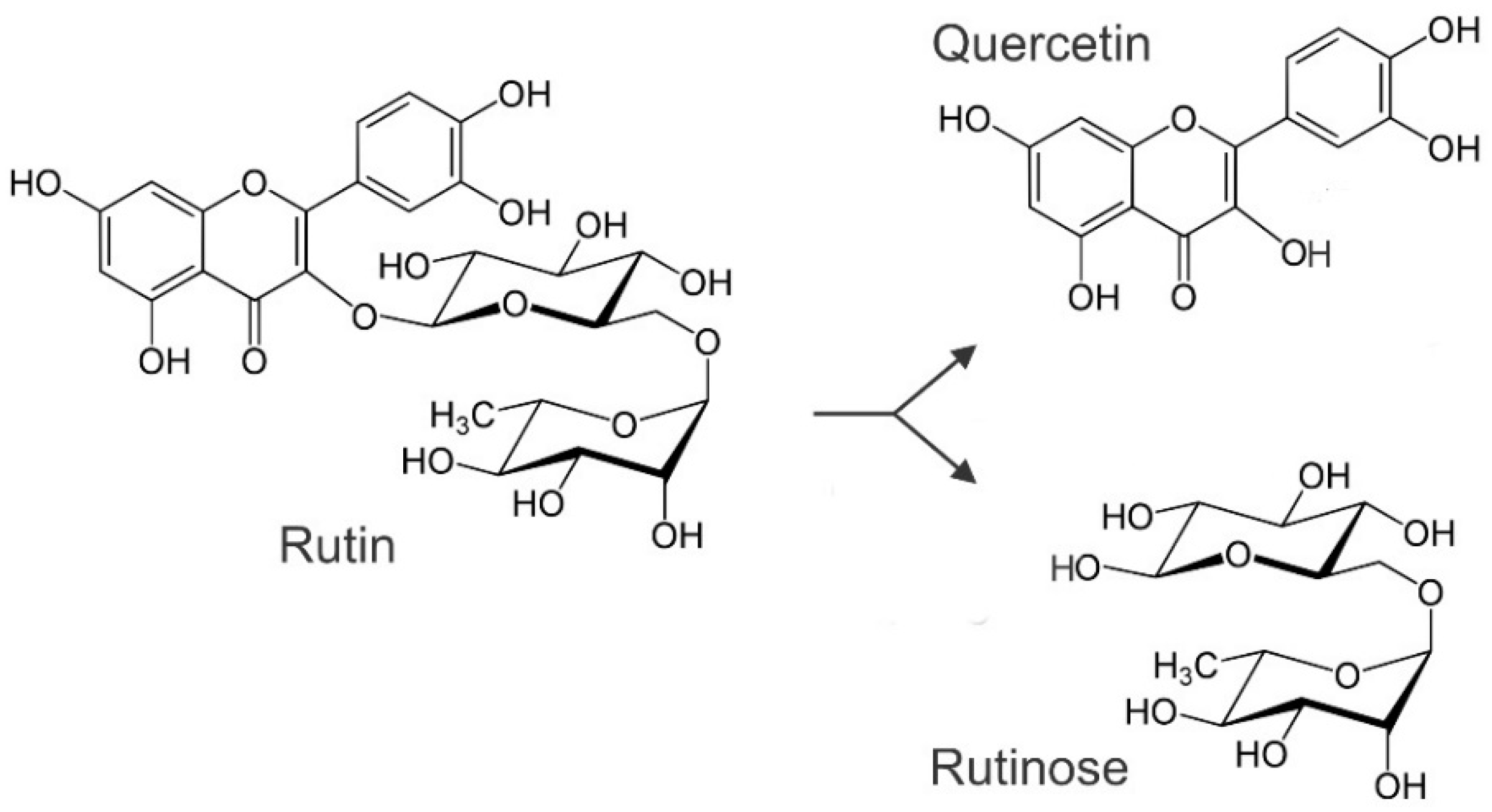
3. Synthesis of Rutin during Germination and Malting
4. Bioactivity of Flavonoids
5. Interaction of Rutin and Its Degradation Products with Proteins
6. Interaction of Flavonoids with Starch
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabjan, N.; Rode, J.; Košir, I.J.; Zhang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercetin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Zhao, Z. Traditional buckwheat growing and utilization in China. In Ethnobotany of Buckwheat; Kreft, I., Chang, K.J., Choi, Y.S., Park, C.H., Eds.; Jinsol Publishing Co.: Seoul, Korea, 2003; pp. 9–20. [Google Scholar]
- Ohnishi, O. Buckwheat in the Himalayan Hills. In Ethnobotany of Buckwheat; Kreft, I., Chang, K.J., Choi, Y.S., Park, C.H., Eds.; Jinsol Publishing Co.: Seoul, Korea, 2003; pp. 21–33. [Google Scholar]
- Ohnishi, O. Search for the wild ancestor of buckwheat—III. The wild ancestor of cultivated common buckwheat, and of tatary buckwheat. Econ. Bot. 1998, 52, 123–133. [Google Scholar] [CrossRef]
- Tsuji, K.; Ohnishi, O. Origin of cultivated Tatary buckwheat (Fagopyrum tataricum Gaertn.) revealed by RAPD analyses. Genet. Resour. Crop Evol. 2000, 47, 431–438. [Google Scholar] [CrossRef]
- Zhang, K.; He, M.; Fan, Y.; Zhao, H.; Gao, B.; Yang, K.; Li, F.; Tang, Y.; Gao, Q.; Lin, T.; et al. Resequencing of global Tartary buckwheat accessions reveals multiple domestication events and key loci associated with agronomic traits. Genome Biol. 2021, 22, 23. [Google Scholar] [CrossRef]
- Tryhub, O.; Burdyga, V.; Kharchenko, Y.; Havrylyanchyk, R. Formation of buckwheat genepool collection in Ukraine and directions of its usage. Fagopyrum 2018, 35, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Tryhub, O. Research results of local buckwheat varieties and forms of Ukrainian origin. Fagopyrum 2019, 36, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.G.; Nagano, M. Buckwheat breeding. Past, present and future. Folia Biol. Geol. 2020, 61, 25–36. [Google Scholar] [CrossRef]
