Lactadherin: From a Well-Known Breast Tumor Marker to a Possible Player in Extracellular Vesicle-Mediated Cancer Progression
Abstract
:1. Introduction
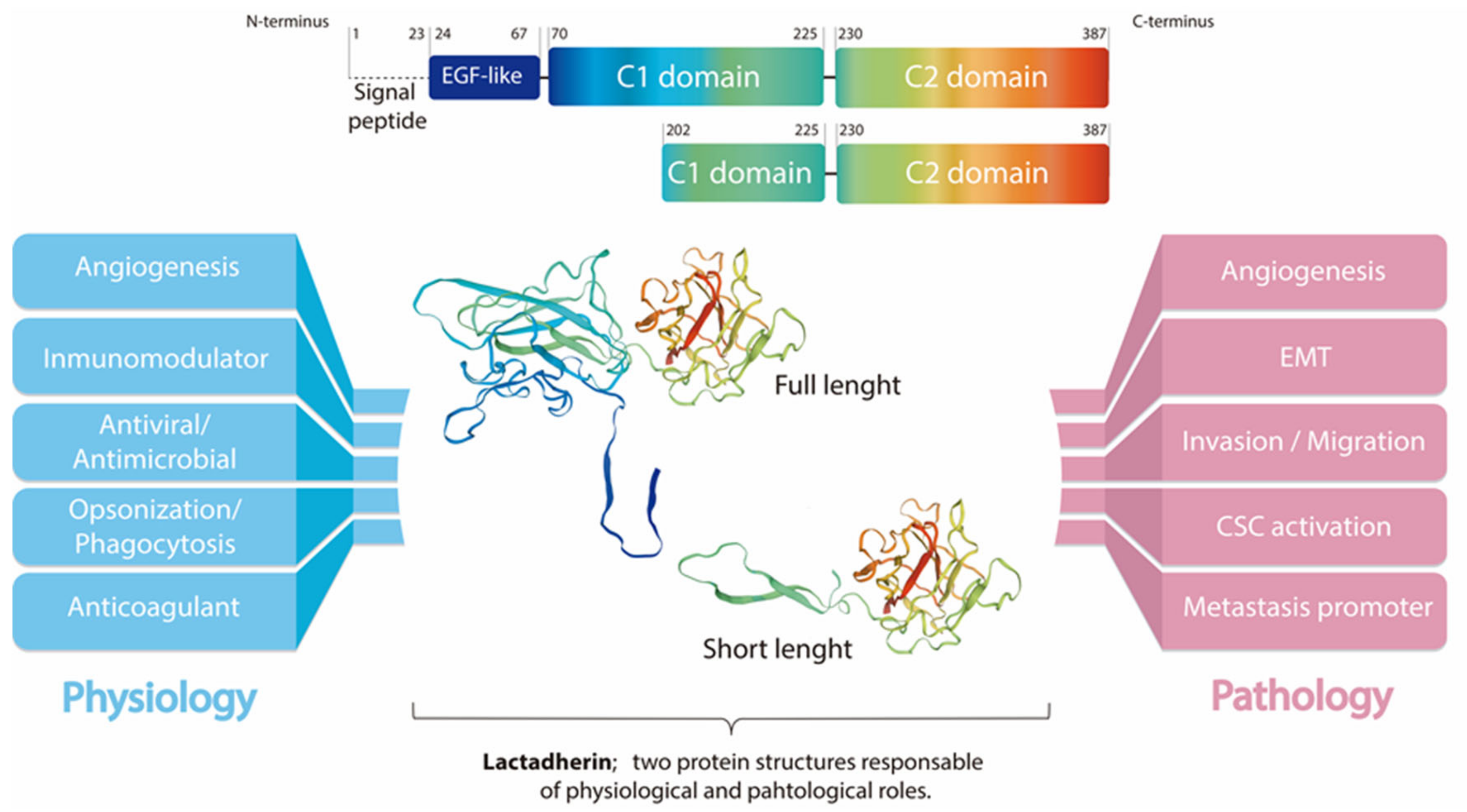
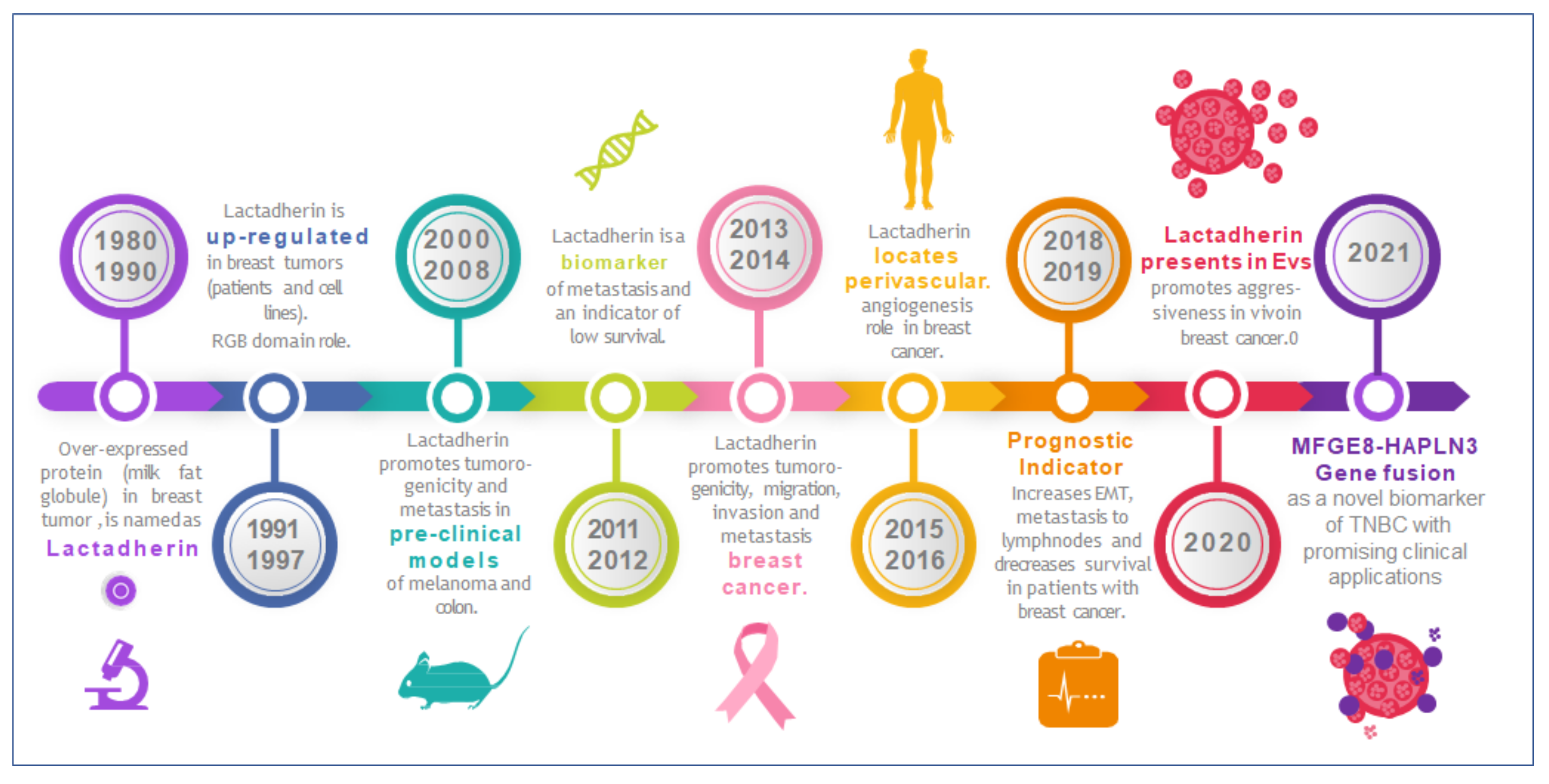
Lactadherin: A Milk Fat Globule Protein Associated with Cancer Development
2. Lactadherin as a Biomarker of Tumor Subtype, Progression and Metastasis
3. Lactadherin Role as Promotor of Tumor Progression
3.1. Tumor Cell Survival/Proliferation and EMT
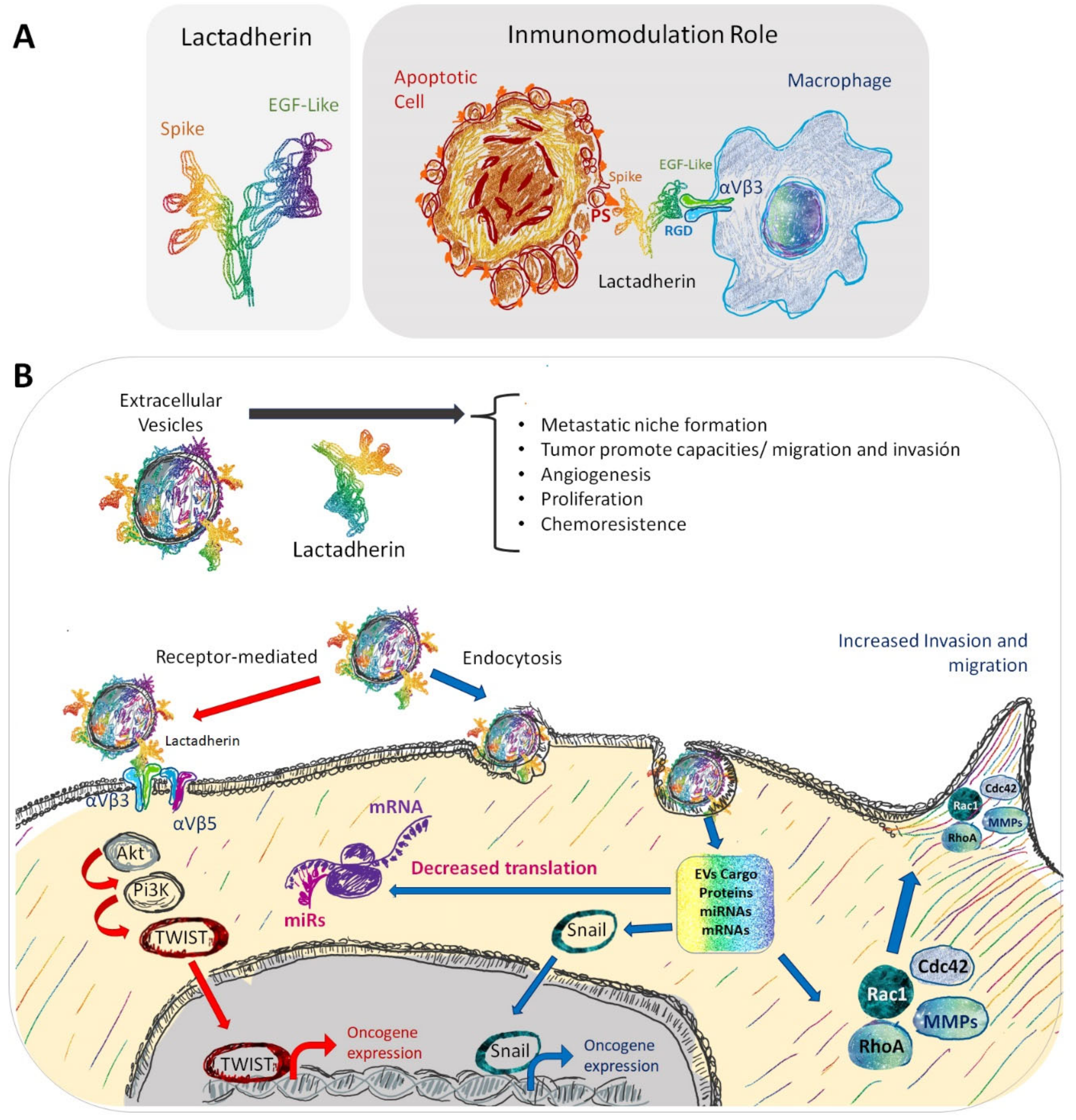
3.2. Lactadherin Immunomodulatory Role
4. Lactadherin as a Possible New Cancer Therapeutic Target
State of Art on Lactadherin Translational Medicine Research and Intellectual Property
5. Extracellular Vesicles and Exosomes as Promotors of Breast Cancer Metastasis
6. Role and Use of Lactadherin in EVs and Exosomes
| Cancer Type | Experimental Strategy | Findings | References |
|---|---|---|---|
| Breastcancer (BC) | Hybridoma clones to obtain MoAbs against HMFG components | MoAbs binds to human epithelial BC cell lines, breast epithelium sections of primary carcinomas and lactating breast. Not reactive against epithelial cell lines of non-breast origin | [26,30] |
| Antisera against HME-Ags to determine its presence in the sera of patients with disseminated BC and others | HME-Ags detected in sera of BC patients, but not in sera from non-BC patients or in normal female controls | [33] | |
| Human mammary epithelial cells to characterize the specificity of MoAbs against HMFG components | Determination of the molecular weight of three different targets of MoAbs. One of them is directed against a 46 kDa protein | [27,30] | |
| MoAbs against Ags of the HMFG to characterize different BC cells | MoAbs are useful in characterizing breast epithelial cells, studying surface alterations in malignancy, and possibly in BC diagnosis and therapy | [32] | |
| MoAbs to select complementary DNAs from an expression library of lactating breast to better characterize the 46 kDa antigen | Detection of one large complementary DNA, encoding a 217 aa peptide. Detection and OE of a single 2.2 kb RNA in a variety of carcinoma cell lines. Sequencing revealed strong homology of the 46 kDa glycoprotein with serum factors VIII and V | [3] | |
| Purified lactadherin to evaluate RGD-dependent cell adhesion | Lactadherin promotes RGD-dependent cell adhesion. This is dependent of integrins, mainly αvβ3 | [2] | |
| Immortalized mammary epithelial cells Analysis of public gene expression data | Lactadherin promotes tumorigenic potential through regulation of cyclins D1/D3 and N-Cadherin Lactadherin is highly expressed in primary and metastatic BC, associated with absent ER expression | [21] | |
| IHC to evaluate lactadherin presence in BC biopsies | Lactadherin OE in BC tissue. OE associated with poor prognosis parameters | [46] | |
| Analysis of TCGA patient genomic data | Detection and validation of MFGE8-HPLN3 in TNBC patient specimens | [47] | |
| EVs secreted by MDA-MB-231 human BC cells. WB analysis | EVs secreted by MDA-MB-231 cell line contain lactadherin | [91] | |
| Proteomic and WB analysis | Exo-WT (secreted by wild type MDA-MB-231) are enriched with lactadherin compared with Exo-1537S Exo-WT (containing lactadherin) promotes tumorigenic capacities on recipient cells and mice | [23] | |
| Pancreatic cancer | Transgenic Rip2-Tag2 mouse model of multistage carcinogenesis | Lactadherin OE in angiogenic islets and tumors of mice compared with normal pancreas, promoting tumor growth | [19] |
| Melanoma | Murine B16 melanoma cells Human primary melanoma cell lines | High expression of lactadherin in the growth phase of melanoma, promoting melanoma progression through Akt/Twist signaling | [39] |
