Enhancing Post-Stroke Rehabilitation and Preventing Exo-Focal Dopaminergic Degeneration in Rats—A Role for Substance P
Abstract
1. Introduction
2. Results
2.1. Intraperitoneal Substance P Application Facilitates Motor Rehabilitation after Stroke
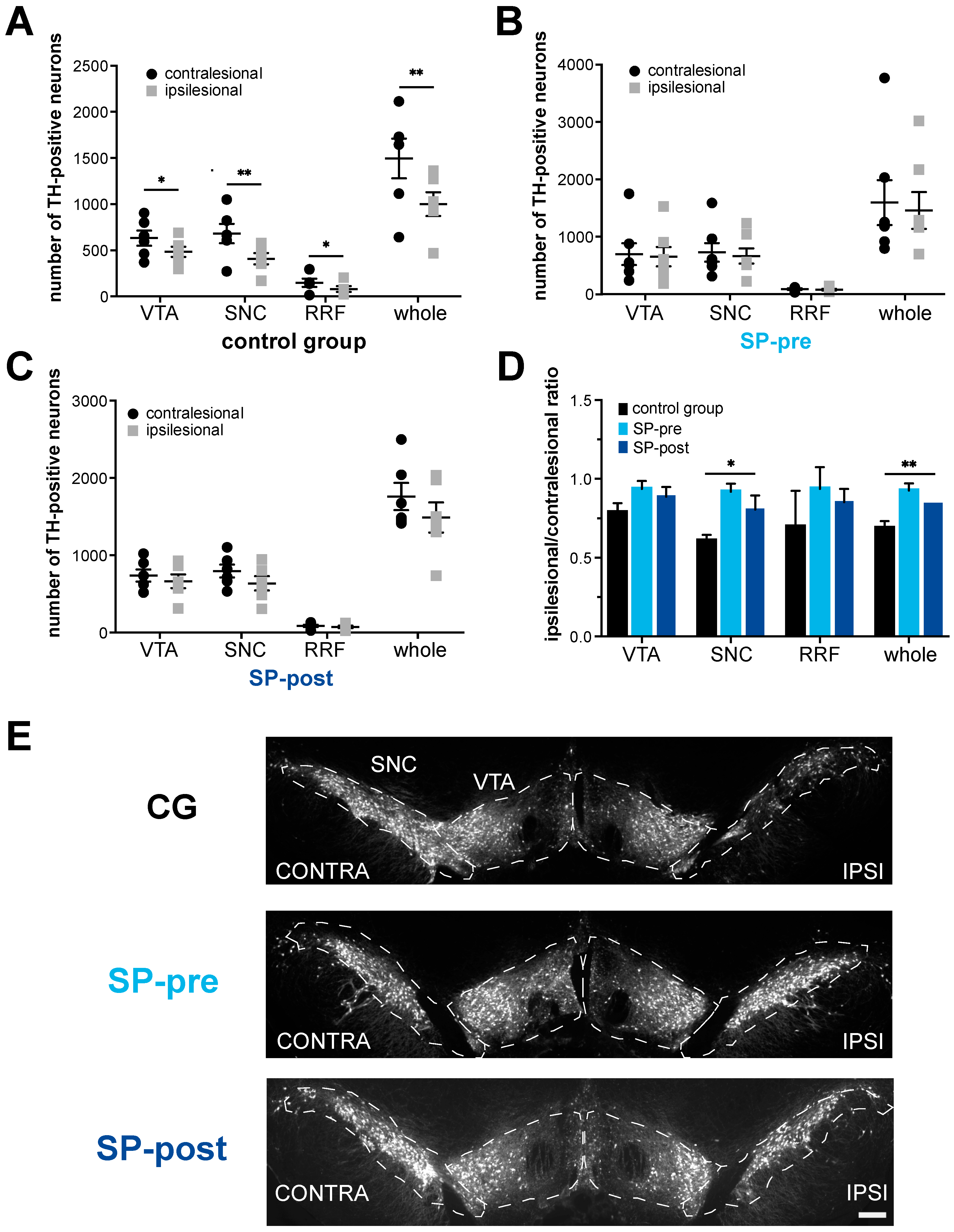
2.2. Dopaminergic Neurodegeneration after Stroke Is Attenuated by Substance P
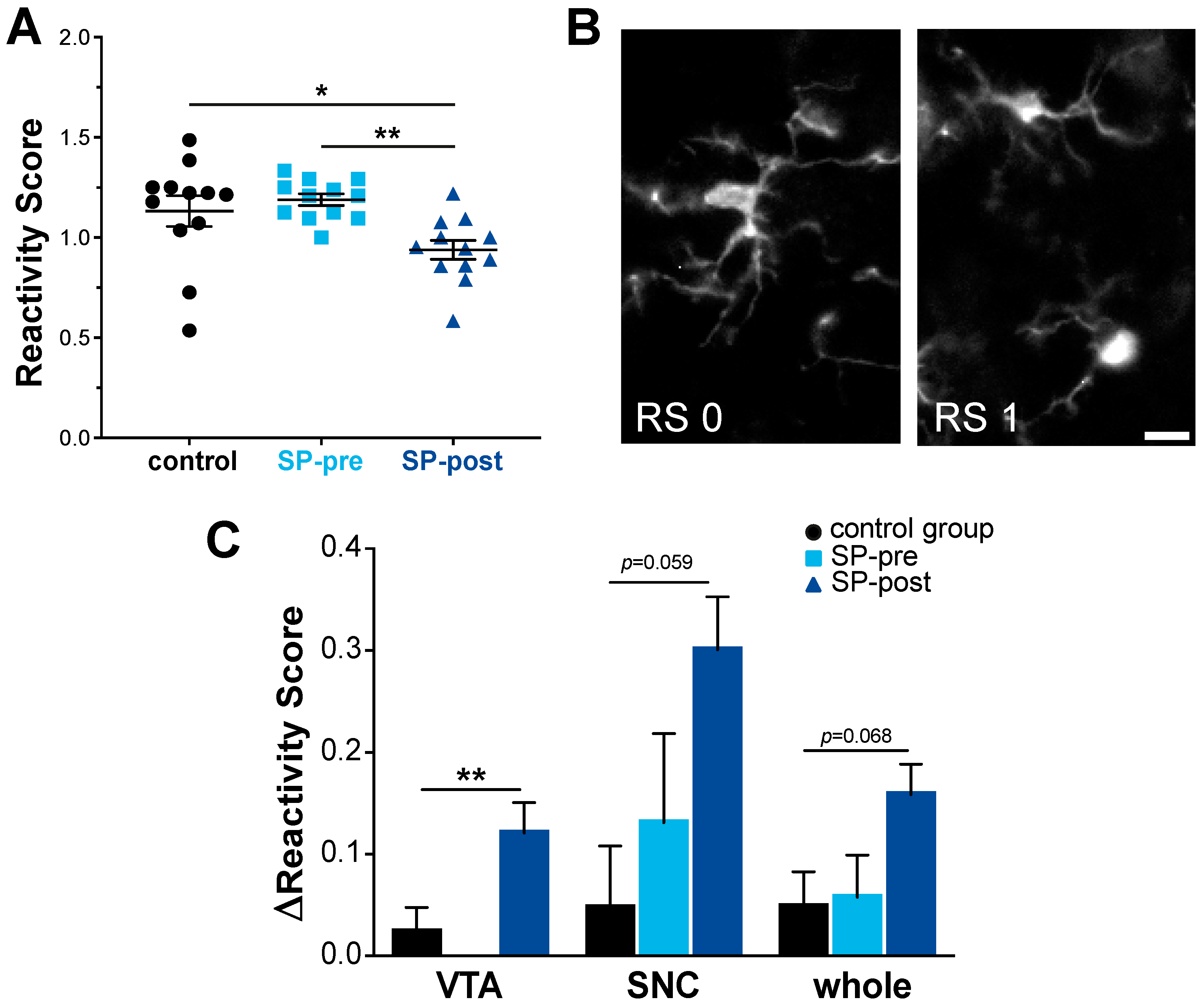
2.3. Microglial Activation in Midbrain Was Not Increased by Substance P
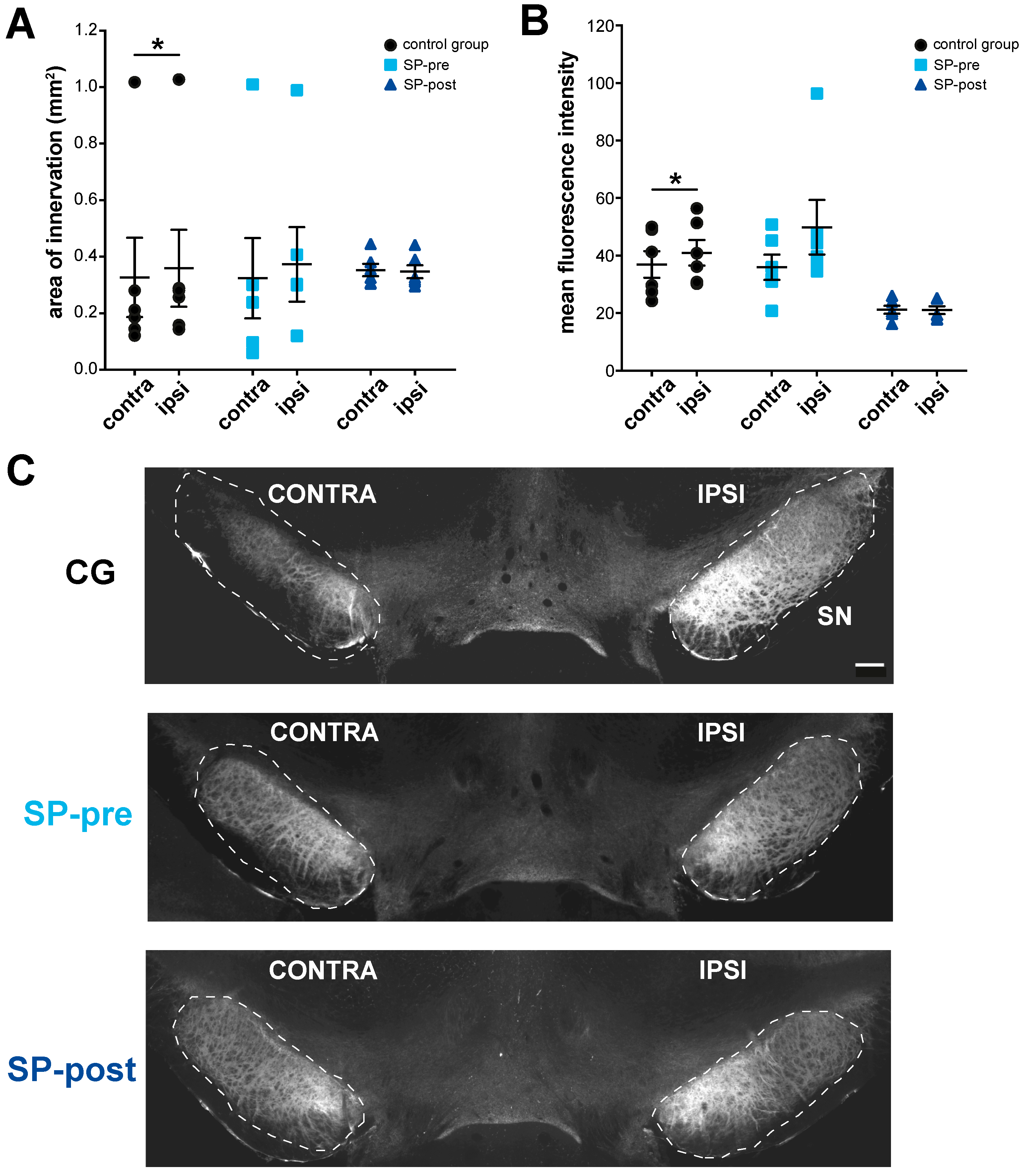
2.4. Substance P Prevents Hypertrophy of Endogenous SPergic Innervation after Stroke
3. Discussion
4. Materials and Methods
4.1. Animals and Experiments
4.2. Induction of Photothrombotic Motor Cortical Stroke
4.3. Behavioral Paradigm, Drug Application and Assessment of Post-Stroke Deficits
4.4. Euthanasia and Tissue Processing
4.5. Histochemistry and Immunohistochemistry
4.6. Histological Analysis
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeGirolami, U.; Crowell, R.M.; Marcoux, F.W. Selective Necrosis and Total Necrosis in Focal Cerebral Ischemia. Neuropathologic Observations on Experimental Middle Cerebral Artery Occlusion in the Macaque Monkey. J. Neuropathol. Exp. Neurol. 1984, 43, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Kogure, K. Exo-Focal Postischemic Neuronal Death in the Rat Brain. Brain Res. 1990, 524, 196–202. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Xing, S.; Liang, Z.; Zeng, J. Secondary Neurodegeneration in Remote Regions after Focal Cerebral Infarction: A New Target for Stroke Management? Stroke 2012, 43, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Yokoyama, H.; Kimoto, H.; Kato, H.; Araki, T. Long-Term Changes in the Ipsilateral Substantia Nigra after Transient Focal Cerebral Ischaemia in Rats. Int. J. Exp. Pathol. 2010, 91, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, G.; Balkaya, M.; Prinz, V.; Gertz, K.; Ji, S.; Kirste, I.; Heuser, I.; Kampmann, B.; Hellmann-Regen, J.; Gass, P.; et al. Exofocal Dopaminergic Degeneration as Antidepressant Target in Mouse Model of Poststroke Depression. Biol. Psychiatry 2012, 72, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Prinz, V.; Hetzer, A.