- Janeš, D.; Prosen, H.; Kreft, I.; Kreft, S. Aroma compounds in buckwheat (Fagopyrum esculentum Moench) groats, flour, bran, and husk. Cereal Chem. 2010, 87, 41–143. [Google Scholar] [CrossRef]
- Janeš, D.; Prosen, H.; Kreft, S. Identification and quantification of aroma compounds of Tartary buckwheat (Fagopyrum tataricum Gaertn.) and some of its milling fractions. J. Food Sci. 2012, 7, 746–751. [Google Scholar] [CrossRef]
- Škrabanja, V.; Laerke, H.; Kreft, I. Effects of hydrothermal processing of buckwheat (Fagopyrum esculentum Moench) groats on starch enzymatic availability In Vitro and In Vivo in rats. J. Cereal Sci. 1998, 28, 209–214. [Google Scholar] [CrossRef]
- Škrabanja, V.; Kreft, I. Resistant starch formation following autoclaving of buckwheat (Fagopyrum esculentum Moench) groats. An in vitro study. J. Agric. Food Chem. 1998, 46, 2020–2023. [Google Scholar] [CrossRef]
- Škrabanja, V.; Laerke, H.N.; Kreft, I. Protein-polyphenol interactions and In Vivo digestibility of buckwheat groat proteins. Pflüg. Arch. Eur. J. Physiol. 2000, 440, R129–R131. [Google Scholar] [CrossRef] [PubMed]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and Tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Škrabanja, V.; Kreft, I.; Golob, T.; Modic, S.; Ikeda, M.; Ikeda, K.; Kreft, S.; Bonafaccia, G.; Knapp, M.; Kosmelj, K. Nutrient content in buckwheat milling fractions. Cereal Chem. 2004, 81, 172–176. [Google Scholar] [CrossRef]
- Vombergar, B.; Luthar, Z. The concentration of flavonoids, tannins and crude proteins in grain fractions of common buckwheat (Fagopyrum esculentum Moench) and Tartary buckwheat (Fagopyrum tataricum Gaertn.). Folia Biol. Geol. 2018, 59, 101–157. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. The Food and Agriculture Organization of the United Nation. Available online: http://www.fao.org/faostat/en/#data (accessed on 9 March 2022).
- CPVO. The Community Plant Variety Office. Available online: https://cpvo.europa.eu/en/applications-and-examinations/cpvo-variety-finder (accessed on 9 March 2022).