| IHC of primary melanoma, metastatic lesions and benign tissue. | High expression of lactadherin in primary and metastatic melanoma High expression associated with tumor progression and worse survival | [40] | |
| Co-injection of B16 melanoma cells and MSC derived from wild type (WT) or MFGE8 KO into mice | Lactadherin promotes melanoma growth through MSCs-induced angiogenesis and M2 polarization of TAMs | [41] | |
| Bladder cancer | Transcriptomic analysis of bladder carcinoma biopsies | Lactadherin OE during tumor development Correlation between lactadherin levels with expression of genes involved in cell adhesion, migration, and immune response, promoting tumor growth | [20] |
| Ovarian cancer (OC) | IHC of human OC biopsies Lactadherin blocking MoAbs effect over tumorigenic capacities of human OC and TNBC cell lines | Lactadherin OE in OC biopsies and in a TNBC cell line Blocking MoAbs efficiently blocked lactadherin tumor-promoting effects, such as survival, migration, and adhesion | [22] |
| Lactadherin and CD133 expression levels were analyzed by IHC in epithelial OC specimens | Lactadherin OE significantly correlated with the presence of CD133 Lactadherin and CD133 levels significantly correlated with bad prognosis parameters in OC | [34] | |
| Colorectal cancer (CRC) | IHC and IF analysis of lactadherin expression in CRC biopsies | Lactadherin OE in CRC samples compared with normal mucosa tissues Lactadherin in close proximity to endothelial cells. Expression correlated with bad prognosis parameters and worse survival | [18] |
| IHC and qRT-PCR analysis of lactadherin expression in CRC biopsies Analysis of CRC RNAseq data from TCGA Use of shRNA and recombinant human lactadherin to investigate its role in CRC cell growth, migration, and invasion | Lactadherin OE in advanced CRC tissues compared with early-stage CRC and adjacent non-cancerous tissues Lactadherin levels correlated with bad prognosis and survival parameters Lactadherin promotes CRC cell migration and invasion, increases levels of MMP-2 and MMP-9, and promotes EMT and promotes progression via AKT/MMPs signaling | [42] | |
| Evaluation of MFG-E8 levels in patient’s tumor specimens Evaluation of antitumor effect of coptisine in vitro and in vivo | OE of MFG-E8 in human CRC tissue samples versus adjacent normal ones Coptisine inhibited CRC growth and progression by downregulating MFG-E8 expression, and inhibiting EMT and expression of MMP-2 and MMP-9 via the PI3K/AKT signaling pathway | [53] | |
| Gastric cancer (GC) | Analysis of GC RNAseq data from TCGA | MGFE8 mRNA level associated with worse survival Establishment of a prognostic predictive model for GC that includes MFGE8 gene measurement | [38] |
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabha, B.H.; Alzahrani, F.; Almehdar, H.A.; Uversky, V.N.; Redwan, E.M. Disorder in milk proteins: Lactadherin multifunctionality and structure. Curr. Protein Pept. Sci. 2018, 19, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.R.; Couto, J.R.; Scallan, C.D.; Ceriani, R.L.; Peterson, J.A. Lactadherin (Formerly BA46), a Membrane-Associated Glycoprotein Expressed in Human Milk and Breast Carcinomas, Promotes Arg-Gly-Asp (RGD)-Dependent Cell Adhesion. DNA Cell Biol. 1997, 16, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Larocca, D.; Peterson, J.A.; Urrea, R.; Kuniyoshi, J.; Bistrain, A.M.; Ceriani, R.L. A Mr 46,000 human milk fat globule protein that is highly expressed in human breast tumors contains Factor VHI-like domains. Cancer Res. 1991, 51, 4994. [Google Scholar] [PubMed]
- Couto, J.R.; Taylor, M.R.; Godwin, S.G.; Ceriani, R.L.; Peterson, J.A. Cloning and Sequence Analysis of Human Breast Epithelial Antigen BA46 Reveals an RGD Cell Adhesion Sequence Presented on an Epidermal Growth Factor-Like Domain. DNA Cell Biol. 1996, 15, 281–286. [Google Scholar] [CrossRef]