-M.; Müller, S.; Balkaya, M.; Leithner, C.; Kronenberg, G.; Endres, M. MRI Heralds Secondary Nigral Lesion after Brain Ischemia in Mice: A Secondary Time Window for Neuroprotection. J. Cereb. Blood Flow Metab. 2015, 35, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Winter, B.; Brunecker, P.; Fiebach, J.B.; Jungehulsing, G.J.; Kronenberg, G.; Endres, M. Striatal Infarction Elicits Secondary Extrafocal MRI Changes in Ipsilateral Substantia Nigra. PLoS ONE 2015, 10, e0136483. [Google Scholar] [CrossRef] [PubMed]
- Forno, L.S. Reaction of the Substantia Nigra to Massive Basal Ganglia Infarction. Acta Neuropathol. 1983, 62, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Hosp, J.A.; Greiner, K.L.; Martinez Arellano, L.; Roth, F.; Löffler, F.; Reis, J.; Fritsch, B. Progressive Secondary Exo-Focal Dopaminergic Neurodegeneration Occurs in Not Directly Connected Midbrain Nuclei after Pure Motor-Cortical Stroke. Exp. Neurol. 2020, 327, 113211. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Jick, S.S.; Meier, C.R. Risk of Stroke in Patients with Idiopathic Parkinson Disease. Parkinsonism Relat. Disord. 2010, 16, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Floel, A.; Hummel, F.; Breitenstein, C.; Knecht, S.; Cohen, L.G. Dopaminergic Effects on Encoding of a Motor Memory in Chronic Stroke. Neurology 2005, 65, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Rösser, N.; Heuschmann, P.; Wersching, H.; Breitenstein, C.; Knecht, S.; Flöel, A. Levodopa Improves Procedural Motor Learning in Chronic Stroke Patients. Arch. Phys. Med. Rehabil. 2008, 89, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Hosp, J.A.; Luft, A.R. Dopaminergic Meso-Cortical Projections to M1: Role in Motor Learning and Motor Cortex Plasticity. Front. Neurol. 2013, 4, 145. [Google Scholar] [CrossRef] [PubMed]
- Hertler, B.; Buitrago, M.M.; Luft, A.R.; Hosp, J.A. Temporal Course of Gene Expression during Motor Memory Formation in Primary Motor Cortex of Rats. Neurobiol. Learn. Mem. 2016, 136, 105–115. [Google Scholar] [CrossRef][Green Version]
- Hosp, J.A.; Pekanovic, A.; Rioult-Pedotti, M.S.; Luft, A.R. Dopaminergic Projections from Midbrain to Primary Motor Cortex Mediate Motor Skill Learning. J. Neurosci. 2011, 31, 2481–2487. [Google Scholar] [CrossRef]