- Chen, L.-H.; Zhang, B.; Xu, Z.-Q. Salt tolerance conferred by overexpression of Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1 in common buckwheat (Fagopyrum esculentum). Transgenic Res. 2008, 17, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Zhang, K.; Wang, C.; Chandora, R.; Khurshid, M.; Li, J.; He, M.; Georgiev, M.; Zhou, M. Strategic enhancement of genetic gain for nutraceutical development in buckwheat: A genomics-driven perspective. Biotechnol. Adv. 2020, 39, 107479. [Google Scholar] [CrossRef]
- Luthar, Z.; Fabjan, P.; Mlinarič, K. Biotechnological methods for buckwheat breeding. Plants 2021, 10, 1547. [Google Scholar] [CrossRef]
- Klykov, A.; Chaikina, E.; Anisimov, M.; Borovaya, S.; Barsukova, E. Rutin content in buckwheat (Fagopyrum esculentum Moench, F. tataricum (L.) Gaertn. and F. cymosum Meissn.) growth in the far east of Russia. Folia Biol. Geol. 2020, 61, 61–68. [Google Scholar] [CrossRef]
- Yu, J.H.; Kwon, S.J.; Choi, J.Y.; Ju, Y.H.; Roy, S.K.; Lee, D.G.; Park, C.H.; Woo, S.H. Variation of rutin and quercetin contents in Tartary buckwheat germplasm. Fagopyrum 2019, 36, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Morishita, T.; Noda, T.; Ishiguro, K.; Otsuka, S.; Katsu, K. Breeding of Buckwheat to Reduce Bitterness and Rutin Hydrolysis. Plants 2021, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Shi, R.; Zhang, J.; Zhang, L. Evaluation of endogenous enzyme-induced chemical transformations of flavonoid glycosides to aglycones and ethyl-rutinoside in different Tartary buckwheat edible tissues. J. Cereal Sci. 2022, 104, 103429. [Google Scholar] [CrossRef]
- Gaberščik, A.; Vončina, M.; Trošt Sedej, T.; Germ, M.; Björn, L.O. Growth and production of buckwheat (Fagopyrum esculentum) treated with reduced, ambient, and enhanced UV-B radiation. J. Photochem. Photobiol. B Biol. 2002, 66, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Kreft, S.; Štrukelj, B.; Gaberščik, A.; Kreft, I. Rutin in buckwheat herbs grown at diferent UV-B radiation levels: Comparison of two UV spectrophotometric and an HPLC method. J. Exp. Bot. 2002, 53, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Breznik, B.; Germ, M.; Gaberščik, A.; Kreft, I. The combined effects of elevated UV-B radiation and selenium on Tartary buckwheat (Fagopyrum tataricum) habitus. Fagopyrum 2004, 21, 59–64. [Google Scholar]
- Germ, M.; Breznik, B.; Dolinar, N.; Kreft, I.; Gaberščik, A. The combined effect of water limitation and UV-B radiation on common and tartary buckwheat. Cereal Res. Commun. 2013, 41, 97–105. [Google Scholar] [CrossRef]
- Vollmannová, A.; Musilová, J.; Lidiková, J.; Árvay, J.; Šnirc, M.; Tóth, T.; Bojňanská, T.; Čičová, I.; Kreft, I.; Germ, M. Concentrations of phenolic acids are differently genetically determined in leaves, flowers, and grain of common buckwheat (Fagopyrum esculentum Moench). Plants 2021, 10, 1142. [Google Scholar] [CrossRef]
- Ozbolt, L.; Kreft, S.; Kreft, I.; Germ, M.; Stibilj, V. Distribution of selenium and phenolics in buckwheat plants grown from seeds soaked in Se solution and under different levels of UV-B radiation. Food Chem. 2008, 110, 691–696. [Google Scholar] [CrossRef]
- Kreft, I.; Fabjan, N.; Germ, M. Rutin in buckwheat—Protection of plants and its importance for the production of functional food. Fagopyrum 2003, 20, 7–11. [Google Scholar]
- Stec, K.; Kordan, B.; Gabryś, B. Quercetin and Rutin as Modifiers of Aphid Probing Behavior. Molecules 2021, 26, 3622. [Google Scholar] [CrossRef]
- Sun, W.; Ma, Z.; Liu, M. Cytochrome P450 family: Genome-wide identification provides insights into the rutin synthesis pathway in Tartary buckwheat and the improvement of agricultural product quality. Int. J. Biol. Macromol. 2020, 164, 4032–4045. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Z.; Xiao, S.; Wang, A.; Hua, X.; Yu, Q.; Liu, Y.; Peng, L.; Yang, Y.; Wang, J. Characterization of abscisic acid (ABA) receptors and analysis of genes that regulate rutin biosynthesis in response to ABA in Fagopyrum tataricum. Plant Physiol. Biochem. 2020, 157, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Baek, S.A.; Kim, N.S.; Sathasivam, R.; Park, J.S.; Kim, J.K.; Park, S.U. Elevated ozone levels affect metabolites and related biosynthetic genes in Tartary buckwheat. J. Agric. Food Chem. 2020, 68, 14758–14767. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Lukšič, L.; Bonafaccia, G.; Timoracka, M.; Vollmannova, A.; Trček, J.; Nyambe, T.K.; Melini, V.; Acquistucci, R.; Germ, M.; Kreft, I. Rutin and quercetin transformation during preparation of buckwheat sourdough bread. J. Cereal Sci. 2016, 69, 71–76. [Google Scholar] [CrossRef]