- Andersen, M.H.; Graversen, H.; Fedosov, S.N.; Petersen, T.E.; Rasmussen, J.T. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry 2000, 39, 6200–6206. [Google Scholar] [CrossRef] [Green Version]
- Giuffrida, M.G.; Cavaletto, M.; Giunta, C.; Conti, A.; Godovac-Zimmermann, J. Isolation and characterization of full and truncated forms of human breast carcinoma protein BA46 from human milk fat globule membranes. J. Protein Chem. 1998, 17, 143–148. [Google Scholar] [CrossRef]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef]
- Newburg, D.S.; Peterson, J.A.; Ruiz-Palacios, G.M.; Matson, D.O.; Morrow, A.L.; Shults, J.; Guerrero, M.L.; Chaturvedi, P.; Newburg, S.O.; Scallan, C.D.; et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 1998, 351, 1160–1164. [Google Scholar] [CrossRef]
- Kvistgaard, A.; Pallesen, L.; Arias, C.; Lopez, S.; Petersen, T.; Heegaard, C.; Rasmussen, J.T. Inhibitory Effects of Human and Bovine Milk Constituents on Rotavirus Infections. J. Dairy Sci. 2004, 87, 4088–4096. [Google Scholar] [CrossRef] [Green Version]
- Jinushi, M.; Chiba, S.; Yoshiyama, H.; Masutomi, K.; Kinoshita, I.; Dosaka-Akita, H.; Yagita, H.; Takaoka, A.; Tahara, H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc. Natl. Acad. Sci. USA 2011, 108, 12425–12430. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, J.-S.; Théry, C.; Hamard, G.; Boddaert, J.; Aguilar, B.; Delcayre, A.; Houbron, C.; Tamarat, R.; Blanc-Brude, O.; Heeneman, S.; et al. Lactadherin promotes VEGF-dependent neovascularization. Nat. Med. 2005, 11, 499–506. [Google Scholar] [CrossRef]
- Zhou, Y.; Bond, A.M.; Shade, J.E.; Zhu, Y.; Davis, C.-H.O.; Wang, X.; Su, Y.; Yoon, K.-J.; Phan, A.T.; Chen, W.J.; et al. Autocrine Mfge8 Signaling Prevents Developmental Exhaustion of the Adult Neural Stem Cell Pool. Cell Stem Cell 2018, 23, 444–452.e4. [Google Scholar] [CrossRef] [Green Version]
- Kamińska, A.; Enguita, F.J.; Stępień, E. Lactadherin: An unappreciated haemostasis regulator and potential therapeutic agent. Vasc. Pharmacol. 2018, 101, 21–28. [Google Scholar] [CrossRef]
- Uchiyama, A.; Yamada, K.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Ishikawa, O.; Motegi, S.-I. Protective Effect of MFG-E8 after Cutaneous Ischemia–Reperfusion Injury. J. Investig. Dermatol. 2015, 135, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Motegi, S.-I.; Leitner, W.; Lu, M.; Tada, Y.; Sárdy, M.; Wu, C.; Chavakis, T.; Udey, M.C. Pericyte-Derived MFG-E8 Regulates Pathologic Angiogenesis. Arter. Thromb. Vasc. Biol. 2011, 31, 2024–2034. [Google Scholar] [CrossRef] [Green Version]
- Motegi, S.; Ishikawa, O. Mesenchymal stem cells: The roles and functions in cutaneous wound healing and tumor growth. J. Dermatol. Sci. 2017, 86, 83–89. [Google Scholar] [CrossRef]
- Nakashima, Y.; Miyagi-Shiohira, C.; Noguchi, H.; Omasa, T. The Healing Effect of Human Milk Fat Globule-EGF Factor 8 Protein (MFG-E8) in A Rat Model of Parkinson’s Disease. Brain Sci. 2018, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Yao, H.; Chen, C.; Wang, Y.; Wang, H.; Cui, T.; Zhu, J. Prognostic Correlation Between MFG-E8 Expression Level and Colorectal Cancer. Arch. Med. Res. 2017, 48, 270–275. [Google Scholar] [CrossRef]
- Neutzner, M.; Lopez, T.; Feng, X.; Bergmann-Leitner, E.S.; Leitner, W.W.; Udey, M.C. MFG-E8/lactadherin promotes tumor growth in an angiogenesis-dependent transgenic mouse model of multistage carcinogenesis. Cancer Res. 2007, 67, 6777–6785. [Google Scholar] [CrossRef] [Green Version]
- Sugano, G.; Bernard-Pierrot, I.; Laé, M.; Battail, C.; Allory, Y.; Stransky, N.; Krumeich, S.; Lepage, M.-L.; Maille, P.; Donnadieu, M.-H.; et al. Milk fat globule–epidermal growth factor–factor VIII (MFGE8)/lactadherin promotes bladder tumor development. Oncogene 2010, 30, 642–653. [Google Scholar] [CrossRef] [Green Version]