- Krishnan, R.V. Relearning toward Motor Recovery in Stroke, Spinal Cord Injury, and Cerebral Palsy: A Cognitive Neural Systems Perspective. Int. J. Neurosci. 2006, 116, 127–140. [Google Scholar] [CrossRef]
- Stinear, C.M. Dopamine for Motor Recovery after Stroke: Where to from Here? Lancet Neurol. 2019, 18, 514–515. [Google Scholar] [CrossRef]
- Scheidtmann, K.; Fries, W.; Müller, F.; Koenig, E. Effect of Levodopa in Combination with Physiotherapy on Functional Motor Recovery after Stroke: A Prospective, Randomised, Double-Blind Study. Lancet 2001, 358, 787–790. [Google Scholar] [CrossRef]
- Lokk, J.; Salman Roghani, R.; Delbari, A. Effect of Methylphenidate and/or Levodopa Coupled with Physiotherapy on Functional and Motor Recovery after Stroke—A Randomized, Double-Blind, Placebo-Controlled Trial. Acta Neurol. Scand. 2011, 123, 266–273. [Google Scholar] [CrossRef]
- Ford, G.A.; Bhakta, B.B.; Cozens, A.; Hartley, S.; Holloway, I.; Meads, D.; Pearn, J.; Ruddock, S.; Sackley, C.M.; Saloniki, E.-C.; et al. Safety and Efficacy of Co-Careldopa as an Add-on Therapy to Occupational and Physical Therapy in Patients after Stroke (DARS): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2019, 18, 530–538. [Google Scholar] [CrossRef]
- Pearson-Fuhrhop, K.M.; Minton, B.; Acevedo, D.; Shahbaba, B.; Cramer, S.C. Genetic Variation in the Human Brain Dopamine System Influences Motor Learning and Its Modulation by L-Dopa. PLoS ONE 2013, 8, e61197. [Google Scholar] [CrossRef]
- Sandweiss, A.J.; Vanderah, T.W. The Pharmacology of Neurokinin Receptors in Addiction: Prospects for Therapy. Subst. Abuse Rehabil. 2015, 6, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Calvo, N.; Reiriz, J.; Pérez-Navarro, E.; Alberch, J. Tachykinins Protect Cholinergic Neurons from Quinolinic Acid Excitotoxicity in Striatal Cultures. Brain Res. 1996, 740, 323–328. [Google Scholar] [CrossRef]
- Lallemend, F.; Lefebvre, P.P.; Hans, G.; Rigo, J.M.; Van de Water, T.R.; Moonen, G.; Malgrange, B. Substance P Protects Spiral Ganglion Neurons from Apoptosis via PKC-Ca2+-MAPK/ERK Pathways. J. Neurochem. 2003, 87, 508–521. [Google Scholar] [CrossRef]