- Lukšič, L.; Árvay, J.; Vollmannová, A.; Tóth, T.; Škrabanja, V.; Trček, J.; Germ, M.; Kreft, I. Hydrothermal treatment of Tartary buckwheat grain hinders the transformation of rutin to quercetin. J. Cereal Sci. 2016, 72, 131–134. [Google Scholar] [CrossRef]
- Germ, M.; Árvay, J.; Vollmannová, A.; Tóth, T.; Golob, A.; Luthar, Z.; Kreft, I. The temperature threshold for the transformation of rutin to quercetin in Tartary buckwheat dough. Food Chem. 2019, 283, 28–31. [Google Scholar] [CrossRef]
- Yasuda, T.; Nakagawa, H. Purification and characterization of the rutin-degrading enzymes in Tartary buckwheat seeds. Phytochemistry 1994, 37, 133–136. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K. Characterization of rutin-rich bread made with ‘Manten-Kirari’, a trace-rutinosidase variety of Tartary buckwheat (Fagopyrum tataricum Gaertn.). Food Sci. Technol. Res. 2015, 21, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Yoshihashi, T. Heat-treatment of Tartary buckwheat (Fagopyrum tataricum Gaertn.) provides dehulled and gelatinized product with denatured rutinosidase. Food Sci. Technol. Res. 2019, 25, 613–618. [Google Scholar] [CrossRef]
- Suzuki, T.; Noda, T.; Morishita, T.; Ishiguro, K.; Otsuka, S.; Brunori, A. Present status and future perspectives of breeding for buckwheat quality. Breed. Sci. 2020, 70, 48–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Fu, G.; Li, R.; Li, Y.; Dong, B.; Liu, C. Effect of thermal processing for rutin preservation on the properties of phenolics & starch in Tartary buckwheat achenes. Int. J. Biol. Macromol. 2020, 164, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Koval, D.; Plockova, M.; Kyselka, J.; Skřivan, P.; Sluková, M.; Horáčková, S. Buckwheat secondary metabolites: Potential antifungal agents. J. Agric. Food Chem. 2020, 68, 11631–11643. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.Y.; Du, W.; Hao, Y.R.; Han, Y.H.; Li, H.Y.; Liu, L.L.; Zhang, K.X.; Zhou, M.L.; Sun, Z.X. Elucidation of the Regulatory Network of Flavonoid Biosynthesis by Profiling the Metabolome and Transcriptome in Tartary Buckwheat. J. Agric. Food Chem. 2021, 69, 7218–7229. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, K.; He, Y.; Zuo, Q.; Zhao, H.; He, M.; Georgiev, M.I.; Park, S.U.; Zhou, M. FtBPM3 modulates the orchestration of FtMYB11-mediated flavonoids biosynthesis in Tartary buckwheat. Plant Biotechnol. J. 2021, 19, 1285–1287. [Google Scholar] [CrossRef]
- Bhinder, S.; Singh, N.; Kaur, A. Impact of germination on nutraceutical, functional and gluten free muffin making properties of Tartary buckwheat (Fagopyrum tataricum). Food Hydrocoll. 2022, 124, 107268. [Google Scholar] [CrossRef]
- Zhao, X.; Li, C.; Jiang, Y.; Wang, M.; Wang, B.; Xiao, L.; Xu, X.; Chai, D.; Dong, L. Metabolite fingerprinting of buckwheat in the malting process. J. Food Meas. Charact. 2021, 15, 1475–1486. [Google Scholar] [CrossRef]
- Živkovic, A.; Polak, T.; Cigić, B.; Požrl, T. Germinated buckwheat: Effects of dehulling on phenolics profile and antioxidant activity of buckwheat seeds. Foods 2021, 10, 740. [Google Scholar] [CrossRef]
- Mravlje, J.; Regvar, M.; Starič, P.; Mozetič, M.; Vogel-Mikuš, K. Cold plasma affects germination and fungal community structure of buckwheat seeds. Plants 2021, 10, 851. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, Z.; Ma, T. The Effects of Plasma-Activated Water Treatment on the Growth of Tartary Buckwheat Sprouts. Front. Nutr. 2022, 9, 849615. [Google Scholar] [CrossRef]
- Molinari, R.; Costantini, L.; Timperio, A.M.; Lelli, V.; Bonafaccia, F.; Bonafaccia, G.; Merendino, N. Tartary buckwheat malt as ingredient of gluten-free cookies. J. Cereal Sci. 2018, 80, 37–43. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of tartary buckwheat, rutin, and quercetin on lipid metabolism in rats during high dietary fat intake. Food Sci. Nutr. 2019, 8, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Henna, T.K.; Raphey, V.R.; Sankar, R.; Ameena Shirin, V.K.; Gangadharappa, H.V.; Pramod, K. Carbon nanostructures: The drug and the delivery system for brain disorders. Int. J. Pharm. 2020, 587, 119701. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, X.; Zhang, Q.; Wang, L.; Li, Y.; Li, Y. Characterization and Evaluation of the Solubility and Oral Bioavailability of Rutin-Ethanolate Solvate. AAPS PharmSciTech 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Al-Qattan, M.N.; Deb, P.K.; Tekade, R.K. Molecular dynamics simulation strategies for designing carbon-nanotube-based targeted drug delivery. Drug Discov. Today 2018, 23, 235–250. [Google Scholar] [CrossRef]