- Carrascosa, C.; Obula, R.G.; Missiaglia, E.; Lehr, H.A.; Delorenzi, M.; Frattini, M.; Rüegg, C.; Mariotti, A. MFG-E8/lactadherin regulates cyclins D1/D3 expression and enhances the tumorigenic potential of mammary epithelial cells. Oncogene 2012, 31, 1521–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibaldi, L.; Leyman, S.; Nicolas, A.; Notebaert, S.; Dewulf, M.; Ngo, T.H.; Zuany-Amorim, C.; Amzallag, N.; Bernard-Pierrot, I.; Sastre-Garau, X.; et al. New Blocking Antibodies Impede Adhesion, Migration and Survival of Ovarian Cancer Cells, Highlighting MFGE8 as a Potential Therapeutic Target of Human Ovarian Carcinoma. PLoS ONE 2013, 8, e72708. [Google Scholar] [CrossRef] [PubMed]
- Lobos-González, L.; Bustos, R.; Campos, A.; Silva, V.; Silva, V.; Jeldes, E.; Salomon, C.; Varas-Godoy, M.; Cáceres-Verschae, A.; Duran, E.; et al. Exosomes released upon mitochondrial ASncmtRNA knockdown reduce tumorigenic properties of malignant breast cancer cells. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crescitelli, R. Subpopulations of extracellular vesicles from human metastatic melanoma tissue identified by quantitative proteomics after optimized isolation. J. Extracell. Vesicles 2020, 9, 1722433. [Google Scholar] [CrossRef]
- Bocca, S.; Anderson, S.; Amaker, B.; Swanson, R.; Franchi, A.; Lattanzio, F.; Oehninger, S. Milk fat globule epidermal growth factor 8 (MFG-E8): A novel protein in the mammalian endometrium with putative roles in implantation and placentation. Placenta 2012, 33, 795–802. [Google Scholar] [CrossRef]
- Arklie, J.; Taytor-Papadimitrou, J.; Bodmer, W.; Egar, M.; Millis, R. Differentiation antigens expressed by epithelial cells in the lactating breast are also detectable in breast cancer. Int. J. Cancer 1981, 28, 23–29. [Google Scholar] [CrossRef]
- Ceriani, R.L.; Peterson, J.A.; Blank, E.W. Breast Cancer Diagnosis with Human Mammary Epithelial Antigens and the Prospective Use of Antibodies against Them in Therapy. In Mechanisms of Cancer Metastasis. Developments in Oncology; Honn, K.V., Powers, W.E., Sloane, B.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 235–257. [Google Scholar]
- UCSC Xena Open Access. Available online: https://xena.ucsc.edu (accessed on 13 May 2020).
- Human Protein Atlas. (Shows Expression of Lactadherin mRNA and Protein in All Female Reproductive Organs). Available online: https://www.proteinatlas.org/ENSG00000140545-MFGE8/tissue (accessed on 13 May 2020).
- Taylor-Papadimitriou, J.; Peterson, J.A.; Arklie, J.; Burchell, J.; Ceriani, R.L.; Bodmer, W.F. Monoclonal antibodies to epithelial-specific components of the human milk fat globule membrane: Production and reaction with cells in culture. Int. J. Cancer 1981, 28, 17–28. [Google Scholar] [CrossRef]
- Sasaki, M.; Peterson, J.A.; Ceriani, R.L. Monoclonal antibodies against breast epithelial cell surface antigens in breast cancer therapy. Hybridoma 1983, 2, 120. [Google Scholar]
- Peterson, J.A.; Zava, D.T.; Duwe, A.K.; Blank, E.W.; Battifora, H.; Ceriani, R.L. Biochemical and histological characterization of antigens preferentially expressed on the surface and cytoplasm of breast carcinoma cells identified by monoclonal antibodies against the human milk fat globule. Hybridoma 1990, 9, 221–235. [Google Scholar] [CrossRef]
- Ceriani, R.L.; Sasaki, M.; Sussman, H.; Wara, W.M.; Blank, E.W. Circulating human mammary epithelial antigens in breast cancer. Proc. Natl. Acad. Sci. USA 1982, 79, 5420–5424. [Google Scholar] [CrossRef] [Green Version]
- Na, L.; Dai, C.; Yang, Y.; Wu, X.; Wang, L.; Wang, P. The expression levels and clinical significance of MFG-E8 and CD133 in epithelial ovarian cancer. Gynecol. Endocrinol. 2020, 36, 803–807. [Google Scholar]