- Salthun-Lassalle, B.; Traver, S.; Hirsch, E.C.; Michel, P.P. Substance P, Neurokinins A and B, and Synthetic Tachykinin Peptides Protect Mesencephalic Dopaminergic Neurons in Culture via an Activity-Dependent Mechanism. Mol. Pharmacol. 2005, 68, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Hasenöhrl, R.U.; Souza-Silva, M.A.; Nikolaus, S.; Tomaz, C.; Brandao, M.L.; Schwarting, R.K.; Huston, J.P. Substance P and Its Role in Neural Mechanisms Governing Learning, Anxiety and Functional Recovery. Neuropeptides 2000, 34, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Huston, J.P.; Hasenöhrl, R.U. The Role of Neuropeptides in Learning: Focus on the Neurokinin Substance P. Behav. Brain Res. 1995, 66, 117–127. [Google Scholar] [CrossRef]
- Barlow, R.B.; Franks, F.M.; Pearson, J.D. Studies on the Stereospecificity of Closely Related Compounds Which Block Postganglionic Acetylcholine Receptors in the Guinea-Pig Ileum. J. Med. Chem. 1973, 16, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Hertler, B.; Hosp, J.A.; Blanco, M.B.; Luft, A.R. Substance P Signalling in Primary Motor Cortex Facilitates Motor Learning in Rats. PLoS ONE 2017, 12, e0189812. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I.; Emson, P.C.; Cuello, A.C. Evidence for the Existence of Substance P-Containing Fibres in Striato-Nigral and Pallido-Nigral Pathways in Rat Brain. Brain Res. 1977, 119, 447–453. [Google Scholar] [CrossRef]
- Rodriguez-Grande, B.; Blackabey, V.; Gittens, B.; Pinteaux, E.; Denes, A. Loss of Substance P and Inflammation Precede Delayed Neurodegeneration in the Substantia Nigra after Cerebral Ischemia. Brain Behav. Immun. 2013, 29, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Pioro, E.P.; Hughes, J.T.; Cuello, A.C. Loss of Substance P and Enkephalin Immunoreactivity in the Human Substantia Nigra after Striato-Pallidal Infarction. Brain Res. 1984, 292, 339–347. [Google Scholar] [CrossRef]
- Corrigan, F.; Vink, R.; Turner, R.J. Inflammation in Acute CNS Injury: A Focus on the Role of Substance P. Br. J. Pharmacol. 2016, 173, 703–715. [Google Scholar] [CrossRef]