- Neto, C.M.S.; Lima, F.C.; Morais, R.P.; de Andrade, L.R.M.; de Lima, R.; Chaud, M.V.; Pereira, M.M.; de Albuquerque Júnior, R.L.C.; Cardoso, J.C.; Zielińska, A.; et al. Rutin-Functionalized Multi-Walled Carbon Nanotubes: Molecular Docking, Physicochemistry and Cytotoxicity in Fibroblasts. Toxics 2021, 9, 173. [Google Scholar] [CrossRef]
- Wu, H.; Su, M.; Jin, H.; Li, X.; Wang, P.; Chen, J.; Chen, J. Rutin-Loaded Silver Nanoparticles With Antithrombotic Function. Front. Bioeng. Biotechnol. 2020, 8, 598977. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Chapter 26—The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: A systematic review and update. In Bioactive Food as Dietary Interventions for Arthritis and Inflammatory Diseases, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 457–479. [Google Scholar] [CrossRef]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as neuroprotective agent: From bench to bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef]
- Çelik, H.; Kandemir, F.M.; Caglayan, C.; Özdemir, S.; Çomaklı, S.; Kucukler, S.; Yardım, A. Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol. Biol. Rep. 2020, 47, 2023–2034. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Li, Y.; Lu, K.; Yin, R.; Ming, J. Phenolics extracted from tartary (Fagopyrum tartaricum L. Gaertn.) buckwheat bran exhibit antioxidant activity, and an antiproliferative effect on human breast cancer MDA-MB-231 cells through the p38/MAP kinase pathway. Food Funct. 2017, 8, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Yue, L.X.; Wang, S.G.; Wang, T.X. Quercitrin restrains the growth and invasion of lung adenocarcinoma cells by regulating gap junction protein beta 2. Bioengineered 2022, 13, 6126–6135. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.C.; Liu, J.X.; Cai, X.Y.; Huang, Y.P.; Xu, P.; Fu, S.P.; Guo, W.J.; Hu, G.Q. Tartary buckwheat flavonoids relieve the tendency of mammary fibrosis induced by HFD during pregnancy and lactation. Aging 2021, 13, 25377–25392. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Rajput, V.S.; Nagpal, P.; Kukrety, H.; Grover, S.; Grover, A. Dual inhibition of SARS-CoV-2 spike and main protease through a repurposed drug, rutin. J. Biomol. Struct. Dyn. 2020, 1–13. [Google Scholar] [CrossRef]
- Rizzuti, B.; Grande, F.; Conforti, F.; Jimenez-Alesanco, A.; Ceballos-Laita, L.; Ortega-Alarcon, D.; Vega, S.; Reyburn, H.T.; Abian, O.; Velazquez-Campoy, A. Rutin Is a Low Micromolar Inhibitor of SARS-CoV-2 Main Protease 3CLpro: Implications for Drug Design of Quercetin Analogs. Biomedicines 2021, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Tabrez, S.; Ali, R.; Alqahtani, A.S.; Ahmed, M.Z.; Rub, A. Molecular docking analysis of rutin reveals possible inhibition of SARS-CoV-2 vital proteins. J. Tradit. Complement. Med. 2021, 11, 173–179. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Moisă, C.; Copolovici, L.; Bungau, S.; Pop, G.; Imbrea, I.; Lupitu, A.-I.; Nemeth, S.; Copolovici, D. Wastes resulting from aromatic plants distillation—Bio-sources of antioxidants and phenolic compounds with biological active principles. Farmacia 2018, 66, 289–295. [Google Scholar]
- Unuk Nahberger, T.; Grebenc, T.; Žlindra, D.; Mrak, T.; Likar, M.; Kraigher, H.; Luthar, Z. Buckwheat milling waste effects on root morphology and mycorrhization of Silver fir seedlings inoculated with Black Summer Truffle (Tuber aestivum Vittad.). Forests 2022, 13, 240. [Google Scholar] [CrossRef]
- Sytar, O.; Chrenkova, M.; Ferencova, J.; Polacikova, M.; Rajsky, M.; Brestic, M. Nutrient capacity of amino acids from buckwheat seeds and sprouts. J. Food Nutr. Res. 2018, 57, 38–47. [Google Scholar]
- Jin, J.; Ohanenye, I.C.; Udenigwe, C.C. Buckwheat proteins: Functionality, safety, bioactivity, and prospects as alternative plant-based proteins in the food industry. Crit. Rev. Food Sci. Nutr. 2020, 62, 1752–1764. [Google Scholar] [CrossRef] [PubMed]
- Annor, G.A.; Tyl, C.; Marcone, M.; Ragaee, S.; Marti, A. Why do millets have slower starch and protein digestibility than other cereals? Trends Food Sci. Technol. 2017, 66, 73–83. [Google Scholar] [CrossRef]