- Okamoto, A.; Sakakura, K.; Takahashi, H.; Motegi, S.I.; Kaira, K.; Yokobori-Kuwabara, Y.; Ishikawa, O.; Chikamatsu, K. Immunological and clinicopathological significance of MFG-E8 expression in patients with oral squamous cell carcinoma. Pathol. Oncol. Res. 2019, 26, 1263–1268. [Google Scholar] [CrossRef]
- Kanemura, T.; Miyata, H.; Makino, T.; Tanaka, K.; Sugimura, K.; Hamada-Uematsu, M.; Mizote, Y.; Uchida, H.; Miyazaki, Y.; Takahashi, T.; et al. Immunoregulatory influence of abundant MFG -E8 expression by esophageal cancer treated with chemotherapy. Cancer Sci. 2018, 109, 3393–3402. [Google Scholar] [CrossRef]
- Fujiwara, C.; Motegi, S.-I.; Ohira, A.; Yamaguchi, S.; Sekiguchi, A.; Yasuda, M.; Nakamura, H.; Makiguchi, T.; Yokoo, S.; Hoshina, D.; et al. The significance of tumor cells-derived MFG-E8 in tumor growth of angiosarcoma. J. Dermatol. Sci. 2019, 96, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Lai, Z.; Li, Y.; Mu, J.; Yang, M.; Xie, J.; Xu, J. Immune signature profiling identified prognostic factors for gastric cancer. Chin. J. Cancer Res. 2019, 31, 463–470. [Google Scholar] [CrossRef]
- Jinushi, M.; Nakazaki, Y.; Carrasco, D.R.; Draganov, D.; Souders, N.; Johnson, M.; Mihm, M.C.; Dranoff, G. Milk Fat Globule EGF-8 Promotes Melanoma Progression through Coordinated Akt and Twist Signaling in the Tumor Microenvironment. Cancer Res. 2008, 68, 8889–8898. [Google Scholar] [CrossRef] [Green Version]
- Oba, J.; Moroi, Y.; Nakahara, T.; Abe, T.; Hagihara, A.; Furue, M. Expression of milk fat globule epidermal growth factor-VIII may be an indicator of poor prognosis in malignant melanoma. Br. J. Dermatol. 2011, 165, 506–512. [Google Scholar] [CrossRef]
- Yamada, K.; Uchiyama, A.; Uehara, A.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Udey, M.C.; Ishikawa, O.; Motegi, S.-I. MFG-E8 Drives Melanoma Growth by Stimulating Mesenchymal Stromal Cell–Induced Angiogenesis and M2 Polarization of Tumor-Associated Macrophages. Cancer Res. 2016, 76, 4283–4292. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Xu, L.; Sun, X.; Zhang, K.; Shen, H.; Tian, Y.; Sun, F.; Li, Y. MFG-E8 overexpression promotes colorectal cancer progression via AKT/MMPs signalling. Tumor Biol. 2017, 39, 1010428317707881. [Google Scholar] [CrossRef] [Green Version]
- Shimagaki, T.; Yoshio, S.; Kawai, H.; Sakamoto, Y.; Doi, H.; Matsuda, M.; Mori, T.; Osawa, Y.; Fukai, M.; Yoshida, T.; et al. Serum milk fat globule-EGF factor 8 (MFG-E8) as a diagnostic and prognostic biomarker in patients with hepatocellular carcinoma. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Ko, D.S.; Kim, S.H.; Park, J.Y.; Lee, G.; Kim, H.J.; Kim, G.; Chi, K.Y.; Kim, I.; Lee, J.; Won, K.-Y.; et al. Milk Fat Globule-EGF Factor 8 Contributes to Progression of Hepatocellular Carcinoma. Cancers 2020, 12, 403. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Hayashida, T.; Forster, N.; Li, C.; Shen, D.; Maheswaran, S.; Chen, L.; Anderson, K.S.; Ellisen, L.W.; Sgroi, D.; et al. The integrin alpha(v)beta(3–5) ligand MFG-E8 is a p63/p73 target gene in triple-negative breast cancers but exhibits suppressive functions in ER(+) and erbB2(+) breast cancers. Cancer Res. 2011, 71, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Zhao, L.; Jia, Z.; Bi, J.; Wei, Q.; Song, X.; Jiang, L.; Lin, S.; Wei, M. MFG-E8 overexpression is associated with poor prognosis in breast cancer patients. Pathol. Res. Pr. 2018, 215, 490–498. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Huang, M.; Wang, C.-Y.; Tang, X.-Y.; Wang, J.-G.; Yang, Y.-D.; Xiong, X.; Gao, C.-W. Transcriptome Analysis Reveals MFGE8-HAPLN3 Fusion as a Novel Biomarker in Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 682021. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Song, Q.; Zhu, K.; Yu, X.; Tian, Y.; Zhang, J. Reduction in milk fat globule-EGF factor 8 inhibits triple-negative breast cancer cell viability and migration. Oncol. Lett. 2019, 17, 3457–3465. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, A.; Zhu, Y.; He, W.; Di, W.; Fang, Y.; Shi, X. MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-κB and PI3K-Akt pathways. J. Cell. Physiol. 2018, 234, 904–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Xu, W.; Ma, Y.; Zhou, S.; Xiao, R. Milk fat globule membrane protein promotes C2C12 cell proliferation through the PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2018, 114, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-Y.; Zhang, Z.-H.; Zhuang, Z.; Lu, Y.; Wu, L.-Y.; Ye, Z.N.; Zhang, X.-S.; Chen, C.-L.; Li, W.; Hang, C.-H. Recombinant milk fat globule-EGF factor-8 reduces apoptosis via integrin β3/FAK/PI3K/AKT signaling pathway in rats after traumatic brain injury. Cell Death Dis. 2018, 9, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusunoki, R.; Ishihara, S.; Tada, Y.; Oka, A.; Sonoyama, H.; Fukuba, N.; Oshima, N.; Moriyama, I.; Yuki, T.; Kawashima, K.; et al. Role of milk fat globule-epidermal growth factor 8 in colonic inflammation and carcinogenesis. J. Gastroenterol. 2015, 50, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Hong, S.; Li, Y.; Chen, H.; Shen, Y.; Shao, K.; Lu, M.; Dai, H.; Ma, S.; Dai, G. Coptisine suppresses tumor growth and progression by down-regulating MFG-E8 in colorectal cancer. RSC Adv. 2018, 8, 30937–30945. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.-Y.; Ma, X.-L.; Li, Z.-M.; Deng, R.; Wang, S.-M.; Shen, G.-B.; Zhang, J.; Wang, F.-T.; Zhang, B.-L.; Wei, Y.-Q. Down-regulation of MFG-E8 by RNA interference combined with doxorubicin triggers melanoma destruction. Clin. Exp. Med. 2014, 15, 127–135. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H.; Cheng, J.; Zhang, L.; Ke, Y.; Zhu, Y.; Wang, C.; Zhang, X.; Zhen, X.; Zheng, L.T. Knockdown of milk-fat globule EGF factor-8 suppresses glioma progression in GL261 glioma cells by repressing microglial M2 polarization. J. Cell. Physiol. 2019, 235, 8679–8690. [Google Scholar] [CrossRef]
- Zhuowang, G.; Chen, Y.; Wang, B.; Zhang, X.; Yan, Y.; Zhou, L.; Zhang, Y.; Xie, Y. MFGE8 attenuates Ang-II-induced atrial fibrosis and vulnerability to atrial fibrillation through inhibition of TGF-β1/Smad2/3 pathway. J. Mol. Cell. Cardiol. 2020, 139, 164–175. [Google Scholar]
- Matsuda, A.; Jacob, A.; Wu, R.; Zhou, M.; Aziz, M.; Wang, P. Milk fat globule–EGF factor VIII ameliorates liver injury after hepatic ischemia-reperfusion. J. Surg. Res. 2012, 180, e37–e46. [Google Scholar] [CrossRef] [Green Version]
- Deng, K.-Q.; Li, J.; She, Z.-G.; Gong, J.; Cheng, W.-L.; Gong, F.-H.; Zhu, X.-Y.; Zhang, Y.; Wang, Z.; Li, H. Restoration of Circulating MFGE8 (Milk Fat Globule-EGF Factor 8) Attenuates Cardiac Hypertrophy Through Inhibition of Akt Pathway. Hypertension 2017, 70, 770–779. [Google Scholar] [CrossRef]
- Ceriani, R.L.; Blank, E.W.; Peterson, J.A. Experimental immunotherapy of human breast carcinomas implanted in nude mice with a mixture of monoclonal antibodies against human milk fat globule components. Cancer Res. 1987, 47, 532–540. [Google Scholar]
- Kotari, C.; Osseni, M.A.; Agbo, L.; Ouellette, G.; Déraspe, M.; Laviolette, F.; Corbeil, J.; Lambert, J.-P.; Diorio, C.; Durocher, F. Machine learning analysis identifies genes differentiating triple negative breast cancers. Sci. Rep. 2020, 10, 10464. [Google Scholar] [CrossRef]
- Tian, J.; Wang, V.; Wang, N.; Khadang, B.; Boudreault, J.; Bakdounes, K.; Ali, S.; Lebrun, J.-J. Identification of MFGE8 and KLK5/7 as mediators of breast tumorigenesis and resistance to COX-2 inhibition. Breast Cancer Res. 2021, 23, 23. [Google Scholar] [CrossRef]
- Tuohy, V.K. A prophylactic vaccine for breast cancer? Why not? Breast Cancer Res. 2010, 12, 405. [Google Scholar] [CrossRef] [Green Version]