- Baloyannis, S.J.; Costa, V.; Deretzi, G.; Michmizos, D. Intraventricular Administration of Substance p Increases the Dendritic Arborisation and the Synaptic Surfaces of Purkinje Cells in Rat’s Cerebellum. Int. J. Neurosci. 2000, 101, 89–107. [Google Scholar] [CrossRef]
- Kato, N.; Yoshimura, H. Facilitatory Effects of Substance P on the Susceptibility to Long-Term Potentiation in the Visual Cortex of Adult Rats. Brain Res. 1993, 617, 353–356. [Google Scholar] [CrossRef]
- Langosch, J.M.; Kupferschmid, S.; Heinen, M.; Walden, J.; Herpfer, I.; Fiebich, B.L.; Lieb, K. Effects of Substance P and Its Antagonist L-733060 on Long Term Potentiation in Guinea Pig Hippocampal Slices. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.; Chahl, L.A. Localization of Tachykinin Receptors and Fos-like Immunoreactivity Induced by Substance P in Guinea-Pig Brain. Clin. Exp. Pharmacol. Physiol. 2000, 27, 943–946. [Google Scholar] [CrossRef]
- Mitsikostas, D.D.; Sanchez del Rio, M. Receptor Systems Mediating C-Fos Expression within Trigeminal Nucleus Caudalis in Animal Models of Migraine. Brain Res. Brain Res. Rev. 2001, 35, 20–35. [Google Scholar] [CrossRef]
- Kleim, J.A.; Lussnig, E.; Schwarz, E.R.; Comery, T.A.; Greenough, W.T. Synaptogenesis and Fos Expression in the Motor Cortex of the Adult Rat after Motor Skill Learning. J. Neurosci. 1996, 16, 4529–4535. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, C.; Zhang, B.; Zhang, Z.; Gao, B.; Liu, Y.; Wang, Y.; Hua, Y.; Hu, J.; Qiu, X.; et al. Constraint Induced Movement Therapy Promotes Contralesional-Oriented Structural and Bihemispheric Functional Neuroplasticity after Stroke. Brain Res. Bull. 2019, 150, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lieb, K.; Ahlvers, K.; Dancker, K.; Strohbusch, S.; Reincke, M.; Feige, B.; Berger, M.; Riemann, D.; Voderholzer, U. Effects of the Neuropeptide Substance P on Sleep, Mood, and Neuroendocrine Measures in Healthy Young Men. Neuropsychopharmacology 2002, 27, 1041–1049. [Google Scholar] [CrossRef]