- Jin, J.; Okagu, O.D.; Yagoub, A.E.A.; Udenigwe, C.C. Effects of sonication on the In Vitro digestibility and structural properties of buckwheat protein isolates. Ultrason. Sonochem. 2021, 70, 105348. [Google Scholar] [CrossRef] [PubMed]
- Radović, S.R.; Maksimović, V.R.; Varkonji-Gašić, E.I. Characterization of buckwheat seed storage proteins. J. Agric. Food Chem. 1996, 44, 972–974. [Google Scholar] [CrossRef]
- Eggum, B.O. The protein quality of buckwheat in comparison with other protein sources of plant or animal origin. In Buckwheat: Genetics, Plant Breeding, Utilization; Kreft, I., Javornik, B., Dolinšek, B., Eds.; Biotechnical Faculty: Ljubljana, Slovenia, 1980; pp. 115–120. [Google Scholar]
- Eggum, B.O.; Kreft, I.; Javornik, B. Chemical composition and protein quality of buckwheat (Fagopyrum esculentum Moench). Plant Foods Hum. Nutr. 1980, 30, 175–179. [Google Scholar] [CrossRef]
- Chen, X.W.; Luo, D.Y.; Chen, Y.J.; Wang, J.M.; Guo, J.; Yang, X.Q. Dry fractionation of surface abrasion for polyphenol-enriched buckwheat protein combined with hydrothermal treatment. Food Chem. 2019, 285, 414–422. [Google Scholar] [CrossRef]
- Kemppainen, K.; Rommi, K.; Holopainen, U.; Kruus, K. Steam explosion of Brewer’s spent grain improves enzymatic digestibility of carbohydrates and affects solubility and stability of proteins. Appl. Biochem. Biotechnol. 2016, 180, 94–108. [Google Scholar] [CrossRef]
- Cirković Veličković, T.D.; Stanić Vučinić, D.J. The role of dietary phenolic compounds in protein digestion and processing technologies to improve their antinutritive properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Kishida, M. Digestibility of proteins in buckwheat seed. Fagopyrum 1993, 13, 21–24. [Google Scholar]
- Zhang, C.N.; Zhang, R.; Li, Y.M.; Liang, N.; Zhao, Y.M.; Zhu, H.Y.; He, Z.Y.; Liu, J.H.; Hao, W.J.; Jiao, R.; et al. Cholesterol-lowering activity of Tartary buckwheat protein. J. Agric. Food Chem. 2017, 65, 1900–1906. [Google Scholar] [CrossRef]
- Zhu, F. Buckwheat proteins and peptides: Biological functions and food applications. Trends Food Sci. Technol. 2021, 110, 155–167. [Google Scholar] [CrossRef]
- Kayashita, J.; Shimaoka, I.; Yamazaki, M.; Kato, N. Buckwheat protein extract ameliorates atropine-induced constipation in rats. Curr. Adv. Buckwheat Res. 1995, 5, 941–946. [Google Scholar]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Kato, N. Feeding of buckwheat protein extract reduces hepatic triglyceride concentration, adipose tissue weight, and hepatic lipogenesis in rats. J. Nutr. Biochem. 1996, 7, 555–559. [Google Scholar] [CrossRef]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Kishida, N.; Kato, N. Consumption of a buckwheat protein extract retards 7,12-dimethylbenz[alpha] anthracene-induced mammary carcinogenesis in rats. Biosci. Biotechnol. Biochem. 1999, 63, 1837–1839. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, K.; Zhang, H.; Yao, H. Anti-tumor activity of a novel protein obtained from tartary buckwheat. Int. J. Mol. Sci. 2010, 11, 5201–5211. [Google Scholar] [CrossRef]
- Vogrinčič, M.; Kreft, I.; Filipič, M.; Žegura, B. Antigenotoxic effect of tartary (Fagopyrum tataricum) and common (Fagopyrum esculentum) buckwheat flour. J. Med. Food 2013, 16, 944–952. [Google Scholar] [CrossRef]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Yamazaki, M.; Kato, N. Consumption of buckwheat protein lowers plasma cholesterol and raises fecal neutral sterols in cholesterol-fed rats because of its low digestibility. J. Nutr. 1997, 127, 1395–1400. [Google Scholar] [CrossRef] [Green Version]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Kondoh, M.; Hayashi, K.; Kato, N. Muscle hypertrophy in rats fed on a buckwheat protein extract. Biosci. Biotechnol. Biochem. 1999, 63, 1242–1245. [Google Scholar] [CrossRef]
- Koyama, M.; Naramoto, K.; Nakajima, T.; Aoyama, T.; Watanabe, M.; Nakamura, K. Purification and identification of antihypertensive peptides from fermented buckwheat sprouts. J. Agric. Food Chem. 2013, 61, 3013–3021. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, L.; Tang, W.; Zhou, Y.; Wang, Q.; Li, Z. A novel buckwheat protein with a beneficial effect in atherosclerosis was purified from Fagopyrum tataricum (L.) Gaertn. Arch. Biol. Sci. 2013, 65, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Leung, E.H.; Ng, T. A relatively stable antifungal peptide from buckwheat seeds with antiproliferative activity toward cancer cells. J. Pept. Sci. 2007, 13, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, J.P.; Vrchotova, N.; Triska, J. Phenolics levels in different parts of common buckwheat (Fagopyrum esculentum) achenes. J. Cereal Sci. 2019, 85, 243–248. [Google Scholar] [CrossRef]