- Tuohy, V.K.; Jaini, R.; Johnson, J.M.; Loya, M.G.; Wilk, D.; Downs-Kelly, E.; Mazumder, S. Targeted Vaccination against Human α-Lactalbumin for Immunotherapy and Primary Immunoprevention of Triple Negative Breast Cancer. Cancers 2016, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Lee, K.W.; Cho, J.A.; Park, H.; Lim, E.H. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int. J. Oncol. 2011, 40, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Gorczynski, R.M.; Erin, N.; Zhu, F. Serum-derived exosomes from mice with highly metastatic breast cancer transfer increased metastatic capacity to a poorly metastatic tumor. Cancer Med. 2016, 5, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Harris, D.A.; Patel, S.H.; Gucek, M.; Hendrix, A.; Westbroek, W.; Taraska, J.W. Exosomes Released from Breast Cancer Carcinomas Stimulate Cell Movement. PLoS ONE 2015, 10, e0117495. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Zhang, C.; Lum, D.; Druso, J.E.; Blank, B.; Wilson, K.F.; Welm, A.; Antonyak, M.A.; Cerione, R.A. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat. Commun. 2017, 8, 14450. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Galindo-Hernandez, O.; Serna-Marquez, N.; Castillo-Sanchez, R.; Salazar, E.P. Extracellular vesicles from MDA-MB-231 breast cancer cells stimulated with linoleic acid promote an EMT-like process in MCF10A cells. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 299–310. [Google Scholar] [CrossRef]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef] [Green Version]
- Joyce, D.P.; Kerin, M.J.; Dwyer, R.M. Exosome-encapsulated microRNAs as circulating biomarkers for breast cancer. Int. J. Cancer 2016, 139, 1443–1448. [Google Scholar] [CrossRef]
- Wong, G.L.; Jalboush, S.A.; Lo, H.-W. Exosomal MicroRNAs and Organotropism in Breast Cancer Metastasis. Cancers 2020, 12, 1827. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Q.; Zhong, H.; Li, L.; Zhang, Q.; Huang, Q.; Yu, Z. Differentially expressed microRNAs in exosomes of patients with breast cancer revealed by next-generation sequencing. Oncol. Rep. 2019, 43, 240–250. [Google Scholar] [CrossRef]
- Sueta, A.; Yamamoto, Y.; Tomiguchi, M.; Takeshita, T.; Yamamoto-Ibusuki, M.; Iwase, H. Differential expression of exosomal miRNAs between breast cancer patients with and without recurrence. Oncotarget 2017, 8, 69934–69944. [Google Scholar] [CrossRef] [Green Version]
- Novikova, S.; Shushkova, N.; Farafonova, T.; Tikhonova, O.; Kamyshinsky, R.; Zgoda, V. Proteomic Approach for Searching for Universal, Tissue-Specific, and Line-Specific Markers of Extracellular Vesicles in Lung and Colorectal Adenocarcinoma Cell Lines. Int. J. Mol. Sci. 2020, 21, 6601. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef] [Green Version]
- Véron, P.; Segura, E.; Sugano, G.; Amigorena, S.; Théry, C. Accumulation of MFG-E8/lactadherin on exosomes from immature dendritic cells. Blood Cells Mol. Dis. 2005, 35, 81–88. [Google Scholar] [CrossRef]
- Kenari, A.N.; Cheng, L.; Hill, A.F. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods 2020, 177, 103–113. [Google Scholar] [CrossRef]
- Miyasaka, K.; Hanayama, R.; Tanaka, M.; Nagata, S. Expression of milk fat globule epidermal growth factor?8 in immature dendritic cells for engulfment of apoptotic cells. Eur. J. Immunol. 2004, 34, 1414–1422. [Google Scholar] [CrossRef]
- Zeelenberg, I.S.; Ostrowski, M.; Krumeich, S.; Bobrie, A.; Jancic, C.; Boissonnas, A.; Delcayre, A.; Le Pecq, J.-B.; Combadière, B.; Amigorena, S.; et al. Targeting Tumor Antigens to Secreted Membrane Vesicles In vivo Induces Efficient Antitumor Immune Responses. Cancer Res. 2008, 68, 1228–1235. [Google Scholar] [CrossRef] [Green Version]
- Komuro, H.; Kawai-Harada, Y.; Aminova, S.; Pascual, N.; Malik, A.; Contag, C.H.; Harada, M. Engineering Extracellular Vesicles to Target Pancreatic Tissue In Vivo. Nanotheranostics 2021, 5, 378–390. [Google Scholar] [CrossRef]