- Leemburg, S.; Canonica, T.; Luft, A. Motor Skill Learning and Reward Consumption Differentially Affect VTA Activation. Sci. Rep. 2018, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Huston, J.P.; Körber, B.; Thiel, C.; Schwarting, R.K. Pretreatment with Neurokinin Substance P but Not with Cholecystokinin-8S Can Alleviate Functional Deficits of Partial Nigrostriatal 6-Hydroxydopamine Lesion. Peptides 1997, 18, 1161–1168. [Google Scholar] [CrossRef]
- Maeno, H.; Kiyama, H.; Tohyama, M. Distribution of the Substance P Receptor (NK-1 Receptor) in the Central Nervous System. Brain Res. Mol. Brain Res. 1993, 18, 43–58. [Google Scholar] [CrossRef]
- Mantyh, P.; Gates, T.; Mantyh, C.; Maggio, J. Autoradiographic Localization and Characterization of Tachykinin Receptor Binding Sites in the Rat Brain and Peripheral Tissues. J. Neurosci. 1989, 9, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.W.; Takahashi, M.; Yatsugi, S.; Shimizu-Sasamata, M.; Yamaguchi, T. Effects of YM872 on Atrophy of Substantia Nigra Reticulata after Focal Ischemia in Rats. Neuroreport 1998, 9, 3719–3724. [Google Scholar] [CrossRef]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The Role of Substance P in Inflammatory Disease. J. Cell Physiol 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Hökfelt, T.; Broberger, C.; Xu, Z.Q.; Sergeyev, V.; Ubink, R.; Diez, M. Neuropeptides—An Overview. Neuropharmacology 2000, 39, 1337–1356. [Google Scholar] [CrossRef]
- Turner, R.J.; Blumbergs, P.C.; Sims, N.R.; Helps, S.C.; Rodgers, K.M.; Vink, R. Increased Substance P Immunoreactivity and Edema Formation Following Reversible Ischemic Stroke. Acta Neurochir. Suppl. 2006, 96, 263–266. [Google Scholar] [CrossRef]
- Turner, R.; Vink, R. Inhibition of Neurogenic Inflammation as a Novel Treatment for Ischemic Stroke. Drug News Perspect. 2007, 20, 221–226. [Google Scholar] [CrossRef]
- Buitrago, M.M.; Ringer, T.; Schulz, J.B.; Dichgans, J.; Luft, A.R. Characterization of Motor Skill and Instrumental Learning Time Scales in a Skilled Reaching Task in Rat. Behav. Brain Res. 2004, 155, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Zhang, J.H.; Noble-Haeusslein, L.J. RIGOR Guidelines: Escalating STAIR and STEPS for Effective Translational Research. Transl. Stroke Res. 2013, 4, 279–285. [Google Scholar] [CrossRef]