- Ikeda, K.; Oku, M.; Kusano, T.; Yasumoto, K. Inhibitory potency of plant antinutrients towards the In Vitro digestibility of buckwheat protein. J. Food Sci. 1986, 51, 1527–1530. [Google Scholar] [CrossRef]
- Oparin, P.B.; Mineev, K.S.; Dunaevsky, Y.E.; Arseniev, A.S.; Belozersky, M.A.; Grishin, E.V.; Egorov, T.A.; Vassilevski, A.A. Buckwheat trypsin inhibitor with helical hairpin structure belongs to a new family of plant defence peptides. Biochem. J. 2012, 446, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Capraro, J.; Benedetti, S.D.; Heinzl, G.C.; Scarafoni, A.; Magni, C. Bioactivities of Pseudocereal Fractionated Seed Proteins and Derived Peptides Relevant for Maintaining Human Well-Being. Int. J. Mol. Sci. 2021, 22, 3543. [Google Scholar] [CrossRef]
- Koyama, M.; Hattori, S.; Amano, Y.; Watanabe, M.; Nakamura, K. Blood pressure-lowering peptides from neo-fermented buckwheat sprouts: A new approach to estimating ACE-inhibitory activity. PLoS ONE 2014, 9, e105802. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, L.; Li, Z.; Zhou, Y.; Chen, Y.; Lu, Y. Advance on the benefits of bioactive peptides from buckwheat. Phytochem. Rev. 2015, 14, 381–388. [Google Scholar] [CrossRef]
- Riedl, K.M.; Hagerman, A.E. Tannin-protein complexes as radical scavengers and radical sinks. J. Agric. Food Chem. 2001, 49, 4917–4923. [Google Scholar] [CrossRef]
- Li, C.H.; Matsui, T.; Matsumoto, K.; Yamasaki, R.; Kawasaki, T. Latent production of angiotensin I-converting enzyme inhibitors from buckwheat protein. J. Pept. Sci. 2002, 8, 267–274. [Google Scholar] [CrossRef]
- Fujimura, M.; Minami, Y.; Watanabe, K.; Tadera, K. Purification, characterization, and sequencing of a novel type of antimicrobial peptides, Fa-AMP1 and Fa-AMP2, from seeds of buckwheat (Fagopyrum esculentum Moench). Biosci. Biotechnol. Biochem. 2003, 67, 1636–1642. [Google Scholar] [CrossRef]
- Tomotake, H.; Shimaoka, I.; Kayashita, J.; Yokoyama, F.; Nakajoh, M.; Kato, N. A buckwheat protein product suppresses gallstone formation and plasma cholesterol more strongly than isolate in hamsters. J. Nutr. 2000, 130, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Tomotake, H.; Yamamoto, N.; Yanaka, N.; Ohinata, H.; Yamazaki, R.; Kayashita, J.; Kato, N. High protein buckwheat flour suppresses hypercholesterolemia in rats and gallstone formation in mice by hypercholesterolemic diet and body fat in rats because of its low protein digestibility. Nutrition 2006, 22, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Tomotake, H.; Yamamoto, N.; Kitabayashi, H.; Kawakami, A.; Kayashita, J.; Ohinata, H.; Karasawa, H.; Kato, N. Preparation of tartary buckwheat protein product and its improving effect on cholesterol metabolism in rats and mice fed cholesterol-enriched diet. J. Food Sci. 2007, 72, S528–S533. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.B.; Warkentin, T.D. Biofortification of Pulse Crops: Status and Future Perspectives. Plants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Pan, R.R.; Obadi, M.; Li, H.T.; Shao, F.; Sun, J.; Wang, Y.F.; Qi, Y.J.; Xu, B. In Vitro starch digestibility of buckwheat cultivars in comparison to wheat: The key role of starch molecular structure. Food Chem. 2022, 368, 130806. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Wang, T.; Li, Z.; Gao, Y.; Cui, S.W.; Qiu, J. Comparison of quercetin and rutin inhibitory influence on Tartary buckwheat starch digestion In Vitro and their differences in binding sites with the digestive enzyme. Food Chem. 2022, 367, 130762. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Q.; Ma, S.; Zhou, X. Effect of quercetin on the In Vitro Tartary buckwheat starch digestibility. Int. J. Biol. Macromol. 2021, 183, 818–830. [Google Scholar] [CrossRef]
- Škrabanja, V.; Liljeberg Elmståhl, H.G.M.; Kreft, I.; Björck, I.M.E. Nutritional properties of starch in buckwheat products: Studies In Vitro and In Vivo. J. Agric. Food Chem. 2001, 49, 490–496. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Liu, H.; Gao, J.; Li, Y.; Wang, M. Effect of rutin and quercetin on the physicochemical properties of Tartary buckwheat starch. Starch-Starke 2018, 70, 1700038. [Google Scholar] [CrossRef]