- Wang, J.H.; Forterre, A.V.; Zhao, J.; Frimannsson, D.O.; Delcayre, A.; Antes, T.J.; Efron, B.; Jeffrey, S.S.; Pegram, M.D.; Matin, A.C. Anti-HER2 scFv-Directed Extracellular Vesicle-Mediated mRNA-Based Gene Delivery Inhibits Growth of HER2-Positive Human Breast Tumor Xenografts by Prodrug Activation. Mol. Cancer Ther. 2018, 17, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Kooijmans, S.A.A.; Gitz-Francois, J.J.J.M.; Schiffelers, R.M.; Vader, P. Recombinant phosphatidylserine-binding nanobodies for targeting of extracellular vesicles to tumor cells: A plug-and-play approach. Nanoscale 2018, 10, 2413–2426. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Liang, X.; Pavlova, S.; Wiklander, O.P.; Corso, G.; Zhao, Y.; Saher, O.; Bost, J.; Zickler, A.M.; Piffko, A.; et al. Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. J. Extracell. Vesicles 2020, 9, 1800222. [Google Scholar] [CrossRef]
- Ooishi, T.; Nadano, D.; Matsuda, T.; Oshima, K. Extracellular vesicle-mediated MFG-E8 localization in the extracellular matrix is required for its integrin-dependent function in mouse mammary epithelial cells. Genes Cells 2017, 22, 885–899. [Google Scholar] [CrossRef] [Green Version]
- Oshima, K.; Aoki, N.; Kato, T.; Kitajima, K.; Matsuda, T. Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. Eur. J. Biochem. 2002, 269, 1209–1218. [Google Scholar] [CrossRef]
- Campos, A.; Salomon, C.; Bustos, R.; Díaz, J.; Martínez, S.; Silva, V.; Reyes, C.; Díiaz-Valdivia, N.; Varas-Godoy, M.; Lobos-González, L.; et al. Caveolin-1-containing extracellular vesicles transport adhesion proteins and promote malignancy in breast cancer cell lines. Nanomedicine 2018, 13, 2597–2609. [Google Scholar] [CrossRef] [Green Version]
- Soki, F.N.; Koh, A.J.; Jones, J.D.; Kim, Y.W.; Dai, J.; Keller, E.T.; Pienta, K.J.; Atabai, K.; Roca, H.; McCauley, L.K. Polarization of Prostate Cancer-associated Macrophages Is Induced by Milk Fat Globule-EGF Factor 8 (MFG-E8)-mediated Efferocytosis. J. Biol. Chem. 2014, 289, 24560–24572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, D.J.; Schnaubelt, M.; Hoti, N.; Hu, Y.; Zhou, Y.; Gooya, M.; Zhang, H. Impact of Increased FUT8 Expression on the Extracellular Vesicle Proteome in Prostate Cancer Cells. J. Proteome Res. 2020, 19, 2195–2205. [Google Scholar] [CrossRef]
- Temoche-Diaz, M.M.; Shurtleff, M.J.; Nottingham, R.M.; Yao, J.; Fadadu, R.P.; Lambowitz, A.M.; Schekman, R. Distinct mechanisms of microRNA sorting into cancer cell-derived extracellular vesicle subtypes. eLife 2019, 8, 47544. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán-Jara, E.; Vera-Tobar, T.; Lobos-González, L.D.L. Lactadherin: From a Well-Known Breast Tumor Marker to a Possible Player in Extracellular Vesicle-Mediated Cancer Progression. Int. J. Mol. Sci. 2022, 23, 3855. https://doi.org/10.3390/ijms23073855
Durán-Jara E, Vera-Tobar T, Lobos-González LDL. Lactadherin: From a Well-Known Breast Tumor Marker to a Possible Player in Extracellular Vesicle-Mediated Cancer Progression. International Journal of Molecular Sciences. 2022; 23(7):3855. https://doi.org/10.3390/ijms23073855
Chicago/Turabian StyleDurán-Jara, Eduardo, Tamara Vera-Tobar, and Lorena De Lourdes Lobos-González. 2022. "Lactadherin: From a Well-Known Breast Tumor Marker to a Possible Player in Extracellular Vesicle-Mediated Cancer Progression" International Journal of Molecular Sciences 23, no. 7: 3855. https://doi.org/10.3390/ijms23073855
APA StyleDurán-Jara, E., Vera-Tobar, T., & Lobos-González, L. D. L. (2022). Lactadherin: From a Well-Known Breast Tumor Marker to a Possible Player in Extracellular Vesicle-Mediated Cancer Progression. International Journal of Molecular Sciences, 23(7), 3855. https://doi.org/10.3390/ijms23073855