- Whishaw, I.Q.; Pellis, S.M. The Structure of Skilled Forelimb Reaching in the Rat: A Proximally Driven Movement with a Single Distal Rotatory Component. Behav. Brain Res. 1990, 41, 49–59. [Google Scholar] [CrossRef]
- Hasenöhrl, R.U.; Frisch, C.; Nikolaus, S.; Huston, J.P. Chronic Administration of Neurokinin SP Improves Maze Performance in Aged Rattus Norvegicus. Behav. Neural Biol. 1994, 62, 110–120. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal Models of Traumatic Brain Injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier Science: Burlington, VT, USA, 2013; ISBN 978-0-12-415752-1. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- Margolis, E.B.; Coker, A.R.; Driscoll, J.R.; Lemaître, A.-I.; Fields, H.L. Reliability in the Identification of Midbrain Dopamine Neurons. PLoS ONE 2010, 5, e15222. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K. Localization of Monoamines in the Lower Brain Stem. Experientia 1964, 20, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Gellner, A.-K.; Reis, J.; Fritsch, B. Glia: A Neglected Player in Non-Invasive Direct Current Brain Stimulation. Front. Cell. Neurosci. 2016, 10, 188. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frase, S.; Löffler, F.; Hosp, J.A. Enhancing Post-Stroke Rehabilitation and Preventing Exo-Focal Dopaminergic Degeneration in Rats—A Role for Substance P. Int. J. Mol. Sci. 2022, 23, 3848. https://doi.org/10.3390/ijms23073848
Frase S, Löffler F, Hosp JA. Enhancing Post-Stroke Rehabilitation and Preventing Exo-Focal Dopaminergic Degeneration in Rats—A Role for Substance P. International Journal of Molecular Sciences. 2022; 23(7):3848. https://doi.org/10.3390/ijms23073848
Chicago/Turabian StyleFrase, Sibylle, Franziska Löffler, and Jonas A. Hosp. 2022. "Enhancing Post-Stroke Rehabilitation and Preventing Exo-Focal Dopaminergic Degeneration in Rats—A Role for Substance P" International Journal of Molecular Sciences 23, no. 7: 3848. https://doi.org/10.3390/ijms23073848
APA StyleFrase, S., Löffler, F., & Hosp, J. A. (2022). Enhancing Post-Stroke Rehabilitation and Preventing Exo-Focal Dopaminergic Degeneration in Rats—A Role for Substance P. International Journal of Molecular Sciences, 23(7), 3848. https://doi.org/10.3390/ijms23073848