- Luo, K.; Zhou, X.; Zhang, G. The impact of Tartary buckwheat extract on the nutritional property of starch in a whole grain context. J. Cereal Sci. 2019, 89, 102798. [Google Scholar] [CrossRef]
- Gao, J.; Kreft, I.; Chao, G.; Wang, Y.; Liu, W.; Wang, L.; Wang, P.; Gao, X.; Feng, B. Tartary buckwheat (Fagopyrum tataricum Gaertn.) starch, a side product in functional food production, as a potential source of retrograded starch. Food Chem. 2016, 190, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Ikeda, S. Buckwheat in Japan. In Ethnobotany of Buckwheat; Kreft, I., Chang, K.J., Choi, Y.S., Park, C.H., Eds.; Jinsol Publishing Co.: Seoul, Korea, 2003; pp. 54–56. [Google Scholar]
- Li, Y.; Gao, S.; Ji, X.; Liu, H.; Liu, N.; Yang, J.; Lu, M.; Han, L.; Wang, M. Evaluation studies on effects of quercetin with different concentrations on the physicochemical properties and In Vitro digestibility of Tartary buckwheat starch. Int. J. Biol. Macromol. 2020, 163, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Boateng, N.A.S.; Ma, H. Ultrasound-induced lipid peroxidation: Effects on phenol content and extraction kinetics and antioxidant activity of Tartary buckwheat (Fagopyrum tataricum) water extract. Food Biosci. 2020, 37, 100719. [Google Scholar] [CrossRef]
- Noda, T.; Ishiguro, K.; Suzuki, T.; Morishita, T. Roasted Tartary Buckwheat Bran as a Material for Producing Rutin-Rich Tea Beverages. Plants 2021, 10, 2662. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Fu, G.M.; Xu, Z.W.; Dong, B.A.; Li, R.Y.; Wan, Y.; Jiang, G.F.; Liu, C.M. In Vitro nutrition properties of whole Tartary buckwheat straight noodles and its amelioration on type 2 diabetic rats. Food Biosci. 2022, 46, 101525. [Google Scholar] [CrossRef]



| Country | Year of Data | Area Harvested (ha) | Yield (t/ha) | Production Quantity (t) |
|---|---|---|---|---|
| Belarus | 2020 | 27,354 | 1.03 | 28,300 |
| Bhutan | 2020 | 2004 | 1.35 | 2701 |
| Bosnia and Herzegovina | 2020 | 833 | 1.56 | 1301 |
| Brazil | 2020 | 46,416 | 1.40 | 65,117 |
| Canada | 2020 | 9800 | 0.91 | 8900 |
| China, mainland | 2020 | 624,780 | 0.81 | 503,988 |
| Croatia | 2017 | 695 | 0.90 | 624 |
| Czech Republic | 2017 | 887 | 2.55 | 2262 |
| Estonia | 2017 | 5278 | 0.64 | 3385 |
| France | 2017 | 74,883 | 3.52 | 263,485 |
| Georgia | 2020 | 106 | 1.11 | 118 |
| Hungary | 2017 | 969 | 0.94 | 909 |
| Japan | 2020 | 66,600 | 0.67 | 44,800 |
| Kazakhstan | 2020 | 55,076 | 0.73 | 40,094 |
| Kyrgyzstan | 2020 | 10 | 1.70 | 17 |
| Latvia | 2017 | 18,300 | 0.93 | 17,100 |
| Lithuania | 2017 | 48,499 | 1.10 | 53,221 |
| Nepal | 2020 | 10,369 | 1.13 | 11,724 |
| Poland | 2027 | 78,027 | 1.45 | 113,113 |
| Korea | 2020 | 1600 | 0.97 | 1549 |
| Republic of Moldova | 2020 | 5 | 0.80 | 4 |
| Russian | 2020 | 821,366 | 1.09 | 892,160 |
| Slovakia | 2017 | 429 | 0.86 | 367 |
| Slovenia | 2017 | 3647 | 0.80 | 2909 |
| South Africa | 2020 | 579 | 0.40 | 234 |
| Ukraine | 2020 | 84,100 | 1.16 | 97,640 |
| United Republic of Tanzania | 2020 | 24,295 | 1.06 | 25,772 |
| USA | 2020 | 81,620 | 1.06 | 86,397 |
| World | 2,088,527 | 1.16 | 2,268,191 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreft, I.; Germ, M.; Golob, A.; Vombergar, B.; Bonafaccia, F.; Luthar, Z. Impact of Rutin and Other Phenolic Substances on the Digestibility of Buckwheat Grain Metabolites. Int. J. Mol. Sci. 2022, 23, 3923. https://doi.org/10.3390/ijms23073923
Kreft I, Germ M, Golob A, Vombergar B, Bonafaccia F, Luthar Z. Impact of Rutin and Other Phenolic Substances on the Digestibility of Buckwheat Grain Metabolites. International Journal of Molecular Sciences. 2022; 23(7):3923. https://doi.org/10.3390/ijms23073923
Chicago/Turabian StyleKreft, Ivan, Mateja Germ, Aleksandra Golob, Blanka Vombergar, Francesco Bonafaccia, and Zlata Luthar. 2022. "Impact of Rutin and Other Phenolic Substances on the Digestibility of Buckwheat Grain Metabolites" International Journal of Molecular Sciences 23, no. 7: 3923. https://doi.org/10.3390/ijms23073923
APA StyleKreft, I., Germ, M., Golob, A., Vombergar, B., Bonafaccia, F., & Luthar, Z. (2022). Impact of Rutin and Other Phenolic Substances on the Digestibility of Buckwheat Grain Metabolites. International Journal of Molecular Sciences, 23(7), 3923. https://doi.org/10.3390/ijms23073923









